User login
Follow-up of Abnormal Metanephrine and Catecholamine Testing: Chasing Missed Neuroendocrine Tumors
From the Department of Medicine, Tufts Medical Center, Boston, MA.
Abstract
- Objective: To measure the frequency of missed pheochromocytoma test results and identify factors related to the risk of failed follow-up.
- Methods: We performed a retrospective review of the medical record to identify patients with abnormal urine or serum metanephrine or catecholamine test results over a 3-year period. We then searched the electronic medical record for documentation that the responsible physician was aware of the test results. We surveyed the physicians in cases where there were abnormal results and no documented follow-up to assess their awareness of the results and any follow-up actions they may have taken.
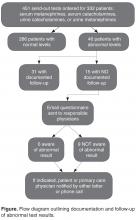
- Results: During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, or urine catecholamines and/or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately (n = 41) or critically elevated values (n = 5). Fifteen of these patients were inpatients when the tests were ordered, and 31 were outpatients. In 15 of 46 abnormal cases, there was no documentation in the electronic medical record that the responsible physician was aware of the result. Of the 15 cases without documentation, 6 of the responsible physicians in such cases were aware of the results.
- Conclusion: One-third of patients with abnormal lab testing for pheochromocytoma did not have clearly documented follow-up in the electronic medical record, and the majority of physicians in such cases were not aware of the results. Changes to the processes at health care institutions and reference laboratories are needed to improve follow-up of send-out lab results.
Delayed or missed follow-up of laboratory tests is a major source of medical harm [1–5]. Testing performed in both the inpatient and outpatient settings is susceptible to lost follow-up, in part because medical testing is a complex process that is vulnerable to multiple process-of-care failures [1,5–7]. In previous studies, the rate of missed follow-up of abnormal medical test results has ranged from 1% to 75% [6]. Laboratory test follow-up is a particularly challenging problem as patients transition between care settings [8,9]. In a study of 86 patients at one academic medical center, Moore and colleagues found that over a 1-year period, 41% of patients who had laboratory tests pending at the time of discharge had no documented follow-up for at least one of those tests [9]. More recently, Roy and colleagues reported that nearly half of 2644 patients discharged from general medicine hospitalist services at 2 academic tertiary care centers had pending laboratory or radiographic results. Nine percent of the pending results were potentially actionable, and a follow-up survey from the study revealed that 61% of physicians were unaware of pending results [10]. Similar findings have been reported in ambulatory care [5,8,11].
Among the universe of laboratory tests, tests performed at reference laboratories outside of the hospital or clinic where care is rendered (ie, “send-out” tests) are particularly susceptible to lost follow-up [12,13]. Because many of these tests are expensive and infrequently ordered, it is most feasible and economical for hospitals and clinics to transport these samples to regional or national laboratories for specialized testing [14,15]. Examples include the serotonin release assay, certain rheumatologic studies, cancer genetics, and advanced endocrine testing. Send-out testing poses several potential risks including accidental ordering of the wrong test, processing or transportation delays, failure of the outside laboratory to receive the specimen, failures of results reporting by the reference laboratory, incorrect result entry into the electronic medical record upon receipt, failure of the clinician to receive or note the result, or failure of clinician to interpret or act on the result [12,13,15]. Although previous studies have identified risk factors associated with missed abnormal test results [1], none to our knowledge have assessed the particular risks associated with samples processed at reference laboratories.
A critical event at our hospital involved a young woman who presented with respiratory failure attributed to a community-acquired pneumonia and systolic congestive heart failure that was thought to be related to her acute illness. Serum and urine metanephrines were ordered in the intensive care unit given the possibility that heart failure in a young patient could be attributed to an occult neuroendocrine tumor. The patient improved clinically and was discharged. Because the discharging service was unaware that the metanephrine tests had been ordered and were being processed at a national reference laboratory, they did not follow up on the test result or include it as pending in the discharge summary. Fortunately, the patient’s primary care physician discovered that the metanephrine levels were elevated and referred the patient for endocrine evaluation and definitive treatment.
Given the risk represented by pending send-out tests raised by this episode, we performed a retrospective study to identify other cases of missed abnormal send-out tests for metanephrines and catecholamines for in- and outpatients over the previous 3 years. We also sought to identify factors that increased the risk of failed follow-up.
Methods
Subjects and Setting
We studied adult in- and outpatients who received care at a 415-bed Boston-based academic medical center.
Project Design and Data Collection
We performed a retrospective record review of a cohort of patients with abnormal send-out laboratory tests for metanephrines and catecholamines. We collected laboratory reports of all results of urine and serum metanephrine and catecholamine tests performed from 1 January 2012 through 31 December 2014. All tests were performed at and reported by Quest Diagnostics in Chantilly, Virginia. The relevant tests were identified using a query of the online Quest Diagnostics system to extract all laboratory results for serum metanephrines, serum catecholamines, urine metanephrines, and urine catecholamines that resulted during this period. Reports were PDF files that were printed and reviewed manually. (Of note, providers typically view lab results directly in the electronic medical record. Reports were extracted from the Quest Diagnostics system for study purposes only.)
We used the reference ranges supplied by the laboratory to sort results into: normal levels, moderately elevated levels (1 to 4 times the upper limit of normal), and critically elevated levels (greater than 4 times the upper limit of normal). A physician (RZ) then reviewed the electronic medical record of each patient with moderately or critically elevated results for evidence that the responsible physician was aware of the results and had documented a follow-up plan. Documentation of physician awareness and follow-up was ascertained by notation and interpretation of the test result in either a discharge summary from the index admission or in an outpatient clinic note. The responsible physician was defined as the ordering physician for tests ordered in ambulatory care and the attending physician at time of discharge for inpatients. In cases where no documentation was identified in the medical record, the responsible physicians received an email questionnaire that asked (1) if they were aware of the abnormal result, (2) if aware of the result, did they notify the primary care physician or referring physician, and (3) if they were aware of any further follow-up or intervention.
Analysis
We stratified the cases into those with normal and abnormal labs values, and then further by those that did and did not have documentation of results and follow-up in the medical record. We then further stratified cases into those in which the responsible physician was aware and those in which they were unaware. If unaware, the patient was contacted directly by the risk management department, primarily for patient safety purposes. If we were unable to contact the patient, the patient’s listed primary care physician was contacted directly. We then performed qualitative analysis of the cases with abnormal results and no documented follow-up, with the goal of identifying common themes.
Results
During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, urine catecholamines, or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately or critically elevated values, while 396 results affecting 286 patients returned within the reference range. Five patients had critically elevated values and 41 patients had moderately elevated values. Fifteen were inpatients when the tests were ordered and 31 were outpatients.
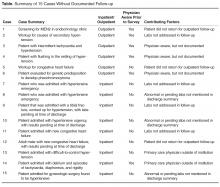
In the survey of the responsible physicians in the 15 cases with no follow-up, all 15 physicians responded. Six were aware of the abnormal result and 9 were not (Figure). Five of the 6 cases in which the physician was aware were outpatients. Eight of the 9 cases in which the physician was not aware were inpatients. In 4 of 15 abnormal cases with no follow-up, the patient was seen at a follow-up appointment but the lab results were not addressed. In 3 of 15 abnormal cases with no follow-up, the patient did not return for a planned follow-up appointment. In 3 of 15 abnormal cases with no follow-up, the physician was aware and addressed the results, but did not document that the results were addressed (all 3 were outpatient cases). In 3 of 15 abnormal cases with no follow-up, lab results for inpatients were pending at time of discharge and there was no documentation of pending results in the designated space for this in the discharge summary. In 2 of 15 abnormal cases with no follow-up, the patient was followed by a primary care physician outside of our institution. In 7 cases, the patient had multiple subspecialists involved in their care. All undocumented abnormal levels were addressed by our institution, either by contacting the patient or primary care physician, or by determining that the abnormality was not clinically relevant.
Discussion
We identified cases in which patients had abnormal results on tests used to diagnose neuroendocrine tumors such as pheochromocytoma over a 3-year period and sought evidence that a responsible clinician had followed up on the abnormal results. In one-third of abnormal test results, we found no documentation in the medical record that the responsible clinician was aware of the result or had communicated it to another clinician or the patient. This occurred most often in cases in which metanephrine and/or catecholamine levels were pending at the time of hospital discharge, and when a patient who was discharged from the hospital or seen in clinic did not return for a scheduled follow-up appointment. When we followed up with the responsible physician, only 6 in 15 were aware of the abnormal results and had either concluded that they were not clinically significant or had addressed the issue without completing documentation.
Previous research has identified vulnerabilities in the follow-up of send-out test results that exceed the challenges with tests performed in-house. These include that send-out tests inherently have more steps and require more manual processes [8], and that these tests are more prone to delay, misinterpretation, and poor documentation. Reference laboratories usually provide non-structured reporting of results, often in the form of paper or PDF files. This can make it difficult for receiving hospitals or clinics to incorporate information into the electronic medical record or to build clinical reminders or alerts for ordering clinicians. Additionally, these data elements are often cryptic in that they provide reference values without necessarily setting parameters for abnormalities. This is a case in point with metanephrine and catecholamine testing, as the results are often variable and poorly reproducible and difficult for clinicians to interpret. There are different cutoffs for moderately elevated and critically elevated values, and how to proceed with patients with moderately elevated values is not clear and may require the expertise of subspecialists. Our study confirmed several issues surrounding vulnerabilities of send-out lab testing.
As a single-institution project with a small cohort of subjects, the generalizability of this project may be limited. However, some process-of-care vulnerabilities noted here are similar to those reported in previous research studies [8]. In addition, hospitals and clinics send specimens to a limited number of regional and national reference laboratories. The challenges that our clinicians encountered in managing these results are likely to be challenges in many other organizations. Also, while our study was limited to tests done to evaluate for pheochromocytoma, our findings are likely applicable to other reference laboratory tests.
Send-out labs continue to represent a major source of lost follow-up and potential patient harm. Creating systems with effective and timely alerts for providers will be useful in preventing missed follow-up. Our study found a lack of clear guidelines designating responsibility for pending lab results, which has been found across institutions in previous studies [8]. Since we conducted this project, our institution has reminded clinicians that discharging attendings are responsible for pending lab results at time of discharge and has developed an automated electronic method for delivering these results. Similar policy interventions at other institutions have shown promise [16]. We hope this will minimize the number of lab results, including those of send-out labs, which are not acted upon in a timely manner. However, other issues, including data interface with the electronic medical record and patients with abnormal results being lost to follow-up, remain barriers for our institution to address.
There are several immediate steps that could be taken by health care organizations and reference labs to reduce patient harm as a result of send-out labs that are not followed up. First, health care organizations can develop better integration between electronic records and lab processing for send-out labs, as well as more electronic alerts. This may help to notify ordering physicians after patients have been discharged and the case may not be front of mind. Reference labs should create robust electronic systems to transmit results as electronic data elements so that health care organizations can easily incorporate results into their electronic medical records, and develop notification systems that flag out-of-bound values. Secure online lab results for send-outs may shorten the delay in reporting. Additionally, creating clear policies establishing the responsible provider is crucial, as has been found by previous research by Singh and others [11,15].
In conclusion, send-out labs are vulnerable to lost follow-up. It is crucial for clinicians to be aware of all send-out lab results and to document their interpretation of abnormal results. Developing policies and systems to facilitate timely follow-up will help to reduce potential patient harm related to send-out labs.
Corresponding author: Richard Zamore, MD, MPH, Tufts Medical Center, 800 Washington St., Boston, MA 02111, rzamore@tuftsmedicalcenter.org.
Financial disclosures: None.
1. Callen J, Georgiou A, Li J, Westbrook JI, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194–9.
2. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32.
3. Bates DW, Leape LL. Doing better with critical test results. Jt Comm J Qual Patient Saf 2005;31:66–7.
4. Schiff GD, Kim S, Krosnjar N, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165:574.
5. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med 2010;123:238–44.
6. Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200.
7. Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med 2009;169:1123–9.
8. Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334–48.
9. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51.
10. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121–8.
11. Singh H, Wilson L, Reis B, et al. Ten strategies to improve management of abnormal test result alerts in the electronic health record. J Patient Saf 2010;6:121–3.
12. Dickerson JA, Cole B, Astion ML. Ten ways to improve the quality of send-out testing. Clin Lab News 2012;38:12–3.
13. Cole B, Dickerson JA, Graber ML, et al. A prospective tool for risk assessment of sendout testing. Clin Chim Acta 2014;434:1–5.
14. MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18:216–9.
15. Krasowski MD, Chudzik D, Dolezal A, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak 2015;15:11.
16. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66.
From the Department of Medicine, Tufts Medical Center, Boston, MA.
Abstract
- Objective: To measure the frequency of missed pheochromocytoma test results and identify factors related to the risk of failed follow-up.
- Methods: We performed a retrospective review of the medical record to identify patients with abnormal urine or serum metanephrine or catecholamine test results over a 3-year period. We then searched the electronic medical record for documentation that the responsible physician was aware of the test results. We surveyed the physicians in cases where there were abnormal results and no documented follow-up to assess their awareness of the results and any follow-up actions they may have taken.
- Results: During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, or urine catecholamines and/or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately (n = 41) or critically elevated values (n = 5). Fifteen of these patients were inpatients when the tests were ordered, and 31 were outpatients. In 15 of 46 abnormal cases, there was no documentation in the electronic medical record that the responsible physician was aware of the result. Of the 15 cases without documentation, 6 of the responsible physicians in such cases were aware of the results.
- Conclusion: One-third of patients with abnormal lab testing for pheochromocytoma did not have clearly documented follow-up in the electronic medical record, and the majority of physicians in such cases were not aware of the results. Changes to the processes at health care institutions and reference laboratories are needed to improve follow-up of send-out lab results.
Delayed or missed follow-up of laboratory tests is a major source of medical harm [1–5]. Testing performed in both the inpatient and outpatient settings is susceptible to lost follow-up, in part because medical testing is a complex process that is vulnerable to multiple process-of-care failures [1,5–7]. In previous studies, the rate of missed follow-up of abnormal medical test results has ranged from 1% to 75% [6]. Laboratory test follow-up is a particularly challenging problem as patients transition between care settings [8,9]. In a study of 86 patients at one academic medical center, Moore and colleagues found that over a 1-year period, 41% of patients who had laboratory tests pending at the time of discharge had no documented follow-up for at least one of those tests [9]. More recently, Roy and colleagues reported that nearly half of 2644 patients discharged from general medicine hospitalist services at 2 academic tertiary care centers had pending laboratory or radiographic results. Nine percent of the pending results were potentially actionable, and a follow-up survey from the study revealed that 61% of physicians were unaware of pending results [10]. Similar findings have been reported in ambulatory care [5,8,11].
Among the universe of laboratory tests, tests performed at reference laboratories outside of the hospital or clinic where care is rendered (ie, “send-out” tests) are particularly susceptible to lost follow-up [12,13]. Because many of these tests are expensive and infrequently ordered, it is most feasible and economical for hospitals and clinics to transport these samples to regional or national laboratories for specialized testing [14,15]. Examples include the serotonin release assay, certain rheumatologic studies, cancer genetics, and advanced endocrine testing. Send-out testing poses several potential risks including accidental ordering of the wrong test, processing or transportation delays, failure of the outside laboratory to receive the specimen, failures of results reporting by the reference laboratory, incorrect result entry into the electronic medical record upon receipt, failure of the clinician to receive or note the result, or failure of clinician to interpret or act on the result [12,13,15]. Although previous studies have identified risk factors associated with missed abnormal test results [1], none to our knowledge have assessed the particular risks associated with samples processed at reference laboratories.
A critical event at our hospital involved a young woman who presented with respiratory failure attributed to a community-acquired pneumonia and systolic congestive heart failure that was thought to be related to her acute illness. Serum and urine metanephrines were ordered in the intensive care unit given the possibility that heart failure in a young patient could be attributed to an occult neuroendocrine tumor. The patient improved clinically and was discharged. Because the discharging service was unaware that the metanephrine tests had been ordered and were being processed at a national reference laboratory, they did not follow up on the test result or include it as pending in the discharge summary. Fortunately, the patient’s primary care physician discovered that the metanephrine levels were elevated and referred the patient for endocrine evaluation and definitive treatment.
Given the risk represented by pending send-out tests raised by this episode, we performed a retrospective study to identify other cases of missed abnormal send-out tests for metanephrines and catecholamines for in- and outpatients over the previous 3 years. We also sought to identify factors that increased the risk of failed follow-up.
Methods
Subjects and Setting
We studied adult in- and outpatients who received care at a 415-bed Boston-based academic medical center.
Project Design and Data Collection
We performed a retrospective record review of a cohort of patients with abnormal send-out laboratory tests for metanephrines and catecholamines. We collected laboratory reports of all results of urine and serum metanephrine and catecholamine tests performed from 1 January 2012 through 31 December 2014. All tests were performed at and reported by Quest Diagnostics in Chantilly, Virginia. The relevant tests were identified using a query of the online Quest Diagnostics system to extract all laboratory results for serum metanephrines, serum catecholamines, urine metanephrines, and urine catecholamines that resulted during this period. Reports were PDF files that were printed and reviewed manually. (Of note, providers typically view lab results directly in the electronic medical record. Reports were extracted from the Quest Diagnostics system for study purposes only.)
We used the reference ranges supplied by the laboratory to sort results into: normal levels, moderately elevated levels (1 to 4 times the upper limit of normal), and critically elevated levels (greater than 4 times the upper limit of normal). A physician (RZ) then reviewed the electronic medical record of each patient with moderately or critically elevated results for evidence that the responsible physician was aware of the results and had documented a follow-up plan. Documentation of physician awareness and follow-up was ascertained by notation and interpretation of the test result in either a discharge summary from the index admission or in an outpatient clinic note. The responsible physician was defined as the ordering physician for tests ordered in ambulatory care and the attending physician at time of discharge for inpatients. In cases where no documentation was identified in the medical record, the responsible physicians received an email questionnaire that asked (1) if they were aware of the abnormal result, (2) if aware of the result, did they notify the primary care physician or referring physician, and (3) if they were aware of any further follow-up or intervention.
Analysis
We stratified the cases into those with normal and abnormal labs values, and then further by those that did and did not have documentation of results and follow-up in the medical record. We then further stratified cases into those in which the responsible physician was aware and those in which they were unaware. If unaware, the patient was contacted directly by the risk management department, primarily for patient safety purposes. If we were unable to contact the patient, the patient’s listed primary care physician was contacted directly. We then performed qualitative analysis of the cases with abnormal results and no documented follow-up, with the goal of identifying common themes.
Results
During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, urine catecholamines, or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately or critically elevated values, while 396 results affecting 286 patients returned within the reference range. Five patients had critically elevated values and 41 patients had moderately elevated values. Fifteen were inpatients when the tests were ordered and 31 were outpatients.
In the survey of the responsible physicians in the 15 cases with no follow-up, all 15 physicians responded. Six were aware of the abnormal result and 9 were not (Figure). Five of the 6 cases in which the physician was aware were outpatients. Eight of the 9 cases in which the physician was not aware were inpatients. In 4 of 15 abnormal cases with no follow-up, the patient was seen at a follow-up appointment but the lab results were not addressed. In 3 of 15 abnormal cases with no follow-up, the patient did not return for a planned follow-up appointment. In 3 of 15 abnormal cases with no follow-up, the physician was aware and addressed the results, but did not document that the results were addressed (all 3 were outpatient cases). In 3 of 15 abnormal cases with no follow-up, lab results for inpatients were pending at time of discharge and there was no documentation of pending results in the designated space for this in the discharge summary. In 2 of 15 abnormal cases with no follow-up, the patient was followed by a primary care physician outside of our institution. In 7 cases, the patient had multiple subspecialists involved in their care. All undocumented abnormal levels were addressed by our institution, either by contacting the patient or primary care physician, or by determining that the abnormality was not clinically relevant.
Discussion
We identified cases in which patients had abnormal results on tests used to diagnose neuroendocrine tumors such as pheochromocytoma over a 3-year period and sought evidence that a responsible clinician had followed up on the abnormal results. In one-third of abnormal test results, we found no documentation in the medical record that the responsible clinician was aware of the result or had communicated it to another clinician or the patient. This occurred most often in cases in which metanephrine and/or catecholamine levels were pending at the time of hospital discharge, and when a patient who was discharged from the hospital or seen in clinic did not return for a scheduled follow-up appointment. When we followed up with the responsible physician, only 6 in 15 were aware of the abnormal results and had either concluded that they were not clinically significant or had addressed the issue without completing documentation.
Previous research has identified vulnerabilities in the follow-up of send-out test results that exceed the challenges with tests performed in-house. These include that send-out tests inherently have more steps and require more manual processes [8], and that these tests are more prone to delay, misinterpretation, and poor documentation. Reference laboratories usually provide non-structured reporting of results, often in the form of paper or PDF files. This can make it difficult for receiving hospitals or clinics to incorporate information into the electronic medical record or to build clinical reminders or alerts for ordering clinicians. Additionally, these data elements are often cryptic in that they provide reference values without necessarily setting parameters for abnormalities. This is a case in point with metanephrine and catecholamine testing, as the results are often variable and poorly reproducible and difficult for clinicians to interpret. There are different cutoffs for moderately elevated and critically elevated values, and how to proceed with patients with moderately elevated values is not clear and may require the expertise of subspecialists. Our study confirmed several issues surrounding vulnerabilities of send-out lab testing.
As a single-institution project with a small cohort of subjects, the generalizability of this project may be limited. However, some process-of-care vulnerabilities noted here are similar to those reported in previous research studies [8]. In addition, hospitals and clinics send specimens to a limited number of regional and national reference laboratories. The challenges that our clinicians encountered in managing these results are likely to be challenges in many other organizations. Also, while our study was limited to tests done to evaluate for pheochromocytoma, our findings are likely applicable to other reference laboratory tests.
Send-out labs continue to represent a major source of lost follow-up and potential patient harm. Creating systems with effective and timely alerts for providers will be useful in preventing missed follow-up. Our study found a lack of clear guidelines designating responsibility for pending lab results, which has been found across institutions in previous studies [8]. Since we conducted this project, our institution has reminded clinicians that discharging attendings are responsible for pending lab results at time of discharge and has developed an automated electronic method for delivering these results. Similar policy interventions at other institutions have shown promise [16]. We hope this will minimize the number of lab results, including those of send-out labs, which are not acted upon in a timely manner. However, other issues, including data interface with the electronic medical record and patients with abnormal results being lost to follow-up, remain barriers for our institution to address.
There are several immediate steps that could be taken by health care organizations and reference labs to reduce patient harm as a result of send-out labs that are not followed up. First, health care organizations can develop better integration between electronic records and lab processing for send-out labs, as well as more electronic alerts. This may help to notify ordering physicians after patients have been discharged and the case may not be front of mind. Reference labs should create robust electronic systems to transmit results as electronic data elements so that health care organizations can easily incorporate results into their electronic medical records, and develop notification systems that flag out-of-bound values. Secure online lab results for send-outs may shorten the delay in reporting. Additionally, creating clear policies establishing the responsible provider is crucial, as has been found by previous research by Singh and others [11,15].
In conclusion, send-out labs are vulnerable to lost follow-up. It is crucial for clinicians to be aware of all send-out lab results and to document their interpretation of abnormal results. Developing policies and systems to facilitate timely follow-up will help to reduce potential patient harm related to send-out labs.
Corresponding author: Richard Zamore, MD, MPH, Tufts Medical Center, 800 Washington St., Boston, MA 02111, rzamore@tuftsmedicalcenter.org.
Financial disclosures: None.
From the Department of Medicine, Tufts Medical Center, Boston, MA.
Abstract
- Objective: To measure the frequency of missed pheochromocytoma test results and identify factors related to the risk of failed follow-up.
- Methods: We performed a retrospective review of the medical record to identify patients with abnormal urine or serum metanephrine or catecholamine test results over a 3-year period. We then searched the electronic medical record for documentation that the responsible physician was aware of the test results. We surveyed the physicians in cases where there were abnormal results and no documented follow-up to assess their awareness of the results and any follow-up actions they may have taken.
- Results: During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, or urine catecholamines and/or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately (n = 41) or critically elevated values (n = 5). Fifteen of these patients were inpatients when the tests were ordered, and 31 were outpatients. In 15 of 46 abnormal cases, there was no documentation in the electronic medical record that the responsible physician was aware of the result. Of the 15 cases without documentation, 6 of the responsible physicians in such cases were aware of the results.
- Conclusion: One-third of patients with abnormal lab testing for pheochromocytoma did not have clearly documented follow-up in the electronic medical record, and the majority of physicians in such cases were not aware of the results. Changes to the processes at health care institutions and reference laboratories are needed to improve follow-up of send-out lab results.
Delayed or missed follow-up of laboratory tests is a major source of medical harm [1–5]. Testing performed in both the inpatient and outpatient settings is susceptible to lost follow-up, in part because medical testing is a complex process that is vulnerable to multiple process-of-care failures [1,5–7]. In previous studies, the rate of missed follow-up of abnormal medical test results has ranged from 1% to 75% [6]. Laboratory test follow-up is a particularly challenging problem as patients transition between care settings [8,9]. In a study of 86 patients at one academic medical center, Moore and colleagues found that over a 1-year period, 41% of patients who had laboratory tests pending at the time of discharge had no documented follow-up for at least one of those tests [9]. More recently, Roy and colleagues reported that nearly half of 2644 patients discharged from general medicine hospitalist services at 2 academic tertiary care centers had pending laboratory or radiographic results. Nine percent of the pending results were potentially actionable, and a follow-up survey from the study revealed that 61% of physicians were unaware of pending results [10]. Similar findings have been reported in ambulatory care [5,8,11].
Among the universe of laboratory tests, tests performed at reference laboratories outside of the hospital or clinic where care is rendered (ie, “send-out” tests) are particularly susceptible to lost follow-up [12,13]. Because many of these tests are expensive and infrequently ordered, it is most feasible and economical for hospitals and clinics to transport these samples to regional or national laboratories for specialized testing [14,15]. Examples include the serotonin release assay, certain rheumatologic studies, cancer genetics, and advanced endocrine testing. Send-out testing poses several potential risks including accidental ordering of the wrong test, processing or transportation delays, failure of the outside laboratory to receive the specimen, failures of results reporting by the reference laboratory, incorrect result entry into the electronic medical record upon receipt, failure of the clinician to receive or note the result, or failure of clinician to interpret or act on the result [12,13,15]. Although previous studies have identified risk factors associated with missed abnormal test results [1], none to our knowledge have assessed the particular risks associated with samples processed at reference laboratories.
A critical event at our hospital involved a young woman who presented with respiratory failure attributed to a community-acquired pneumonia and systolic congestive heart failure that was thought to be related to her acute illness. Serum and urine metanephrines were ordered in the intensive care unit given the possibility that heart failure in a young patient could be attributed to an occult neuroendocrine tumor. The patient improved clinically and was discharged. Because the discharging service was unaware that the metanephrine tests had been ordered and were being processed at a national reference laboratory, they did not follow up on the test result or include it as pending in the discharge summary. Fortunately, the patient’s primary care physician discovered that the metanephrine levels were elevated and referred the patient for endocrine evaluation and definitive treatment.
Given the risk represented by pending send-out tests raised by this episode, we performed a retrospective study to identify other cases of missed abnormal send-out tests for metanephrines and catecholamines for in- and outpatients over the previous 3 years. We also sought to identify factors that increased the risk of failed follow-up.
Methods
Subjects and Setting
We studied adult in- and outpatients who received care at a 415-bed Boston-based academic medical center.
Project Design and Data Collection
We performed a retrospective record review of a cohort of patients with abnormal send-out laboratory tests for metanephrines and catecholamines. We collected laboratory reports of all results of urine and serum metanephrine and catecholamine tests performed from 1 January 2012 through 31 December 2014. All tests were performed at and reported by Quest Diagnostics in Chantilly, Virginia. The relevant tests were identified using a query of the online Quest Diagnostics system to extract all laboratory results for serum metanephrines, serum catecholamines, urine metanephrines, and urine catecholamines that resulted during this period. Reports were PDF files that were printed and reviewed manually. (Of note, providers typically view lab results directly in the electronic medical record. Reports were extracted from the Quest Diagnostics system for study purposes only.)
We used the reference ranges supplied by the laboratory to sort results into: normal levels, moderately elevated levels (1 to 4 times the upper limit of normal), and critically elevated levels (greater than 4 times the upper limit of normal). A physician (RZ) then reviewed the electronic medical record of each patient with moderately or critically elevated results for evidence that the responsible physician was aware of the results and had documented a follow-up plan. Documentation of physician awareness and follow-up was ascertained by notation and interpretation of the test result in either a discharge summary from the index admission or in an outpatient clinic note. The responsible physician was defined as the ordering physician for tests ordered in ambulatory care and the attending physician at time of discharge for inpatients. In cases where no documentation was identified in the medical record, the responsible physicians received an email questionnaire that asked (1) if they were aware of the abnormal result, (2) if aware of the result, did they notify the primary care physician or referring physician, and (3) if they were aware of any further follow-up or intervention.
Analysis
We stratified the cases into those with normal and abnormal labs values, and then further by those that did and did not have documentation of results and follow-up in the medical record. We then further stratified cases into those in which the responsible physician was aware and those in which they were unaware. If unaware, the patient was contacted directly by the risk management department, primarily for patient safety purposes. If we were unable to contact the patient, the patient’s listed primary care physician was contacted directly. We then performed qualitative analysis of the cases with abnormal results and no documented follow-up, with the goal of identifying common themes.
Results
During the 3-year look-back period, 451 send-out tests for 332 patients were ordered for serum metanephrines, serum catecholamines, urine catecholamines, or metanephrines. Fifty-five tests affecting 46 patients returned with either moderately or critically elevated values, while 396 results affecting 286 patients returned within the reference range. Five patients had critically elevated values and 41 patients had moderately elevated values. Fifteen were inpatients when the tests were ordered and 31 were outpatients.
In the survey of the responsible physicians in the 15 cases with no follow-up, all 15 physicians responded. Six were aware of the abnormal result and 9 were not (Figure). Five of the 6 cases in which the physician was aware were outpatients. Eight of the 9 cases in which the physician was not aware were inpatients. In 4 of 15 abnormal cases with no follow-up, the patient was seen at a follow-up appointment but the lab results were not addressed. In 3 of 15 abnormal cases with no follow-up, the patient did not return for a planned follow-up appointment. In 3 of 15 abnormal cases with no follow-up, the physician was aware and addressed the results, but did not document that the results were addressed (all 3 were outpatient cases). In 3 of 15 abnormal cases with no follow-up, lab results for inpatients were pending at time of discharge and there was no documentation of pending results in the designated space for this in the discharge summary. In 2 of 15 abnormal cases with no follow-up, the patient was followed by a primary care physician outside of our institution. In 7 cases, the patient had multiple subspecialists involved in their care. All undocumented abnormal levels were addressed by our institution, either by contacting the patient or primary care physician, or by determining that the abnormality was not clinically relevant.
Discussion
We identified cases in which patients had abnormal results on tests used to diagnose neuroendocrine tumors such as pheochromocytoma over a 3-year period and sought evidence that a responsible clinician had followed up on the abnormal results. In one-third of abnormal test results, we found no documentation in the medical record that the responsible clinician was aware of the result or had communicated it to another clinician or the patient. This occurred most often in cases in which metanephrine and/or catecholamine levels were pending at the time of hospital discharge, and when a patient who was discharged from the hospital or seen in clinic did not return for a scheduled follow-up appointment. When we followed up with the responsible physician, only 6 in 15 were aware of the abnormal results and had either concluded that they were not clinically significant or had addressed the issue without completing documentation.
Previous research has identified vulnerabilities in the follow-up of send-out test results that exceed the challenges with tests performed in-house. These include that send-out tests inherently have more steps and require more manual processes [8], and that these tests are more prone to delay, misinterpretation, and poor documentation. Reference laboratories usually provide non-structured reporting of results, often in the form of paper or PDF files. This can make it difficult for receiving hospitals or clinics to incorporate information into the electronic medical record or to build clinical reminders or alerts for ordering clinicians. Additionally, these data elements are often cryptic in that they provide reference values without necessarily setting parameters for abnormalities. This is a case in point with metanephrine and catecholamine testing, as the results are often variable and poorly reproducible and difficult for clinicians to interpret. There are different cutoffs for moderately elevated and critically elevated values, and how to proceed with patients with moderately elevated values is not clear and may require the expertise of subspecialists. Our study confirmed several issues surrounding vulnerabilities of send-out lab testing.
As a single-institution project with a small cohort of subjects, the generalizability of this project may be limited. However, some process-of-care vulnerabilities noted here are similar to those reported in previous research studies [8]. In addition, hospitals and clinics send specimens to a limited number of regional and national reference laboratories. The challenges that our clinicians encountered in managing these results are likely to be challenges in many other organizations. Also, while our study was limited to tests done to evaluate for pheochromocytoma, our findings are likely applicable to other reference laboratory tests.
Send-out labs continue to represent a major source of lost follow-up and potential patient harm. Creating systems with effective and timely alerts for providers will be useful in preventing missed follow-up. Our study found a lack of clear guidelines designating responsibility for pending lab results, which has been found across institutions in previous studies [8]. Since we conducted this project, our institution has reminded clinicians that discharging attendings are responsible for pending lab results at time of discharge and has developed an automated electronic method for delivering these results. Similar policy interventions at other institutions have shown promise [16]. We hope this will minimize the number of lab results, including those of send-out labs, which are not acted upon in a timely manner. However, other issues, including data interface with the electronic medical record and patients with abnormal results being lost to follow-up, remain barriers for our institution to address.
There are several immediate steps that could be taken by health care organizations and reference labs to reduce patient harm as a result of send-out labs that are not followed up. First, health care organizations can develop better integration between electronic records and lab processing for send-out labs, as well as more electronic alerts. This may help to notify ordering physicians after patients have been discharged and the case may not be front of mind. Reference labs should create robust electronic systems to transmit results as electronic data elements so that health care organizations can easily incorporate results into their electronic medical records, and develop notification systems that flag out-of-bound values. Secure online lab results for send-outs may shorten the delay in reporting. Additionally, creating clear policies establishing the responsible provider is crucial, as has been found by previous research by Singh and others [11,15].
In conclusion, send-out labs are vulnerable to lost follow-up. It is crucial for clinicians to be aware of all send-out lab results and to document their interpretation of abnormal results. Developing policies and systems to facilitate timely follow-up will help to reduce potential patient harm related to send-out labs.
Corresponding author: Richard Zamore, MD, MPH, Tufts Medical Center, 800 Washington St., Boston, MA 02111, rzamore@tuftsmedicalcenter.org.
Financial disclosures: None.
1. Callen J, Georgiou A, Li J, Westbrook JI, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194–9.
2. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32.
3. Bates DW, Leape LL. Doing better with critical test results. Jt Comm J Qual Patient Saf 2005;31:66–7.
4. Schiff GD, Kim S, Krosnjar N, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165:574.
5. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med 2010;123:238–44.
6. Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200.
7. Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med 2009;169:1123–9.
8. Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334–48.
9. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51.
10. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121–8.
11. Singh H, Wilson L, Reis B, et al. Ten strategies to improve management of abnormal test result alerts in the electronic health record. J Patient Saf 2010;6:121–3.
12. Dickerson JA, Cole B, Astion ML. Ten ways to improve the quality of send-out testing. Clin Lab News 2012;38:12–3.
13. Cole B, Dickerson JA, Graber ML, et al. A prospective tool for risk assessment of sendout testing. Clin Chim Acta 2014;434:1–5.
14. MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18:216–9.
15. Krasowski MD, Chudzik D, Dolezal A, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak 2015;15:11.
16. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66.
1. Callen J, Georgiou A, Li J, Westbrook JI, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194–9.
2. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32.
3. Bates DW, Leape LL. Doing better with critical test results. Jt Comm J Qual Patient Saf 2005;31:66–7.
4. Schiff GD, Kim S, Krosnjar N, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165:574.
5. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med 2010;123:238–44.
6. Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200.
7. Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med 2009;169:1123–9.
8. Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334–48.
9. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51.
10. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121–8.
11. Singh H, Wilson L, Reis B, et al. Ten strategies to improve management of abnormal test result alerts in the electronic health record. J Patient Saf 2010;6:121–3.
12. Dickerson JA, Cole B, Astion ML. Ten ways to improve the quality of send-out testing. Clin Lab News 2012;38:12–3.
13. Cole B, Dickerson JA, Graber ML, et al. A prospective tool for risk assessment of sendout testing. Clin Chim Acta 2014;434:1–5.
14. MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18:216–9.
15. Krasowski MD, Chudzik D, Dolezal A, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak 2015;15:11.
16. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66.