User login
Nivolumab Use for First-Line Management of Hepatocellular Carcinoma: Results of a Real-World Cohort of Patients
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
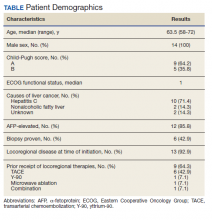
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252