User login
Modern Indications, Results, and Global Trends in the Use of Unicompartmental Knee Arthroplasty and High Tibial Osteotomy in the Treatment of Isolated Medial Compartment Osteoarthritis
An increasingly number of patients with symptomatic isolated medial unicompartmental knee osteoarthritis (OA) are too young and too functionally active to be ideal candidates for total knee arthroplasty (TKA). Isolated medial compartment OA occurs in 10% to 29.5% of all cases, whereas the isolated lateral variant is less common, with a reported incidence of 1% to 7%.1,2 In 1961, Jackson and Waugh3 introduced the high tibial osteotomy (HTO) as a surgical treatment for single-compartment OA. This procedure is designed to increase the life span of articular cartilage by unloading and redistributing the mechanical forces over the nonaffected compartment. Unicompartmental knee arthroplasty (UKA) was introduced in the 1970s as an alternative to TKA or HTO for single-compartment OA.
Since the introduction of these methods, there has been debate about which patients are appropriate candidates for each procedure. Improved surgical techniques and implant designs have led surgeons to reexamine the selection criteria and contraindications for these procedures. Furthermore, given the increasing popularity and use of UKA, the question arises as to whether HTO still has a role in clinical practice in the surgical treatment of medial OA of the knee.
To clarify current ambiguities, we review the modern indications, subjective outcome scores, and survivorship results of UKA and HTO in the treatment of isolated medial compartment degeneration of the knee. In addition, in a thorough review of the literature, we evaluate global trends in the use of both methods.
High Tibial Osteotomy for Medial Compartment OA
Indications
Before the introduction of TKA and UKA for single-compartment OA, surgical management consisted of HTO. When the mechanical axis is slightly overcorrected, the medial compartment is decompressed, ensuring tissue viability and delaying progressive compartment degeneration.
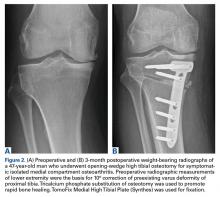
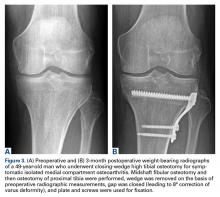
Traditionally, HTO is indicated for young (age <60 years), normal-weight, active patients with radiographic single-compartment OA.6 The knee should be stable and have good range of motion (ROM; flexion >120°), and pain should be localized to the tibiofemoral joint line.
Over the past few decades, numerous authors have reported similar inclusion criteria, clarifying their definition. This definition should be further refined in order to optimize survivorship and clinical outcomes.
Confirming age as an inclusion criterion for HTO, Trieb and colleagues7 found that the risk of failure was significantly (P = .046) higher for HTO patients older than 65 years than for those younger than 65 years (relative risk, 1.5). This finding agrees with findings of other studies, which suggests that, in particular, young patients benefit from HTO.8-11
Moreover, there is a clear relation between HTO survival and obesity. In a study of 159 CWHTOs, Akizuki and colleagues12 reported that preoperative body mass index (BMI) higher than 27.5 kg/m2 was a significant risk factor for early failure. Using BMI higher than 30 kg/m2 as a threshold, Howells and colleagues9 found significantly inferior Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) results for the obese group 5 years after HTO.
Radiographic evidence of severe preoperative compartment degeneration has been associated with early conversion to TKA. Flecher and colleagues11 and van Raaij and colleagues13 both concluded the best long-term survival grades are achieved in HTO patients with mild compartment OA (Ahlbäck14 grade I). The question then becomes whether these patients should be treated nonoperatively instead.15,16The literature supports strict adherence to inclusion criteria in the selection of a potential HTO candidate. Age, BMI, and the preoperative state of OA should be taken into account in order to optimize clinical outcome and survivorship results in patients about to undergo HTO.
Outcomes
Multiple authors have described or compared the midterm or long-term results of the various surgical HTO techniques. Howells and colleagues9 noted overall survival rates of 87% (5 years after CWHTO) and 79% (10 years after CWHTO). Over the 10-year postoperative period, there was significant deterioration in clinical outcome scores and survivorship. Others authors have had similar findings.17-19 van Raaij and colleagues13 found that the 10-year probability of survival after CWHTO was 75%. In 455 patients who underwent lateral CWHTO, Hui and colleagues8 found that 5-year probability of survival was 95%, 10-year probability was 79%, and 15-year probability was 56%. Niinimäki and colleagues10 used the Finnish Arthroplasty Register to report HTO survivorship at a national level. Using conversion to TKA as a cutoff, they noted 5-year survivorship of 89% and 10-year survivorship of 73%. To our knowledge, 2 groups, both in Japan, have reported substantially higher 15-year survival rates: 90%12 and 93%.20 The authors acknowledged that their results were significantly better than in other countries and that Japanese lifestyle, culture, and body habitus therefore require further investigation. At this time, it is not possible to compare their results with Western results.
In an attempt to compare the different survival rates of the various HTO techniques, Schallberger and colleagues21 conducted a retrospective study of OWHTOs and CWHTOs. At median follow-up of 16.5 years, comparative survival rates showed a trend of deterioration. Although data were limited, there were no significant differences in survival or functional outcome between the 2 techniques. In a recent randomized clinical trial, Duivenvoorden and colleagues5 compared these techniques’ midterm results (mean follow-up, 6 years). Clinical outcomes were not significantly different. There were more complications in the OWHTO group and more conversions to TKA in the CWHTO group. Considering these results, the authors suggested OWHTO without autologous bone graft is the best HTO treatment strategy for medial gonarthritis with varus malalignment of <12°.
The HTO results noted in these studies show a similar deteriorating trend; expected 10-year survivorship is 75%. Although modern implants and surgical techniques are being used, evidence supporting use of one surgical HTO method over another is lacking.
UKA for Medial Compartment OA
Indications
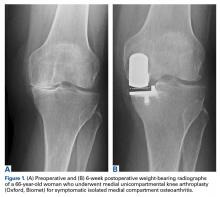
Since it was first introduced in the 1970s, use of UKA for single-compartment OA has been a subject of debate. The high failure rates reported at the time raised skepticism about the new treatment.22 Kozinn and Scott23 defined classic indications and contraindications. Indications included isolated medial or lateral compartment OA or osteonecrosis of the knee, age over 60 years, and weight under 82 kg. In addition, the angular deformity of the affected lower extremity had to be <15° and passively correctable to neutral at time of surgery. Last, the flexion contracture had to be <5°, and ideal ROM was 90°. Contraindications included high activity, age under 60 years, and inflammatory arthritis. Strict adherence led to improved implant survival and lower revision rates. Because of improved surgical techniques, modern implant designs, and accumulating experience with the procedure, the surgical indications for UKA have expanded. Exact thresholds for UKA inclusion, however, remain unclear.
The modern literature is overturning the traditional idea that UKA is not indicated for patients under age 60 years.23 Using KSS, Thompson and colleagues24 found that younger patients did better than older patients 2 years after UKA using various types of implants. Analyzing survivorship results, Heyse and colleagues25 concluded that UKA can be successful in patients under age 60 years and reported a 15-year survivorship rate of 85.6% and excellent outcome scores. Other authors have had similar findings.26-28
Evaluating the influence of weight, Thompson and colleagues24 found obese patients did not have a higher revision rate but did have slower progression of improvement 2 years after UKA. Cavaignac and colleagues29 concluded that, at minimum follow-up of 7 years (range, 7-22 years), weight did not influence UKA survivorship. Other authors30-33 have found no significant influence of BMI on survival.
Reports on preoperative radiographic parameters that can potentially influence UKA results are limited. In 113 medial UKAs studied by Niinimäki and colleagues,34 mild medial compartment degeneration, seen on preoperative radiographs, was associated with significantly higher failure rates. The authors concluded that other treatment options should be favored in the absence of severe isolated compartment OA.
Although the classic indications defined by Kozinn and Scott23 have yielded good to excellent UKA results, improvements in implants and surgical techniques35-38 have extended the criteria. The modern literature demonstrates that age and BMI should not be used as criteria for excluding UKA candidates. Radiographically, there should be significant isolated compartment degeneration in order to optimize patient-reported outcome and survivorship.
Outcomes
Improved implant designs and modern minimally invasive techniques have effected a change in outcome results and a renewed interest in implants. Over the past decade, multiple authors have described the various modern UKA implants and their survivorship. Reports published since UKA was introduced in the 1970s show a continual increase in implant survival. Koskinen and colleagues,39 using Finnish Arthroplasty Register data on 1819 UKAs performed between 1985 and 2003, found 10-year survival rates of 81% for Oxford implants (Zimmer Biomet), 79% for Miller-Galante II (Zimmer Biomet), 78% for Duracon (Howmedica), and 53% for PCA unicompartmental knee (Howmedica). Heyse and colleagues25 reported 10- and 15-year survivorship data (93.5% and 86.3%, respectively) for 223 patients under age 60 years at the time of their index surgery (Genesis Unicondylar implant, Smith & Nephew), performed between 1993 and 2005. KSS was good to excellent. Similar numbers in cohorts under age 60 years were reported by Schai and colleagues26 using the PFC system (Johnson & Johnson) and by Price and colleagues27 using the medial Oxford UKA. Both groups reported excellent survivorship rates: 93% at 2- to 6-year follow-up and 91% at 10-year follow-up. The outcome in older patients seems satisfactory as well. In another multicenter report, by Price and colleagues,40 medial Oxford UKAs had a 15-year survival rate of 93%. Berger and colleagues41 reported similar numbers for the Miller-Galante prosthesis. Survival rates were 98% (10 years) and 95.7% (13 years), and 92% of patients had good to excellent Hospital for Special Surgery knee scores.
Although various modern implants have had good to excellent results, the historical question of what type of UKA to use (mobile or fixed-bearing) remains unanswered. To try to address it, Peersman and colleagues42 performed a systematic review of 44 papers (9463 knees). The 2 implant types had comparable revision rates. Another recent retrospective study tried to determine what is crucial for implant survival: implant design or surgeon experience.43 The authors concluded that prosthetic component positioning is key. Other authors have reported high-volume centers are crucial for satisfactory UKA results and lower revision rates.44-46
Results of these studies indicate that, where UKAs are being performed in volume, 10-year survivorship rates higher than 90% and good to excellent outcomes can be expected.
UKA vs HTO
Cohort studies that have directly compared the 2 treatment modalities are scarce, and most have been retrospective. In a prospective study, Stukenborg-Colsman and colleagues47 randomized patients with medial compartment OA to undergo either CWHTO (32 patients) with a technique reported by Coventry48 or UKA (28 patients) with the unicondylar knee sliding prosthesis, Tübingen pattern (Aesculap), between 1988 and 1991. Patients were assessed 2.5, 4.5, and 7.5 years after surgery. More postoperative complications were noted in the HTO group. At 7- to 10-year follow-up, 71% of the HTO group and 65% of the UKA group had excellent KSS. Mean ROM was 103° after UKA (range, 35°-140°) and 117° after HTO (range, 85°-135°) during the same assessment. Although differences were not significant, Kaplan-Meier survival analysis was 60% for HTO and 77% for UKA at 10 years. Results were not promising for the implants used, compared with other implants, but the authors concluded that, because of improvements in implant designs and image-guided techniques, better long-term success can be expected with UKA than with HTO.
In another prospective study, Börjesson and colleagues49 evaluated pain during walking, ROM, British Orthopaedic Association (BOA) scores, and gait variables at 1- and 5-year follow-up. Patients with moderate medial OA (Ahlbäck14 grade I-III) were randomly selected to undergo CWHTO or UKA (Brigham, DePuy). There were no significant differences in BOA scores, ROM, or pain during walking between the 2 groups at 3 months, 1 year, and 5 years after surgery. Gait analysis showed a significant difference in favor of UKA only at 3 months after surgery. At 1- and 5-year follow-up, no significant differences were noted.
To clarify current ambiguities, Fu and colleagues50 performed a systematic review of all (11) comparative studies. These studies had a total of 5840 (5081 UKA, 759 HTO) patients. Although ROM was significantly better for the HTO group than the UKA group, the UKA group had significantly better functional results. Walking after surgery was significantly faster for the UKA group. The authors suggested the difference might be attributed to the different postoperative regimens—HTO patients wore a whole-leg plaster cast for 6 weeks, and UKA patients were allowed immediate postoperative weight-bearing. Regarding rates of survival and complications, pooled data showed no significant differences. Despite these results, the authors acknowledged the limitation of available randomized clinical trials and the multiple techniques and implants used. We share their assertion that larger prospective controlled trials are needed. These are crucial to getting a definitive answer regarding which of the 2 treatment strategies should be used for isolated compartment OA.
Current Trends in Use of UKA and HTO
Evaluation of national registries and recent reports showed a global shift in use of both HTO and UKA. Despite the lack of national HTO registries, a few reports have described use of TKA, UKA, and HTO in Western populations over the past 2 decades. Using 1998-2007 data from the Swedish Knee Arthroplasty Register, W-Dahl and colleagues51 found a 3-fold increase in UKA use, whereas HTO use was halved over the same period. Niinimäki and colleagues52 reported similar findings with the Finnish National Hospital Discharge Register. They noted a steady 6.8% annual decrease in osteotomies, whereas UKA use increased sharply after the Oxford UKA was introduced (Phase 3; Biomet). These findings are consistent with several reports from North America. In their epidemiologic analysis covering the period 1985-1990, Wright and colleagues53 found an 11% to 14% annual decrease in osteotomies among the elderly, compared with an annual decrease of only 3% to 4% among patients younger than 65 years. Nwachukwu and colleagues54 recently compared UKA and HTO practice patterns between 2007 and 2011, using data from a large US private payer insurance database. They noted an annual growth rate of 4.7% in UKA use, compared with an annual 3.9% decrease in HTO use. Furthermore, based on their subgroup analysis, they speculated there was a demographic shift toward UKA, as opposed to TKA, particularly in older women. Bolognesi and colleagues55 investigated further. Evaluating all Medicare beneficiaries who underwent knee arthroplasty in the United States between 2000 and 2009, they noted a 1.7-fold increase in TKA use and a 6.2-fold increase in UKA use. As there were no substantial changes in patient characteristics over that period, the authors hypothesized that a possible broadening of inclusion criteria may have led to the increased use of UKA.
There is a possible multifactorial explanation for the current global shift in favor of UKA. First, UKA was once a technically demanding procedure, but improved surgical techniques, image guidance, and robot assistance56 have made it relatively less difficult. Second, UKA surgery is associated with lower reported perioperative morbidities.57 We think these factors have contributed to the global trend of less HTO use and more UKA use in the treatment of unicompartmental OA.
Conclusion
The modern literature suggests the inclusion criteria for HTO have been well investigated and defined; the UKA criteria remain a matter of debate but seem to be expanding. Long-term survival results seem to favor UKA, though patient satisfaction with both procedures is good to excellent. The broadening range of inclusion criteria and consistent reports of durable outcomes, coupled with excellent patient satisfaction, likely explain the shift toward UKA in the treatment of isolated compartment degeneration.
Am J Orthop. 2016;45(6):E355-E361. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7): 520-526.
2. Wise BL, Niu J, Yang M, et al; Multicenter Osteoarthritis (MOST) Group. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 2012;64(6): 847-852.
3. Jackson JP, Waugh W. Tibial osteotomy for osteoarthritis of the knee. J Bone Joint Surg Br. 1961;43:746-751.
4. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88(11):1454-1459.
5. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425-1432.
6. Hutchison CR, Cho B, Wong N, Agnidis Z, Gross AE. Proximal valgus tibial osteotomy for osteoarthritis of the knee. Instr Course Lect. 1999;48:131-134.
7. Trieb K, Grohs J, Hanslik-Schnabel B, Stulnig T, Panotopoulos J, Wanivenhaus A. Age predicts outcome of high-tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):149-152.
8. Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64-70.
9. Howells NR, Salmon L, Waller A, Scanelli J, Pinczewski LA. The outcome at ten years of lateral closing-wedge high tibial osteotomy: determinants of survival and functional outcome. Bone Joint J Br. 2014;96(11):1491-1497.
10. Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J. Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: Finnish registry-based study of 3195 knees. J Bone Joint Surg Br. 2012;94(11):1517-1521.
11. Flecher X, Parratte S, Aubaniac JM, Argenson JN. A 12-28-year followup study of closing wedge high tibial osteotomy. Clin Orthop Relat Res. 2006;(452):91-96.
12. Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90(5):592-596.
13. van Raaij T, Reijman M, Brouwer RW, Jakma TS, Verhaar JN. Survival of closing-wedge high tibial osteotomy: good outcome in men with low-grade osteoarthritis after 10-16 years. Acta Orthop. 2008;79:230-234.
14. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
15. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-1711.
16. Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;(390):173-181.
17. Naudie D, Bourne RB, Rorabeck CH, Bourne TJ. The Install Award. Survivorship of the high tibial valgus osteotomy. A 10- to -22-year followup study. Clin Orthop Relat Res. 1999;(367):18-27.
18. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Br. 2003;85(3):469-474.
19. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
20. Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11(6):439-444.
21. Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):122-127.
22. Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62(8):1329-1337.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
24. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
25. Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19(5):585-591.
26. Schai PA, Suh JT, Thornhill TS, Scott RD. Unicompartmental knee arthroplasty in middle-aged patients: a 2- to 6-year follow-up evaluation. J Arthroplasty. 1998;13(4):365-372.
27. Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87(11):1488-1492.
28. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2003;85(10):1968-1973.
29. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J Br. 2013;95(8):1064-1068.
30. Tabor OB Jr, Tabor OB, Bernard M, Wan JY. Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv. 2005;14(2):59-63.
31. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(5 suppl):19-23.
32. Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25(5):369-373.
33. Plate JF, Augart MA, Seyler TM, et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty [published online April 12, 2015]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3597-5.
34. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
35. Carlsson LV, Albrektsson BE, Regnér LR. Minimally invasive surgery vs conventional exposure using the Miller-Galante unicompartmental knee arthroplasty: a randomized radiostereometric study. J Arthroplasty. 2006;21(2):151-156.
36. Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282-286.
37. Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54-60.
38. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
39. Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78(1):128-135.
40. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
41. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
42. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3296-3305.
43. Zambianchi F, Digennaro V, Giorgini A, et al. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2074-2080.
44. Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45-49.
45. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-525.
46. Robertsson O, Lidgren L. The short-term results of 3 common UKA implants during different periods in Sweden. J Arthroplasty. 2008;23(6):801-807.
47. Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7-10-year follow-up prospective randomised study. Knee. 2001;8(3):187-194.
48. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55(1):23-48.
49. Börjesson M, Weidenhielm L, Mattsson E, Olsson E. Gait and clinical measurements in patients with knee osteoarthritis after surgery: a prospective 5-year follow-up study. Knee. 2005;12(2):121-127.
50. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013;28(5):759-765.
51. W-Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2010;81(2):161-164.
52. Niinimäki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399-1402.
53. Wright J, Heck D, Hawker G, et al. Rates of tibial osteotomies in Canada and the United States. Clin Orthop Relat Res. 1995;(319):266-275.
54. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
55. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
56. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
57. Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8 suppl):86-90.
An increasingly number of patients with symptomatic isolated medial unicompartmental knee osteoarthritis (OA) are too young and too functionally active to be ideal candidates for total knee arthroplasty (TKA). Isolated medial compartment OA occurs in 10% to 29.5% of all cases, whereas the isolated lateral variant is less common, with a reported incidence of 1% to 7%.1,2 In 1961, Jackson and Waugh3 introduced the high tibial osteotomy (HTO) as a surgical treatment for single-compartment OA. This procedure is designed to increase the life span of articular cartilage by unloading and redistributing the mechanical forces over the nonaffected compartment. Unicompartmental knee arthroplasty (UKA) was introduced in the 1970s as an alternative to TKA or HTO for single-compartment OA.
Since the introduction of these methods, there has been debate about which patients are appropriate candidates for each procedure. Improved surgical techniques and implant designs have led surgeons to reexamine the selection criteria and contraindications for these procedures. Furthermore, given the increasing popularity and use of UKA, the question arises as to whether HTO still has a role in clinical practice in the surgical treatment of medial OA of the knee.
To clarify current ambiguities, we review the modern indications, subjective outcome scores, and survivorship results of UKA and HTO in the treatment of isolated medial compartment degeneration of the knee. In addition, in a thorough review of the literature, we evaluate global trends in the use of both methods.
High Tibial Osteotomy for Medial Compartment OA
Indications
Before the introduction of TKA and UKA for single-compartment OA, surgical management consisted of HTO. When the mechanical axis is slightly overcorrected, the medial compartment is decompressed, ensuring tissue viability and delaying progressive compartment degeneration.
Traditionally, HTO is indicated for young (age <60 years), normal-weight, active patients with radiographic single-compartment OA.6 The knee should be stable and have good range of motion (ROM; flexion >120°), and pain should be localized to the tibiofemoral joint line.
Over the past few decades, numerous authors have reported similar inclusion criteria, clarifying their definition. This definition should be further refined in order to optimize survivorship and clinical outcomes.
Confirming age as an inclusion criterion for HTO, Trieb and colleagues7 found that the risk of failure was significantly (P = .046) higher for HTO patients older than 65 years than for those younger than 65 years (relative risk, 1.5). This finding agrees with findings of other studies, which suggests that, in particular, young patients benefit from HTO.8-11
Moreover, there is a clear relation between HTO survival and obesity. In a study of 159 CWHTOs, Akizuki and colleagues12 reported that preoperative body mass index (BMI) higher than 27.5 kg/m2 was a significant risk factor for early failure. Using BMI higher than 30 kg/m2 as a threshold, Howells and colleagues9 found significantly inferior Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) results for the obese group 5 years after HTO.
Radiographic evidence of severe preoperative compartment degeneration has been associated with early conversion to TKA. Flecher and colleagues11 and van Raaij and colleagues13 both concluded the best long-term survival grades are achieved in HTO patients with mild compartment OA (Ahlbäck14 grade I). The question then becomes whether these patients should be treated nonoperatively instead.15,16The literature supports strict adherence to inclusion criteria in the selection of a potential HTO candidate. Age, BMI, and the preoperative state of OA should be taken into account in order to optimize clinical outcome and survivorship results in patients about to undergo HTO.
Outcomes
Multiple authors have described or compared the midterm or long-term results of the various surgical HTO techniques. Howells and colleagues9 noted overall survival rates of 87% (5 years after CWHTO) and 79% (10 years after CWHTO). Over the 10-year postoperative period, there was significant deterioration in clinical outcome scores and survivorship. Others authors have had similar findings.17-19 van Raaij and colleagues13 found that the 10-year probability of survival after CWHTO was 75%. In 455 patients who underwent lateral CWHTO, Hui and colleagues8 found that 5-year probability of survival was 95%, 10-year probability was 79%, and 15-year probability was 56%. Niinimäki and colleagues10 used the Finnish Arthroplasty Register to report HTO survivorship at a national level. Using conversion to TKA as a cutoff, they noted 5-year survivorship of 89% and 10-year survivorship of 73%. To our knowledge, 2 groups, both in Japan, have reported substantially higher 15-year survival rates: 90%12 and 93%.20 The authors acknowledged that their results were significantly better than in other countries and that Japanese lifestyle, culture, and body habitus therefore require further investigation. At this time, it is not possible to compare their results with Western results.
In an attempt to compare the different survival rates of the various HTO techniques, Schallberger and colleagues21 conducted a retrospective study of OWHTOs and CWHTOs. At median follow-up of 16.5 years, comparative survival rates showed a trend of deterioration. Although data were limited, there were no significant differences in survival or functional outcome between the 2 techniques. In a recent randomized clinical trial, Duivenvoorden and colleagues5 compared these techniques’ midterm results (mean follow-up, 6 years). Clinical outcomes were not significantly different. There were more complications in the OWHTO group and more conversions to TKA in the CWHTO group. Considering these results, the authors suggested OWHTO without autologous bone graft is the best HTO treatment strategy for medial gonarthritis with varus malalignment of <12°.
The HTO results noted in these studies show a similar deteriorating trend; expected 10-year survivorship is 75%. Although modern implants and surgical techniques are being used, evidence supporting use of one surgical HTO method over another is lacking.
UKA for Medial Compartment OA
Indications
Since it was first introduced in the 1970s, use of UKA for single-compartment OA has been a subject of debate. The high failure rates reported at the time raised skepticism about the new treatment.22 Kozinn and Scott23 defined classic indications and contraindications. Indications included isolated medial or lateral compartment OA or osteonecrosis of the knee, age over 60 years, and weight under 82 kg. In addition, the angular deformity of the affected lower extremity had to be <15° and passively correctable to neutral at time of surgery. Last, the flexion contracture had to be <5°, and ideal ROM was 90°. Contraindications included high activity, age under 60 years, and inflammatory arthritis. Strict adherence led to improved implant survival and lower revision rates. Because of improved surgical techniques, modern implant designs, and accumulating experience with the procedure, the surgical indications for UKA have expanded. Exact thresholds for UKA inclusion, however, remain unclear.
The modern literature is overturning the traditional idea that UKA is not indicated for patients under age 60 years.23 Using KSS, Thompson and colleagues24 found that younger patients did better than older patients 2 years after UKA using various types of implants. Analyzing survivorship results, Heyse and colleagues25 concluded that UKA can be successful in patients under age 60 years and reported a 15-year survivorship rate of 85.6% and excellent outcome scores. Other authors have had similar findings.26-28
Evaluating the influence of weight, Thompson and colleagues24 found obese patients did not have a higher revision rate but did have slower progression of improvement 2 years after UKA. Cavaignac and colleagues29 concluded that, at minimum follow-up of 7 years (range, 7-22 years), weight did not influence UKA survivorship. Other authors30-33 have found no significant influence of BMI on survival.
Reports on preoperative radiographic parameters that can potentially influence UKA results are limited. In 113 medial UKAs studied by Niinimäki and colleagues,34 mild medial compartment degeneration, seen on preoperative radiographs, was associated with significantly higher failure rates. The authors concluded that other treatment options should be favored in the absence of severe isolated compartment OA.
Although the classic indications defined by Kozinn and Scott23 have yielded good to excellent UKA results, improvements in implants and surgical techniques35-38 have extended the criteria. The modern literature demonstrates that age and BMI should not be used as criteria for excluding UKA candidates. Radiographically, there should be significant isolated compartment degeneration in order to optimize patient-reported outcome and survivorship.
Outcomes
Improved implant designs and modern minimally invasive techniques have effected a change in outcome results and a renewed interest in implants. Over the past decade, multiple authors have described the various modern UKA implants and their survivorship. Reports published since UKA was introduced in the 1970s show a continual increase in implant survival. Koskinen and colleagues,39 using Finnish Arthroplasty Register data on 1819 UKAs performed between 1985 and 2003, found 10-year survival rates of 81% for Oxford implants (Zimmer Biomet), 79% for Miller-Galante II (Zimmer Biomet), 78% for Duracon (Howmedica), and 53% for PCA unicompartmental knee (Howmedica). Heyse and colleagues25 reported 10- and 15-year survivorship data (93.5% and 86.3%, respectively) for 223 patients under age 60 years at the time of their index surgery (Genesis Unicondylar implant, Smith & Nephew), performed between 1993 and 2005. KSS was good to excellent. Similar numbers in cohorts under age 60 years were reported by Schai and colleagues26 using the PFC system (Johnson & Johnson) and by Price and colleagues27 using the medial Oxford UKA. Both groups reported excellent survivorship rates: 93% at 2- to 6-year follow-up and 91% at 10-year follow-up. The outcome in older patients seems satisfactory as well. In another multicenter report, by Price and colleagues,40 medial Oxford UKAs had a 15-year survival rate of 93%. Berger and colleagues41 reported similar numbers for the Miller-Galante prosthesis. Survival rates were 98% (10 years) and 95.7% (13 years), and 92% of patients had good to excellent Hospital for Special Surgery knee scores.
Although various modern implants have had good to excellent results, the historical question of what type of UKA to use (mobile or fixed-bearing) remains unanswered. To try to address it, Peersman and colleagues42 performed a systematic review of 44 papers (9463 knees). The 2 implant types had comparable revision rates. Another recent retrospective study tried to determine what is crucial for implant survival: implant design or surgeon experience.43 The authors concluded that prosthetic component positioning is key. Other authors have reported high-volume centers are crucial for satisfactory UKA results and lower revision rates.44-46
Results of these studies indicate that, where UKAs are being performed in volume, 10-year survivorship rates higher than 90% and good to excellent outcomes can be expected.
UKA vs HTO
Cohort studies that have directly compared the 2 treatment modalities are scarce, and most have been retrospective. In a prospective study, Stukenborg-Colsman and colleagues47 randomized patients with medial compartment OA to undergo either CWHTO (32 patients) with a technique reported by Coventry48 or UKA (28 patients) with the unicondylar knee sliding prosthesis, Tübingen pattern (Aesculap), between 1988 and 1991. Patients were assessed 2.5, 4.5, and 7.5 years after surgery. More postoperative complications were noted in the HTO group. At 7- to 10-year follow-up, 71% of the HTO group and 65% of the UKA group had excellent KSS. Mean ROM was 103° after UKA (range, 35°-140°) and 117° after HTO (range, 85°-135°) during the same assessment. Although differences were not significant, Kaplan-Meier survival analysis was 60% for HTO and 77% for UKA at 10 years. Results were not promising for the implants used, compared with other implants, but the authors concluded that, because of improvements in implant designs and image-guided techniques, better long-term success can be expected with UKA than with HTO.
In another prospective study, Börjesson and colleagues49 evaluated pain during walking, ROM, British Orthopaedic Association (BOA) scores, and gait variables at 1- and 5-year follow-up. Patients with moderate medial OA (Ahlbäck14 grade I-III) were randomly selected to undergo CWHTO or UKA (Brigham, DePuy). There were no significant differences in BOA scores, ROM, or pain during walking between the 2 groups at 3 months, 1 year, and 5 years after surgery. Gait analysis showed a significant difference in favor of UKA only at 3 months after surgery. At 1- and 5-year follow-up, no significant differences were noted.
To clarify current ambiguities, Fu and colleagues50 performed a systematic review of all (11) comparative studies. These studies had a total of 5840 (5081 UKA, 759 HTO) patients. Although ROM was significantly better for the HTO group than the UKA group, the UKA group had significantly better functional results. Walking after surgery was significantly faster for the UKA group. The authors suggested the difference might be attributed to the different postoperative regimens—HTO patients wore a whole-leg plaster cast for 6 weeks, and UKA patients were allowed immediate postoperative weight-bearing. Regarding rates of survival and complications, pooled data showed no significant differences. Despite these results, the authors acknowledged the limitation of available randomized clinical trials and the multiple techniques and implants used. We share their assertion that larger prospective controlled trials are needed. These are crucial to getting a definitive answer regarding which of the 2 treatment strategies should be used for isolated compartment OA.
Current Trends in Use of UKA and HTO
Evaluation of national registries and recent reports showed a global shift in use of both HTO and UKA. Despite the lack of national HTO registries, a few reports have described use of TKA, UKA, and HTO in Western populations over the past 2 decades. Using 1998-2007 data from the Swedish Knee Arthroplasty Register, W-Dahl and colleagues51 found a 3-fold increase in UKA use, whereas HTO use was halved over the same period. Niinimäki and colleagues52 reported similar findings with the Finnish National Hospital Discharge Register. They noted a steady 6.8% annual decrease in osteotomies, whereas UKA use increased sharply after the Oxford UKA was introduced (Phase 3; Biomet). These findings are consistent with several reports from North America. In their epidemiologic analysis covering the period 1985-1990, Wright and colleagues53 found an 11% to 14% annual decrease in osteotomies among the elderly, compared with an annual decrease of only 3% to 4% among patients younger than 65 years. Nwachukwu and colleagues54 recently compared UKA and HTO practice patterns between 2007 and 2011, using data from a large US private payer insurance database. They noted an annual growth rate of 4.7% in UKA use, compared with an annual 3.9% decrease in HTO use. Furthermore, based on their subgroup analysis, they speculated there was a demographic shift toward UKA, as opposed to TKA, particularly in older women. Bolognesi and colleagues55 investigated further. Evaluating all Medicare beneficiaries who underwent knee arthroplasty in the United States between 2000 and 2009, they noted a 1.7-fold increase in TKA use and a 6.2-fold increase in UKA use. As there were no substantial changes in patient characteristics over that period, the authors hypothesized that a possible broadening of inclusion criteria may have led to the increased use of UKA.
There is a possible multifactorial explanation for the current global shift in favor of UKA. First, UKA was once a technically demanding procedure, but improved surgical techniques, image guidance, and robot assistance56 have made it relatively less difficult. Second, UKA surgery is associated with lower reported perioperative morbidities.57 We think these factors have contributed to the global trend of less HTO use and more UKA use in the treatment of unicompartmental OA.
Conclusion
The modern literature suggests the inclusion criteria for HTO have been well investigated and defined; the UKA criteria remain a matter of debate but seem to be expanding. Long-term survival results seem to favor UKA, though patient satisfaction with both procedures is good to excellent. The broadening range of inclusion criteria and consistent reports of durable outcomes, coupled with excellent patient satisfaction, likely explain the shift toward UKA in the treatment of isolated compartment degeneration.
Am J Orthop. 2016;45(6):E355-E361. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
An increasingly number of patients with symptomatic isolated medial unicompartmental knee osteoarthritis (OA) are too young and too functionally active to be ideal candidates for total knee arthroplasty (TKA). Isolated medial compartment OA occurs in 10% to 29.5% of all cases, whereas the isolated lateral variant is less common, with a reported incidence of 1% to 7%.1,2 In 1961, Jackson and Waugh3 introduced the high tibial osteotomy (HTO) as a surgical treatment for single-compartment OA. This procedure is designed to increase the life span of articular cartilage by unloading and redistributing the mechanical forces over the nonaffected compartment. Unicompartmental knee arthroplasty (UKA) was introduced in the 1970s as an alternative to TKA or HTO for single-compartment OA.
Since the introduction of these methods, there has been debate about which patients are appropriate candidates for each procedure. Improved surgical techniques and implant designs have led surgeons to reexamine the selection criteria and contraindications for these procedures. Furthermore, given the increasing popularity and use of UKA, the question arises as to whether HTO still has a role in clinical practice in the surgical treatment of medial OA of the knee.
To clarify current ambiguities, we review the modern indications, subjective outcome scores, and survivorship results of UKA and HTO in the treatment of isolated medial compartment degeneration of the knee. In addition, in a thorough review of the literature, we evaluate global trends in the use of both methods.
High Tibial Osteotomy for Medial Compartment OA
Indications
Before the introduction of TKA and UKA for single-compartment OA, surgical management consisted of HTO. When the mechanical axis is slightly overcorrected, the medial compartment is decompressed, ensuring tissue viability and delaying progressive compartment degeneration.
Traditionally, HTO is indicated for young (age <60 years), normal-weight, active patients with radiographic single-compartment OA.6 The knee should be stable and have good range of motion (ROM; flexion >120°), and pain should be localized to the tibiofemoral joint line.
Over the past few decades, numerous authors have reported similar inclusion criteria, clarifying their definition. This definition should be further refined in order to optimize survivorship and clinical outcomes.
Confirming age as an inclusion criterion for HTO, Trieb and colleagues7 found that the risk of failure was significantly (P = .046) higher for HTO patients older than 65 years than for those younger than 65 years (relative risk, 1.5). This finding agrees with findings of other studies, which suggests that, in particular, young patients benefit from HTO.8-11
Moreover, there is a clear relation between HTO survival and obesity. In a study of 159 CWHTOs, Akizuki and colleagues12 reported that preoperative body mass index (BMI) higher than 27.5 kg/m2 was a significant risk factor for early failure. Using BMI higher than 30 kg/m2 as a threshold, Howells and colleagues9 found significantly inferior Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) results for the obese group 5 years after HTO.
Radiographic evidence of severe preoperative compartment degeneration has been associated with early conversion to TKA. Flecher and colleagues11 and van Raaij and colleagues13 both concluded the best long-term survival grades are achieved in HTO patients with mild compartment OA (Ahlbäck14 grade I). The question then becomes whether these patients should be treated nonoperatively instead.15,16The literature supports strict adherence to inclusion criteria in the selection of a potential HTO candidate. Age, BMI, and the preoperative state of OA should be taken into account in order to optimize clinical outcome and survivorship results in patients about to undergo HTO.
Outcomes
Multiple authors have described or compared the midterm or long-term results of the various surgical HTO techniques. Howells and colleagues9 noted overall survival rates of 87% (5 years after CWHTO) and 79% (10 years after CWHTO). Over the 10-year postoperative period, there was significant deterioration in clinical outcome scores and survivorship. Others authors have had similar findings.17-19 van Raaij and colleagues13 found that the 10-year probability of survival after CWHTO was 75%. In 455 patients who underwent lateral CWHTO, Hui and colleagues8 found that 5-year probability of survival was 95%, 10-year probability was 79%, and 15-year probability was 56%. Niinimäki and colleagues10 used the Finnish Arthroplasty Register to report HTO survivorship at a national level. Using conversion to TKA as a cutoff, they noted 5-year survivorship of 89% and 10-year survivorship of 73%. To our knowledge, 2 groups, both in Japan, have reported substantially higher 15-year survival rates: 90%12 and 93%.20 The authors acknowledged that their results were significantly better than in other countries and that Japanese lifestyle, culture, and body habitus therefore require further investigation. At this time, it is not possible to compare their results with Western results.
In an attempt to compare the different survival rates of the various HTO techniques, Schallberger and colleagues21 conducted a retrospective study of OWHTOs and CWHTOs. At median follow-up of 16.5 years, comparative survival rates showed a trend of deterioration. Although data were limited, there were no significant differences in survival or functional outcome between the 2 techniques. In a recent randomized clinical trial, Duivenvoorden and colleagues5 compared these techniques’ midterm results (mean follow-up, 6 years). Clinical outcomes were not significantly different. There were more complications in the OWHTO group and more conversions to TKA in the CWHTO group. Considering these results, the authors suggested OWHTO without autologous bone graft is the best HTO treatment strategy for medial gonarthritis with varus malalignment of <12°.
The HTO results noted in these studies show a similar deteriorating trend; expected 10-year survivorship is 75%. Although modern implants and surgical techniques are being used, evidence supporting use of one surgical HTO method over another is lacking.
UKA for Medial Compartment OA
Indications
Since it was first introduced in the 1970s, use of UKA for single-compartment OA has been a subject of debate. The high failure rates reported at the time raised skepticism about the new treatment.22 Kozinn and Scott23 defined classic indications and contraindications. Indications included isolated medial or lateral compartment OA or osteonecrosis of the knee, age over 60 years, and weight under 82 kg. In addition, the angular deformity of the affected lower extremity had to be <15° and passively correctable to neutral at time of surgery. Last, the flexion contracture had to be <5°, and ideal ROM was 90°. Contraindications included high activity, age under 60 years, and inflammatory arthritis. Strict adherence led to improved implant survival and lower revision rates. Because of improved surgical techniques, modern implant designs, and accumulating experience with the procedure, the surgical indications for UKA have expanded. Exact thresholds for UKA inclusion, however, remain unclear.
The modern literature is overturning the traditional idea that UKA is not indicated for patients under age 60 years.23 Using KSS, Thompson and colleagues24 found that younger patients did better than older patients 2 years after UKA using various types of implants. Analyzing survivorship results, Heyse and colleagues25 concluded that UKA can be successful in patients under age 60 years and reported a 15-year survivorship rate of 85.6% and excellent outcome scores. Other authors have had similar findings.26-28
Evaluating the influence of weight, Thompson and colleagues24 found obese patients did not have a higher revision rate but did have slower progression of improvement 2 years after UKA. Cavaignac and colleagues29 concluded that, at minimum follow-up of 7 years (range, 7-22 years), weight did not influence UKA survivorship. Other authors30-33 have found no significant influence of BMI on survival.
Reports on preoperative radiographic parameters that can potentially influence UKA results are limited. In 113 medial UKAs studied by Niinimäki and colleagues,34 mild medial compartment degeneration, seen on preoperative radiographs, was associated with significantly higher failure rates. The authors concluded that other treatment options should be favored in the absence of severe isolated compartment OA.
Although the classic indications defined by Kozinn and Scott23 have yielded good to excellent UKA results, improvements in implants and surgical techniques35-38 have extended the criteria. The modern literature demonstrates that age and BMI should not be used as criteria for excluding UKA candidates. Radiographically, there should be significant isolated compartment degeneration in order to optimize patient-reported outcome and survivorship.
Outcomes
Improved implant designs and modern minimally invasive techniques have effected a change in outcome results and a renewed interest in implants. Over the past decade, multiple authors have described the various modern UKA implants and their survivorship. Reports published since UKA was introduced in the 1970s show a continual increase in implant survival. Koskinen and colleagues,39 using Finnish Arthroplasty Register data on 1819 UKAs performed between 1985 and 2003, found 10-year survival rates of 81% for Oxford implants (Zimmer Biomet), 79% for Miller-Galante II (Zimmer Biomet), 78% for Duracon (Howmedica), and 53% for PCA unicompartmental knee (Howmedica). Heyse and colleagues25 reported 10- and 15-year survivorship data (93.5% and 86.3%, respectively) for 223 patients under age 60 years at the time of their index surgery (Genesis Unicondylar implant, Smith & Nephew), performed between 1993 and 2005. KSS was good to excellent. Similar numbers in cohorts under age 60 years were reported by Schai and colleagues26 using the PFC system (Johnson & Johnson) and by Price and colleagues27 using the medial Oxford UKA. Both groups reported excellent survivorship rates: 93% at 2- to 6-year follow-up and 91% at 10-year follow-up. The outcome in older patients seems satisfactory as well. In another multicenter report, by Price and colleagues,40 medial Oxford UKAs had a 15-year survival rate of 93%. Berger and colleagues41 reported similar numbers for the Miller-Galante prosthesis. Survival rates were 98% (10 years) and 95.7% (13 years), and 92% of patients had good to excellent Hospital for Special Surgery knee scores.
Although various modern implants have had good to excellent results, the historical question of what type of UKA to use (mobile or fixed-bearing) remains unanswered. To try to address it, Peersman and colleagues42 performed a systematic review of 44 papers (9463 knees). The 2 implant types had comparable revision rates. Another recent retrospective study tried to determine what is crucial for implant survival: implant design or surgeon experience.43 The authors concluded that prosthetic component positioning is key. Other authors have reported high-volume centers are crucial for satisfactory UKA results and lower revision rates.44-46
Results of these studies indicate that, where UKAs are being performed in volume, 10-year survivorship rates higher than 90% and good to excellent outcomes can be expected.
UKA vs HTO
Cohort studies that have directly compared the 2 treatment modalities are scarce, and most have been retrospective. In a prospective study, Stukenborg-Colsman and colleagues47 randomized patients with medial compartment OA to undergo either CWHTO (32 patients) with a technique reported by Coventry48 or UKA (28 patients) with the unicondylar knee sliding prosthesis, Tübingen pattern (Aesculap), between 1988 and 1991. Patients were assessed 2.5, 4.5, and 7.5 years after surgery. More postoperative complications were noted in the HTO group. At 7- to 10-year follow-up, 71% of the HTO group and 65% of the UKA group had excellent KSS. Mean ROM was 103° after UKA (range, 35°-140°) and 117° after HTO (range, 85°-135°) during the same assessment. Although differences were not significant, Kaplan-Meier survival analysis was 60% for HTO and 77% for UKA at 10 years. Results were not promising for the implants used, compared with other implants, but the authors concluded that, because of improvements in implant designs and image-guided techniques, better long-term success can be expected with UKA than with HTO.
In another prospective study, Börjesson and colleagues49 evaluated pain during walking, ROM, British Orthopaedic Association (BOA) scores, and gait variables at 1- and 5-year follow-up. Patients with moderate medial OA (Ahlbäck14 grade I-III) were randomly selected to undergo CWHTO or UKA (Brigham, DePuy). There were no significant differences in BOA scores, ROM, or pain during walking between the 2 groups at 3 months, 1 year, and 5 years after surgery. Gait analysis showed a significant difference in favor of UKA only at 3 months after surgery. At 1- and 5-year follow-up, no significant differences were noted.
To clarify current ambiguities, Fu and colleagues50 performed a systematic review of all (11) comparative studies. These studies had a total of 5840 (5081 UKA, 759 HTO) patients. Although ROM was significantly better for the HTO group than the UKA group, the UKA group had significantly better functional results. Walking after surgery was significantly faster for the UKA group. The authors suggested the difference might be attributed to the different postoperative regimens—HTO patients wore a whole-leg plaster cast for 6 weeks, and UKA patients were allowed immediate postoperative weight-bearing. Regarding rates of survival and complications, pooled data showed no significant differences. Despite these results, the authors acknowledged the limitation of available randomized clinical trials and the multiple techniques and implants used. We share their assertion that larger prospective controlled trials are needed. These are crucial to getting a definitive answer regarding which of the 2 treatment strategies should be used for isolated compartment OA.
Current Trends in Use of UKA and HTO
Evaluation of national registries and recent reports showed a global shift in use of both HTO and UKA. Despite the lack of national HTO registries, a few reports have described use of TKA, UKA, and HTO in Western populations over the past 2 decades. Using 1998-2007 data from the Swedish Knee Arthroplasty Register, W-Dahl and colleagues51 found a 3-fold increase in UKA use, whereas HTO use was halved over the same period. Niinimäki and colleagues52 reported similar findings with the Finnish National Hospital Discharge Register. They noted a steady 6.8% annual decrease in osteotomies, whereas UKA use increased sharply after the Oxford UKA was introduced (Phase 3; Biomet). These findings are consistent with several reports from North America. In their epidemiologic analysis covering the period 1985-1990, Wright and colleagues53 found an 11% to 14% annual decrease in osteotomies among the elderly, compared with an annual decrease of only 3% to 4% among patients younger than 65 years. Nwachukwu and colleagues54 recently compared UKA and HTO practice patterns between 2007 and 2011, using data from a large US private payer insurance database. They noted an annual growth rate of 4.7% in UKA use, compared with an annual 3.9% decrease in HTO use. Furthermore, based on their subgroup analysis, they speculated there was a demographic shift toward UKA, as opposed to TKA, particularly in older women. Bolognesi and colleagues55 investigated further. Evaluating all Medicare beneficiaries who underwent knee arthroplasty in the United States between 2000 and 2009, they noted a 1.7-fold increase in TKA use and a 6.2-fold increase in UKA use. As there were no substantial changes in patient characteristics over that period, the authors hypothesized that a possible broadening of inclusion criteria may have led to the increased use of UKA.
There is a possible multifactorial explanation for the current global shift in favor of UKA. First, UKA was once a technically demanding procedure, but improved surgical techniques, image guidance, and robot assistance56 have made it relatively less difficult. Second, UKA surgery is associated with lower reported perioperative morbidities.57 We think these factors have contributed to the global trend of less HTO use and more UKA use in the treatment of unicompartmental OA.
Conclusion
The modern literature suggests the inclusion criteria for HTO have been well investigated and defined; the UKA criteria remain a matter of debate but seem to be expanding. Long-term survival results seem to favor UKA, though patient satisfaction with both procedures is good to excellent. The broadening range of inclusion criteria and consistent reports of durable outcomes, coupled with excellent patient satisfaction, likely explain the shift toward UKA in the treatment of isolated compartment degeneration.
Am J Orthop. 2016;45(6):E355-E361. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7): 520-526.
2. Wise BL, Niu J, Yang M, et al; Multicenter Osteoarthritis (MOST) Group. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 2012;64(6): 847-852.
3. Jackson JP, Waugh W. Tibial osteotomy for osteoarthritis of the knee. J Bone Joint Surg Br. 1961;43:746-751.
4. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88(11):1454-1459.
5. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425-1432.
6. Hutchison CR, Cho B, Wong N, Agnidis Z, Gross AE. Proximal valgus tibial osteotomy for osteoarthritis of the knee. Instr Course Lect. 1999;48:131-134.
7. Trieb K, Grohs J, Hanslik-Schnabel B, Stulnig T, Panotopoulos J, Wanivenhaus A. Age predicts outcome of high-tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):149-152.
8. Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64-70.
9. Howells NR, Salmon L, Waller A, Scanelli J, Pinczewski LA. The outcome at ten years of lateral closing-wedge high tibial osteotomy: determinants of survival and functional outcome. Bone Joint J Br. 2014;96(11):1491-1497.
10. Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J. Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: Finnish registry-based study of 3195 knees. J Bone Joint Surg Br. 2012;94(11):1517-1521.
11. Flecher X, Parratte S, Aubaniac JM, Argenson JN. A 12-28-year followup study of closing wedge high tibial osteotomy. Clin Orthop Relat Res. 2006;(452):91-96.
12. Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90(5):592-596.
13. van Raaij T, Reijman M, Brouwer RW, Jakma TS, Verhaar JN. Survival of closing-wedge high tibial osteotomy: good outcome in men with low-grade osteoarthritis after 10-16 years. Acta Orthop. 2008;79:230-234.
14. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
15. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-1711.
16. Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;(390):173-181.
17. Naudie D, Bourne RB, Rorabeck CH, Bourne TJ. The Install Award. Survivorship of the high tibial valgus osteotomy. A 10- to -22-year followup study. Clin Orthop Relat Res. 1999;(367):18-27.
18. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Br. 2003;85(3):469-474.
19. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
20. Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11(6):439-444.
21. Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):122-127.
22. Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62(8):1329-1337.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
24. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
25. Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19(5):585-591.
26. Schai PA, Suh JT, Thornhill TS, Scott RD. Unicompartmental knee arthroplasty in middle-aged patients: a 2- to 6-year follow-up evaluation. J Arthroplasty. 1998;13(4):365-372.
27. Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87(11):1488-1492.
28. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2003;85(10):1968-1973.
29. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J Br. 2013;95(8):1064-1068.
30. Tabor OB Jr, Tabor OB, Bernard M, Wan JY. Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv. 2005;14(2):59-63.
31. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(5 suppl):19-23.
32. Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25(5):369-373.
33. Plate JF, Augart MA, Seyler TM, et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty [published online April 12, 2015]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3597-5.
34. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
35. Carlsson LV, Albrektsson BE, Regnér LR. Minimally invasive surgery vs conventional exposure using the Miller-Galante unicompartmental knee arthroplasty: a randomized radiostereometric study. J Arthroplasty. 2006;21(2):151-156.
36. Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282-286.
37. Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54-60.
38. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
39. Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78(1):128-135.
40. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
41. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
42. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3296-3305.
43. Zambianchi F, Digennaro V, Giorgini A, et al. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2074-2080.
44. Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45-49.
45. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-525.
46. Robertsson O, Lidgren L. The short-term results of 3 common UKA implants during different periods in Sweden. J Arthroplasty. 2008;23(6):801-807.
47. Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7-10-year follow-up prospective randomised study. Knee. 2001;8(3):187-194.
48. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55(1):23-48.
49. Börjesson M, Weidenhielm L, Mattsson E, Olsson E. Gait and clinical measurements in patients with knee osteoarthritis after surgery: a prospective 5-year follow-up study. Knee. 2005;12(2):121-127.
50. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013;28(5):759-765.
51. W-Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2010;81(2):161-164.
52. Niinimäki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399-1402.
53. Wright J, Heck D, Hawker G, et al. Rates of tibial osteotomies in Canada and the United States. Clin Orthop Relat Res. 1995;(319):266-275.
54. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
55. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
56. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
57. Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8 suppl):86-90.
1. Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7): 520-526.
2. Wise BL, Niu J, Yang M, et al; Multicenter Osteoarthritis (MOST) Group. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 2012;64(6): 847-852.
3. Jackson JP, Waugh W. Tibial osteotomy for osteoarthritis of the knee. J Bone Joint Surg Br. 1961;43:746-751.
4. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88(11):1454-1459.
5. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425-1432.
6. Hutchison CR, Cho B, Wong N, Agnidis Z, Gross AE. Proximal valgus tibial osteotomy for osteoarthritis of the knee. Instr Course Lect. 1999;48:131-134.
7. Trieb K, Grohs J, Hanslik-Schnabel B, Stulnig T, Panotopoulos J, Wanivenhaus A. Age predicts outcome of high-tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):149-152.
8. Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64-70.
9. Howells NR, Salmon L, Waller A, Scanelli J, Pinczewski LA. The outcome at ten years of lateral closing-wedge high tibial osteotomy: determinants of survival and functional outcome. Bone Joint J Br. 2014;96(11):1491-1497.
10. Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J. Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: Finnish registry-based study of 3195 knees. J Bone Joint Surg Br. 2012;94(11):1517-1521.
11. Flecher X, Parratte S, Aubaniac JM, Argenson JN. A 12-28-year followup study of closing wedge high tibial osteotomy. Clin Orthop Relat Res. 2006;(452):91-96.
12. Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90(5):592-596.
13. van Raaij T, Reijman M, Brouwer RW, Jakma TS, Verhaar JN. Survival of closing-wedge high tibial osteotomy: good outcome in men with low-grade osteoarthritis after 10-16 years. Acta Orthop. 2008;79:230-234.
14. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
15. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-1711.
16. Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;(390):173-181.
17. Naudie D, Bourne RB, Rorabeck CH, Bourne TJ. The Install Award. Survivorship of the high tibial valgus osteotomy. A 10- to -22-year followup study. Clin Orthop Relat Res. 1999;(367):18-27.
18. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Br. 2003;85(3):469-474.
19. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
20. Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11(6):439-444.
21. Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):122-127.
22. Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62(8):1329-1337.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
24. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
25. Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19(5):585-591.
26. Schai PA, Suh JT, Thornhill TS, Scott RD. Unicompartmental knee arthroplasty in middle-aged patients: a 2- to 6-year follow-up evaluation. J Arthroplasty. 1998;13(4):365-372.
27. Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87(11):1488-1492.
28. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2003;85(10):1968-1973.
29. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J Br. 2013;95(8):1064-1068.
30. Tabor OB Jr, Tabor OB, Bernard M, Wan JY. Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv. 2005;14(2):59-63.
31. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(5 suppl):19-23.
32. Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25(5):369-373.
33. Plate JF, Augart MA, Seyler TM, et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty [published online April 12, 2015]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3597-5.
34. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
35. Carlsson LV, Albrektsson BE, Regnér LR. Minimally invasive surgery vs conventional exposure using the Miller-Galante unicompartmental knee arthroplasty: a randomized radiostereometric study. J Arthroplasty. 2006;21(2):151-156.
36. Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282-286.
37. Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54-60.
38. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
39. Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78(1):128-135.
40. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
41. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
42. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3296-3305.
43. Zambianchi F, Digennaro V, Giorgini A, et al. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2074-2080.
44. Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45-49.
45. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-525.
46. Robertsson O, Lidgren L. The short-term results of 3 common UKA implants during different periods in Sweden. J Arthroplasty. 2008;23(6):801-807.
47. Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7-10-year follow-up prospective randomised study. Knee. 2001;8(3):187-194.
48. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55(1):23-48.
49. Börjesson M, Weidenhielm L, Mattsson E, Olsson E. Gait and clinical measurements in patients with knee osteoarthritis after surgery: a prospective 5-year follow-up study. Knee. 2005;12(2):121-127.
50. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013;28(5):759-765.
51. W-Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2010;81(2):161-164.
52. Niinimäki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399-1402.
53. Wright J, Heck D, Hawker G, et al. Rates of tibial osteotomies in Canada and the United States. Clin Orthop Relat Res. 1995;(319):266-275.
54. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
55. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
56. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
57. Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8 suppl):86-90.
Concussions in American Football
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
Exertional Heat Stroke and American Football: What the Team Physician Needs to Know
Football, one of the most popular sports in the United States, is additionally recognized as a leading contributor to sports injury secondary to the contact collision nature of the endeavor. There are an estimated 1.1 million high school football players with another 100,000 participants combined in the National Football League (NFL), college, junior college, Arena Football League, and semipro levels of play.1 USA Football estimates that an additional 3 million youth participate in community football leagues.1 The National Center for Catastrophic Sports Injury Research recently calculated a fatality rate of 0.14 per 100,000 participants in 2014 for the 4.2 million who play football at all levels—and 0.45 per 100,000 in high school.1 While direct deaths from head and spine injury remain a significant contributor to the number of catastrophic injuries, indirect deaths (systemic failure) predominate. Exertional heat stroke (EHS) has emerged as one of the leading indirect causes of death in high school and collegiate football. Boden and colleagues2 reported that high school and college football players sustain approximately 12 fatalities annually, with indirect systemic causes being twice as common as direct blunt trauma.2The most common indirect causes identified included cardiac failure, heat illness, and complications of sickle cell trait (SCT). It was also noted that the risk of SCT, heat-related, and cardiac deaths increased during the second decade of the study, indicating these conditions may require a greater emphasis on diagnosis, treatment, and prevention. This review details for the team physician the unique challenge of exercising in the heat to the football player, and the prevention, diagnosis, management and return-to-play issues pertinent to exertional heat illness (EHI).
The Challenge
EHS represents the most severe manifestation of EHI—a gamut of diseases commonly encountered during the hot summer months when American football season begins. The breadth of EHI includes several important clinical diagnoses: exercise-associated muscle cramps (heat cramps); heat exhaustion with and without syncope; heat injury with evidence of end organ injury (eg, rhabdomyolysis); and EHS. EHS is defined as “a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi-organ dysfunction in which encephalopathy predominates.”3 EHS, if left untreated, or even if clinical treatment is delayed, may result in significant end organ morbidity and/or mortality.
During exercise, the human thermoregulatory system mitigates heat gain by increasing skin blood flow and sweating, causing an increased dissipation of heat to the surrounding environment by leveraging conduction, convection, and evaporation.4,5 Elevated environmental temperatures, increased humidity, and dehydration can impede the body’s ability to dissipate heat at a rate needed to maintain thermoregulation. This imbalance can result in hyperthermia secondary to uncompensated heat stress,5 which in turn can lead to EHI. Football players have unique challenges that make them particularly vulnerable to EHI. The summer heat during early-season participation and the requirement for equipment that covers nearly 60% of body surfaces pose increased risk of volume losses and hyperthermia that trigger the onset of EHI.6 Football athletes’ body compositions and physical size are additional contributing risk factors; the relatively high muscle and fat content increase thermogenicity, which require their bodies to dissipate more heat.7
An estimated 9000 cases of EHI occur annually across all high school sports,8 with an incidence of 1.6:100,000 athlete-exposures.8,9 Studies have demonstrated, however, that EHI occurs in football 11.4 times more often than in all other high school sports combined.10 The incidence of nonfatal EHI in all levels of football is 4.42-5:100,000.8,9 Between 2000 and 2014, 41 football players died from EHS.1 In football, approximately 75% of all EHI events occurred during practices, while only 25% of incidents occurred during games.8
Given these potentially deadly consequences, it is important that football team physicians are not only alert to the early symptoms of heat illness and prepared to intervene to prevent the progression to EHS, but are critical leaders in educating coaches and players in evidence-based EHI prevention practices and policies.
Prevention
EHS is a preventable condition, arguably the most common cause of preventable nontraumatic exertional death in young athletes in the United States. Close attention to mitigating risk factors should begin prior to the onset of preseason practice and continue through the early season, where athletes are at the highest risk of developing heat illness.
Primary Prevention
Primary prevention is fundamental to minimizing the occurrences of EHI. It focuses on the following methods: recognition of inherent risk factors, acclimatization, hydration, and avoidance of inciting substances (including supplements).
Pre-Participation Examination. The purpose of the pre-participation examination (PPE) is to maximize an athlete’s safety by identifying medical conditions that place the athlete at risk.11,12 The Preparticipation Physical Evaluation, 4th edition, the most widely used consensus publication, specifically queries if an athlete has a previous history of heat injury. However, it only indirectly addresses intrinsic risk factors that may predispose an athlete to EHI who has never had an EHI before. Therefore, providers should take the opportunity of the PPE to inquire about additional risk factors that may make an athlete high risk for sustaining a heat injury. Common risk factors for EHI are listed in Table 1.
Heat Acclimatization. The risk of EHI escalates significantly when athletes are subjected to multiple stressors during periods of heat exposure, such as sudden increases in intensity or duration of exercise; prolonged new exposures to heat; dehydration; and sleep loss.5 When football season begins in late summer, athletes are least conditioned as temperatures reach their seasonal peak, causing increased risk of EHI.15 Planning for heat acclimatization is vital for all athletes who exercise in hot environments. Acclimatization procedures place progressively mounting physiologic strains on the body to improve athletes’ ability to dissipate heat, diminishing thermoregulatory and cardiovascular exertion.4,5 Acclimatization begins with expansion of plasma volume on days 3 to 6, causing improvements in cardiac efficiency and resulting in an overall decrease in basal internal body temperature.4,5,15 This process results in improvements in heat tolerance and exercise performance, evolving over 10 to 14 days of gradual escalation of exercise intensity and duration.5,10,11,16 However, poor fitness levels and extreme temperatures can prolong this period, requiring up to 2 to 3 months to fully take effect.5,7
The National Athletic Trainers Association (NATA) and National Collegiate Athletic Association (NCAA) have released consensus guidelines regarding heat acclimatization protocols for football athletes at the high school and college levels (Tables 3 and 4). Each of these guidelines involves an initial period without use of protective equipment, followed by a gradual addition of further equipment.11,16
Secondary Prevention
Despite physicians’ best efforts to prevent all cases of EHI, athletes will still experience the effects of exercise-induced hyperthermia. The goal of secondary prevention is to slow the progression of this hyperthermia so that it does not progress to more dangerous EHI.
Hydration. Dehydration is an important risk factor for EHI. Sweat maintains thermoregulation by dissipating heat generated during exercise; however, it also contributes to body water losses. Furthermore, intravascular depletion decreases stroke volume, thereby increasing cardiovascular strain. It is estimated that for every 1% loss in body mass from dehydration, body temperature rises 0.22°C in comparison to a euhydrated state.6 Dehydration occurs more rapidly in hot environments, as fluid is lost through increased sweat production.7 After approximately 6% to 10% body weight volume loss, cardiac output cannot be maintained, diminishing sweat production and blood flow to both skin and muscle and causing diminished performance and a significant risk of heat exhaustion.7 If left unchecked, these physiologic changes result in further elevations in body temperature and increased cardiovascular strain, ultimately placing the athlete at significant risk for development of EHS.
Adequate hydration to maintain euvolemia is an important step in avoiding possible EHI. Multiple studies have shown that football players experience a baseline hypovolemia during their competition season,6 a deficit that is most marked during the first week of practices.17 This deficit is multifactorial, as football players expend a significant amount of fluid through sweat, are not able to adequately replace these losses during practice, and do not appropriately hydrate off the field.6,18 Some players, especially linemen, sweat at a higher rate than their teammates, posing a possible risk of significant dehydration.6 Coaches and players alike should be educated on the importance of adequate hydration to meet their fluid needs.
The goal of hydration during exercise is to prevent large fluid losses that can adversely affect performance and increase risk of EHI;6 it may be unrealistic to replace all fluid losses during the practice period. Instead, athletes should target complete volume replacement over the post-exercise period.6 Some recommend hydrating based upon thirst drive; however, thirst is activated following a volume loss of approximately 2% body mass, the same degree of losses that place athletes at an increased risk for performance impairment and EHI.4,6,11,12 Individuals should have access to fluids throughout practice and competition and be encouraged to hydrate as needed.6,12,15 Furthermore, staff should modify their practices based upon WBGT and acclimatization status to provide more frequent hydration breaks.
Hyperhydration and Salt Intake. Of note, there are inherent risks to hyperhydration. Athletes with low sweat rates have an increased risk of overhydration and the development of exercise-associated hyponatremia (EAH),6 a condition whose presentation is very similar to EHS. In addition, inadequate sodium intake and excessive sweating can also contribute to the development of EAH. EAH has been implicated in the deaths of 2 football players in 2014.1,6 Establishing team hydration guidelines and educating players and staff on appropriate hydration and dietary salt intake is essential to reduce the risk of both dehydration and hyperhydration and their complications.6Intra-Event Cooling. During exercise, team physicians can employ strategies for cooling athletes during exertion to mitigate their risk of EHI by decreasing thermal and cardiovascular strain.4,19 Cooling during exercise is hypothesized to allow for accelerated heat dissipation, where heat is lost from the body more effectively. This accelerated loss enables athletes to maintain a higher heat storage capacity over the duration of exercise, avoiding uncompensated heat stresses that ultimately cause EHI.19
Some intra-event cooling strategies include the use of cooling garments, cooling packs, and cold water/slurry ingestion. Cooling garments lower skin temperature, which in turn can decrease thermoregulatory strains;4 a recent meta-analysis of intra-event cooling modalities revealed that wearing an ice vest during exercise resulted in the greatest decrease in thermal heat strain.19 Internal cooling strategies—namely ingestion of cold fluids/ice slurry—have shown some mild benefit in decreasing internal temperatures; however, some studies have demonstrated some decrease in sweat production associated with cold oral intake used in isolation.19 Overall, studies have shown that combining external (cooling clothing, ice packs, fanning) and internal (cold water, ice slurry) cooling methods result in a greater cooling effect than a use of a single method.4
Tertiary Prevention
The goal of tertiary prevention is to mitigate the risk of long-term adverse outcomes following an EHS event. The most effective means of reducing risk for morbidity and mortality is rapid identification and treatment of EHS as well as close evaluation of an athlete’s return to activity in heat. This process is spearheaded by an effective and well-rehearsed emergency action plan.
Diagnosis and Management
Rapid identification and treatment of EHS is crucial to minimizing the risk of poor outcomes.7 Any delay in the treatment of EHS can dramatically increase the likelihood of associated morbidity and mortality.20
EHS is diagnosed by an elevated rectal temperature ≥40°C (104°F) and associated central nervous system (CNS) dysfunction.21 EHS should be strongly suspected in any athlete exercising in heat who exhibits signs of CNS dysfunction, including disorientation, confusion, dizziness, erratic behavior, irritability, headache, loss of coordination, delirium, collapse, or seizures.7,12,15 EHS may also present with symptoms of heat exhaustion, including fatigue, hyperventilation, tachycardia, vomiting, diarrhea, and hypotension.7,12,15
Rectal temperature should be taken for any athlete with suspected EHS, as other modalities—oral, skin, axillary, and aural—can be inaccurate and easily modified by ambient confounders such as ambient and skin temperature, athlete hyperventilation, and consumption of liquids.7,11,12 Athletes exhibiting CNS symptoms with moderately elevated rectal temperatures that do not exceed 40°C should also be assumed to be suffering from EHS and treated with rapid cooling.11 On the other hand, athletes with CNS symptoms who are normothermic should be assumed to have EAH until ruled out by electrolyte assessment; IV fluids should be at no more than keep vein open (KVO) pending this determination.11 In some cases, an athlete may initially present with altered mental status but return to “normal.” However, this improvement may represent a “lucid period”; evaluation should continue with rectal temperature and treatment, as EHS in these cases may progress quickly.15
Treatment is centered on rapid, whole body cooling initiated at the first sign of heat illness.7,22 The goal of treatment is to achieve a rectal temperature <38.9°C within 30 minutes of the onset of EHS.15 Upon diagnosis, the athlete should be quickly placed in a tub of ice water to facilitate cold water immersion (CWI) therapy. Some guidelines suggest the athlete’s clothing be removed to potentiate evaporative cooling during CWI;12 however, cooling should not be delayed due to difficulties in removing equipment. CWI, where a heat stroke victim is submerged in ice water up to their neck while water is continuously circulated, is generally considered to be the gold standard treatment as it is the modality with the highest recorded cooling rates and the lowest rate of morbidity and mortality.7,20,21 Multiple studies of CWI have shown that survival nears 100% when aggressive cooling starts within 5 minutes of collapse or identification of EHS.20,21,22
If whole body CWI is unavailable, alternative methods of rapid cooling should be employed. Partial CWI, with torso immersion being preferable to the extremities, has been shown to achieve an acceptable rate of cooling to achieve sufficient drops in internal body temperature.20,23 However, one popular treatment—applying ice packs to the whole body, in particular to the groin and axillae—has not been shown to be sufficient to achieve standard cooling goals.20 None of these methods have been shown to be as effective as CWI.23
Intravenous access should be initiated with fluid resuscitation dictated by the provider’s assessment. Normal saline is recommended as the resuscitative fluid of choice, with the rate dictated by clinical judgment and adjusted as guided by electrolyte determination and clinical response. It cannot be overstated that in normothermic patients with confusion, EAH is the diagnosis of exclusion and aggressive fluid resuscitation should be withheld until electrolyte determination.
Once rectal temperature descends appropriately (~38.9°C), the cooling process should stop and the individual should be transported to a hospital for further observation20 and evaluation of possible sequelae, including rhabdomyolysis and renal injury, cardiac dysfunction and arrhythmia, severe electrolyte abnormalities, acute respiratory distress syndrome, lactic acidosis, and other forms of end-organ failure (Figure).
Rapid cooling is more crucial than transport; transport poses a risk of delayed cooling, which can dramatically increase an individual’s risk of morbidity and mortality.20,23 In situations where a patient can be cooled on-site, physicians should pursue cooling before transporting the patient to a medical treatment facility.
Emergency Action Plan
Team physicians should be proactive in developing an emergency action plan to address possible EHS events. These plans should be site-specific, addressing procedures for all practice and home competition locations.12 All competition venues should have a CWI tub on-site in events where there is an increased risk of EHS.12,15,20 This tub should be set up and functional for all high-risk activities, including practices.12
Following recognition of a potential case of EHS, treatment teams should have procedures in place to transport athletes to the treatment area, obtain rectal temperature, initiate rapid cooling, and stabilize the athlete for transport to an emergency department (ED) for further evaluation.12,15 A written record of treatments and medications provided during athlete stabilization should be maintained and transported with the athlete to the ED.15 A list of helpful equipment and supplies for treatment of EHS can be found in Table 5.
EHS is a unique life-threatening situation where it is best to treat the patient on the sideline before transport.15 Those athletes transported before cooling risk spending an increased amount of time above critical temperatures for cell damage, which has been associated with increased morbidity and mortality. This mantra of “cool first, transport second” cannot be overemphasized, as those individuals with EHS who present to the ED with a persisting rectal temperature >41°F may risk up to an 80% mortality rate.24 Conversely, a recent large, retrospective study of 274 EHS events sustained during the Falmouth Road Race found a 100% survival rate when athletes were rapidly identified via rectal thermometry and treated with aggressive, rapid cooling through CWI.21
Return to Play
Perhaps the most challenging and important role the team physician has is determining an athlete’s return to play following EHI, as there currently are no evidence-based guidelines for return to activity for these athletes.7 The decisions surrounding return to play are highly individualized, as recovery from EHS and heat injury is associated with the duration of internal body temperature elevation above the critical level (40°C).7,20 Guidelines for return to activity following recovery from EHI differ among experts and institutions.7,25 The general consensus from these guidelines is that, at minimum, athletes should not participate in any physical activity until they are asymptomatic and all blood tests have normalized.11 Following this asymptomatic period, most guidelines advocate for a slow, deliberate return to activity.11 The American College of Sports Medicine (ACSM) offers one reasonable approach to the returning athlete following EHS:7
- No exercise for at least 7 days following release from medical care.
- Follow-up with a physician 1 week after release from medical care for physical examination and any warranted lab or radiologic studies (based upon organ systems affected during EHS).
- Once cleared to return to activity, the athlete begins exercise in a cool environment, gradually increasing the duration, intensity, and heat exposure over 2 weeks to demonstrate heat tolerance and acclimatization.
- Athletes who cannot resume vigorous activity due to recurrent symptoms (eg, excessive fatigue) should be reevaluated after 4 weeks. Laboratory exercise-heat tolerance testing may be useful in this setting.
- The athlete may resume full competition once they are able to participate in full training in the heat for 2 to 4 weeks without adverse effects.
Heat tolerance testing (HTT) in these athletes remains controversial.5 26 The ACSM recommends that HTT be considered only for those unable to return to vigorous activity after a suitable period (approximately 4 weeks). In contrast, the Israeli Defense Force (IDF) uses HTT to evaluate soldiers following EHS to guide decision-making about return to duty.27 The IDF HTT assumes that individuals will respond differently to heat stresses. They identify individuals who are “heat intolerant” as being unable to tolerate specific heat challenges, indicated by increases in body temperature occurring more rapidly than normal responders under identical environmental and exercise conditions. However, despite being used for more than 30 years, there is no clear evidence that HTT adequately predicts who will experience subsequent episodes of EHS.
Conclusion
While the recognized cornerstone of being a team physician is the provision of medical care, the ACSM Team Physician Consensus Statement28 further delineates the medical and administrative responsibilities as both (1) understanding medical management and prevention of injury and illness in athletes; and (2) awareness of or involvement in the development and rehearsal of an emergency action plan. These tenets are critical for the team physician who accepts the responsibility to cover sports at the high school level or higher. Football team physicians play an essential role in mitigating risk of EHI in their athletes. Through development and execution of both comprehensive prevention strategies and emergency action plans, physicians can work to minimize athletes’ risk of both developing and experiencing significant adverse outcomes from an EHI.
Am J Orthop. 2016;45(6):340-348. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kucera KL, Klossner D, Colgate B, Cantu RC. Annual Survey of Football Injury Research: 1931-2014. National Center for Catastrophic Sport Injury Research Web site. https://nccsir.unc.edu/files/2013/10/Annual-Football-2014-Fatalities-Final.pdf. Accessed May 31, 2016.
2. Boden BP, Breit I, Beachler JA, Williams A, Mueller FO. Fatalities in high school and college football players. Am J Sports Med. 2013;41(5):1108-1116.
3. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978-1988.
4. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25 Suppl 1:6-19.
5. Pryor RR, Casa DJ, Adams WM, et al. Maximizing athletic performance in the heat. Strength Cond J. 2013;35(6):24-33.
6. Adams WM, Casa DJ. Hydration for football athletes. Sports Sci Exchange. 2015;28(141):1-5.
7. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
8. Yard EE, Gilchrist J, Haileyesus T, et al. Heat illness among high school athletes--United States, 2005-2009. J Safety Res. 2010;41(6):471-474.
9. Huffman EA, Yard EE, Fields SK, Collins CL, Comstock RD. Epidemiology of rare injuries and conditions among United States high school athletes during the 2005-2006 and 2006-2007 school years. J Athl Train. 2008;43(6):624-630.
10. Kerr ZY, Casa DJ, Marshall SW, Comstock RD. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8-14.
11. Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986-1000.
12. Casa DJ, Almquist J, Anderson SA. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. J Athl Train. 2013;48(4):546-553.
13. Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28(8):939-944.
14. Gundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometerol. 2012;56(1):11-20.
15. Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117-127.
16. Casa DJ, Csillan D; Inter-Association Task Force for Preseason Secondary School Athletics Participants, et al. Preseason heat-acclimatization guidelines for secondary school athletics. J Athl Train. 2009;44(3):332-333.
17. Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843-851.
18. Stover EA, Zachwieja J, Stofan J, Murray R, Horswill CA. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330-335.
19. Bongers CC, Thijssen DH, Veltmeijer MTW, Hopman MT, Eijsvogels TM. Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br J Sports Med. 2015;49(6):377-384.
20. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141-149.
21. DeMartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47(2):240-245.
22. Casa DJ, Kenny GP, Taylor NA. Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc. 2010;42(7):1246-1252.
23. Casa DJ, Armstrong LE, Kenny GP, O’Connor FG, Huggins RA. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11(3):115-122.
24. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heat stroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
25. O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine Roundtable on exertional heat stroke--return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314-321.
26. Kazman JB, Heled Y, Lisman PJ, Druyan A, Deuster PA, O’Connor FG. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101-105.
27. Moran DS, Heled Y, Still L, Laor A, Shapiro Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):CR252-CR257.
28. Herring SA, Kibler WB, Putukian M. Team Physician Consensus Statement: 2013 update. Med Sci Sports Exerc. 2013;45(8):1618-1622.
29. Heat stroke treatment. Korey Stringer Institute University of Connecticut Web site. http://ksi.uconn.edu/emergency-conditions/heat-illnesses/exertional-heat-stroke/heat-stroke-treatment/. Accessed June 14, 2016.
30. Headquarters, Department of the Army and the Air Force. Heat Stress Control and Heat Casualty Management. Technical Bulletin Medical 507. http://www.dir.ca.gov/oshsb/documents/Heat_illness_prevention_tbmed507.pdf. Published March 7, 2003. Accessed June 14, 2016.
Football, one of the most popular sports in the United States, is additionally recognized as a leading contributor to sports injury secondary to the contact collision nature of the endeavor. There are an estimated 1.1 million high school football players with another 100,000 participants combined in the National Football League (NFL), college, junior college, Arena Football League, and semipro levels of play.1 USA Football estimates that an additional 3 million youth participate in community football leagues.1 The National Center for Catastrophic Sports Injury Research recently calculated a fatality rate of 0.14 per 100,000 participants in 2014 for the 4.2 million who play football at all levels—and 0.45 per 100,000 in high school.1 While direct deaths from head and spine injury remain a significant contributor to the number of catastrophic injuries, indirect deaths (systemic failure) predominate. Exertional heat stroke (EHS) has emerged as one of the leading indirect causes of death in high school and collegiate football. Boden and colleagues2 reported that high school and college football players sustain approximately 12 fatalities annually, with indirect systemic causes being twice as common as direct blunt trauma.2The most common indirect causes identified included cardiac failure, heat illness, and complications of sickle cell trait (SCT). It was also noted that the risk of SCT, heat-related, and cardiac deaths increased during the second decade of the study, indicating these conditions may require a greater emphasis on diagnosis, treatment, and prevention. This review details for the team physician the unique challenge of exercising in the heat to the football player, and the prevention, diagnosis, management and return-to-play issues pertinent to exertional heat illness (EHI).
The Challenge
EHS represents the most severe manifestation of EHI—a gamut of diseases commonly encountered during the hot summer months when American football season begins. The breadth of EHI includes several important clinical diagnoses: exercise-associated muscle cramps (heat cramps); heat exhaustion with and without syncope; heat injury with evidence of end organ injury (eg, rhabdomyolysis); and EHS. EHS is defined as “a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi-organ dysfunction in which encephalopathy predominates.”3 EHS, if left untreated, or even if clinical treatment is delayed, may result in significant end organ morbidity and/or mortality.
During exercise, the human thermoregulatory system mitigates heat gain by increasing skin blood flow and sweating, causing an increased dissipation of heat to the surrounding environment by leveraging conduction, convection, and evaporation.4,5 Elevated environmental temperatures, increased humidity, and dehydration can impede the body’s ability to dissipate heat at a rate needed to maintain thermoregulation. This imbalance can result in hyperthermia secondary to uncompensated heat stress,5 which in turn can lead to EHI. Football players have unique challenges that make them particularly vulnerable to EHI. The summer heat during early-season participation and the requirement for equipment that covers nearly 60% of body surfaces pose increased risk of volume losses and hyperthermia that trigger the onset of EHI.6 Football athletes’ body compositions and physical size are additional contributing risk factors; the relatively high muscle and fat content increase thermogenicity, which require their bodies to dissipate more heat.7
An estimated 9000 cases of EHI occur annually across all high school sports,8 with an incidence of 1.6:100,000 athlete-exposures.8,9 Studies have demonstrated, however, that EHI occurs in football 11.4 times more often than in all other high school sports combined.10 The incidence of nonfatal EHI in all levels of football is 4.42-5:100,000.8,9 Between 2000 and 2014, 41 football players died from EHS.1 In football, approximately 75% of all EHI events occurred during practices, while only 25% of incidents occurred during games.8
Given these potentially deadly consequences, it is important that football team physicians are not only alert to the early symptoms of heat illness and prepared to intervene to prevent the progression to EHS, but are critical leaders in educating coaches and players in evidence-based EHI prevention practices and policies.
Prevention
EHS is a preventable condition, arguably the most common cause of preventable nontraumatic exertional death in young athletes in the United States. Close attention to mitigating risk factors should begin prior to the onset of preseason practice and continue through the early season, where athletes are at the highest risk of developing heat illness.
Primary Prevention
Primary prevention is fundamental to minimizing the occurrences of EHI. It focuses on the following methods: recognition of inherent risk factors, acclimatization, hydration, and avoidance of inciting substances (including supplements).
Pre-Participation Examination. The purpose of the pre-participation examination (PPE) is to maximize an athlete’s safety by identifying medical conditions that place the athlete at risk.11,12 The Preparticipation Physical Evaluation, 4th edition, the most widely used consensus publication, specifically queries if an athlete has a previous history of heat injury. However, it only indirectly addresses intrinsic risk factors that may predispose an athlete to EHI who has never had an EHI before. Therefore, providers should take the opportunity of the PPE to inquire about additional risk factors that may make an athlete high risk for sustaining a heat injury. Common risk factors for EHI are listed in Table 1.
Heat Acclimatization. The risk of EHI escalates significantly when athletes are subjected to multiple stressors during periods of heat exposure, such as sudden increases in intensity or duration of exercise; prolonged new exposures to heat; dehydration; and sleep loss.5 When football season begins in late summer, athletes are least conditioned as temperatures reach their seasonal peak, causing increased risk of EHI.15 Planning for heat acclimatization is vital for all athletes who exercise in hot environments. Acclimatization procedures place progressively mounting physiologic strains on the body to improve athletes’ ability to dissipate heat, diminishing thermoregulatory and cardiovascular exertion.4,5 Acclimatization begins with expansion of plasma volume on days 3 to 6, causing improvements in cardiac efficiency and resulting in an overall decrease in basal internal body temperature.4,5,15 This process results in improvements in heat tolerance and exercise performance, evolving over 10 to 14 days of gradual escalation of exercise intensity and duration.5,10,11,16 However, poor fitness levels and extreme temperatures can prolong this period, requiring up to 2 to 3 months to fully take effect.5,7
The National Athletic Trainers Association (NATA) and National Collegiate Athletic Association (NCAA) have released consensus guidelines regarding heat acclimatization protocols for football athletes at the high school and college levels (Tables 3 and 4). Each of these guidelines involves an initial period without use of protective equipment, followed by a gradual addition of further equipment.11,16
Secondary Prevention
Despite physicians’ best efforts to prevent all cases of EHI, athletes will still experience the effects of exercise-induced hyperthermia. The goal of secondary prevention is to slow the progression of this hyperthermia so that it does not progress to more dangerous EHI.
Hydration. Dehydration is an important risk factor for EHI. Sweat maintains thermoregulation by dissipating heat generated during exercise; however, it also contributes to body water losses. Furthermore, intravascular depletion decreases stroke volume, thereby increasing cardiovascular strain. It is estimated that for every 1% loss in body mass from dehydration, body temperature rises 0.22°C in comparison to a euhydrated state.6 Dehydration occurs more rapidly in hot environments, as fluid is lost through increased sweat production.7 After approximately 6% to 10% body weight volume loss, cardiac output cannot be maintained, diminishing sweat production and blood flow to both skin and muscle and causing diminished performance and a significant risk of heat exhaustion.7 If left unchecked, these physiologic changes result in further elevations in body temperature and increased cardiovascular strain, ultimately placing the athlete at significant risk for development of EHS.
Adequate hydration to maintain euvolemia is an important step in avoiding possible EHI. Multiple studies have shown that football players experience a baseline hypovolemia during their competition season,6 a deficit that is most marked during the first week of practices.17 This deficit is multifactorial, as football players expend a significant amount of fluid through sweat, are not able to adequately replace these losses during practice, and do not appropriately hydrate off the field.6,18 Some players, especially linemen, sweat at a higher rate than their teammates, posing a possible risk of significant dehydration.6 Coaches and players alike should be educated on the importance of adequate hydration to meet their fluid needs.
The goal of hydration during exercise is to prevent large fluid losses that can adversely affect performance and increase risk of EHI;6 it may be unrealistic to replace all fluid losses during the practice period. Instead, athletes should target complete volume replacement over the post-exercise period.6 Some recommend hydrating based upon thirst drive; however, thirst is activated following a volume loss of approximately 2% body mass, the same degree of losses that place athletes at an increased risk for performance impairment and EHI.4,6,11,12 Individuals should have access to fluids throughout practice and competition and be encouraged to hydrate as needed.6,12,15 Furthermore, staff should modify their practices based upon WBGT and acclimatization status to provide more frequent hydration breaks.
Hyperhydration and Salt Intake. Of note, there are inherent risks to hyperhydration. Athletes with low sweat rates have an increased risk of overhydration and the development of exercise-associated hyponatremia (EAH),6 a condition whose presentation is very similar to EHS. In addition, inadequate sodium intake and excessive sweating can also contribute to the development of EAH. EAH has been implicated in the deaths of 2 football players in 2014.1,6 Establishing team hydration guidelines and educating players and staff on appropriate hydration and dietary salt intake is essential to reduce the risk of both dehydration and hyperhydration and their complications.6Intra-Event Cooling. During exercise, team physicians can employ strategies for cooling athletes during exertion to mitigate their risk of EHI by decreasing thermal and cardiovascular strain.4,19 Cooling during exercise is hypothesized to allow for accelerated heat dissipation, where heat is lost from the body more effectively. This accelerated loss enables athletes to maintain a higher heat storage capacity over the duration of exercise, avoiding uncompensated heat stresses that ultimately cause EHI.19
Some intra-event cooling strategies include the use of cooling garments, cooling packs, and cold water/slurry ingestion. Cooling garments lower skin temperature, which in turn can decrease thermoregulatory strains;4 a recent meta-analysis of intra-event cooling modalities revealed that wearing an ice vest during exercise resulted in the greatest decrease in thermal heat strain.19 Internal cooling strategies—namely ingestion of cold fluids/ice slurry—have shown some mild benefit in decreasing internal temperatures; however, some studies have demonstrated some decrease in sweat production associated with cold oral intake used in isolation.19 Overall, studies have shown that combining external (cooling clothing, ice packs, fanning) and internal (cold water, ice slurry) cooling methods result in a greater cooling effect than a use of a single method.4
Tertiary Prevention
The goal of tertiary prevention is to mitigate the risk of long-term adverse outcomes following an EHS event. The most effective means of reducing risk for morbidity and mortality is rapid identification and treatment of EHS as well as close evaluation of an athlete’s return to activity in heat. This process is spearheaded by an effective and well-rehearsed emergency action plan.
Diagnosis and Management
Rapid identification and treatment of EHS is crucial to minimizing the risk of poor outcomes.7 Any delay in the treatment of EHS can dramatically increase the likelihood of associated morbidity and mortality.20
EHS is diagnosed by an elevated rectal temperature ≥40°C (104°F) and associated central nervous system (CNS) dysfunction.21 EHS should be strongly suspected in any athlete exercising in heat who exhibits signs of CNS dysfunction, including disorientation, confusion, dizziness, erratic behavior, irritability, headache, loss of coordination, delirium, collapse, or seizures.7,12,15 EHS may also present with symptoms of heat exhaustion, including fatigue, hyperventilation, tachycardia, vomiting, diarrhea, and hypotension.7,12,15
Rectal temperature should be taken for any athlete with suspected EHS, as other modalities—oral, skin, axillary, and aural—can be inaccurate and easily modified by ambient confounders such as ambient and skin temperature, athlete hyperventilation, and consumption of liquids.7,11,12 Athletes exhibiting CNS symptoms with moderately elevated rectal temperatures that do not exceed 40°C should also be assumed to be suffering from EHS and treated with rapid cooling.11 On the other hand, athletes with CNS symptoms who are normothermic should be assumed to have EAH until ruled out by electrolyte assessment; IV fluids should be at no more than keep vein open (KVO) pending this determination.11 In some cases, an athlete may initially present with altered mental status but return to “normal.” However, this improvement may represent a “lucid period”; evaluation should continue with rectal temperature and treatment, as EHS in these cases may progress quickly.15
Treatment is centered on rapid, whole body cooling initiated at the first sign of heat illness.7,22 The goal of treatment is to achieve a rectal temperature <38.9°C within 30 minutes of the onset of EHS.15 Upon diagnosis, the athlete should be quickly placed in a tub of ice water to facilitate cold water immersion (CWI) therapy. Some guidelines suggest the athlete’s clothing be removed to potentiate evaporative cooling during CWI;12 however, cooling should not be delayed due to difficulties in removing equipment. CWI, where a heat stroke victim is submerged in ice water up to their neck while water is continuously circulated, is generally considered to be the gold standard treatment as it is the modality with the highest recorded cooling rates and the lowest rate of morbidity and mortality.7,20,21 Multiple studies of CWI have shown that survival nears 100% when aggressive cooling starts within 5 minutes of collapse or identification of EHS.20,21,22
If whole body CWI is unavailable, alternative methods of rapid cooling should be employed. Partial CWI, with torso immersion being preferable to the extremities, has been shown to achieve an acceptable rate of cooling to achieve sufficient drops in internal body temperature.20,23 However, one popular treatment—applying ice packs to the whole body, in particular to the groin and axillae—has not been shown to be sufficient to achieve standard cooling goals.20 None of these methods have been shown to be as effective as CWI.23
Intravenous access should be initiated with fluid resuscitation dictated by the provider’s assessment. Normal saline is recommended as the resuscitative fluid of choice, with the rate dictated by clinical judgment and adjusted as guided by electrolyte determination and clinical response. It cannot be overstated that in normothermic patients with confusion, EAH is the diagnosis of exclusion and aggressive fluid resuscitation should be withheld until electrolyte determination.
Once rectal temperature descends appropriately (~38.9°C), the cooling process should stop and the individual should be transported to a hospital for further observation20 and evaluation of possible sequelae, including rhabdomyolysis and renal injury, cardiac dysfunction and arrhythmia, severe electrolyte abnormalities, acute respiratory distress syndrome, lactic acidosis, and other forms of end-organ failure (Figure).
Rapid cooling is more crucial than transport; transport poses a risk of delayed cooling, which can dramatically increase an individual’s risk of morbidity and mortality.20,23 In situations where a patient can be cooled on-site, physicians should pursue cooling before transporting the patient to a medical treatment facility.
Emergency Action Plan
Team physicians should be proactive in developing an emergency action plan to address possible EHS events. These plans should be site-specific, addressing procedures for all practice and home competition locations.12 All competition venues should have a CWI tub on-site in events where there is an increased risk of EHS.12,15,20 This tub should be set up and functional for all high-risk activities, including practices.12
Following recognition of a potential case of EHS, treatment teams should have procedures in place to transport athletes to the treatment area, obtain rectal temperature, initiate rapid cooling, and stabilize the athlete for transport to an emergency department (ED) for further evaluation.12,15 A written record of treatments and medications provided during athlete stabilization should be maintained and transported with the athlete to the ED.15 A list of helpful equipment and supplies for treatment of EHS can be found in Table 5.
EHS is a unique life-threatening situation where it is best to treat the patient on the sideline before transport.15 Those athletes transported before cooling risk spending an increased amount of time above critical temperatures for cell damage, which has been associated with increased morbidity and mortality. This mantra of “cool first, transport second” cannot be overemphasized, as those individuals with EHS who present to the ED with a persisting rectal temperature >41°F may risk up to an 80% mortality rate.24 Conversely, a recent large, retrospective study of 274 EHS events sustained during the Falmouth Road Race found a 100% survival rate when athletes were rapidly identified via rectal thermometry and treated with aggressive, rapid cooling through CWI.21
Return to Play
Perhaps the most challenging and important role the team physician has is determining an athlete’s return to play following EHI, as there currently are no evidence-based guidelines for return to activity for these athletes.7 The decisions surrounding return to play are highly individualized, as recovery from EHS and heat injury is associated with the duration of internal body temperature elevation above the critical level (40°C).7,20 Guidelines for return to activity following recovery from EHI differ among experts and institutions.7,25 The general consensus from these guidelines is that, at minimum, athletes should not participate in any physical activity until they are asymptomatic and all blood tests have normalized.11 Following this asymptomatic period, most guidelines advocate for a slow, deliberate return to activity.11 The American College of Sports Medicine (ACSM) offers one reasonable approach to the returning athlete following EHS:7
- No exercise for at least 7 days following release from medical care.
- Follow-up with a physician 1 week after release from medical care for physical examination and any warranted lab or radiologic studies (based upon organ systems affected during EHS).
- Once cleared to return to activity, the athlete begins exercise in a cool environment, gradually increasing the duration, intensity, and heat exposure over 2 weeks to demonstrate heat tolerance and acclimatization.
- Athletes who cannot resume vigorous activity due to recurrent symptoms (eg, excessive fatigue) should be reevaluated after 4 weeks. Laboratory exercise-heat tolerance testing may be useful in this setting.
- The athlete may resume full competition once they are able to participate in full training in the heat for 2 to 4 weeks without adverse effects.
Heat tolerance testing (HTT) in these athletes remains controversial.5 26 The ACSM recommends that HTT be considered only for those unable to return to vigorous activity after a suitable period (approximately 4 weeks). In contrast, the Israeli Defense Force (IDF) uses HTT to evaluate soldiers following EHS to guide decision-making about return to duty.27 The IDF HTT assumes that individuals will respond differently to heat stresses. They identify individuals who are “heat intolerant” as being unable to tolerate specific heat challenges, indicated by increases in body temperature occurring more rapidly than normal responders under identical environmental and exercise conditions. However, despite being used for more than 30 years, there is no clear evidence that HTT adequately predicts who will experience subsequent episodes of EHS.
Conclusion
While the recognized cornerstone of being a team physician is the provision of medical care, the ACSM Team Physician Consensus Statement28 further delineates the medical and administrative responsibilities as both (1) understanding medical management and prevention of injury and illness in athletes; and (2) awareness of or involvement in the development and rehearsal of an emergency action plan. These tenets are critical for the team physician who accepts the responsibility to cover sports at the high school level or higher. Football team physicians play an essential role in mitigating risk of EHI in their athletes. Through development and execution of both comprehensive prevention strategies and emergency action plans, physicians can work to minimize athletes’ risk of both developing and experiencing significant adverse outcomes from an EHI.
Am J Orthop. 2016;45(6):340-348. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football, one of the most popular sports in the United States, is additionally recognized as a leading contributor to sports injury secondary to the contact collision nature of the endeavor. There are an estimated 1.1 million high school football players with another 100,000 participants combined in the National Football League (NFL), college, junior college, Arena Football League, and semipro levels of play.1 USA Football estimates that an additional 3 million youth participate in community football leagues.1 The National Center for Catastrophic Sports Injury Research recently calculated a fatality rate of 0.14 per 100,000 participants in 2014 for the 4.2 million who play football at all levels—and 0.45 per 100,000 in high school.1 While direct deaths from head and spine injury remain a significant contributor to the number of catastrophic injuries, indirect deaths (systemic failure) predominate. Exertional heat stroke (EHS) has emerged as one of the leading indirect causes of death in high school and collegiate football. Boden and colleagues2 reported that high school and college football players sustain approximately 12 fatalities annually, with indirect systemic causes being twice as common as direct blunt trauma.2The most common indirect causes identified included cardiac failure, heat illness, and complications of sickle cell trait (SCT). It was also noted that the risk of SCT, heat-related, and cardiac deaths increased during the second decade of the study, indicating these conditions may require a greater emphasis on diagnosis, treatment, and prevention. This review details for the team physician the unique challenge of exercising in the heat to the football player, and the prevention, diagnosis, management and return-to-play issues pertinent to exertional heat illness (EHI).
The Challenge
EHS represents the most severe manifestation of EHI—a gamut of diseases commonly encountered during the hot summer months when American football season begins. The breadth of EHI includes several important clinical diagnoses: exercise-associated muscle cramps (heat cramps); heat exhaustion with and without syncope; heat injury with evidence of end organ injury (eg, rhabdomyolysis); and EHS. EHS is defined as “a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi-organ dysfunction in which encephalopathy predominates.”3 EHS, if left untreated, or even if clinical treatment is delayed, may result in significant end organ morbidity and/or mortality.
During exercise, the human thermoregulatory system mitigates heat gain by increasing skin blood flow and sweating, causing an increased dissipation of heat to the surrounding environment by leveraging conduction, convection, and evaporation.4,5 Elevated environmental temperatures, increased humidity, and dehydration can impede the body’s ability to dissipate heat at a rate needed to maintain thermoregulation. This imbalance can result in hyperthermia secondary to uncompensated heat stress,5 which in turn can lead to EHI. Football players have unique challenges that make them particularly vulnerable to EHI. The summer heat during early-season participation and the requirement for equipment that covers nearly 60% of body surfaces pose increased risk of volume losses and hyperthermia that trigger the onset of EHI.6 Football athletes’ body compositions and physical size are additional contributing risk factors; the relatively high muscle and fat content increase thermogenicity, which require their bodies to dissipate more heat.7
An estimated 9000 cases of EHI occur annually across all high school sports,8 with an incidence of 1.6:100,000 athlete-exposures.8,9 Studies have demonstrated, however, that EHI occurs in football 11.4 times more often than in all other high school sports combined.10 The incidence of nonfatal EHI in all levels of football is 4.42-5:100,000.8,9 Between 2000 and 2014, 41 football players died from EHS.1 In football, approximately 75% of all EHI events occurred during practices, while only 25% of incidents occurred during games.8
Given these potentially deadly consequences, it is important that football team physicians are not only alert to the early symptoms of heat illness and prepared to intervene to prevent the progression to EHS, but are critical leaders in educating coaches and players in evidence-based EHI prevention practices and policies.
Prevention
EHS is a preventable condition, arguably the most common cause of preventable nontraumatic exertional death in young athletes in the United States. Close attention to mitigating risk factors should begin prior to the onset of preseason practice and continue through the early season, where athletes are at the highest risk of developing heat illness.
Primary Prevention
Primary prevention is fundamental to minimizing the occurrences of EHI. It focuses on the following methods: recognition of inherent risk factors, acclimatization, hydration, and avoidance of inciting substances (including supplements).
Pre-Participation Examination. The purpose of the pre-participation examination (PPE) is to maximize an athlete’s safety by identifying medical conditions that place the athlete at risk.11,12 The Preparticipation Physical Evaluation, 4th edition, the most widely used consensus publication, specifically queries if an athlete has a previous history of heat injury. However, it only indirectly addresses intrinsic risk factors that may predispose an athlete to EHI who has never had an EHI before. Therefore, providers should take the opportunity of the PPE to inquire about additional risk factors that may make an athlete high risk for sustaining a heat injury. Common risk factors for EHI are listed in Table 1.
Heat Acclimatization. The risk of EHI escalates significantly when athletes are subjected to multiple stressors during periods of heat exposure, such as sudden increases in intensity or duration of exercise; prolonged new exposures to heat; dehydration; and sleep loss.5 When football season begins in late summer, athletes are least conditioned as temperatures reach their seasonal peak, causing increased risk of EHI.15 Planning for heat acclimatization is vital for all athletes who exercise in hot environments. Acclimatization procedures place progressively mounting physiologic strains on the body to improve athletes’ ability to dissipate heat, diminishing thermoregulatory and cardiovascular exertion.4,5 Acclimatization begins with expansion of plasma volume on days 3 to 6, causing improvements in cardiac efficiency and resulting in an overall decrease in basal internal body temperature.4,5,15 This process results in improvements in heat tolerance and exercise performance, evolving over 10 to 14 days of gradual escalation of exercise intensity and duration.5,10,11,16 However, poor fitness levels and extreme temperatures can prolong this period, requiring up to 2 to 3 months to fully take effect.5,7
The National Athletic Trainers Association (NATA) and National Collegiate Athletic Association (NCAA) have released consensus guidelines regarding heat acclimatization protocols for football athletes at the high school and college levels (Tables 3 and 4). Each of these guidelines involves an initial period without use of protective equipment, followed by a gradual addition of further equipment.11,16
Secondary Prevention
Despite physicians’ best efforts to prevent all cases of EHI, athletes will still experience the effects of exercise-induced hyperthermia. The goal of secondary prevention is to slow the progression of this hyperthermia so that it does not progress to more dangerous EHI.
Hydration. Dehydration is an important risk factor for EHI. Sweat maintains thermoregulation by dissipating heat generated during exercise; however, it also contributes to body water losses. Furthermore, intravascular depletion decreases stroke volume, thereby increasing cardiovascular strain. It is estimated that for every 1% loss in body mass from dehydration, body temperature rises 0.22°C in comparison to a euhydrated state.6 Dehydration occurs more rapidly in hot environments, as fluid is lost through increased sweat production.7 After approximately 6% to 10% body weight volume loss, cardiac output cannot be maintained, diminishing sweat production and blood flow to both skin and muscle and causing diminished performance and a significant risk of heat exhaustion.7 If left unchecked, these physiologic changes result in further elevations in body temperature and increased cardiovascular strain, ultimately placing the athlete at significant risk for development of EHS.
Adequate hydration to maintain euvolemia is an important step in avoiding possible EHI. Multiple studies have shown that football players experience a baseline hypovolemia during their competition season,6 a deficit that is most marked during the first week of practices.17 This deficit is multifactorial, as football players expend a significant amount of fluid through sweat, are not able to adequately replace these losses during practice, and do not appropriately hydrate off the field.6,18 Some players, especially linemen, sweat at a higher rate than their teammates, posing a possible risk of significant dehydration.6 Coaches and players alike should be educated on the importance of adequate hydration to meet their fluid needs.
The goal of hydration during exercise is to prevent large fluid losses that can adversely affect performance and increase risk of EHI;6 it may be unrealistic to replace all fluid losses during the practice period. Instead, athletes should target complete volume replacement over the post-exercise period.6 Some recommend hydrating based upon thirst drive; however, thirst is activated following a volume loss of approximately 2% body mass, the same degree of losses that place athletes at an increased risk for performance impairment and EHI.4,6,11,12 Individuals should have access to fluids throughout practice and competition and be encouraged to hydrate as needed.6,12,15 Furthermore, staff should modify their practices based upon WBGT and acclimatization status to provide more frequent hydration breaks.
Hyperhydration and Salt Intake. Of note, there are inherent risks to hyperhydration. Athletes with low sweat rates have an increased risk of overhydration and the development of exercise-associated hyponatremia (EAH),6 a condition whose presentation is very similar to EHS. In addition, inadequate sodium intake and excessive sweating can also contribute to the development of EAH. EAH has been implicated in the deaths of 2 football players in 2014.1,6 Establishing team hydration guidelines and educating players and staff on appropriate hydration and dietary salt intake is essential to reduce the risk of both dehydration and hyperhydration and their complications.6Intra-Event Cooling. During exercise, team physicians can employ strategies for cooling athletes during exertion to mitigate their risk of EHI by decreasing thermal and cardiovascular strain.4,19 Cooling during exercise is hypothesized to allow for accelerated heat dissipation, where heat is lost from the body more effectively. This accelerated loss enables athletes to maintain a higher heat storage capacity over the duration of exercise, avoiding uncompensated heat stresses that ultimately cause EHI.19
Some intra-event cooling strategies include the use of cooling garments, cooling packs, and cold water/slurry ingestion. Cooling garments lower skin temperature, which in turn can decrease thermoregulatory strains;4 a recent meta-analysis of intra-event cooling modalities revealed that wearing an ice vest during exercise resulted in the greatest decrease in thermal heat strain.19 Internal cooling strategies—namely ingestion of cold fluids/ice slurry—have shown some mild benefit in decreasing internal temperatures; however, some studies have demonstrated some decrease in sweat production associated with cold oral intake used in isolation.19 Overall, studies have shown that combining external (cooling clothing, ice packs, fanning) and internal (cold water, ice slurry) cooling methods result in a greater cooling effect than a use of a single method.4
Tertiary Prevention
The goal of tertiary prevention is to mitigate the risk of long-term adverse outcomes following an EHS event. The most effective means of reducing risk for morbidity and mortality is rapid identification and treatment of EHS as well as close evaluation of an athlete’s return to activity in heat. This process is spearheaded by an effective and well-rehearsed emergency action plan.
Diagnosis and Management
Rapid identification and treatment of EHS is crucial to minimizing the risk of poor outcomes.7 Any delay in the treatment of EHS can dramatically increase the likelihood of associated morbidity and mortality.20
EHS is diagnosed by an elevated rectal temperature ≥40°C (104°F) and associated central nervous system (CNS) dysfunction.21 EHS should be strongly suspected in any athlete exercising in heat who exhibits signs of CNS dysfunction, including disorientation, confusion, dizziness, erratic behavior, irritability, headache, loss of coordination, delirium, collapse, or seizures.7,12,15 EHS may also present with symptoms of heat exhaustion, including fatigue, hyperventilation, tachycardia, vomiting, diarrhea, and hypotension.7,12,15
Rectal temperature should be taken for any athlete with suspected EHS, as other modalities—oral, skin, axillary, and aural—can be inaccurate and easily modified by ambient confounders such as ambient and skin temperature, athlete hyperventilation, and consumption of liquids.7,11,12 Athletes exhibiting CNS symptoms with moderately elevated rectal temperatures that do not exceed 40°C should also be assumed to be suffering from EHS and treated with rapid cooling.11 On the other hand, athletes with CNS symptoms who are normothermic should be assumed to have EAH until ruled out by electrolyte assessment; IV fluids should be at no more than keep vein open (KVO) pending this determination.11 In some cases, an athlete may initially present with altered mental status but return to “normal.” However, this improvement may represent a “lucid period”; evaluation should continue with rectal temperature and treatment, as EHS in these cases may progress quickly.15
Treatment is centered on rapid, whole body cooling initiated at the first sign of heat illness.7,22 The goal of treatment is to achieve a rectal temperature <38.9°C within 30 minutes of the onset of EHS.15 Upon diagnosis, the athlete should be quickly placed in a tub of ice water to facilitate cold water immersion (CWI) therapy. Some guidelines suggest the athlete’s clothing be removed to potentiate evaporative cooling during CWI;12 however, cooling should not be delayed due to difficulties in removing equipment. CWI, where a heat stroke victim is submerged in ice water up to their neck while water is continuously circulated, is generally considered to be the gold standard treatment as it is the modality with the highest recorded cooling rates and the lowest rate of morbidity and mortality.7,20,21 Multiple studies of CWI have shown that survival nears 100% when aggressive cooling starts within 5 minutes of collapse or identification of EHS.20,21,22
If whole body CWI is unavailable, alternative methods of rapid cooling should be employed. Partial CWI, with torso immersion being preferable to the extremities, has been shown to achieve an acceptable rate of cooling to achieve sufficient drops in internal body temperature.20,23 However, one popular treatment—applying ice packs to the whole body, in particular to the groin and axillae—has not been shown to be sufficient to achieve standard cooling goals.20 None of these methods have been shown to be as effective as CWI.23
Intravenous access should be initiated with fluid resuscitation dictated by the provider’s assessment. Normal saline is recommended as the resuscitative fluid of choice, with the rate dictated by clinical judgment and adjusted as guided by electrolyte determination and clinical response. It cannot be overstated that in normothermic patients with confusion, EAH is the diagnosis of exclusion and aggressive fluid resuscitation should be withheld until electrolyte determination.
Once rectal temperature descends appropriately (~38.9°C), the cooling process should stop and the individual should be transported to a hospital for further observation20 and evaluation of possible sequelae, including rhabdomyolysis and renal injury, cardiac dysfunction and arrhythmia, severe electrolyte abnormalities, acute respiratory distress syndrome, lactic acidosis, and other forms of end-organ failure (Figure).
Rapid cooling is more crucial than transport; transport poses a risk of delayed cooling, which can dramatically increase an individual’s risk of morbidity and mortality.20,23 In situations where a patient can be cooled on-site, physicians should pursue cooling before transporting the patient to a medical treatment facility.
Emergency Action Plan
Team physicians should be proactive in developing an emergency action plan to address possible EHS events. These plans should be site-specific, addressing procedures for all practice and home competition locations.12 All competition venues should have a CWI tub on-site in events where there is an increased risk of EHS.12,15,20 This tub should be set up and functional for all high-risk activities, including practices.12
Following recognition of a potential case of EHS, treatment teams should have procedures in place to transport athletes to the treatment area, obtain rectal temperature, initiate rapid cooling, and stabilize the athlete for transport to an emergency department (ED) for further evaluation.12,15 A written record of treatments and medications provided during athlete stabilization should be maintained and transported with the athlete to the ED.15 A list of helpful equipment and supplies for treatment of EHS can be found in Table 5.
EHS is a unique life-threatening situation where it is best to treat the patient on the sideline before transport.15 Those athletes transported before cooling risk spending an increased amount of time above critical temperatures for cell damage, which has been associated with increased morbidity and mortality. This mantra of “cool first, transport second” cannot be overemphasized, as those individuals with EHS who present to the ED with a persisting rectal temperature >41°F may risk up to an 80% mortality rate.24 Conversely, a recent large, retrospective study of 274 EHS events sustained during the Falmouth Road Race found a 100% survival rate when athletes were rapidly identified via rectal thermometry and treated with aggressive, rapid cooling through CWI.21
Return to Play
Perhaps the most challenging and important role the team physician has is determining an athlete’s return to play following EHI, as there currently are no evidence-based guidelines for return to activity for these athletes.7 The decisions surrounding return to play are highly individualized, as recovery from EHS and heat injury is associated with the duration of internal body temperature elevation above the critical level (40°C).7,20 Guidelines for return to activity following recovery from EHI differ among experts and institutions.7,25 The general consensus from these guidelines is that, at minimum, athletes should not participate in any physical activity until they are asymptomatic and all blood tests have normalized.11 Following this asymptomatic period, most guidelines advocate for a slow, deliberate return to activity.11 The American College of Sports Medicine (ACSM) offers one reasonable approach to the returning athlete following EHS:7
- No exercise for at least 7 days following release from medical care.
- Follow-up with a physician 1 week after release from medical care for physical examination and any warranted lab or radiologic studies (based upon organ systems affected during EHS).
- Once cleared to return to activity, the athlete begins exercise in a cool environment, gradually increasing the duration, intensity, and heat exposure over 2 weeks to demonstrate heat tolerance and acclimatization.
- Athletes who cannot resume vigorous activity due to recurrent symptoms (eg, excessive fatigue) should be reevaluated after 4 weeks. Laboratory exercise-heat tolerance testing may be useful in this setting.
- The athlete may resume full competition once they are able to participate in full training in the heat for 2 to 4 weeks without adverse effects.
Heat tolerance testing (HTT) in these athletes remains controversial.5 26 The ACSM recommends that HTT be considered only for those unable to return to vigorous activity after a suitable period (approximately 4 weeks). In contrast, the Israeli Defense Force (IDF) uses HTT to evaluate soldiers following EHS to guide decision-making about return to duty.27 The IDF HTT assumes that individuals will respond differently to heat stresses. They identify individuals who are “heat intolerant” as being unable to tolerate specific heat challenges, indicated by increases in body temperature occurring more rapidly than normal responders under identical environmental and exercise conditions. However, despite being used for more than 30 years, there is no clear evidence that HTT adequately predicts who will experience subsequent episodes of EHS.
Conclusion
While the recognized cornerstone of being a team physician is the provision of medical care, the ACSM Team Physician Consensus Statement28 further delineates the medical and administrative responsibilities as both (1) understanding medical management and prevention of injury and illness in athletes; and (2) awareness of or involvement in the development and rehearsal of an emergency action plan. These tenets are critical for the team physician who accepts the responsibility to cover sports at the high school level or higher. Football team physicians play an essential role in mitigating risk of EHI in their athletes. Through development and execution of both comprehensive prevention strategies and emergency action plans, physicians can work to minimize athletes’ risk of both developing and experiencing significant adverse outcomes from an EHI.
Am J Orthop. 2016;45(6):340-348. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kucera KL, Klossner D, Colgate B, Cantu RC. Annual Survey of Football Injury Research: 1931-2014. National Center for Catastrophic Sport Injury Research Web site. https://nccsir.unc.edu/files/2013/10/Annual-Football-2014-Fatalities-Final.pdf. Accessed May 31, 2016.
2. Boden BP, Breit I, Beachler JA, Williams A, Mueller FO. Fatalities in high school and college football players. Am J Sports Med. 2013;41(5):1108-1116.
3. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978-1988.
4. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25 Suppl 1:6-19.
5. Pryor RR, Casa DJ, Adams WM, et al. Maximizing athletic performance in the heat. Strength Cond J. 2013;35(6):24-33.
6. Adams WM, Casa DJ. Hydration for football athletes. Sports Sci Exchange. 2015;28(141):1-5.
7. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
8. Yard EE, Gilchrist J, Haileyesus T, et al. Heat illness among high school athletes--United States, 2005-2009. J Safety Res. 2010;41(6):471-474.
9. Huffman EA, Yard EE, Fields SK, Collins CL, Comstock RD. Epidemiology of rare injuries and conditions among United States high school athletes during the 2005-2006 and 2006-2007 school years. J Athl Train. 2008;43(6):624-630.
10. Kerr ZY, Casa DJ, Marshall SW, Comstock RD. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8-14.
11. Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986-1000.
12. Casa DJ, Almquist J, Anderson SA. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. J Athl Train. 2013;48(4):546-553.
13. Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28(8):939-944.
14. Gundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometerol. 2012;56(1):11-20.
15. Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117-127.
16. Casa DJ, Csillan D; Inter-Association Task Force for Preseason Secondary School Athletics Participants, et al. Preseason heat-acclimatization guidelines for secondary school athletics. J Athl Train. 2009;44(3):332-333.
17. Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843-851.
18. Stover EA, Zachwieja J, Stofan J, Murray R, Horswill CA. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330-335.
19. Bongers CC, Thijssen DH, Veltmeijer MTW, Hopman MT, Eijsvogels TM. Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br J Sports Med. 2015;49(6):377-384.
20. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141-149.
21. DeMartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47(2):240-245.
22. Casa DJ, Kenny GP, Taylor NA. Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc. 2010;42(7):1246-1252.
23. Casa DJ, Armstrong LE, Kenny GP, O’Connor FG, Huggins RA. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11(3):115-122.
24. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heat stroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
25. O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine Roundtable on exertional heat stroke--return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314-321.
26. Kazman JB, Heled Y, Lisman PJ, Druyan A, Deuster PA, O’Connor FG. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101-105.
27. Moran DS, Heled Y, Still L, Laor A, Shapiro Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):CR252-CR257.
28. Herring SA, Kibler WB, Putukian M. Team Physician Consensus Statement: 2013 update. Med Sci Sports Exerc. 2013;45(8):1618-1622.
29. Heat stroke treatment. Korey Stringer Institute University of Connecticut Web site. http://ksi.uconn.edu/emergency-conditions/heat-illnesses/exertional-heat-stroke/heat-stroke-treatment/. Accessed June 14, 2016.
30. Headquarters, Department of the Army and the Air Force. Heat Stress Control and Heat Casualty Management. Technical Bulletin Medical 507. http://www.dir.ca.gov/oshsb/documents/Heat_illness_prevention_tbmed507.pdf. Published March 7, 2003. Accessed June 14, 2016.
1. Kucera KL, Klossner D, Colgate B, Cantu RC. Annual Survey of Football Injury Research: 1931-2014. National Center for Catastrophic Sport Injury Research Web site. https://nccsir.unc.edu/files/2013/10/Annual-Football-2014-Fatalities-Final.pdf. Accessed May 31, 2016.
2. Boden BP, Breit I, Beachler JA, Williams A, Mueller FO. Fatalities in high school and college football players. Am J Sports Med. 2013;41(5):1108-1116.
3. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978-1988.
4. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25 Suppl 1:6-19.
5. Pryor RR, Casa DJ, Adams WM, et al. Maximizing athletic performance in the heat. Strength Cond J. 2013;35(6):24-33.
6. Adams WM, Casa DJ. Hydration for football athletes. Sports Sci Exchange. 2015;28(141):1-5.
7. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
8. Yard EE, Gilchrist J, Haileyesus T, et al. Heat illness among high school athletes--United States, 2005-2009. J Safety Res. 2010;41(6):471-474.
9. Huffman EA, Yard EE, Fields SK, Collins CL, Comstock RD. Epidemiology of rare injuries and conditions among United States high school athletes during the 2005-2006 and 2006-2007 school years. J Athl Train. 2008;43(6):624-630.
10. Kerr ZY, Casa DJ, Marshall SW, Comstock RD. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8-14.
11. Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986-1000.
12. Casa DJ, Almquist J, Anderson SA. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. J Athl Train. 2013;48(4):546-553.
13. Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28(8):939-944.
14. Gundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometerol. 2012;56(1):11-20.
15. Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117-127.
16. Casa DJ, Csillan D; Inter-Association Task Force for Preseason Secondary School Athletics Participants, et al. Preseason heat-acclimatization guidelines for secondary school athletics. J Athl Train. 2009;44(3):332-333.
17. Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843-851.
18. Stover EA, Zachwieja J, Stofan J, Murray R, Horswill CA. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330-335.
19. Bongers CC, Thijssen DH, Veltmeijer MTW, Hopman MT, Eijsvogels TM. Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br J Sports Med. 2015;49(6):377-384.
20. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141-149.
21. DeMartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47(2):240-245.
22. Casa DJ, Kenny GP, Taylor NA. Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc. 2010;42(7):1246-1252.
23. Casa DJ, Armstrong LE, Kenny GP, O’Connor FG, Huggins RA. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11(3):115-122.
24. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heat stroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
25. O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine Roundtable on exertional heat stroke--return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314-321.
26. Kazman JB, Heled Y, Lisman PJ, Druyan A, Deuster PA, O’Connor FG. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101-105.
27. Moran DS, Heled Y, Still L, Laor A, Shapiro Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):CR252-CR257.
28. Herring SA, Kibler WB, Putukian M. Team Physician Consensus Statement: 2013 update. Med Sci Sports Exerc. 2013;45(8):1618-1622.
29. Heat stroke treatment. Korey Stringer Institute University of Connecticut Web site. http://ksi.uconn.edu/emergency-conditions/heat-illnesses/exertional-heat-stroke/heat-stroke-treatment/. Accessed June 14, 2016.
30. Headquarters, Department of the Army and the Air Force. Heat Stress Control and Heat Casualty Management. Technical Bulletin Medical 507. http://www.dir.ca.gov/oshsb/documents/Heat_illness_prevention_tbmed507.pdf. Published March 7, 2003. Accessed June 14, 2016.
Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
Alectinib provides a new option for ALK-positive NSCLC patients after progression on crizotinib
In December 2015, alectinib became the third ALK inhibitor approved by the United States Food and Drug Administration for the treatment of non-small-cell lung cancer (NSCLC) that displays rearrangements of the anaplastic lymphoma kinase (ALK) gene. Alectinib is a second-generation small molecule inhibitor of the ALK protein that joins ceritinib in providing a useful treatment option for patients who have progressed on crizotinib, as a result of its ability to target crizotinib-resistant mutant forms of the ALK protein. Alectinib also displays enhanced penetrance of the blood-brain barrier, which improves efficacy against central nervous system (CNS) metastases.
Click on the PDF icon at the top of this introduction to read the full article.
In December 2015, alectinib became the third ALK inhibitor approved by the United States Food and Drug Administration for the treatment of non-small-cell lung cancer (NSCLC) that displays rearrangements of the anaplastic lymphoma kinase (ALK) gene. Alectinib is a second-generation small molecule inhibitor of the ALK protein that joins ceritinib in providing a useful treatment option for patients who have progressed on crizotinib, as a result of its ability to target crizotinib-resistant mutant forms of the ALK protein. Alectinib also displays enhanced penetrance of the blood-brain barrier, which improves efficacy against central nervous system (CNS) metastases.
Click on the PDF icon at the top of this introduction to read the full article.
In December 2015, alectinib became the third ALK inhibitor approved by the United States Food and Drug Administration for the treatment of non-small-cell lung cancer (NSCLC) that displays rearrangements of the anaplastic lymphoma kinase (ALK) gene. Alectinib is a second-generation small molecule inhibitor of the ALK protein that joins ceritinib in providing a useful treatment option for patients who have progressed on crizotinib, as a result of its ability to target crizotinib-resistant mutant forms of the ALK protein. Alectinib also displays enhanced penetrance of the blood-brain barrier, which improves efficacy against central nervous system (CNS) metastases.
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma: newly approved drugs forge paradigm shift toward chronic disease
The pace of drug development for multiple myeloma was dizzying in 2015, with 5 regulatory approvals for the treatment of relapsed/refractory disease, 3 in a single month. As we stand on the brink of another paradigm shift in the management of this disease, we discuss the new classes of drugs and how they are shaping standard of care with the potential to make multiple myeloma a chronic disease.
Click on the PDF icon at the top of this introduction to read the full article.
The pace of drug development for multiple myeloma was dizzying in 2015, with 5 regulatory approvals for the treatment of relapsed/refractory disease, 3 in a single month. As we stand on the brink of another paradigm shift in the management of this disease, we discuss the new classes of drugs and how they are shaping standard of care with the potential to make multiple myeloma a chronic disease.
Click on the PDF icon at the top of this introduction to read the full article.
The pace of drug development for multiple myeloma was dizzying in 2015, with 5 regulatory approvals for the treatment of relapsed/refractory disease, 3 in a single month. As we stand on the brink of another paradigm shift in the management of this disease, we discuss the new classes of drugs and how they are shaping standard of care with the potential to make multiple myeloma a chronic disease.
Click on the PDF icon at the top of this introduction to read the full article.
Panobinostat: a novel mechanism of action shows promise in multiple myeloma
Following an initial “no” vote from the Oncologic Drugs Advisory Committee (ODAC) in late 2014, the US Food and Drug Administration eventually awarded accelerated approval in February 2015 to the histone deacetylase (HDAC) inhibitor panobinostat for use in select patients with relapsed multiple myeloma. Panobinostat has a novel mechanism of action that demonstrates synergy with the proteasome inhibitor bortezomib and the immunomodulatory agent dexamethasone, which translated into improved progression-free survival (PFS) for patients with multiple myeloma who had received at least 2 prior therapies, according to data from a prespecified subgroup analysis from the Panorama-1 trial.
Click on the PDF icon at the top of this introduction to read the full article.
Following an initial “no” vote from the Oncologic Drugs Advisory Committee (ODAC) in late 2014, the US Food and Drug Administration eventually awarded accelerated approval in February 2015 to the histone deacetylase (HDAC) inhibitor panobinostat for use in select patients with relapsed multiple myeloma. Panobinostat has a novel mechanism of action that demonstrates synergy with the proteasome inhibitor bortezomib and the immunomodulatory agent dexamethasone, which translated into improved progression-free survival (PFS) for patients with multiple myeloma who had received at least 2 prior therapies, according to data from a prespecified subgroup analysis from the Panorama-1 trial.
Click on the PDF icon at the top of this introduction to read the full article.
Following an initial “no” vote from the Oncologic Drugs Advisory Committee (ODAC) in late 2014, the US Food and Drug Administration eventually awarded accelerated approval in February 2015 to the histone deacetylase (HDAC) inhibitor panobinostat for use in select patients with relapsed multiple myeloma. Panobinostat has a novel mechanism of action that demonstrates synergy with the proteasome inhibitor bortezomib and the immunomodulatory agent dexamethasone, which translated into improved progression-free survival (PFS) for patients with multiple myeloma who had received at least 2 prior therapies, according to data from a prespecified subgroup analysis from the Panorama-1 trial.
Click on the PDF icon at the top of this introduction to read the full article.
Unplanned Exubations in the ICU: Risk Factors and Strategies for Reducing Adverse Events
From the MetroHealth System, Cleveland, OH.
Abstract
- Objective: To describe risk factors for unplanned extubation (UE) among critically ill adults requiring mechanical ventilation and to identify strategies to reduce the occurrence of this adverse event.
- Methods: Review of the literature.
- Results: Inadvertent removal of an endotracheal tube, or a UE, occurs in 7% to 22.5% of mechanically ventilated adult patients and is often due to deliberate patient removal. Despite the multitude of research examining risk factors and predictors of UE, rates have remained unchanged for the past 2 decades. Risk factors can be classified by intensive care unit (ICU) type, including medical ICUs, surgical ICUs, and mixed medical-surgical ICUs. The majority of risk factors for UEs across ICUs may be amenable to changes in unit processes, such as programs for agitation management, use of weaning protocols, increased surveillance of patients, and ongoing education for patients and health care staff.
- Conclusion: Prevention of UE remains an elusive target. Changes in unit processes that target identified risk factors may be an effective method to decrease prevalence of UE.
Unplanned extubation (UE) is the inadvertent removal of an endotracheal tube, either by a patient (deliberate self-extubation), or by a member of the health care team providing routine care such as repositioning, suctioning, or procedures (accidental extubation). Approximately 7% to 22.5% of mechanically ventilated patients in the intensive care unit (ICU) experience UE [1–7]. Estimates are likely higher, as current regulatory and accreditation standards do not include mandatory reporting of this event. Despite numerous studies investigating risk factors associated with UE, it remains a prevalent problem with adverse outcomes for patients and hospitals. The purpose of this review is to provide a summary of the literature on risk factors for UE, review effects on patient and organizational outcomes, and identify evidence-based strategies for reducing occurrence of UE among mechanically ventilated patients.
Prevalence of Unplanned Exubation
There is substantial heterogeneity in how UE is calculated and reported in the research literature. UE is calculated as the number of UE events per 100 or 1000 patient days, or the number of UE per total ventilator days. Rates of UE are also reported as the proportion of patients who experience UE out of all intubated patients over a set time period [8]. Despite efforts aimed at mitigating risk factors for UE, rates have remained static over the past 2 decades. Reported UE rates from 1994–2002 were 2.6% to 14% [3,6,9–11], while rates from 2004–2014 ranged from 1% to 22% [3–5,8,12–15]. Interventions utilizing a multidisciplinary approach have been implemented with the aim of decreasing UE, yet few have proven successful on improving rates nationally.
Unplanned self-extubation by the patient (deliberate self-extubation) is the most common type of UE [3,10,12,16–18]. A multicenter trial of 426 patients from 11 medical centers indicates that 46 patients experienced UE, with 36 of these (78.2%) caused by patient self-extubation [6]. Prospective single-site studies report similar or higher estimates of patient self-extubation, ranging from 75.8% to 91.7% [3,5], while a multisite study of 10,112 patients revealed 32 of 35 UE (91.4%) were due to patient self-extubation [12]. Similarly, a 4-year analysis of 85 UEs reported 82 incidences (96.5%) were a result of deliberate patient removal [13]. Patients either physically pull out the endotracheal tube or use their tongue or coughing/gagging maneuvers to displace or intentionally remove the endotracheal tube [5]. Only 3% to 8% of UEs are caused by inadvertent removal by health care staff [3,5,12,13].
Effects on Patient and Organizational Outcomes
Regardless of the cause of the UE, there are adverse consequences for both patients and hospitals. Some patients who experience UE have higher rates of in-hospital mortality; however, this is often due to contributing factors associated with severity of injury, the need for reintubation, and underlying chronic diseases [13]. Patients who experience accidental UE have higher incidence of nosocomial pneumonia (27.6% vs. 138%, P = 0.002) [11], longer duration of mechanical ventilation, and increased length of stay (LOS) [7,13]. While some studies report UE can result in serious consequences such as respiratory distress, hypoxia [13], and even death [6,12], others report lower mortality and length of stay when UE occurs, likely due to the fact that many patients are ready for liberation from mechanical ventilation at the time of UE [5,15].
Despite the emergent nature of UE, not all patients experience immediate reintubation. Many instances of UE occur during patient weaning trials or in preparation for planned extubations [5,11], which explains why only 10% to 60% of patients require reintubation [3,5,10,11,15,19,20]. When reintubation is necessary, it results in increased number of ventilator days [10,11], and increased ICU and hospital LOS [1,11]. There is little evidence directly linking reintubation with in-hospital mortality; however, it can cause serious complications such as hypotension, hypertension, arrhythmias, and airway trauma [21]. For hospitals and health care organizations, the need for reintubation results in increased hospital costs, estimated to be $1000 per reintubation event [17,22]. This estimate does not take into account additional costs incurred with increased ICU care, longer periods of mechanical ventilation, and increased LOS. Estimates of these additional costs in pediatric patients are approximately $36,000 [23]. Costs are likely higher in adult patients, due to multiple comorbidities that often accompany the need for mechanical ventilation, as well as increased pharmacy, lab, and diagnostic charges [1].
Risk Factors for Unplanned Extubation
Medical ICU Risk Factors
MICUs traditionally have the highest rates of UE [4,8]. Data from a national prevalence study indicated that there were 23.4 episodes of UE in MICUs per 1000 ventilator days [4]. Approximately 9.5% to 15% of all ventilated patients in the MICU experience UE [4,5,8]. Patients in the MICU who require mechanical ventilation often have complex chronic illness with underlying respiratory disease, which can result in prolonged periods of ventilation and increased risk of UE. Specific risk factors investigated in UE research include patient specific factors (age, gender, diagnosis, comorbidities, agitation, level of consciousness, laboratory values), ventilatory factors (ventilator type and setting, type of tracheal tube, method of tube fixation), as well as type of sedation and use of protocols [5,6,24]. Surprisingly, few variables emerge as significant risk factors for UE among MICU patients. Risk factors associated with UE have included male gender [24], presence of chronic obstructive pulmonary disease (COPD) [24], increased level of consciousness [25], and use of weaning protocols [5]. While gender, COPD, and level of consciousness increase risk of UE, the presence of weaning protocols is shown to decrease risk of UE [5]. Although UE are reported most often in MICUs, few risk factors consistently emerge for this specific cohort, making definitive recommendations for prevention of UE difficult.
Surgical ICU Risk Factors
The prevalence of UE for mechanically ventilated patients in the SICU tend to be lower than those for MICU cohorts. Prevalence of UE in the SICU is reported at 1.41 episodes per 100 ventilator days [13], or 6.8 episodes per 1000 ventilator days [4]. Percentages of UE in the SICU range from 2% to 6% [4,8,19]. Similar to MICU patients, critically ill patients in the SICU often have specific risk factors placing them at risk for UE. Causative factors examined in research studies with this population include gender, age, sedation scale scores, need for reintubation, time from intubation to extubation, use of sedatives/analgesics, restraints, ICU nurse experience, location of staff at time of UE, and criteria for extubation [17,19]. Similar to MICU cohorts, few variables are identified as predictors of UE. Significant predictors include use of restraints, decreased sedation [17], and meeting criteria for extubation [19]. Among patients who experienced an UE, 87% were restrained at the time of the UE [17], and most had low levels of sedation (mean Ramsay sedation scale score = 2.42 in the hour preceding the UE). Approximately 64% of patients who experienced UE met criteria for planned extubation and did not require re-intubation [19], suggesting many patients were essentially ready for planned extubation.
Mixed ICU Risk Factors
The majority of research investigating risk factors for UE is conducted within medical-surgical or mixed/general ICUs. The prevalence of UE within this type of unit is reported at 1.59 episodes per 100 patient days [6], or approximately 2% to 10% [4,6,7]. Among this population, potential risk factors are similar to those included in solely MICU or SICU studies. Because of the high number of studies investigating UE in a mixed ICU setting, there are significantly more variables included in as potential risk factors. Variables include patient age, gender, admission diagnosis, injury severity using Acute Physiological and Chronic Health Evaluation (APACHE II), ICU and hospital LOS, patient level of consciousness, agitation, days of mechanical ventilation, ventilator settings, nosocomial infection, sedation, physical restraints, vital signs [7,14,26], laboratory values, medication types, and body mass index [15,26]. One study also included time of UE and ICU nurse level of experience [3]. Among all factors, several were significant predictors of UE: male gender [15], decreased sedation and increased level of consciousness [8], agitation [3,19,26], use of restraints [3,7], sedation practices (particularly use of benzodiazapines) [3,7,15,26,27], lack of strong tube fixation, absence of IV sedation, and orotracheal intubation [6]. UE were more likely to occur on the night shift and among staff that included nurses with fewer years of experience [3]. Many episodes of UE occurred during weaning [10] or among patients who could communicate and were alert [3]. One study reports 57% of patients who intentionally self-extubated explained they simply removed the tube because it was uncomfortable [3].
Strategies for Reducing Adverse Events
Agitation Management
The majority of studies cited agitation, altered level of consciousness, or inadequate sedation as risk factors for UE [3,6–8,15,17,18,25,26,28,29]. These factors directly impact restraint use, another common risk factor for UE [3,7,17]. A key recommendation for agitation management is to identify the source of agitation, which is often caused by delirium onset in the ICU [30–32]. Prevalence of delirium in the ICU ranges from 20% to 80% [33–35]. ICU patients are at high risk for delirium due to sleep deprivation, older age, restraints, abnormal lab values, medications, infection, and respiratory complications [31]. Treatment for delirium centers on prevention, early recognition, interdisciplinary and pharmacologic protocols, increased nursing presence, and use of short-acting sedation when necessary [30–32,36]. While there is no research specifically linking delirium to UE, a quality analysis of risk factors present at the time of UE using bow-tie analysis methods identified delirium as a key factor present in the majority of UE cases [36]. It is possible that agitation reported in other studies investigating risk factors for UE may actually be reflective of underlying delirium. Routine screening using validated tools, such as the Confusion Assessment Method-ICU (CAM-ICU) [37] would aid in early detection and management of delirium, and would provide a standardized method for exploring the relationship of delirium and UE in future trials.
Integration of Weaning Protocols
Protocol-directed weaning is beneficial for decreasing ventilator days, time to wean from mechanical ventilation, and ICU LOS [38]. A systematic review including 7 trials (2434 patients) comparing protocol/non-protocol for weaning from mechanical ventilation reported a 26% decrease in the mean duration of mechanical ventilation for the protocol groups (95% CI 13%–37%, P < 0.001), a 70% reduction in time to wean, (95% CI 27%–88%, P = 0.009), and a decrease in ICU LOS by 11% (95% CI 3%–19%, P = 0.01). Weaning protocols are also an important risk factor for UE [5]. Findings from a prospective cohort study specifically identify the presence of weaning protocols as an important factor for reducing UE; patients who had weaning protocols ordered and followed were least likely to experience UE (P = 0.02) [5]. A separate quality improvement initiative demonstrated an overall decrease in the number of UEs (from 5.2% to 0.9%) after implementing weaning protocols as standard of care [39]. Considering many UEs occur during weaning [10], integration of weaning protocols aids in expediting the process and ensuring timely extubation.
Increased Surveillance
Increasing surveillance and monitoring of ventilated patients is a recommendation based on risk factors presented at the time of UE. Specifically, staffing levels and shifts and the use of physical restraints are variables associated with UE that are amendable to changes in unit processes based on increased surveillance. It is reported that 40% to 76% of UEs occurred during the night shift [14,17,24,40]; many more occur during change of shift or when there is not a nurse present at the bedside [3,17]. Recent trends towards mandatory bedside reporting is a specific intervention that may positively impact UE among patients in the ICU [41]. Meta-analyses of observational studies investigating the effect of nurse staffing on hospital outcomes indicate that increasing the number of RNs is associated with decreased risk of adverse patient outcomes, including UE [42,43]. The addition of 1 additional nurse per patient day can result in a 51% decrease in UE, while a decrease in nursing workload could result in a 45% decrease in UE [42]. Data from a national prevalence study reports ICUs with fewer available resources, including staff, experienced a higher number of UEs [4].
Increasing surveillance by nursing and health care staff may also impact prevalence of physical restraint use. A significant number of patients who experience UE are physically restrained at the time of the incident, ranging from 40% to 90% of intubated patients [5–7,14,17,40]. It is well documented that UE continue to occur despite the use of restraints [5,7,28,29,44] Patients who are physically restrained often experience higher rates of unplanned extubation (42.9% vs. 16.5% , P < 0.001 in Chang et al’s study [7]), and longer ICU LOS (20.3 days vs. 15.8 days, P = 0.009) [7]. Soft wrist restraints are commonly used to prevent pulling of the endotracheal tube; however, research evidence on UE demonstrates this is not always an effective intervention. Increasing surveillance of ventilated patients, treating their agitation and screening for underlying delirium, and integration of weaning protocols are all interventions that may decrease UE and the need for routine use of physical restraints.
Ongoing Education for Patients and Health Care Staff
Initial and ongoing education about UE, risk factors, and effective interventions is beneficial for patients and health care staff. Although there are no trials investigating effects of educational interventions for patients on UE outcomes, pre-education of surgical patients regarding what to expect while intubated may aid in decreasing delirium risk, agitation, physical restraint use, and possibly UE. Verbal and written educational information during pre-admission testing is a feasible method easily integrated into pre-operative programs.
Because UEs often occur more frequently among less experienced staff, initial education about risk factors for UE is crucial to include in ICU staff orientation programs [3,7]. Educational initiatives should incorporate training on routine delirium screening and avoidance of agitation, use of protocols, and increased surveillance of patients receiving mechanical ventilation [5,15,17,39,45]. Ongoing education of staff regarding ventilatory equipment and risk factors for UE can be particularly effective in decreasing UE [46]. Initial educational efforts should be followed by routine updates for all members of the healthcare team about ongoing quality improvement efforts to monitor UE. Associated factors for UE that may be unit- or process-specific, including methods for endotracheal tube securement and intra-hospital transport, should be communicated with all individuals involved in patient care. Integration of continuous quality improvement programs can decrease UE rates by 22% to 53% [16]. Quality efforts typically focus on standardization of reporting and tracking tools, protocol implementations, and ongoing monitoring, auditing, and recording of UE.
Current Trends and Future Directions
Recent trends in critical care recommendations may mitigate potential risk factors identified in UE research. Integration of lightened sedation and daily wake up periods for intubated patients may decrease prevalence of risk factors for UE, specifically agitation, physical restraint use, and altered level of consciousness [30], while routine weaning protocols may improve ventilatory outcomes, including UE [5,38,40]. Nursing bedside report and purposeful hourly rounding are quickly emerging as mainstays of professional nursing care [41]. Inherent in these 2 initiatives are increased surveillance and vigilance by health care staff, which can result in timely extubation of those who indicate readiness, as well as decreased incidence of adverse events. Delirium remains a key factor that may be a likely cause for UE; recent trends towards early detection and proper management of delirium among ICU staff may result in improved ventilatory outcomes, including weaning, planned extubation, and the prevalence of UE.
Another important trend in critical care is the emergence of a neurocritical care specialty and routine admission of neurocritically ill patients to neuroscience ICUs [47,48]. However, there are no studies investigating prevalence of UE among these patients, who often have higher rates of agitation or restlessness due to cognitive impairment. Among general ICUs, patients with a primary respiratory diagnosis accounted for 23% of all UE in one study, while those with a neurological diagnosis accounted for the second highest percentage (12%) among the study population [15]. A separate study concluded that presence of neurological injury with a concomitant nosocomial infection increased risk of UE among patients in a mixed ICU [7]. A recent systematic review of weaning protocols highlights positive effects on ventilatory outcomes but cites lack of evidence for effectiveness of protocols among those with neurological injury [38]. Areas for future UE research should include factors specific to this patient population, as they may be at higher risk for adverse ventilatory outcomes due to the nature of the neurological injury.
Conclusion
Prevention of UE remains an elusive target, evidenced by little change in reported rates over 2 decades. Research provides data on risk factors that may be patient, unit, or process related. Structuring prevention efforts around modifiable risk factors for UE is a feasible approach amenable to ongoing monitoring for effectiveness. Integration of current trends in health care safety and quality may produce an added benefit of reducing the occurrence of UE in critical care units. Future research evaluating these trends and the prevalence of UE in subspecialty populations is warranted.
Corresponding author: Molly McNett, PhD, RN, CNRN, Attn: NBO, MetroHealth Medical Center, 2500 MetroHealth Drive; Cleveland, OH 44109, mmcnett@metrohealth.org.
Financial disclosures: None.
1. Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560–6.
2. Coppolo DP, May JJ. Self-extubations. A 12-month experience. Chest 1990;98:165–9.
3. Yeh SH, Lee LN, Ho TH, et al. Implications of nursing care in the occurrence and consequences of unplanned extubation in adult intensive care units. Int J Nurs Stud 2004;41:255–62.
4. Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med 2007;35:2714–20.
5. Jarachovic M, Mason M, Kerber K. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care 2011;20:304–11.
6. Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Resp Crit Care Med 1998;157(4 Pt 1):1131–7.
7. Chang LY, Wang KW, Chao YF. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care 2008;17:408–15.
8. Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med 2004;30:1348–55.
9. Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med 1996;22:1269–71.
10. Betbese AJ, Perez M, Bak E, et al. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med 1998;26:1180–6.
11. de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology 2002;97:148–56.
12. Kapadia FN, Tekawade PC, Nath SS, et al. A prolonged observational study of tracheal tube displacements: Benchmarking an incidence <0.5-1% in a medical-surgical adult intensive care unit. Ind J Crit Care Med 2014;18:273–7.
13. Lee JH, Lee HC, Jeon YT, et al. Clinical outcomes after unplanned extubation in a surgical intensive care population. World J Surg 2014;38:203–10.
14. Chang LC, Liu PF, Huang YL, et al. Risk factors associated with unplanned endotracheal self-extubation of hospitalized intubated patients: a 3-year retrospective case-control study. Appl Nurs Res 2011;24:188–92.
15. de Groot RI, Dekkers OM, Herold IH, et al. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15:R19.
16. da Silva PS, Fonseca MC. Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesth Analg 2012;114:1003–14.
17. Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care 2008;17:45–51.
18. Christie JM, Dethlefsen M, Cane RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 1996;8:289–93.
19. Huang YT. Factors leading to self-extubation of endotracheal tubes in the intensive care unit. Nurs Crit Care 2009;14:68–74.
20. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34.
21. Mort TC. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg 1998;86:1171–6.
22. Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest 2005;128:2749–57.
23. Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Ped Crit Care Med 2015.
24. Bouza C, Garcia E, Diaz M, et al. Unplanned extubation in orally intubated medical patients in the intensive care unit: a prospective cohort study. Heart Lung 2007;36:270–6.
25. Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med 1993;19:340-342.
26. Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001;13:24–9.
27. Tanios M, Epstein S, Grzeskowiak M, et al. Influence of sedation strategies on unplanned extubation in a mixed intensive care unit. Am J Crit Care 2014;23:306–14.
28. Atkins PM, Mion LC, Mendelson W, et al. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest 1997;112:1317–23.
29. Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med 1998;26:1049–53.
30. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
31. Morandi A, Jackson JC. Delirium in the intensive care unit: a review. Neurol Clin 2011;29:749–63.
32. Banerjee A, Vasilevskis, EE, Pandharipande, P. Strategies to improve delirium assessment practices in the intensive care unit. J Clin Outcomes Manag 2010;17:459–68.
33. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
34. Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32:106–12.
35. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591–8.
36. Kerckhoffs MC, van der Sluijs AF, Binnekade JM, Dongelmans DA. Improving patient safety in the ICU by prospective identification of missing safety barriers using the bow-tie prospective risk analysis model. J Patient Safe 2013;9:154–9.
37. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8.
38. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11:CD006904.
39. Chia PL, Santos DR, Tan TC, et al. Clinical quality improvement: eliminating unplanned extubation in the CCU. Int J Health Care Qual Ass 2013;26:642–52.
40. Balon JA. Common factors of spontaneous self-extubation in a critical care setting. Int J Trauma Nurs 2001;7:93–9.
41. Gregory S, Tan D, Tilrico M, et al. Bedside shift reports: what does the evidence say? J Nurs Admin 2014;44:541–5.
42. Kane RL, Shamliyan TA, Mueller C, et al. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care 2007;45:1195–204.
43. Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med 2010;38:1521–8; quiz 1529.
44. Tindol GA, Jr., DiBenedetto RJ, Kosciuk L. Unplanned extubations. Chest 1994;105:1804–7.
45. Chen CM CK, Fong Y, Hsing SC, et al. Age is an important predictor of failed unplanned extubation. Int J Gerontol 2010;4:120–9.
46. Richmond AL, Jarog DL, Hanson VM. Unplanned extubation in adult critical care. Quality improvement and education payoff. Crit Care Nurs 2004;24:32–7.
47. Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 2011;15:477–80.
48. McNett MM, Horowitz DA. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit Care 2014;21 Suppl 2:S215–28.
49. Gardner A, Hughes, D, Cook R, et al. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Database of abstracts of reivews of effects (DARE): Quality-assessed reviews. 2005. York: Center for Reviews and Dissemination.
50. Hofso K, Coyer FM. Part 1: Chemical and physical restraints in the management of mechanically ventliated patients in the ICU: Contributing factors. Intensive Crit Care Nurs 2007; 23:249–55.
51. Kiekkas P, Diamanto A, Panteli E, et al. Unplanned extubation in critially ill adults: Clinical reviews. Nurs Crit Care 2012;18:123–34.
52. King JN, Elliiot VA. Self/unplanned extubation: Safety, surveillance, and monitoring of the mechanically ventilated patient. Crit Care Nurs Clin North Am 2012;24:469–79.
From the MetroHealth System, Cleveland, OH.
Abstract
- Objective: To describe risk factors for unplanned extubation (UE) among critically ill adults requiring mechanical ventilation and to identify strategies to reduce the occurrence of this adverse event.
- Methods: Review of the literature.
- Results: Inadvertent removal of an endotracheal tube, or a UE, occurs in 7% to 22.5% of mechanically ventilated adult patients and is often due to deliberate patient removal. Despite the multitude of research examining risk factors and predictors of UE, rates have remained unchanged for the past 2 decades. Risk factors can be classified by intensive care unit (ICU) type, including medical ICUs, surgical ICUs, and mixed medical-surgical ICUs. The majority of risk factors for UEs across ICUs may be amenable to changes in unit processes, such as programs for agitation management, use of weaning protocols, increased surveillance of patients, and ongoing education for patients and health care staff.
- Conclusion: Prevention of UE remains an elusive target. Changes in unit processes that target identified risk factors may be an effective method to decrease prevalence of UE.
Unplanned extubation (UE) is the inadvertent removal of an endotracheal tube, either by a patient (deliberate self-extubation), or by a member of the health care team providing routine care such as repositioning, suctioning, or procedures (accidental extubation). Approximately 7% to 22.5% of mechanically ventilated patients in the intensive care unit (ICU) experience UE [1–7]. Estimates are likely higher, as current regulatory and accreditation standards do not include mandatory reporting of this event. Despite numerous studies investigating risk factors associated with UE, it remains a prevalent problem with adverse outcomes for patients and hospitals. The purpose of this review is to provide a summary of the literature on risk factors for UE, review effects on patient and organizational outcomes, and identify evidence-based strategies for reducing occurrence of UE among mechanically ventilated patients.
Prevalence of Unplanned Exubation
There is substantial heterogeneity in how UE is calculated and reported in the research literature. UE is calculated as the number of UE events per 100 or 1000 patient days, or the number of UE per total ventilator days. Rates of UE are also reported as the proportion of patients who experience UE out of all intubated patients over a set time period [8]. Despite efforts aimed at mitigating risk factors for UE, rates have remained static over the past 2 decades. Reported UE rates from 1994–2002 were 2.6% to 14% [3,6,9–11], while rates from 2004–2014 ranged from 1% to 22% [3–5,8,12–15]. Interventions utilizing a multidisciplinary approach have been implemented with the aim of decreasing UE, yet few have proven successful on improving rates nationally.
Unplanned self-extubation by the patient (deliberate self-extubation) is the most common type of UE [3,10,12,16–18]. A multicenter trial of 426 patients from 11 medical centers indicates that 46 patients experienced UE, with 36 of these (78.2%) caused by patient self-extubation [6]. Prospective single-site studies report similar or higher estimates of patient self-extubation, ranging from 75.8% to 91.7% [3,5], while a multisite study of 10,112 patients revealed 32 of 35 UE (91.4%) were due to patient self-extubation [12]. Similarly, a 4-year analysis of 85 UEs reported 82 incidences (96.5%) were a result of deliberate patient removal [13]. Patients either physically pull out the endotracheal tube or use their tongue or coughing/gagging maneuvers to displace or intentionally remove the endotracheal tube [5]. Only 3% to 8% of UEs are caused by inadvertent removal by health care staff [3,5,12,13].
Effects on Patient and Organizational Outcomes
Regardless of the cause of the UE, there are adverse consequences for both patients and hospitals. Some patients who experience UE have higher rates of in-hospital mortality; however, this is often due to contributing factors associated with severity of injury, the need for reintubation, and underlying chronic diseases [13]. Patients who experience accidental UE have higher incidence of nosocomial pneumonia (27.6% vs. 138%, P = 0.002) [11], longer duration of mechanical ventilation, and increased length of stay (LOS) [7,13]. While some studies report UE can result in serious consequences such as respiratory distress, hypoxia [13], and even death [6,12], others report lower mortality and length of stay when UE occurs, likely due to the fact that many patients are ready for liberation from mechanical ventilation at the time of UE [5,15].
Despite the emergent nature of UE, not all patients experience immediate reintubation. Many instances of UE occur during patient weaning trials or in preparation for planned extubations [5,11], which explains why only 10% to 60% of patients require reintubation [3,5,10,11,15,19,20]. When reintubation is necessary, it results in increased number of ventilator days [10,11], and increased ICU and hospital LOS [1,11]. There is little evidence directly linking reintubation with in-hospital mortality; however, it can cause serious complications such as hypotension, hypertension, arrhythmias, and airway trauma [21]. For hospitals and health care organizations, the need for reintubation results in increased hospital costs, estimated to be $1000 per reintubation event [17,22]. This estimate does not take into account additional costs incurred with increased ICU care, longer periods of mechanical ventilation, and increased LOS. Estimates of these additional costs in pediatric patients are approximately $36,000 [23]. Costs are likely higher in adult patients, due to multiple comorbidities that often accompany the need for mechanical ventilation, as well as increased pharmacy, lab, and diagnostic charges [1].
Risk Factors for Unplanned Extubation
Medical ICU Risk Factors
MICUs traditionally have the highest rates of UE [4,8]. Data from a national prevalence study indicated that there were 23.4 episodes of UE in MICUs per 1000 ventilator days [4]. Approximately 9.5% to 15% of all ventilated patients in the MICU experience UE [4,5,8]. Patients in the MICU who require mechanical ventilation often have complex chronic illness with underlying respiratory disease, which can result in prolonged periods of ventilation and increased risk of UE. Specific risk factors investigated in UE research include patient specific factors (age, gender, diagnosis, comorbidities, agitation, level of consciousness, laboratory values), ventilatory factors (ventilator type and setting, type of tracheal tube, method of tube fixation), as well as type of sedation and use of protocols [5,6,24]. Surprisingly, few variables emerge as significant risk factors for UE among MICU patients. Risk factors associated with UE have included male gender [24], presence of chronic obstructive pulmonary disease (COPD) [24], increased level of consciousness [25], and use of weaning protocols [5]. While gender, COPD, and level of consciousness increase risk of UE, the presence of weaning protocols is shown to decrease risk of UE [5]. Although UE are reported most often in MICUs, few risk factors consistently emerge for this specific cohort, making definitive recommendations for prevention of UE difficult.
Surgical ICU Risk Factors
The prevalence of UE for mechanically ventilated patients in the SICU tend to be lower than those for MICU cohorts. Prevalence of UE in the SICU is reported at 1.41 episodes per 100 ventilator days [13], or 6.8 episodes per 1000 ventilator days [4]. Percentages of UE in the SICU range from 2% to 6% [4,8,19]. Similar to MICU patients, critically ill patients in the SICU often have specific risk factors placing them at risk for UE. Causative factors examined in research studies with this population include gender, age, sedation scale scores, need for reintubation, time from intubation to extubation, use of sedatives/analgesics, restraints, ICU nurse experience, location of staff at time of UE, and criteria for extubation [17,19]. Similar to MICU cohorts, few variables are identified as predictors of UE. Significant predictors include use of restraints, decreased sedation [17], and meeting criteria for extubation [19]. Among patients who experienced an UE, 87% were restrained at the time of the UE [17], and most had low levels of sedation (mean Ramsay sedation scale score = 2.42 in the hour preceding the UE). Approximately 64% of patients who experienced UE met criteria for planned extubation and did not require re-intubation [19], suggesting many patients were essentially ready for planned extubation.
Mixed ICU Risk Factors
The majority of research investigating risk factors for UE is conducted within medical-surgical or mixed/general ICUs. The prevalence of UE within this type of unit is reported at 1.59 episodes per 100 patient days [6], or approximately 2% to 10% [4,6,7]. Among this population, potential risk factors are similar to those included in solely MICU or SICU studies. Because of the high number of studies investigating UE in a mixed ICU setting, there are significantly more variables included in as potential risk factors. Variables include patient age, gender, admission diagnosis, injury severity using Acute Physiological and Chronic Health Evaluation (APACHE II), ICU and hospital LOS, patient level of consciousness, agitation, days of mechanical ventilation, ventilator settings, nosocomial infection, sedation, physical restraints, vital signs [7,14,26], laboratory values, medication types, and body mass index [15,26]. One study also included time of UE and ICU nurse level of experience [3]. Among all factors, several were significant predictors of UE: male gender [15], decreased sedation and increased level of consciousness [8], agitation [3,19,26], use of restraints [3,7], sedation practices (particularly use of benzodiazapines) [3,7,15,26,27], lack of strong tube fixation, absence of IV sedation, and orotracheal intubation [6]. UE were more likely to occur on the night shift and among staff that included nurses with fewer years of experience [3]. Many episodes of UE occurred during weaning [10] or among patients who could communicate and were alert [3]. One study reports 57% of patients who intentionally self-extubated explained they simply removed the tube because it was uncomfortable [3].
Strategies for Reducing Adverse Events
Agitation Management
The majority of studies cited agitation, altered level of consciousness, or inadequate sedation as risk factors for UE [3,6–8,15,17,18,25,26,28,29]. These factors directly impact restraint use, another common risk factor for UE [3,7,17]. A key recommendation for agitation management is to identify the source of agitation, which is often caused by delirium onset in the ICU [30–32]. Prevalence of delirium in the ICU ranges from 20% to 80% [33–35]. ICU patients are at high risk for delirium due to sleep deprivation, older age, restraints, abnormal lab values, medications, infection, and respiratory complications [31]. Treatment for delirium centers on prevention, early recognition, interdisciplinary and pharmacologic protocols, increased nursing presence, and use of short-acting sedation when necessary [30–32,36]. While there is no research specifically linking delirium to UE, a quality analysis of risk factors present at the time of UE using bow-tie analysis methods identified delirium as a key factor present in the majority of UE cases [36]. It is possible that agitation reported in other studies investigating risk factors for UE may actually be reflective of underlying delirium. Routine screening using validated tools, such as the Confusion Assessment Method-ICU (CAM-ICU) [37] would aid in early detection and management of delirium, and would provide a standardized method for exploring the relationship of delirium and UE in future trials.
Integration of Weaning Protocols
Protocol-directed weaning is beneficial for decreasing ventilator days, time to wean from mechanical ventilation, and ICU LOS [38]. A systematic review including 7 trials (2434 patients) comparing protocol/non-protocol for weaning from mechanical ventilation reported a 26% decrease in the mean duration of mechanical ventilation for the protocol groups (95% CI 13%–37%, P < 0.001), a 70% reduction in time to wean, (95% CI 27%–88%, P = 0.009), and a decrease in ICU LOS by 11% (95% CI 3%–19%, P = 0.01). Weaning protocols are also an important risk factor for UE [5]. Findings from a prospective cohort study specifically identify the presence of weaning protocols as an important factor for reducing UE; patients who had weaning protocols ordered and followed were least likely to experience UE (P = 0.02) [5]. A separate quality improvement initiative demonstrated an overall decrease in the number of UEs (from 5.2% to 0.9%) after implementing weaning protocols as standard of care [39]. Considering many UEs occur during weaning [10], integration of weaning protocols aids in expediting the process and ensuring timely extubation.
Increased Surveillance
Increasing surveillance and monitoring of ventilated patients is a recommendation based on risk factors presented at the time of UE. Specifically, staffing levels and shifts and the use of physical restraints are variables associated with UE that are amendable to changes in unit processes based on increased surveillance. It is reported that 40% to 76% of UEs occurred during the night shift [14,17,24,40]; many more occur during change of shift or when there is not a nurse present at the bedside [3,17]. Recent trends towards mandatory bedside reporting is a specific intervention that may positively impact UE among patients in the ICU [41]. Meta-analyses of observational studies investigating the effect of nurse staffing on hospital outcomes indicate that increasing the number of RNs is associated with decreased risk of adverse patient outcomes, including UE [42,43]. The addition of 1 additional nurse per patient day can result in a 51% decrease in UE, while a decrease in nursing workload could result in a 45% decrease in UE [42]. Data from a national prevalence study reports ICUs with fewer available resources, including staff, experienced a higher number of UEs [4].
Increasing surveillance by nursing and health care staff may also impact prevalence of physical restraint use. A significant number of patients who experience UE are physically restrained at the time of the incident, ranging from 40% to 90% of intubated patients [5–7,14,17,40]. It is well documented that UE continue to occur despite the use of restraints [5,7,28,29,44] Patients who are physically restrained often experience higher rates of unplanned extubation (42.9% vs. 16.5% , P < 0.001 in Chang et al’s study [7]), and longer ICU LOS (20.3 days vs. 15.8 days, P = 0.009) [7]. Soft wrist restraints are commonly used to prevent pulling of the endotracheal tube; however, research evidence on UE demonstrates this is not always an effective intervention. Increasing surveillance of ventilated patients, treating their agitation and screening for underlying delirium, and integration of weaning protocols are all interventions that may decrease UE and the need for routine use of physical restraints.
Ongoing Education for Patients and Health Care Staff
Initial and ongoing education about UE, risk factors, and effective interventions is beneficial for patients and health care staff. Although there are no trials investigating effects of educational interventions for patients on UE outcomes, pre-education of surgical patients regarding what to expect while intubated may aid in decreasing delirium risk, agitation, physical restraint use, and possibly UE. Verbal and written educational information during pre-admission testing is a feasible method easily integrated into pre-operative programs.
Because UEs often occur more frequently among less experienced staff, initial education about risk factors for UE is crucial to include in ICU staff orientation programs [3,7]. Educational initiatives should incorporate training on routine delirium screening and avoidance of agitation, use of protocols, and increased surveillance of patients receiving mechanical ventilation [5,15,17,39,45]. Ongoing education of staff regarding ventilatory equipment and risk factors for UE can be particularly effective in decreasing UE [46]. Initial educational efforts should be followed by routine updates for all members of the healthcare team about ongoing quality improvement efforts to monitor UE. Associated factors for UE that may be unit- or process-specific, including methods for endotracheal tube securement and intra-hospital transport, should be communicated with all individuals involved in patient care. Integration of continuous quality improvement programs can decrease UE rates by 22% to 53% [16]. Quality efforts typically focus on standardization of reporting and tracking tools, protocol implementations, and ongoing monitoring, auditing, and recording of UE.
Current Trends and Future Directions
Recent trends in critical care recommendations may mitigate potential risk factors identified in UE research. Integration of lightened sedation and daily wake up periods for intubated patients may decrease prevalence of risk factors for UE, specifically agitation, physical restraint use, and altered level of consciousness [30], while routine weaning protocols may improve ventilatory outcomes, including UE [5,38,40]. Nursing bedside report and purposeful hourly rounding are quickly emerging as mainstays of professional nursing care [41]. Inherent in these 2 initiatives are increased surveillance and vigilance by health care staff, which can result in timely extubation of those who indicate readiness, as well as decreased incidence of adverse events. Delirium remains a key factor that may be a likely cause for UE; recent trends towards early detection and proper management of delirium among ICU staff may result in improved ventilatory outcomes, including weaning, planned extubation, and the prevalence of UE.
Another important trend in critical care is the emergence of a neurocritical care specialty and routine admission of neurocritically ill patients to neuroscience ICUs [47,48]. However, there are no studies investigating prevalence of UE among these patients, who often have higher rates of agitation or restlessness due to cognitive impairment. Among general ICUs, patients with a primary respiratory diagnosis accounted for 23% of all UE in one study, while those with a neurological diagnosis accounted for the second highest percentage (12%) among the study population [15]. A separate study concluded that presence of neurological injury with a concomitant nosocomial infection increased risk of UE among patients in a mixed ICU [7]. A recent systematic review of weaning protocols highlights positive effects on ventilatory outcomes but cites lack of evidence for effectiveness of protocols among those with neurological injury [38]. Areas for future UE research should include factors specific to this patient population, as they may be at higher risk for adverse ventilatory outcomes due to the nature of the neurological injury.
Conclusion
Prevention of UE remains an elusive target, evidenced by little change in reported rates over 2 decades. Research provides data on risk factors that may be patient, unit, or process related. Structuring prevention efforts around modifiable risk factors for UE is a feasible approach amenable to ongoing monitoring for effectiveness. Integration of current trends in health care safety and quality may produce an added benefit of reducing the occurrence of UE in critical care units. Future research evaluating these trends and the prevalence of UE in subspecialty populations is warranted.
Corresponding author: Molly McNett, PhD, RN, CNRN, Attn: NBO, MetroHealth Medical Center, 2500 MetroHealth Drive; Cleveland, OH 44109, mmcnett@metrohealth.org.
Financial disclosures: None.
From the MetroHealth System, Cleveland, OH.
Abstract
- Objective: To describe risk factors for unplanned extubation (UE) among critically ill adults requiring mechanical ventilation and to identify strategies to reduce the occurrence of this adverse event.
- Methods: Review of the literature.
- Results: Inadvertent removal of an endotracheal tube, or a UE, occurs in 7% to 22.5% of mechanically ventilated adult patients and is often due to deliberate patient removal. Despite the multitude of research examining risk factors and predictors of UE, rates have remained unchanged for the past 2 decades. Risk factors can be classified by intensive care unit (ICU) type, including medical ICUs, surgical ICUs, and mixed medical-surgical ICUs. The majority of risk factors for UEs across ICUs may be amenable to changes in unit processes, such as programs for agitation management, use of weaning protocols, increased surveillance of patients, and ongoing education for patients and health care staff.
- Conclusion: Prevention of UE remains an elusive target. Changes in unit processes that target identified risk factors may be an effective method to decrease prevalence of UE.
Unplanned extubation (UE) is the inadvertent removal of an endotracheal tube, either by a patient (deliberate self-extubation), or by a member of the health care team providing routine care such as repositioning, suctioning, or procedures (accidental extubation). Approximately 7% to 22.5% of mechanically ventilated patients in the intensive care unit (ICU) experience UE [1–7]. Estimates are likely higher, as current regulatory and accreditation standards do not include mandatory reporting of this event. Despite numerous studies investigating risk factors associated with UE, it remains a prevalent problem with adverse outcomes for patients and hospitals. The purpose of this review is to provide a summary of the literature on risk factors for UE, review effects on patient and organizational outcomes, and identify evidence-based strategies for reducing occurrence of UE among mechanically ventilated patients.
Prevalence of Unplanned Exubation
There is substantial heterogeneity in how UE is calculated and reported in the research literature. UE is calculated as the number of UE events per 100 or 1000 patient days, or the number of UE per total ventilator days. Rates of UE are also reported as the proportion of patients who experience UE out of all intubated patients over a set time period [8]. Despite efforts aimed at mitigating risk factors for UE, rates have remained static over the past 2 decades. Reported UE rates from 1994–2002 were 2.6% to 14% [3,6,9–11], while rates from 2004–2014 ranged from 1% to 22% [3–5,8,12–15]. Interventions utilizing a multidisciplinary approach have been implemented with the aim of decreasing UE, yet few have proven successful on improving rates nationally.
Unplanned self-extubation by the patient (deliberate self-extubation) is the most common type of UE [3,10,12,16–18]. A multicenter trial of 426 patients from 11 medical centers indicates that 46 patients experienced UE, with 36 of these (78.2%) caused by patient self-extubation [6]. Prospective single-site studies report similar or higher estimates of patient self-extubation, ranging from 75.8% to 91.7% [3,5], while a multisite study of 10,112 patients revealed 32 of 35 UE (91.4%) were due to patient self-extubation [12]. Similarly, a 4-year analysis of 85 UEs reported 82 incidences (96.5%) were a result of deliberate patient removal [13]. Patients either physically pull out the endotracheal tube or use their tongue or coughing/gagging maneuvers to displace or intentionally remove the endotracheal tube [5]. Only 3% to 8% of UEs are caused by inadvertent removal by health care staff [3,5,12,13].
Effects on Patient and Organizational Outcomes
Regardless of the cause of the UE, there are adverse consequences for both patients and hospitals. Some patients who experience UE have higher rates of in-hospital mortality; however, this is often due to contributing factors associated with severity of injury, the need for reintubation, and underlying chronic diseases [13]. Patients who experience accidental UE have higher incidence of nosocomial pneumonia (27.6% vs. 138%, P = 0.002) [11], longer duration of mechanical ventilation, and increased length of stay (LOS) [7,13]. While some studies report UE can result in serious consequences such as respiratory distress, hypoxia [13], and even death [6,12], others report lower mortality and length of stay when UE occurs, likely due to the fact that many patients are ready for liberation from mechanical ventilation at the time of UE [5,15].
Despite the emergent nature of UE, not all patients experience immediate reintubation. Many instances of UE occur during patient weaning trials or in preparation for planned extubations [5,11], which explains why only 10% to 60% of patients require reintubation [3,5,10,11,15,19,20]. When reintubation is necessary, it results in increased number of ventilator days [10,11], and increased ICU and hospital LOS [1,11]. There is little evidence directly linking reintubation with in-hospital mortality; however, it can cause serious complications such as hypotension, hypertension, arrhythmias, and airway trauma [21]. For hospitals and health care organizations, the need for reintubation results in increased hospital costs, estimated to be $1000 per reintubation event [17,22]. This estimate does not take into account additional costs incurred with increased ICU care, longer periods of mechanical ventilation, and increased LOS. Estimates of these additional costs in pediatric patients are approximately $36,000 [23]. Costs are likely higher in adult patients, due to multiple comorbidities that often accompany the need for mechanical ventilation, as well as increased pharmacy, lab, and diagnostic charges [1].
Risk Factors for Unplanned Extubation
Medical ICU Risk Factors
MICUs traditionally have the highest rates of UE [4,8]. Data from a national prevalence study indicated that there were 23.4 episodes of UE in MICUs per 1000 ventilator days [4]. Approximately 9.5% to 15% of all ventilated patients in the MICU experience UE [4,5,8]. Patients in the MICU who require mechanical ventilation often have complex chronic illness with underlying respiratory disease, which can result in prolonged periods of ventilation and increased risk of UE. Specific risk factors investigated in UE research include patient specific factors (age, gender, diagnosis, comorbidities, agitation, level of consciousness, laboratory values), ventilatory factors (ventilator type and setting, type of tracheal tube, method of tube fixation), as well as type of sedation and use of protocols [5,6,24]. Surprisingly, few variables emerge as significant risk factors for UE among MICU patients. Risk factors associated with UE have included male gender [24], presence of chronic obstructive pulmonary disease (COPD) [24], increased level of consciousness [25], and use of weaning protocols [5]. While gender, COPD, and level of consciousness increase risk of UE, the presence of weaning protocols is shown to decrease risk of UE [5]. Although UE are reported most often in MICUs, few risk factors consistently emerge for this specific cohort, making definitive recommendations for prevention of UE difficult.
Surgical ICU Risk Factors
The prevalence of UE for mechanically ventilated patients in the SICU tend to be lower than those for MICU cohorts. Prevalence of UE in the SICU is reported at 1.41 episodes per 100 ventilator days [13], or 6.8 episodes per 1000 ventilator days [4]. Percentages of UE in the SICU range from 2% to 6% [4,8,19]. Similar to MICU patients, critically ill patients in the SICU often have specific risk factors placing them at risk for UE. Causative factors examined in research studies with this population include gender, age, sedation scale scores, need for reintubation, time from intubation to extubation, use of sedatives/analgesics, restraints, ICU nurse experience, location of staff at time of UE, and criteria for extubation [17,19]. Similar to MICU cohorts, few variables are identified as predictors of UE. Significant predictors include use of restraints, decreased sedation [17], and meeting criteria for extubation [19]. Among patients who experienced an UE, 87% were restrained at the time of the UE [17], and most had low levels of sedation (mean Ramsay sedation scale score = 2.42 in the hour preceding the UE). Approximately 64% of patients who experienced UE met criteria for planned extubation and did not require re-intubation [19], suggesting many patients were essentially ready for planned extubation.
Mixed ICU Risk Factors
The majority of research investigating risk factors for UE is conducted within medical-surgical or mixed/general ICUs. The prevalence of UE within this type of unit is reported at 1.59 episodes per 100 patient days [6], or approximately 2% to 10% [4,6,7]. Among this population, potential risk factors are similar to those included in solely MICU or SICU studies. Because of the high number of studies investigating UE in a mixed ICU setting, there are significantly more variables included in as potential risk factors. Variables include patient age, gender, admission diagnosis, injury severity using Acute Physiological and Chronic Health Evaluation (APACHE II), ICU and hospital LOS, patient level of consciousness, agitation, days of mechanical ventilation, ventilator settings, nosocomial infection, sedation, physical restraints, vital signs [7,14,26], laboratory values, medication types, and body mass index [15,26]. One study also included time of UE and ICU nurse level of experience [3]. Among all factors, several were significant predictors of UE: male gender [15], decreased sedation and increased level of consciousness [8], agitation [3,19,26], use of restraints [3,7], sedation practices (particularly use of benzodiazapines) [3,7,15,26,27], lack of strong tube fixation, absence of IV sedation, and orotracheal intubation [6]. UE were more likely to occur on the night shift and among staff that included nurses with fewer years of experience [3]. Many episodes of UE occurred during weaning [10] or among patients who could communicate and were alert [3]. One study reports 57% of patients who intentionally self-extubated explained they simply removed the tube because it was uncomfortable [3].
Strategies for Reducing Adverse Events
Agitation Management
The majority of studies cited agitation, altered level of consciousness, or inadequate sedation as risk factors for UE [3,6–8,15,17,18,25,26,28,29]. These factors directly impact restraint use, another common risk factor for UE [3,7,17]. A key recommendation for agitation management is to identify the source of agitation, which is often caused by delirium onset in the ICU [30–32]. Prevalence of delirium in the ICU ranges from 20% to 80% [33–35]. ICU patients are at high risk for delirium due to sleep deprivation, older age, restraints, abnormal lab values, medications, infection, and respiratory complications [31]. Treatment for delirium centers on prevention, early recognition, interdisciplinary and pharmacologic protocols, increased nursing presence, and use of short-acting sedation when necessary [30–32,36]. While there is no research specifically linking delirium to UE, a quality analysis of risk factors present at the time of UE using bow-tie analysis methods identified delirium as a key factor present in the majority of UE cases [36]. It is possible that agitation reported in other studies investigating risk factors for UE may actually be reflective of underlying delirium. Routine screening using validated tools, such as the Confusion Assessment Method-ICU (CAM-ICU) [37] would aid in early detection and management of delirium, and would provide a standardized method for exploring the relationship of delirium and UE in future trials.
Integration of Weaning Protocols
Protocol-directed weaning is beneficial for decreasing ventilator days, time to wean from mechanical ventilation, and ICU LOS [38]. A systematic review including 7 trials (2434 patients) comparing protocol/non-protocol for weaning from mechanical ventilation reported a 26% decrease in the mean duration of mechanical ventilation for the protocol groups (95% CI 13%–37%, P < 0.001), a 70% reduction in time to wean, (95% CI 27%–88%, P = 0.009), and a decrease in ICU LOS by 11% (95% CI 3%–19%, P = 0.01). Weaning protocols are also an important risk factor for UE [5]. Findings from a prospective cohort study specifically identify the presence of weaning protocols as an important factor for reducing UE; patients who had weaning protocols ordered and followed were least likely to experience UE (P = 0.02) [5]. A separate quality improvement initiative demonstrated an overall decrease in the number of UEs (from 5.2% to 0.9%) after implementing weaning protocols as standard of care [39]. Considering many UEs occur during weaning [10], integration of weaning protocols aids in expediting the process and ensuring timely extubation.
Increased Surveillance
Increasing surveillance and monitoring of ventilated patients is a recommendation based on risk factors presented at the time of UE. Specifically, staffing levels and shifts and the use of physical restraints are variables associated with UE that are amendable to changes in unit processes based on increased surveillance. It is reported that 40% to 76% of UEs occurred during the night shift [14,17,24,40]; many more occur during change of shift or when there is not a nurse present at the bedside [3,17]. Recent trends towards mandatory bedside reporting is a specific intervention that may positively impact UE among patients in the ICU [41]. Meta-analyses of observational studies investigating the effect of nurse staffing on hospital outcomes indicate that increasing the number of RNs is associated with decreased risk of adverse patient outcomes, including UE [42,43]. The addition of 1 additional nurse per patient day can result in a 51% decrease in UE, while a decrease in nursing workload could result in a 45% decrease in UE [42]. Data from a national prevalence study reports ICUs with fewer available resources, including staff, experienced a higher number of UEs [4].
Increasing surveillance by nursing and health care staff may also impact prevalence of physical restraint use. A significant number of patients who experience UE are physically restrained at the time of the incident, ranging from 40% to 90% of intubated patients [5–7,14,17,40]. It is well documented that UE continue to occur despite the use of restraints [5,7,28,29,44] Patients who are physically restrained often experience higher rates of unplanned extubation (42.9% vs. 16.5% , P < 0.001 in Chang et al’s study [7]), and longer ICU LOS (20.3 days vs. 15.8 days, P = 0.009) [7]. Soft wrist restraints are commonly used to prevent pulling of the endotracheal tube; however, research evidence on UE demonstrates this is not always an effective intervention. Increasing surveillance of ventilated patients, treating their agitation and screening for underlying delirium, and integration of weaning protocols are all interventions that may decrease UE and the need for routine use of physical restraints.
Ongoing Education for Patients and Health Care Staff
Initial and ongoing education about UE, risk factors, and effective interventions is beneficial for patients and health care staff. Although there are no trials investigating effects of educational interventions for patients on UE outcomes, pre-education of surgical patients regarding what to expect while intubated may aid in decreasing delirium risk, agitation, physical restraint use, and possibly UE. Verbal and written educational information during pre-admission testing is a feasible method easily integrated into pre-operative programs.
Because UEs often occur more frequently among less experienced staff, initial education about risk factors for UE is crucial to include in ICU staff orientation programs [3,7]. Educational initiatives should incorporate training on routine delirium screening and avoidance of agitation, use of protocols, and increased surveillance of patients receiving mechanical ventilation [5,15,17,39,45]. Ongoing education of staff regarding ventilatory equipment and risk factors for UE can be particularly effective in decreasing UE [46]. Initial educational efforts should be followed by routine updates for all members of the healthcare team about ongoing quality improvement efforts to monitor UE. Associated factors for UE that may be unit- or process-specific, including methods for endotracheal tube securement and intra-hospital transport, should be communicated with all individuals involved in patient care. Integration of continuous quality improvement programs can decrease UE rates by 22% to 53% [16]. Quality efforts typically focus on standardization of reporting and tracking tools, protocol implementations, and ongoing monitoring, auditing, and recording of UE.
Current Trends and Future Directions
Recent trends in critical care recommendations may mitigate potential risk factors identified in UE research. Integration of lightened sedation and daily wake up periods for intubated patients may decrease prevalence of risk factors for UE, specifically agitation, physical restraint use, and altered level of consciousness [30], while routine weaning protocols may improve ventilatory outcomes, including UE [5,38,40]. Nursing bedside report and purposeful hourly rounding are quickly emerging as mainstays of professional nursing care [41]. Inherent in these 2 initiatives are increased surveillance and vigilance by health care staff, which can result in timely extubation of those who indicate readiness, as well as decreased incidence of adverse events. Delirium remains a key factor that may be a likely cause for UE; recent trends towards early detection and proper management of delirium among ICU staff may result in improved ventilatory outcomes, including weaning, planned extubation, and the prevalence of UE.
Another important trend in critical care is the emergence of a neurocritical care specialty and routine admission of neurocritically ill patients to neuroscience ICUs [47,48]. However, there are no studies investigating prevalence of UE among these patients, who often have higher rates of agitation or restlessness due to cognitive impairment. Among general ICUs, patients with a primary respiratory diagnosis accounted for 23% of all UE in one study, while those with a neurological diagnosis accounted for the second highest percentage (12%) among the study population [15]. A separate study concluded that presence of neurological injury with a concomitant nosocomial infection increased risk of UE among patients in a mixed ICU [7]. A recent systematic review of weaning protocols highlights positive effects on ventilatory outcomes but cites lack of evidence for effectiveness of protocols among those with neurological injury [38]. Areas for future UE research should include factors specific to this patient population, as they may be at higher risk for adverse ventilatory outcomes due to the nature of the neurological injury.
Conclusion
Prevention of UE remains an elusive target, evidenced by little change in reported rates over 2 decades. Research provides data on risk factors that may be patient, unit, or process related. Structuring prevention efforts around modifiable risk factors for UE is a feasible approach amenable to ongoing monitoring for effectiveness. Integration of current trends in health care safety and quality may produce an added benefit of reducing the occurrence of UE in critical care units. Future research evaluating these trends and the prevalence of UE in subspecialty populations is warranted.
Corresponding author: Molly McNett, PhD, RN, CNRN, Attn: NBO, MetroHealth Medical Center, 2500 MetroHealth Drive; Cleveland, OH 44109, mmcnett@metrohealth.org.
Financial disclosures: None.
1. Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560–6.
2. Coppolo DP, May JJ. Self-extubations. A 12-month experience. Chest 1990;98:165–9.
3. Yeh SH, Lee LN, Ho TH, et al. Implications of nursing care in the occurrence and consequences of unplanned extubation in adult intensive care units. Int J Nurs Stud 2004;41:255–62.
4. Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med 2007;35:2714–20.
5. Jarachovic M, Mason M, Kerber K. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care 2011;20:304–11.
6. Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Resp Crit Care Med 1998;157(4 Pt 1):1131–7.
7. Chang LY, Wang KW, Chao YF. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care 2008;17:408–15.
8. Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med 2004;30:1348–55.
9. Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med 1996;22:1269–71.
10. Betbese AJ, Perez M, Bak E, et al. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med 1998;26:1180–6.
11. de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology 2002;97:148–56.
12. Kapadia FN, Tekawade PC, Nath SS, et al. A prolonged observational study of tracheal tube displacements: Benchmarking an incidence <0.5-1% in a medical-surgical adult intensive care unit. Ind J Crit Care Med 2014;18:273–7.
13. Lee JH, Lee HC, Jeon YT, et al. Clinical outcomes after unplanned extubation in a surgical intensive care population. World J Surg 2014;38:203–10.
14. Chang LC, Liu PF, Huang YL, et al. Risk factors associated with unplanned endotracheal self-extubation of hospitalized intubated patients: a 3-year retrospective case-control study. Appl Nurs Res 2011;24:188–92.
15. de Groot RI, Dekkers OM, Herold IH, et al. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15:R19.
16. da Silva PS, Fonseca MC. Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesth Analg 2012;114:1003–14.
17. Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care 2008;17:45–51.
18. Christie JM, Dethlefsen M, Cane RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 1996;8:289–93.
19. Huang YT. Factors leading to self-extubation of endotracheal tubes in the intensive care unit. Nurs Crit Care 2009;14:68–74.
20. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34.
21. Mort TC. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg 1998;86:1171–6.
22. Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest 2005;128:2749–57.
23. Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Ped Crit Care Med 2015.
24. Bouza C, Garcia E, Diaz M, et al. Unplanned extubation in orally intubated medical patients in the intensive care unit: a prospective cohort study. Heart Lung 2007;36:270–6.
25. Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med 1993;19:340-342.
26. Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001;13:24–9.
27. Tanios M, Epstein S, Grzeskowiak M, et al. Influence of sedation strategies on unplanned extubation in a mixed intensive care unit. Am J Crit Care 2014;23:306–14.
28. Atkins PM, Mion LC, Mendelson W, et al. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest 1997;112:1317–23.
29. Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med 1998;26:1049–53.
30. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
31. Morandi A, Jackson JC. Delirium in the intensive care unit: a review. Neurol Clin 2011;29:749–63.
32. Banerjee A, Vasilevskis, EE, Pandharipande, P. Strategies to improve delirium assessment practices in the intensive care unit. J Clin Outcomes Manag 2010;17:459–68.
33. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
34. Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32:106–12.
35. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591–8.
36. Kerckhoffs MC, van der Sluijs AF, Binnekade JM, Dongelmans DA. Improving patient safety in the ICU by prospective identification of missing safety barriers using the bow-tie prospective risk analysis model. J Patient Safe 2013;9:154–9.
37. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8.
38. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11:CD006904.
39. Chia PL, Santos DR, Tan TC, et al. Clinical quality improvement: eliminating unplanned extubation in the CCU. Int J Health Care Qual Ass 2013;26:642–52.
40. Balon JA. Common factors of spontaneous self-extubation in a critical care setting. Int J Trauma Nurs 2001;7:93–9.
41. Gregory S, Tan D, Tilrico M, et al. Bedside shift reports: what does the evidence say? J Nurs Admin 2014;44:541–5.
42. Kane RL, Shamliyan TA, Mueller C, et al. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care 2007;45:1195–204.
43. Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med 2010;38:1521–8; quiz 1529.
44. Tindol GA, Jr., DiBenedetto RJ, Kosciuk L. Unplanned extubations. Chest 1994;105:1804–7.
45. Chen CM CK, Fong Y, Hsing SC, et al. Age is an important predictor of failed unplanned extubation. Int J Gerontol 2010;4:120–9.
46. Richmond AL, Jarog DL, Hanson VM. Unplanned extubation in adult critical care. Quality improvement and education payoff. Crit Care Nurs 2004;24:32–7.
47. Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 2011;15:477–80.
48. McNett MM, Horowitz DA. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit Care 2014;21 Suppl 2:S215–28.
49. Gardner A, Hughes, D, Cook R, et al. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Database of abstracts of reivews of effects (DARE): Quality-assessed reviews. 2005. York: Center for Reviews and Dissemination.
50. Hofso K, Coyer FM. Part 1: Chemical and physical restraints in the management of mechanically ventliated patients in the ICU: Contributing factors. Intensive Crit Care Nurs 2007; 23:249–55.
51. Kiekkas P, Diamanto A, Panteli E, et al. Unplanned extubation in critially ill adults: Clinical reviews. Nurs Crit Care 2012;18:123–34.
52. King JN, Elliiot VA. Self/unplanned extubation: Safety, surveillance, and monitoring of the mechanically ventilated patient. Crit Care Nurs Clin North Am 2012;24:469–79.
1. Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560–6.
2. Coppolo DP, May JJ. Self-extubations. A 12-month experience. Chest 1990;98:165–9.
3. Yeh SH, Lee LN, Ho TH, et al. Implications of nursing care in the occurrence and consequences of unplanned extubation in adult intensive care units. Int J Nurs Stud 2004;41:255–62.
4. Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med 2007;35:2714–20.
5. Jarachovic M, Mason M, Kerber K. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care 2011;20:304–11.
6. Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Resp Crit Care Med 1998;157(4 Pt 1):1131–7.
7. Chang LY, Wang KW, Chao YF. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care 2008;17:408–15.
8. Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med 2004;30:1348–55.
9. Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med 1996;22:1269–71.
10. Betbese AJ, Perez M, Bak E, et al. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med 1998;26:1180–6.
11. de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology 2002;97:148–56.
12. Kapadia FN, Tekawade PC, Nath SS, et al. A prolonged observational study of tracheal tube displacements: Benchmarking an incidence <0.5-1% in a medical-surgical adult intensive care unit. Ind J Crit Care Med 2014;18:273–7.
13. Lee JH, Lee HC, Jeon YT, et al. Clinical outcomes after unplanned extubation in a surgical intensive care population. World J Surg 2014;38:203–10.
14. Chang LC, Liu PF, Huang YL, et al. Risk factors associated with unplanned endotracheal self-extubation of hospitalized intubated patients: a 3-year retrospective case-control study. Appl Nurs Res 2011;24:188–92.
15. de Groot RI, Dekkers OM, Herold IH, et al. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15:R19.
16. da Silva PS, Fonseca MC. Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesth Analg 2012;114:1003–14.
17. Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care 2008;17:45–51.
18. Christie JM, Dethlefsen M, Cane RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 1996;8:289–93.
19. Huang YT. Factors leading to self-extubation of endotracheal tubes in the intensive care unit. Nurs Crit Care 2009;14:68–74.
20. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34.
21. Mort TC. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg 1998;86:1171–6.
22. Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest 2005;128:2749–57.
23. Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Ped Crit Care Med 2015.
24. Bouza C, Garcia E, Diaz M, et al. Unplanned extubation in orally intubated medical patients in the intensive care unit: a prospective cohort study. Heart Lung 2007;36:270–6.
25. Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med 1993;19:340-342.
26. Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001;13:24–9.
27. Tanios M, Epstein S, Grzeskowiak M, et al. Influence of sedation strategies on unplanned extubation in a mixed intensive care unit. Am J Crit Care 2014;23:306–14.
28. Atkins PM, Mion LC, Mendelson W, et al. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest 1997;112:1317–23.
29. Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med 1998;26:1049–53.
30. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
31. Morandi A, Jackson JC. Delirium in the intensive care unit: a review. Neurol Clin 2011;29:749–63.
32. Banerjee A, Vasilevskis, EE, Pandharipande, P. Strategies to improve delirium assessment practices in the intensive care unit. J Clin Outcomes Manag 2010;17:459–68.
33. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
34. Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32:106–12.
35. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591–8.
36. Kerckhoffs MC, van der Sluijs AF, Binnekade JM, Dongelmans DA. Improving patient safety in the ICU by prospective identification of missing safety barriers using the bow-tie prospective risk analysis model. J Patient Safe 2013;9:154–9.
37. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8.
38. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11:CD006904.
39. Chia PL, Santos DR, Tan TC, et al. Clinical quality improvement: eliminating unplanned extubation in the CCU. Int J Health Care Qual Ass 2013;26:642–52.
40. Balon JA. Common factors of spontaneous self-extubation in a critical care setting. Int J Trauma Nurs 2001;7:93–9.
41. Gregory S, Tan D, Tilrico M, et al. Bedside shift reports: what does the evidence say? J Nurs Admin 2014;44:541–5.
42. Kane RL, Shamliyan TA, Mueller C, et al. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care 2007;45:1195–204.
43. Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med 2010;38:1521–8; quiz 1529.
44. Tindol GA, Jr., DiBenedetto RJ, Kosciuk L. Unplanned extubations. Chest 1994;105:1804–7.
45. Chen CM CK, Fong Y, Hsing SC, et al. Age is an important predictor of failed unplanned extubation. Int J Gerontol 2010;4:120–9.
46. Richmond AL, Jarog DL, Hanson VM. Unplanned extubation in adult critical care. Quality improvement and education payoff. Crit Care Nurs 2004;24:32–7.
47. Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 2011;15:477–80.
48. McNett MM, Horowitz DA. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit Care 2014;21 Suppl 2:S215–28.
49. Gardner A, Hughes, D, Cook R, et al. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Database of abstracts of reivews of effects (DARE): Quality-assessed reviews. 2005. York: Center for Reviews and Dissemination.
50. Hofso K, Coyer FM. Part 1: Chemical and physical restraints in the management of mechanically ventliated patients in the ICU: Contributing factors. Intensive Crit Care Nurs 2007; 23:249–55.
51. Kiekkas P, Diamanto A, Panteli E, et al. Unplanned extubation in critially ill adults: Clinical reviews. Nurs Crit Care 2012;18:123–34.
52. King JN, Elliiot VA. Self/unplanned extubation: Safety, surveillance, and monitoring of the mechanically ventilated patient. Crit Care Nurs Clin North Am 2012;24:469–79.
New Treatments for Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is a slow-growing malignancy of B lymphocytes (B cells) that tends to affect older people and men more than women. More than 17,000 new cases of CLL are reported every year. Patients with CLL do not need treatment with chemotherapy until they become symptomatic or display evidence of rapid progression of the disease. In multiple studies and a meta-analysis, early initiation of chemotherapy has failed to show benefit in managing CLL; indeed, it may increase mortality.1,2
The combination chemotherapy fludarabine, cyclophosphamide, and rituximab (FCR) is often the initial choice for treatment. Other chemotherapy drugs used are chlorambucil, bendamustine, pentostatin or cladribine, rituximab, ofatumumab, and alemtuzumab. Although chlorambucil is a forgotten drug in the U.S., it is still used first line in elderly, fragile populations in Europe, which make up the bulk of true CLL cases.3
Various combination regimens used in CLL treatment have shown improved response rates in several randomized trials but have failed to show any survival advantage until recently. The treatment of patients with CLL has undergone a dramatic transformation and has changed the management paradigm since the FDA approved new, targeted agents. This article includes a brief discussion of these new agents and the pipeline for new agents.
Newly Approved Treatments
Obinutuzumab is a CD20-directed cytolytic antibody, which on binding to CD20, mediates B-cell lysis. Mediation may be (1) through engagement of immune effector cells; (2) by directly activating intracellular deathsignaling pathways; and/or (3) by activation of the complement. The FDA approved obinutuzumab in November 2013 for previously untreated CLL in combination with chlorambucil based on a pivotal phase 3 trial in 356 previously untreated patients with CLL (mean age, 73 years). Those who received obinutuzumab in combination with chlorambucil had significantly better median progression-free survival (PFS) than did those treated with chlorambucil alone (23 months vs 11.1 months; P < .0001). These results effectively end the use of chlorambucil as monotherapy.4
Ibrutinib is a Bruton’s tyrosine kinase (BTK) inhibitor that forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. The BTK is a signaling molecule of the B-cell antigen receptor and cytokine receptor pathways. Accelerated approval of ibrutinib was based on of a clinical study of participants with CLL who had received 4 previous therapies. At 26 months, estimated PFS was 75%, and the rate of overall survival (OS) was 83%.5 In January 2014, the RESONATE trial was stopped early because of a positive interim analysis showing statistically significant improvement in PFS as well as in OS with oral ibrutinib compared with IV ofatumumab. The RESONATE trial was a phase 3, multicenter study involving 391 patients with relapsed or refractory CLL who had received at least 1 previous therapy.6 At 6 months, 88% of patients treated with ibrutinib were progression free compared with 65% with ofatumumab. At 12 months, the OS rate was 90% for patients treated with ibrutinib compared with 81% for patients in the ofatumumab group. By traditional response criteria, the overall response rate (ORR) with ibrutinib was 43% compared with 4% for ofatumumab. Ibrutinib is now approved for chemotherapy-naïve CLL patients with a 17p13.1 deletion, a genetic abnormality that generally portends a poor prognosis.
On July 23, 2014, the FDA approved an oral PI3-kinase delta (PI3K d) inhibitor called idelalisib in combination with rituximab used as a treatment for patients with high-risk CLL. In supporting data from a phase 3 study, the addition of idelalisib to rituximab improved OS by 72% and PFS by 82% vs placebo and rituximab. At 24 weeks, 90% of patients treated with idelalisib remained progression free compared with 50% of patients treated with the placebo. Approval was based on a placebo-controlled study of 220 patients in which patients treated with idelalisib plus rituximab showed significantly longer PFS (10.7 months) than did those who received placebo plus rituximab (5.5 months).7
Lenalidomide is an immunomodulatory drug (IMiD) currently approved for use in multiple myeloma and myelodysplastic syndrome with deletion of chromosome 5q. Studies have used this medication in treatment of patients with relapsed and refractory CLL. Response rates of 47% to 38% with complete response rates of 9% and elimination of minimal residual disease (MRD) have also been reported.8
CLL Pipeline
Clinical trials continue to explore new agents, with the most promising being the PI3K δ and γ inhibitor duvelisib (IPI-145) and the BCL-2 inhibitor ABT-199. In
a phase 1 study exploring duvelisib in patients with relapsed or refractory CLL, the ORR was 47%.9 Additionally, 98% of patients with refractory disease had nodal responses, which did not differ between patients with or without the 17p deletion or p53 mutation.
ABT-199 demonstrated efficacy in a phase 1b study when administered to patients with relapsed/refractory CLL in combination with rituximab.10 In 18 evaluable patients, 39% achieved complete remission (CR) or CR with incomplete blood count recovery and 39% achieved partial remissions (78% ORR). Altogether, 22% were deemed MRD-negative. In evaluable patients with 17p deletions, 81% achieved a response to ABT-199. In patients with fludarabine-refractory CLL, 78% achieved a response.
Although it is advantageous to have so many newer effective, targeted drugs for relapsed/refractory advanced CLL, in early-stage CLL when a watch and wait approach might be best, it may become a challenge for the patient as well as for the treating physician. All these drugs are expensive and carry risks. Although PFS is promising, physicians have to make a judgment call in balancing cost and toxicity.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Bosch F, Ferrer A, Villamor N, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res. 2008;14(1):155-161.
2. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24(3):437-443.
3. Eichhorst BF, Busch R, Stilgenbauer S, et al; German CLL Study Group (GCLLSG). First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382-3391.
4. Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756-1765.
5. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
6. Byrd JC, Brown JR, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223.
7. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007.
8. Molica S. Immunomodulatory drugs in chronic lymphocytic leukemia: a new
treatment paradigm. Leuk Lymphoma. 2007;48(5):866-869.
9. O’Brien S, Patel M, et al. Duvelisib (IPI-145), a PI3K-d,g inhibitor, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. Paper presented at: the 56th ASH Annual Meeting and Exposition; December 7, 2014; San Francisco, CA. Abstract 3334.
10. Ma S, Seymour JF, Lanasa MC, et al. ABT-199 (GDC-0199) combined with rituximab (R) in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): interim results of a phase 1b study. J Clin Oncol. 2014;32 (suppl; abstr 7013):5s.
Chronic lymphocytic leukemia (CLL) is a slow-growing malignancy of B lymphocytes (B cells) that tends to affect older people and men more than women. More than 17,000 new cases of CLL are reported every year. Patients with CLL do not need treatment with chemotherapy until they become symptomatic or display evidence of rapid progression of the disease. In multiple studies and a meta-analysis, early initiation of chemotherapy has failed to show benefit in managing CLL; indeed, it may increase mortality.1,2
The combination chemotherapy fludarabine, cyclophosphamide, and rituximab (FCR) is often the initial choice for treatment. Other chemotherapy drugs used are chlorambucil, bendamustine, pentostatin or cladribine, rituximab, ofatumumab, and alemtuzumab. Although chlorambucil is a forgotten drug in the U.S., it is still used first line in elderly, fragile populations in Europe, which make up the bulk of true CLL cases.3
Various combination regimens used in CLL treatment have shown improved response rates in several randomized trials but have failed to show any survival advantage until recently. The treatment of patients with CLL has undergone a dramatic transformation and has changed the management paradigm since the FDA approved new, targeted agents. This article includes a brief discussion of these new agents and the pipeline for new agents.
Newly Approved Treatments
Obinutuzumab is a CD20-directed cytolytic antibody, which on binding to CD20, mediates B-cell lysis. Mediation may be (1) through engagement of immune effector cells; (2) by directly activating intracellular deathsignaling pathways; and/or (3) by activation of the complement. The FDA approved obinutuzumab in November 2013 for previously untreated CLL in combination with chlorambucil based on a pivotal phase 3 trial in 356 previously untreated patients with CLL (mean age, 73 years). Those who received obinutuzumab in combination with chlorambucil had significantly better median progression-free survival (PFS) than did those treated with chlorambucil alone (23 months vs 11.1 months; P < .0001). These results effectively end the use of chlorambucil as monotherapy.4
Ibrutinib is a Bruton’s tyrosine kinase (BTK) inhibitor that forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. The BTK is a signaling molecule of the B-cell antigen receptor and cytokine receptor pathways. Accelerated approval of ibrutinib was based on of a clinical study of participants with CLL who had received 4 previous therapies. At 26 months, estimated PFS was 75%, and the rate of overall survival (OS) was 83%.5 In January 2014, the RESONATE trial was stopped early because of a positive interim analysis showing statistically significant improvement in PFS as well as in OS with oral ibrutinib compared with IV ofatumumab. The RESONATE trial was a phase 3, multicenter study involving 391 patients with relapsed or refractory CLL who had received at least 1 previous therapy.6 At 6 months, 88% of patients treated with ibrutinib were progression free compared with 65% with ofatumumab. At 12 months, the OS rate was 90% for patients treated with ibrutinib compared with 81% for patients in the ofatumumab group. By traditional response criteria, the overall response rate (ORR) with ibrutinib was 43% compared with 4% for ofatumumab. Ibrutinib is now approved for chemotherapy-naïve CLL patients with a 17p13.1 deletion, a genetic abnormality that generally portends a poor prognosis.
On July 23, 2014, the FDA approved an oral PI3-kinase delta (PI3K d) inhibitor called idelalisib in combination with rituximab used as a treatment for patients with high-risk CLL. In supporting data from a phase 3 study, the addition of idelalisib to rituximab improved OS by 72% and PFS by 82% vs placebo and rituximab. At 24 weeks, 90% of patients treated with idelalisib remained progression free compared with 50% of patients treated with the placebo. Approval was based on a placebo-controlled study of 220 patients in which patients treated with idelalisib plus rituximab showed significantly longer PFS (10.7 months) than did those who received placebo plus rituximab (5.5 months).7
Lenalidomide is an immunomodulatory drug (IMiD) currently approved for use in multiple myeloma and myelodysplastic syndrome with deletion of chromosome 5q. Studies have used this medication in treatment of patients with relapsed and refractory CLL. Response rates of 47% to 38% with complete response rates of 9% and elimination of minimal residual disease (MRD) have also been reported.8
CLL Pipeline
Clinical trials continue to explore new agents, with the most promising being the PI3K δ and γ inhibitor duvelisib (IPI-145) and the BCL-2 inhibitor ABT-199. In
a phase 1 study exploring duvelisib in patients with relapsed or refractory CLL, the ORR was 47%.9 Additionally, 98% of patients with refractory disease had nodal responses, which did not differ between patients with or without the 17p deletion or p53 mutation.
ABT-199 demonstrated efficacy in a phase 1b study when administered to patients with relapsed/refractory CLL in combination with rituximab.10 In 18 evaluable patients, 39% achieved complete remission (CR) or CR with incomplete blood count recovery and 39% achieved partial remissions (78% ORR). Altogether, 22% were deemed MRD-negative. In evaluable patients with 17p deletions, 81% achieved a response to ABT-199. In patients with fludarabine-refractory CLL, 78% achieved a response.
Although it is advantageous to have so many newer effective, targeted drugs for relapsed/refractory advanced CLL, in early-stage CLL when a watch and wait approach might be best, it may become a challenge for the patient as well as for the treating physician. All these drugs are expensive and carry risks. Although PFS is promising, physicians have to make a judgment call in balancing cost and toxicity.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
Chronic lymphocytic leukemia (CLL) is a slow-growing malignancy of B lymphocytes (B cells) that tends to affect older people and men more than women. More than 17,000 new cases of CLL are reported every year. Patients with CLL do not need treatment with chemotherapy until they become symptomatic or display evidence of rapid progression of the disease. In multiple studies and a meta-analysis, early initiation of chemotherapy has failed to show benefit in managing CLL; indeed, it may increase mortality.1,2
The combination chemotherapy fludarabine, cyclophosphamide, and rituximab (FCR) is often the initial choice for treatment. Other chemotherapy drugs used are chlorambucil, bendamustine, pentostatin or cladribine, rituximab, ofatumumab, and alemtuzumab. Although chlorambucil is a forgotten drug in the U.S., it is still used first line in elderly, fragile populations in Europe, which make up the bulk of true CLL cases.3
Various combination regimens used in CLL treatment have shown improved response rates in several randomized trials but have failed to show any survival advantage until recently. The treatment of patients with CLL has undergone a dramatic transformation and has changed the management paradigm since the FDA approved new, targeted agents. This article includes a brief discussion of these new agents and the pipeline for new agents.
Newly Approved Treatments
Obinutuzumab is a CD20-directed cytolytic antibody, which on binding to CD20, mediates B-cell lysis. Mediation may be (1) through engagement of immune effector cells; (2) by directly activating intracellular deathsignaling pathways; and/or (3) by activation of the complement. The FDA approved obinutuzumab in November 2013 for previously untreated CLL in combination with chlorambucil based on a pivotal phase 3 trial in 356 previously untreated patients with CLL (mean age, 73 years). Those who received obinutuzumab in combination with chlorambucil had significantly better median progression-free survival (PFS) than did those treated with chlorambucil alone (23 months vs 11.1 months; P < .0001). These results effectively end the use of chlorambucil as monotherapy.4
Ibrutinib is a Bruton’s tyrosine kinase (BTK) inhibitor that forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. The BTK is a signaling molecule of the B-cell antigen receptor and cytokine receptor pathways. Accelerated approval of ibrutinib was based on of a clinical study of participants with CLL who had received 4 previous therapies. At 26 months, estimated PFS was 75%, and the rate of overall survival (OS) was 83%.5 In January 2014, the RESONATE trial was stopped early because of a positive interim analysis showing statistically significant improvement in PFS as well as in OS with oral ibrutinib compared with IV ofatumumab. The RESONATE trial was a phase 3, multicenter study involving 391 patients with relapsed or refractory CLL who had received at least 1 previous therapy.6 At 6 months, 88% of patients treated with ibrutinib were progression free compared with 65% with ofatumumab. At 12 months, the OS rate was 90% for patients treated with ibrutinib compared with 81% for patients in the ofatumumab group. By traditional response criteria, the overall response rate (ORR) with ibrutinib was 43% compared with 4% for ofatumumab. Ibrutinib is now approved for chemotherapy-naïve CLL patients with a 17p13.1 deletion, a genetic abnormality that generally portends a poor prognosis.
On July 23, 2014, the FDA approved an oral PI3-kinase delta (PI3K d) inhibitor called idelalisib in combination with rituximab used as a treatment for patients with high-risk CLL. In supporting data from a phase 3 study, the addition of idelalisib to rituximab improved OS by 72% and PFS by 82% vs placebo and rituximab. At 24 weeks, 90% of patients treated with idelalisib remained progression free compared with 50% of patients treated with the placebo. Approval was based on a placebo-controlled study of 220 patients in which patients treated with idelalisib plus rituximab showed significantly longer PFS (10.7 months) than did those who received placebo plus rituximab (5.5 months).7
Lenalidomide is an immunomodulatory drug (IMiD) currently approved for use in multiple myeloma and myelodysplastic syndrome with deletion of chromosome 5q. Studies have used this medication in treatment of patients with relapsed and refractory CLL. Response rates of 47% to 38% with complete response rates of 9% and elimination of minimal residual disease (MRD) have also been reported.8
CLL Pipeline
Clinical trials continue to explore new agents, with the most promising being the PI3K δ and γ inhibitor duvelisib (IPI-145) and the BCL-2 inhibitor ABT-199. In
a phase 1 study exploring duvelisib in patients with relapsed or refractory CLL, the ORR was 47%.9 Additionally, 98% of patients with refractory disease had nodal responses, which did not differ between patients with or without the 17p deletion or p53 mutation.
ABT-199 demonstrated efficacy in a phase 1b study when administered to patients with relapsed/refractory CLL in combination with rituximab.10 In 18 evaluable patients, 39% achieved complete remission (CR) or CR with incomplete blood count recovery and 39% achieved partial remissions (78% ORR). Altogether, 22% were deemed MRD-negative. In evaluable patients with 17p deletions, 81% achieved a response to ABT-199. In patients with fludarabine-refractory CLL, 78% achieved a response.
Although it is advantageous to have so many newer effective, targeted drugs for relapsed/refractory advanced CLL, in early-stage CLL when a watch and wait approach might be best, it may become a challenge for the patient as well as for the treating physician. All these drugs are expensive and carry risks. Although PFS is promising, physicians have to make a judgment call in balancing cost and toxicity.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Bosch F, Ferrer A, Villamor N, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res. 2008;14(1):155-161.
2. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24(3):437-443.
3. Eichhorst BF, Busch R, Stilgenbauer S, et al; German CLL Study Group (GCLLSG). First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382-3391.
4. Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756-1765.
5. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
6. Byrd JC, Brown JR, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223.
7. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007.
8. Molica S. Immunomodulatory drugs in chronic lymphocytic leukemia: a new
treatment paradigm. Leuk Lymphoma. 2007;48(5):866-869.
9. O’Brien S, Patel M, et al. Duvelisib (IPI-145), a PI3K-d,g inhibitor, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. Paper presented at: the 56th ASH Annual Meeting and Exposition; December 7, 2014; San Francisco, CA. Abstract 3334.
10. Ma S, Seymour JF, Lanasa MC, et al. ABT-199 (GDC-0199) combined with rituximab (R) in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): interim results of a phase 1b study. J Clin Oncol. 2014;32 (suppl; abstr 7013):5s.
1. Bosch F, Ferrer A, Villamor N, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res. 2008;14(1):155-161.
2. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24(3):437-443.
3. Eichhorst BF, Busch R, Stilgenbauer S, et al; German CLL Study Group (GCLLSG). First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382-3391.
4. Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756-1765.
5. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
6. Byrd JC, Brown JR, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223.
7. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007.
8. Molica S. Immunomodulatory drugs in chronic lymphocytic leukemia: a new
treatment paradigm. Leuk Lymphoma. 2007;48(5):866-869.
9. O’Brien S, Patel M, et al. Duvelisib (IPI-145), a PI3K-d,g inhibitor, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. Paper presented at: the 56th ASH Annual Meeting and Exposition; December 7, 2014; San Francisco, CA. Abstract 3334.
10. Ma S, Seymour JF, Lanasa MC, et al. ABT-199 (GDC-0199) combined with rituximab (R) in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): interim results of a phase 1b study. J Clin Oncol. 2014;32 (suppl; abstr 7013):5s.
Palonosetron and netupitant for prevention of chemotherapy-induced nausea and vomiting
The US Food and Drug Administration (FDA) recently approved NEPA, an oral fixed-dose combination of netupitant and palonosetron for treatment of chemotherapy-induced nausea and vomiting (CINV). Palonosetron is a pharmacologically distinct, best-in-class serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist, which prevents CINV during the acute phase (0-24 h) after administration of chemotherapy, and netupitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist, which prevents CINV during both the acute and delayed (25-120 h) phases. The 2 agents have also been shown potentially to act synergistically in inhibiting NK-1 receptor activity.
Click on the PDF icon at the top of this introduction to read the full article.
The US Food and Drug Administration (FDA) recently approved NEPA, an oral fixed-dose combination of netupitant and palonosetron for treatment of chemotherapy-induced nausea and vomiting (CINV). Palonosetron is a pharmacologically distinct, best-in-class serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist, which prevents CINV during the acute phase (0-24 h) after administration of chemotherapy, and netupitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist, which prevents CINV during both the acute and delayed (25-120 h) phases. The 2 agents have also been shown potentially to act synergistically in inhibiting NK-1 receptor activity.
Click on the PDF icon at the top of this introduction to read the full article.
The US Food and Drug Administration (FDA) recently approved NEPA, an oral fixed-dose combination of netupitant and palonosetron for treatment of chemotherapy-induced nausea and vomiting (CINV). Palonosetron is a pharmacologically distinct, best-in-class serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist, which prevents CINV during the acute phase (0-24 h) after administration of chemotherapy, and netupitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist, which prevents CINV during both the acute and delayed (25-120 h) phases. The 2 agents have also been shown potentially to act synergistically in inhibiting NK-1 receptor activity.
Click on the PDF icon at the top of this introduction to read the full article.