User login
Management of Do Not Resuscitate Orders Before Invasive Procedures
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
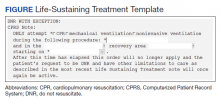
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
Quality of Chronic Obstructive Pulmonary Disease-Related Health Care in Rural and Urban Veterans Affairs Clinics
Chronic obstructive pulmonary disease (COPD) affects between 11 and 24 million people in the U.S. and is the third leading cause of death in this country.1,2 Airflow obstruction on spirometry in addition to respiratory symptoms is required to establish a diagnosis of COPD.3,4 As many as 40% of patients with a clinical diagnosis of COPD have not had spirometry or have spirometry results inconsistent with the diagnosis of COPD.5,6 In addition to recommended spirometry, many patients with COPD do not receive other evidence-based therapies.7,8
About 50% of patients in the Minneapolis VA Health Care System (MVAHCS) receive care in its rural community-based outreach clinics (CBOCs). Data regarding the quality of general medical care between rural and urban populations are sparse; however, studies suggest that the quality of care delivered in rural clinics may be lower than the care provided in an urban setting.9-12 Care for patients with COPD in an urban setting is suboptimal with only 58% of patients receiving guideline-based care, and there are no comparative data for penetrance in the rural setting.8 Most published studies on patients with COPD treated in rural vs urban locations are outcomes studies that queried statewide or national registry data evaluating the frequency of emergency department (ED) visits or hospital admissions for COPD exacerbations, all-cause mortality, or COPD exacerbation-related mortality.13-18 There are no studies examining potential differences in the quality of health care received by patients with COPD in rural vs urban locations or whether these potential differences are associated with changes in health care utilization.
The authors sought to determine whether patients with the diagnosis of COPD treated in the MVAHCS and its 13 CBOCs receive similar quality of disease-related health care in rural vs urban primary care clinic locations. The authors hypothesized that patients who receive their primary care in rural clinics would be less likely to have had spirometry or to receive respiratory immunizations and short- or long-acting inhalers and that discrepancies would be associated with increased health care utilization in rural areas as measured by prescriptions for systemic corticosteroids, antibiotics, ED visits, or hospital admissions for COPD exacerbations.
Methods
The MVAHCS has 14 primary care locations; these locations were designated as rural or urban based on the Rural-Urban Commuting Area codes.19,20 There were 4 urban locations and 10 rural clinics; all rural clinics were farther than 40 miles from the main Minneapolis VAMC.
Patient Selection
The authors performed a retrospective chart review after receiving an institutional review board waiver for this quality assessment study. All patients who had a prior ICD-9 encounter diagnosis of COPD (codes: 491.0, 491.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496) and who were seen in primary care during March 2015 were identified. Each subject’s first visit during that month was used as the start of the retrospective 1-year look-back period. All eligible subjects were sorted based on their rural or urban location and a randomly assigned number. Patients were then selected according to ascending numbers from each rural and urban clinic in proportion to the clinic’s representation among all eligible patients.
Outcomes
The primary outcomes—possible discrepancies in quality of health care for patients with COPD in rural vs urban primary care clinics—were assessed by (1) prior spirometry; (2) any prior pneumonia vaccination; (3) an influenza vaccination within the past year; (4) prescriptions within the past year for a short-acting beta agonist (SABA) metered-dose inhaler; and (5) prescriptions for a long-acting inhalers, including long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or inhaled corticosteroids (ICSs).
Secondary outcomes included (1) an active prescription for home oxygen within the past calendar year; (2) health care utilization assessed via prescriptions for intermittent courses of oral corticosteroids; (3) prescriptions for respiratory antibiotics (macrolides, tetracyclines, fluoroquinolones) within the past year for COPD exacerbations; (4) ED visits; (5) hospital admissions (and need for mechanical ventilation) for COPD exacerbations within the past year; and (6) whether patients were seen by either VA or Non-VA pulmonology providers.
Data Collection
Patients’ demographic data and comorbidities were collected via chart review. A 1-year prescription medication list was obtained by an electronic database search of the MVAHCS electronic medical record (EMR). Additional antibiotics and corticosteroid prescriptions for COPD exacerbations paid for by the VA but filled at a local pharmacy were manually searched from a separate database to supplement the electronic prescription list. Comparison of the electronic prescription list and pharmacy records in 25% of patients found 100% concordance in the prescription lists. The investigator manually reviewed and extracted the following data from the EMR, scanned-in records, and a Midwest VA COPD registry database: most recent spirometry results; immunization status for influenza in the past year; prior pneumonia vaccination; home oxygen prescription; whether the patient received respiratory antibiotic or intermittent oral corticosteroid treatment for COPD exacerbations; whether the patient had a ED visit or hospital admission for COPD exacerbation with or without need for mechanical ventilation; and whether the patient had been seen by a pulmonology provider. The investigator reviewed all primary care provider notes in the past year for documentation of non-VA ED visits or hospitalizations that were not present in the EMR, Midwest VA COPD registry database, or scanned patient records.
Data Analysis
Results are described as mean ± standard deviation, median (interquartile range) or proportion, expressed as a percentage as appropriate for the level of measurement and distribution. The proportions meeting the COPD quality of health care outcomes in the urban and rural groups were compared using a chi-square test of proportions, and 95% confidence intervals (CI) on the differences were estimated. Samples of 400 patients each from the rural and urban groups were estimated to provide a 95% 2-sided CI on the differences of about ± 0.05 (5%), assuming the proportion meeting the quality of care outcomes in the urban group would be at least 0.8 (80%).
Results
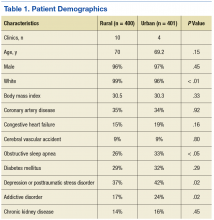
The authors identified 1,538 patients with a previous encounter diagnosis of COPD who were seen in a primary care clinic in the MVAHCS in March of 2015. The authors reviewed the medical records of 801 randomly selected patients: 400 rural clinic patients and 401 urban clinic patients. Demographic characteristics and major comorbidities of rural and urban patients were similar except more rural patients were white, and fewer had a record of obstructive sleep apnea, alcoholism, or addictive disorders (Table 1). Prescriptions for common chronic medical conditions were similar for rural and urban groups, including medications for depression (31% vs 33%) or diabetes mellitus (25% vs 28%). In patients who had spirometry, the severity of COPD, as assessed by mean forced expiratory volume (FEV1), was similar between rural and urban patients (2.06 L vs 2.10 L).
Quality of COPD Care
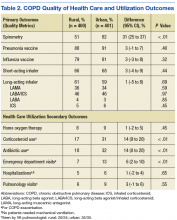
Spirometry was documented in fewer rural clinic patients than in urban clinic patients (51% vs 82%; difference 31%, 95% CI: 25% to 37%) (Table 2).
COPD Outcomes
Home oxygen prescription rates were similar for rural and urban clinic patients (8% vs 9%; difference 1%, 95% CI: -2% to 5%). Rural patients received fewer prescriptions for intermittent oral corticosteroids (17% vs 31%; difference 14%, 95% CI: 9% to 20%) and antibiotics for COPD exacerbations (18% vs 32%; difference 14%, 95% CI: 8% to 20%). Rural patients had fewer ED visits for COPD exacerbations (7% vs 13%; difference 6%, 95% CI: 2% to 10%), and similar admission rates for COPD exacerbations (5% vs 6%; difference 1%, 95% CI: -2% to 4%). Of the few patients hospitalized for COPD exacerbations, none required mechanical ventilation. There was no significant difference in the number of rural vs urban patients seen by a pulmonologist in the calendar year of the study (6% vs 9%; difference 3%, 95% CI:-1% to 6%), with the majority seen by VA providers: 20/24 rural patients and 35/35 urban patients.
Discussion
Fewer rural patients had prior spirometry; otherwise, the COPD-related quality metrics were similar between rural and urban patient groups in the MVAHCS, including immunizations for pneumonia and influenza, and prescribing rates for short- and long-acting inhaler therapy. Despite the similarity in these COPD quality measures, rural clinic patients seemed to have less health care utilization related to COPD exacerbations.
Spirometry with airflow obstruction in the presence of respiratory symptoms is required for accurate diagnosis of COPD.3,4 Spirometry has been available at the MVAHCS hospital-based clinic for years. Efforts to address this disparity led to implementation of on-site spirometry at all rural and urban clinics about 2 years prior to the patient enrollment visit date for the study. Fewer rural patients had spirometry, which is possibly from prior disparity in resources; yet rates of spirometry in all patients with a clinical diagnosis of COPD in the MVAHCS are higher (rural 51%, urban 82%) than previously reported. A nationwide study of 94,000 veterans with recent clinical diagnosis of COPD found only 37% had spirometry within 2.5 years of diagnosis,21 and another non-VA study (n = 553) showed only 31% of patients discharged from a hospital with a diagnosis of COPD exacerbation had spirometry performed within a 8-year period prior to hospitalization.22
Annual influenza vaccines are recommended for everyone aged > 6 months, and the pneumonia vaccine is recommended for all patients with COPD in order to reduce the risk of COPD exacerbations and pneumonias.23,24 The rates of vaccination at MVAHCS rural and urban clinics for both influenza (79% vs 81%) and pneumococcus (88% vs 91%) are higher than previously published studies of patients with COPD for influenza vaccination (30%-51%) and pneumonia vaccination (21%-51%) and did not differ between rural and urban clinics.7,25-28 The observed high vaccination rates may be due to EMR prompts and requirements to document vaccination status and offer recommended vaccinations.
Long-acting inhalers have been shown to reduce rates of COPD exacerbations and improve patients’ quality of life.29 The authors found no disparity in the prescription rate of short- or long-acting inhalers between rural and urban patients, and no difference in the severity of COPD, as indicated by FEV1, that might influence prescription rates.
The authors attempted to evaluate health care resource utilization as an indicator of health care quality and outcomes. Based on previous reports, the authors expected to find lower quality of care and increased utilization in rural patients. Previous studies have shown rural patients can be more symptomatic with a higher body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE index) than are patients in urban settings.16,30 Statewide and national registry data have shown rural patients have higher rates of primary care visits, ER visits, and hospitalizations for COPD exacerbations.
Rural patients also have been shown to have higher mortality rates and were more likely to be in a long-term care center and less likely to have home care or palliative care than were their urban counterparts.13-18,31 If the severity of illness is similar in rural and urban areas, higher health care utilization related to COPD would suggest that patients in rural settings may be receiving inferior quality of health care. The authors could not find any previous reports of the quality of COPD care delivered in rural vs urban settings.
In this study the only difference in quality of care was the lower proportion of rural patients with a record of spirometry that is needed to confirm the diagnosis. The observed differences in the quality of care measures wouldn’t be expected to lead to large differences in the outcome measures. Contrary to the literature and the observed similarity in quality of care, rural patients had better COPD outcomes perhaps due to unmeasured differences in risk or failure to capture medical visits outside of the VA system. The severity of COPD based on FEV1 and concurrent diagnoses, such as heart failure, did not suggest that rural patients in this comparison had a higher burden of illness or risk of poor COPD outcomes.
More than 70,000 patients spanning a large geographic region receive primary care at MVAHCS, which provides comparable care to all COPD patients, regardless of location, by using the same EMR system, providing evidence-based order sets for disease management, proactively offering on-site and remote COPD case managers for high-risk patients, and more recently, implementing on-site spirometry testing in all clinics. This approach as opposed to the traditional outreach clinic model may in part explain the similarity in quality of care in urban and rural clinics that was not reported in previous studies.
Limitations
This study was performed retrospectively, increasing the potential of missing data, especially from outside the VA health care system. Patients were not randomly assigned to rural or urban clinics, so differences in patient characteristics could exist. Alcohol and addictive disorders were more common in urban patients, which might affect adherence to prescribed medications. In addition, lower rate of obstructive sleep apnea was found in the rural population, which has been linked to increased airway inflammation and COPD exacerbations resulting in hospitalization.32,33 Mortality was not assessed as all patients were alive and seen in clinic at time of enrollment.
The authors were not able to record a patients’ residence in a long-term care facility or institutionalization, use of home care, or palliative care services due to limitations in the EMR system. Whether patients received comanaged primary care or underwent pulmonary rehabilitation could not be obtained from the EMR. Most patients never had lung volumes or diffusion capacity and thus were not included. The authors could not report whether inhaler therapy was appropriate compared with the Global Initiative for Chronic Obstructive Lund Disease(GOLD)severity score because most of the spirometry was done at a discordant time to when inhaler therapy was assessed, and new GOLD guidelines include patient symptoms that were not reliably recorded in the EMR. Last, the authors had hoped to include smoking status and cessation practices as part of the quality measures, but due to significant variability in patients’ documented smoking status in the same period, the data were deemed unreliable.
Conclusion
No disparities were found between rural and urban clinics in the quality of health care for patients with COPD in the MVAHCS except that fewer rural patients had prior spirometry; a difference that is likely due to the fact that only recently has spirometry been implemented in the MVAHCS rural clinics. Overall the quality of COPD care was high and above the previously reported rates. Further larger studies of rural and urban quality of health care for patients with COPD are needed in other VA and non-VA systems to determine whether disparities exist and whether they are associated with clinical outcomes, including ED visits, hospitalizations, and mortality.
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1-161.
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-1276.
5. Ghattas C, Dai A, Gemmel DJ, Awad MH. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis. 2013;8:545-549.
6. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171.
7. Lopez-Campos JL, Abad Arranz M, Calero-Acuña C, et al. Guideline adherence in outpatient clinics for chronic obstructive pulmonary disease: results from a clinical audit. PLoS One. 2016;11(3):e0151896.
8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645.
9. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: U.S. Department of Veteran Affairs; 2011.
10. Weeks WB, Wallace AE, Wang S, Lee A, Kazis LE. Rural-urban disparities in health-related quality of life within disease categories of Veterans. J Rural Health. 2006;22(3):204-211.
11. Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57(6):851-856.
12. Meit M, Knudson A, Gilbert T, et al; Rural Health Reform Policy Research Center. The 2014 update of the rural-urban chartbook. https://ruralhealth.und .edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published October 2014. Accessed April 18, 2017.
13. Jackson BE, Suzuki S, Coultas D, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563-571.
14. Jackson BE, Suzuki S, Lo K, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105(5):734-739.15. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006-2011: statistical brief #179. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Published September 2014. Accessed May 9, 2017.
16. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(suppl 1):62S-69S.
17. Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19-e29.
18. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155(2):80-86.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155.
20. U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#U20K1F50H0A. Updated October 12, 2016. Accessed May 9, 2017.
21. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38-45.
22. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120-1124.
23. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818-825.
24. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2016. Ann Intern Med. 2016;164(3):184-194.
25. Cimen P, Unlu M, Kirakli C, et al. Should patients with COPD be vaccinated? Respir Care. 2015;60(2):239-243.
26. Mowls DS, Cheruvu VK, Zullo MD. Influenza vaccination in adults with chronic obstructive pulmonary disease: the impact of a diagnostic breathing test on vaccination rates PLoS One. 2013;8(6):e67600.
27. Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405-e413.
28. Arinez-Fernandez MC, Carrasco-Garrido P, Garcia-Carballo M, Hernandez-Barrera V, de Miguel AG, Jimenez-Garcia R. Determinants of pneumococcal vaccination among patients with chronic obstructive pulmonary disease in Spain. Hum Vaccin. 2006;2(3):99-104.29. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
30. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.
31. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10(2):1349.
32. Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123-1130.
33. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331
Chronic obstructive pulmonary disease (COPD) affects between 11 and 24 million people in the U.S. and is the third leading cause of death in this country.1,2 Airflow obstruction on spirometry in addition to respiratory symptoms is required to establish a diagnosis of COPD.3,4 As many as 40% of patients with a clinical diagnosis of COPD have not had spirometry or have spirometry results inconsistent with the diagnosis of COPD.5,6 In addition to recommended spirometry, many patients with COPD do not receive other evidence-based therapies.7,8
About 50% of patients in the Minneapolis VA Health Care System (MVAHCS) receive care in its rural community-based outreach clinics (CBOCs). Data regarding the quality of general medical care between rural and urban populations are sparse; however, studies suggest that the quality of care delivered in rural clinics may be lower than the care provided in an urban setting.9-12 Care for patients with COPD in an urban setting is suboptimal with only 58% of patients receiving guideline-based care, and there are no comparative data for penetrance in the rural setting.8 Most published studies on patients with COPD treated in rural vs urban locations are outcomes studies that queried statewide or national registry data evaluating the frequency of emergency department (ED) visits or hospital admissions for COPD exacerbations, all-cause mortality, or COPD exacerbation-related mortality.13-18 There are no studies examining potential differences in the quality of health care received by patients with COPD in rural vs urban locations or whether these potential differences are associated with changes in health care utilization.
The authors sought to determine whether patients with the diagnosis of COPD treated in the MVAHCS and its 13 CBOCs receive similar quality of disease-related health care in rural vs urban primary care clinic locations. The authors hypothesized that patients who receive their primary care in rural clinics would be less likely to have had spirometry or to receive respiratory immunizations and short- or long-acting inhalers and that discrepancies would be associated with increased health care utilization in rural areas as measured by prescriptions for systemic corticosteroids, antibiotics, ED visits, or hospital admissions for COPD exacerbations.
Methods
The MVAHCS has 14 primary care locations; these locations were designated as rural or urban based on the Rural-Urban Commuting Area codes.19,20 There were 4 urban locations and 10 rural clinics; all rural clinics were farther than 40 miles from the main Minneapolis VAMC.
Patient Selection
The authors performed a retrospective chart review after receiving an institutional review board waiver for this quality assessment study. All patients who had a prior ICD-9 encounter diagnosis of COPD (codes: 491.0, 491.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496) and who were seen in primary care during March 2015 were identified. Each subject’s first visit during that month was used as the start of the retrospective 1-year look-back period. All eligible subjects were sorted based on their rural or urban location and a randomly assigned number. Patients were then selected according to ascending numbers from each rural and urban clinic in proportion to the clinic’s representation among all eligible patients.
Outcomes
The primary outcomes—possible discrepancies in quality of health care for patients with COPD in rural vs urban primary care clinics—were assessed by (1) prior spirometry; (2) any prior pneumonia vaccination; (3) an influenza vaccination within the past year; (4) prescriptions within the past year for a short-acting beta agonist (SABA) metered-dose inhaler; and (5) prescriptions for a long-acting inhalers, including long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or inhaled corticosteroids (ICSs).
Secondary outcomes included (1) an active prescription for home oxygen within the past calendar year; (2) health care utilization assessed via prescriptions for intermittent courses of oral corticosteroids; (3) prescriptions for respiratory antibiotics (macrolides, tetracyclines, fluoroquinolones) within the past year for COPD exacerbations; (4) ED visits; (5) hospital admissions (and need for mechanical ventilation) for COPD exacerbations within the past year; and (6) whether patients were seen by either VA or Non-VA pulmonology providers.
Data Collection
Patients’ demographic data and comorbidities were collected via chart review. A 1-year prescription medication list was obtained by an electronic database search of the MVAHCS electronic medical record (EMR). Additional antibiotics and corticosteroid prescriptions for COPD exacerbations paid for by the VA but filled at a local pharmacy were manually searched from a separate database to supplement the electronic prescription list. Comparison of the electronic prescription list and pharmacy records in 25% of patients found 100% concordance in the prescription lists. The investigator manually reviewed and extracted the following data from the EMR, scanned-in records, and a Midwest VA COPD registry database: most recent spirometry results; immunization status for influenza in the past year; prior pneumonia vaccination; home oxygen prescription; whether the patient received respiratory antibiotic or intermittent oral corticosteroid treatment for COPD exacerbations; whether the patient had a ED visit or hospital admission for COPD exacerbation with or without need for mechanical ventilation; and whether the patient had been seen by a pulmonology provider. The investigator reviewed all primary care provider notes in the past year for documentation of non-VA ED visits or hospitalizations that were not present in the EMR, Midwest VA COPD registry database, or scanned patient records.
Data Analysis
Results are described as mean ± standard deviation, median (interquartile range) or proportion, expressed as a percentage as appropriate for the level of measurement and distribution. The proportions meeting the COPD quality of health care outcomes in the urban and rural groups were compared using a chi-square test of proportions, and 95% confidence intervals (CI) on the differences were estimated. Samples of 400 patients each from the rural and urban groups were estimated to provide a 95% 2-sided CI on the differences of about ± 0.05 (5%), assuming the proportion meeting the quality of care outcomes in the urban group would be at least 0.8 (80%).
Results
The authors identified 1,538 patients with a previous encounter diagnosis of COPD who were seen in a primary care clinic in the MVAHCS in March of 2015. The authors reviewed the medical records of 801 randomly selected patients: 400 rural clinic patients and 401 urban clinic patients. Demographic characteristics and major comorbidities of rural and urban patients were similar except more rural patients were white, and fewer had a record of obstructive sleep apnea, alcoholism, or addictive disorders (Table 1). Prescriptions for common chronic medical conditions were similar for rural and urban groups, including medications for depression (31% vs 33%) or diabetes mellitus (25% vs 28%). In patients who had spirometry, the severity of COPD, as assessed by mean forced expiratory volume (FEV1), was similar between rural and urban patients (2.06 L vs 2.10 L).
Quality of COPD Care
Spirometry was documented in fewer rural clinic patients than in urban clinic patients (51% vs 82%; difference 31%, 95% CI: 25% to 37%) (Table 2).
COPD Outcomes
Home oxygen prescription rates were similar for rural and urban clinic patients (8% vs 9%; difference 1%, 95% CI: -2% to 5%). Rural patients received fewer prescriptions for intermittent oral corticosteroids (17% vs 31%; difference 14%, 95% CI: 9% to 20%) and antibiotics for COPD exacerbations (18% vs 32%; difference 14%, 95% CI: 8% to 20%). Rural patients had fewer ED visits for COPD exacerbations (7% vs 13%; difference 6%, 95% CI: 2% to 10%), and similar admission rates for COPD exacerbations (5% vs 6%; difference 1%, 95% CI: -2% to 4%). Of the few patients hospitalized for COPD exacerbations, none required mechanical ventilation. There was no significant difference in the number of rural vs urban patients seen by a pulmonologist in the calendar year of the study (6% vs 9%; difference 3%, 95% CI:-1% to 6%), with the majority seen by VA providers: 20/24 rural patients and 35/35 urban patients.
Discussion
Fewer rural patients had prior spirometry; otherwise, the COPD-related quality metrics were similar between rural and urban patient groups in the MVAHCS, including immunizations for pneumonia and influenza, and prescribing rates for short- and long-acting inhaler therapy. Despite the similarity in these COPD quality measures, rural clinic patients seemed to have less health care utilization related to COPD exacerbations.
Spirometry with airflow obstruction in the presence of respiratory symptoms is required for accurate diagnosis of COPD.3,4 Spirometry has been available at the MVAHCS hospital-based clinic for years. Efforts to address this disparity led to implementation of on-site spirometry at all rural and urban clinics about 2 years prior to the patient enrollment visit date for the study. Fewer rural patients had spirometry, which is possibly from prior disparity in resources; yet rates of spirometry in all patients with a clinical diagnosis of COPD in the MVAHCS are higher (rural 51%, urban 82%) than previously reported. A nationwide study of 94,000 veterans with recent clinical diagnosis of COPD found only 37% had spirometry within 2.5 years of diagnosis,21 and another non-VA study (n = 553) showed only 31% of patients discharged from a hospital with a diagnosis of COPD exacerbation had spirometry performed within a 8-year period prior to hospitalization.22
Annual influenza vaccines are recommended for everyone aged > 6 months, and the pneumonia vaccine is recommended for all patients with COPD in order to reduce the risk of COPD exacerbations and pneumonias.23,24 The rates of vaccination at MVAHCS rural and urban clinics for both influenza (79% vs 81%) and pneumococcus (88% vs 91%) are higher than previously published studies of patients with COPD for influenza vaccination (30%-51%) and pneumonia vaccination (21%-51%) and did not differ between rural and urban clinics.7,25-28 The observed high vaccination rates may be due to EMR prompts and requirements to document vaccination status and offer recommended vaccinations.
Long-acting inhalers have been shown to reduce rates of COPD exacerbations and improve patients’ quality of life.29 The authors found no disparity in the prescription rate of short- or long-acting inhalers between rural and urban patients, and no difference in the severity of COPD, as indicated by FEV1, that might influence prescription rates.
The authors attempted to evaluate health care resource utilization as an indicator of health care quality and outcomes. Based on previous reports, the authors expected to find lower quality of care and increased utilization in rural patients. Previous studies have shown rural patients can be more symptomatic with a higher body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE index) than are patients in urban settings.16,30 Statewide and national registry data have shown rural patients have higher rates of primary care visits, ER visits, and hospitalizations for COPD exacerbations.
Rural patients also have been shown to have higher mortality rates and were more likely to be in a long-term care center and less likely to have home care or palliative care than were their urban counterparts.13-18,31 If the severity of illness is similar in rural and urban areas, higher health care utilization related to COPD would suggest that patients in rural settings may be receiving inferior quality of health care. The authors could not find any previous reports of the quality of COPD care delivered in rural vs urban settings.
In this study the only difference in quality of care was the lower proportion of rural patients with a record of spirometry that is needed to confirm the diagnosis. The observed differences in the quality of care measures wouldn’t be expected to lead to large differences in the outcome measures. Contrary to the literature and the observed similarity in quality of care, rural patients had better COPD outcomes perhaps due to unmeasured differences in risk or failure to capture medical visits outside of the VA system. The severity of COPD based on FEV1 and concurrent diagnoses, such as heart failure, did not suggest that rural patients in this comparison had a higher burden of illness or risk of poor COPD outcomes.
More than 70,000 patients spanning a large geographic region receive primary care at MVAHCS, which provides comparable care to all COPD patients, regardless of location, by using the same EMR system, providing evidence-based order sets for disease management, proactively offering on-site and remote COPD case managers for high-risk patients, and more recently, implementing on-site spirometry testing in all clinics. This approach as opposed to the traditional outreach clinic model may in part explain the similarity in quality of care in urban and rural clinics that was not reported in previous studies.
Limitations
This study was performed retrospectively, increasing the potential of missing data, especially from outside the VA health care system. Patients were not randomly assigned to rural or urban clinics, so differences in patient characteristics could exist. Alcohol and addictive disorders were more common in urban patients, which might affect adherence to prescribed medications. In addition, lower rate of obstructive sleep apnea was found in the rural population, which has been linked to increased airway inflammation and COPD exacerbations resulting in hospitalization.32,33 Mortality was not assessed as all patients were alive and seen in clinic at time of enrollment.
The authors were not able to record a patients’ residence in a long-term care facility or institutionalization, use of home care, or palliative care services due to limitations in the EMR system. Whether patients received comanaged primary care or underwent pulmonary rehabilitation could not be obtained from the EMR. Most patients never had lung volumes or diffusion capacity and thus were not included. The authors could not report whether inhaler therapy was appropriate compared with the Global Initiative for Chronic Obstructive Lund Disease(GOLD)severity score because most of the spirometry was done at a discordant time to when inhaler therapy was assessed, and new GOLD guidelines include patient symptoms that were not reliably recorded in the EMR. Last, the authors had hoped to include smoking status and cessation practices as part of the quality measures, but due to significant variability in patients’ documented smoking status in the same period, the data were deemed unreliable.
Conclusion
No disparities were found between rural and urban clinics in the quality of health care for patients with COPD in the MVAHCS except that fewer rural patients had prior spirometry; a difference that is likely due to the fact that only recently has spirometry been implemented in the MVAHCS rural clinics. Overall the quality of COPD care was high and above the previously reported rates. Further larger studies of rural and urban quality of health care for patients with COPD are needed in other VA and non-VA systems to determine whether disparities exist and whether they are associated with clinical outcomes, including ED visits, hospitalizations, and mortality.
Chronic obstructive pulmonary disease (COPD) affects between 11 and 24 million people in the U.S. and is the third leading cause of death in this country.1,2 Airflow obstruction on spirometry in addition to respiratory symptoms is required to establish a diagnosis of COPD.3,4 As many as 40% of patients with a clinical diagnosis of COPD have not had spirometry or have spirometry results inconsistent with the diagnosis of COPD.5,6 In addition to recommended spirometry, many patients with COPD do not receive other evidence-based therapies.7,8
About 50% of patients in the Minneapolis VA Health Care System (MVAHCS) receive care in its rural community-based outreach clinics (CBOCs). Data regarding the quality of general medical care between rural and urban populations are sparse; however, studies suggest that the quality of care delivered in rural clinics may be lower than the care provided in an urban setting.9-12 Care for patients with COPD in an urban setting is suboptimal with only 58% of patients receiving guideline-based care, and there are no comparative data for penetrance in the rural setting.8 Most published studies on patients with COPD treated in rural vs urban locations are outcomes studies that queried statewide or national registry data evaluating the frequency of emergency department (ED) visits or hospital admissions for COPD exacerbations, all-cause mortality, or COPD exacerbation-related mortality.13-18 There are no studies examining potential differences in the quality of health care received by patients with COPD in rural vs urban locations or whether these potential differences are associated with changes in health care utilization.
The authors sought to determine whether patients with the diagnosis of COPD treated in the MVAHCS and its 13 CBOCs receive similar quality of disease-related health care in rural vs urban primary care clinic locations. The authors hypothesized that patients who receive their primary care in rural clinics would be less likely to have had spirometry or to receive respiratory immunizations and short- or long-acting inhalers and that discrepancies would be associated with increased health care utilization in rural areas as measured by prescriptions for systemic corticosteroids, antibiotics, ED visits, or hospital admissions for COPD exacerbations.
Methods
The MVAHCS has 14 primary care locations; these locations were designated as rural or urban based on the Rural-Urban Commuting Area codes.19,20 There were 4 urban locations and 10 rural clinics; all rural clinics were farther than 40 miles from the main Minneapolis VAMC.
Patient Selection
The authors performed a retrospective chart review after receiving an institutional review board waiver for this quality assessment study. All patients who had a prior ICD-9 encounter diagnosis of COPD (codes: 491.0, 491.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496) and who were seen in primary care during March 2015 were identified. Each subject’s first visit during that month was used as the start of the retrospective 1-year look-back period. All eligible subjects were sorted based on their rural or urban location and a randomly assigned number. Patients were then selected according to ascending numbers from each rural and urban clinic in proportion to the clinic’s representation among all eligible patients.
Outcomes
The primary outcomes—possible discrepancies in quality of health care for patients with COPD in rural vs urban primary care clinics—were assessed by (1) prior spirometry; (2) any prior pneumonia vaccination; (3) an influenza vaccination within the past year; (4) prescriptions within the past year for a short-acting beta agonist (SABA) metered-dose inhaler; and (5) prescriptions for a long-acting inhalers, including long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or inhaled corticosteroids (ICSs).
Secondary outcomes included (1) an active prescription for home oxygen within the past calendar year; (2) health care utilization assessed via prescriptions for intermittent courses of oral corticosteroids; (3) prescriptions for respiratory antibiotics (macrolides, tetracyclines, fluoroquinolones) within the past year for COPD exacerbations; (4) ED visits; (5) hospital admissions (and need for mechanical ventilation) for COPD exacerbations within the past year; and (6) whether patients were seen by either VA or Non-VA pulmonology providers.
Data Collection
Patients’ demographic data and comorbidities were collected via chart review. A 1-year prescription medication list was obtained by an electronic database search of the MVAHCS electronic medical record (EMR). Additional antibiotics and corticosteroid prescriptions for COPD exacerbations paid for by the VA but filled at a local pharmacy were manually searched from a separate database to supplement the electronic prescription list. Comparison of the electronic prescription list and pharmacy records in 25% of patients found 100% concordance in the prescription lists. The investigator manually reviewed and extracted the following data from the EMR, scanned-in records, and a Midwest VA COPD registry database: most recent spirometry results; immunization status for influenza in the past year; prior pneumonia vaccination; home oxygen prescription; whether the patient received respiratory antibiotic or intermittent oral corticosteroid treatment for COPD exacerbations; whether the patient had a ED visit or hospital admission for COPD exacerbation with or without need for mechanical ventilation; and whether the patient had been seen by a pulmonology provider. The investigator reviewed all primary care provider notes in the past year for documentation of non-VA ED visits or hospitalizations that were not present in the EMR, Midwest VA COPD registry database, or scanned patient records.
Data Analysis
Results are described as mean ± standard deviation, median (interquartile range) or proportion, expressed as a percentage as appropriate for the level of measurement and distribution. The proportions meeting the COPD quality of health care outcomes in the urban and rural groups were compared using a chi-square test of proportions, and 95% confidence intervals (CI) on the differences were estimated. Samples of 400 patients each from the rural and urban groups were estimated to provide a 95% 2-sided CI on the differences of about ± 0.05 (5%), assuming the proportion meeting the quality of care outcomes in the urban group would be at least 0.8 (80%).
Results
The authors identified 1,538 patients with a previous encounter diagnosis of COPD who were seen in a primary care clinic in the MVAHCS in March of 2015. The authors reviewed the medical records of 801 randomly selected patients: 400 rural clinic patients and 401 urban clinic patients. Demographic characteristics and major comorbidities of rural and urban patients were similar except more rural patients were white, and fewer had a record of obstructive sleep apnea, alcoholism, or addictive disorders (Table 1). Prescriptions for common chronic medical conditions were similar for rural and urban groups, including medications for depression (31% vs 33%) or diabetes mellitus (25% vs 28%). In patients who had spirometry, the severity of COPD, as assessed by mean forced expiratory volume (FEV1), was similar between rural and urban patients (2.06 L vs 2.10 L).
Quality of COPD Care
Spirometry was documented in fewer rural clinic patients than in urban clinic patients (51% vs 82%; difference 31%, 95% CI: 25% to 37%) (Table 2).
COPD Outcomes
Home oxygen prescription rates were similar for rural and urban clinic patients (8% vs 9%; difference 1%, 95% CI: -2% to 5%). Rural patients received fewer prescriptions for intermittent oral corticosteroids (17% vs 31%; difference 14%, 95% CI: 9% to 20%) and antibiotics for COPD exacerbations (18% vs 32%; difference 14%, 95% CI: 8% to 20%). Rural patients had fewer ED visits for COPD exacerbations (7% vs 13%; difference 6%, 95% CI: 2% to 10%), and similar admission rates for COPD exacerbations (5% vs 6%; difference 1%, 95% CI: -2% to 4%). Of the few patients hospitalized for COPD exacerbations, none required mechanical ventilation. There was no significant difference in the number of rural vs urban patients seen by a pulmonologist in the calendar year of the study (6% vs 9%; difference 3%, 95% CI:-1% to 6%), with the majority seen by VA providers: 20/24 rural patients and 35/35 urban patients.
Discussion
Fewer rural patients had prior spirometry; otherwise, the COPD-related quality metrics were similar between rural and urban patient groups in the MVAHCS, including immunizations for pneumonia and influenza, and prescribing rates for short- and long-acting inhaler therapy. Despite the similarity in these COPD quality measures, rural clinic patients seemed to have less health care utilization related to COPD exacerbations.
Spirometry with airflow obstruction in the presence of respiratory symptoms is required for accurate diagnosis of COPD.3,4 Spirometry has been available at the MVAHCS hospital-based clinic for years. Efforts to address this disparity led to implementation of on-site spirometry at all rural and urban clinics about 2 years prior to the patient enrollment visit date for the study. Fewer rural patients had spirometry, which is possibly from prior disparity in resources; yet rates of spirometry in all patients with a clinical diagnosis of COPD in the MVAHCS are higher (rural 51%, urban 82%) than previously reported. A nationwide study of 94,000 veterans with recent clinical diagnosis of COPD found only 37% had spirometry within 2.5 years of diagnosis,21 and another non-VA study (n = 553) showed only 31% of patients discharged from a hospital with a diagnosis of COPD exacerbation had spirometry performed within a 8-year period prior to hospitalization.22
Annual influenza vaccines are recommended for everyone aged > 6 months, and the pneumonia vaccine is recommended for all patients with COPD in order to reduce the risk of COPD exacerbations and pneumonias.23,24 The rates of vaccination at MVAHCS rural and urban clinics for both influenza (79% vs 81%) and pneumococcus (88% vs 91%) are higher than previously published studies of patients with COPD for influenza vaccination (30%-51%) and pneumonia vaccination (21%-51%) and did not differ between rural and urban clinics.7,25-28 The observed high vaccination rates may be due to EMR prompts and requirements to document vaccination status and offer recommended vaccinations.
Long-acting inhalers have been shown to reduce rates of COPD exacerbations and improve patients’ quality of life.29 The authors found no disparity in the prescription rate of short- or long-acting inhalers between rural and urban patients, and no difference in the severity of COPD, as indicated by FEV1, that might influence prescription rates.
The authors attempted to evaluate health care resource utilization as an indicator of health care quality and outcomes. Based on previous reports, the authors expected to find lower quality of care and increased utilization in rural patients. Previous studies have shown rural patients can be more symptomatic with a higher body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE index) than are patients in urban settings.16,30 Statewide and national registry data have shown rural patients have higher rates of primary care visits, ER visits, and hospitalizations for COPD exacerbations.
Rural patients also have been shown to have higher mortality rates and were more likely to be in a long-term care center and less likely to have home care or palliative care than were their urban counterparts.13-18,31 If the severity of illness is similar in rural and urban areas, higher health care utilization related to COPD would suggest that patients in rural settings may be receiving inferior quality of health care. The authors could not find any previous reports of the quality of COPD care delivered in rural vs urban settings.
In this study the only difference in quality of care was the lower proportion of rural patients with a record of spirometry that is needed to confirm the diagnosis. The observed differences in the quality of care measures wouldn’t be expected to lead to large differences in the outcome measures. Contrary to the literature and the observed similarity in quality of care, rural patients had better COPD outcomes perhaps due to unmeasured differences in risk or failure to capture medical visits outside of the VA system. The severity of COPD based on FEV1 and concurrent diagnoses, such as heart failure, did not suggest that rural patients in this comparison had a higher burden of illness or risk of poor COPD outcomes.
More than 70,000 patients spanning a large geographic region receive primary care at MVAHCS, which provides comparable care to all COPD patients, regardless of location, by using the same EMR system, providing evidence-based order sets for disease management, proactively offering on-site and remote COPD case managers for high-risk patients, and more recently, implementing on-site spirometry testing in all clinics. This approach as opposed to the traditional outreach clinic model may in part explain the similarity in quality of care in urban and rural clinics that was not reported in previous studies.
Limitations
This study was performed retrospectively, increasing the potential of missing data, especially from outside the VA health care system. Patients were not randomly assigned to rural or urban clinics, so differences in patient characteristics could exist. Alcohol and addictive disorders were more common in urban patients, which might affect adherence to prescribed medications. In addition, lower rate of obstructive sleep apnea was found in the rural population, which has been linked to increased airway inflammation and COPD exacerbations resulting in hospitalization.32,33 Mortality was not assessed as all patients were alive and seen in clinic at time of enrollment.
The authors were not able to record a patients’ residence in a long-term care facility or institutionalization, use of home care, or palliative care services due to limitations in the EMR system. Whether patients received comanaged primary care or underwent pulmonary rehabilitation could not be obtained from the EMR. Most patients never had lung volumes or diffusion capacity and thus were not included. The authors could not report whether inhaler therapy was appropriate compared with the Global Initiative for Chronic Obstructive Lund Disease(GOLD)severity score because most of the spirometry was done at a discordant time to when inhaler therapy was assessed, and new GOLD guidelines include patient symptoms that were not reliably recorded in the EMR. Last, the authors had hoped to include smoking status and cessation practices as part of the quality measures, but due to significant variability in patients’ documented smoking status in the same period, the data were deemed unreliable.
Conclusion
No disparities were found between rural and urban clinics in the quality of health care for patients with COPD in the MVAHCS except that fewer rural patients had prior spirometry; a difference that is likely due to the fact that only recently has spirometry been implemented in the MVAHCS rural clinics. Overall the quality of COPD care was high and above the previously reported rates. Further larger studies of rural and urban quality of health care for patients with COPD are needed in other VA and non-VA systems to determine whether disparities exist and whether they are associated with clinical outcomes, including ED visits, hospitalizations, and mortality.
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1-161.
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-1276.
5. Ghattas C, Dai A, Gemmel DJ, Awad MH. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis. 2013;8:545-549.
6. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171.
7. Lopez-Campos JL, Abad Arranz M, Calero-Acuña C, et al. Guideline adherence in outpatient clinics for chronic obstructive pulmonary disease: results from a clinical audit. PLoS One. 2016;11(3):e0151896.
8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645.
9. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: U.S. Department of Veteran Affairs; 2011.
10. Weeks WB, Wallace AE, Wang S, Lee A, Kazis LE. Rural-urban disparities in health-related quality of life within disease categories of Veterans. J Rural Health. 2006;22(3):204-211.
11. Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57(6):851-856.
12. Meit M, Knudson A, Gilbert T, et al; Rural Health Reform Policy Research Center. The 2014 update of the rural-urban chartbook. https://ruralhealth.und .edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published October 2014. Accessed April 18, 2017.
13. Jackson BE, Suzuki S, Coultas D, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563-571.
14. Jackson BE, Suzuki S, Lo K, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105(5):734-739.15. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006-2011: statistical brief #179. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Published September 2014. Accessed May 9, 2017.
16. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(suppl 1):62S-69S.
17. Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19-e29.
18. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155(2):80-86.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155.
20. U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#U20K1F50H0A. Updated October 12, 2016. Accessed May 9, 2017.
21. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38-45.
22. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120-1124.
23. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818-825.
24. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2016. Ann Intern Med. 2016;164(3):184-194.
25. Cimen P, Unlu M, Kirakli C, et al. Should patients with COPD be vaccinated? Respir Care. 2015;60(2):239-243.
26. Mowls DS, Cheruvu VK, Zullo MD. Influenza vaccination in adults with chronic obstructive pulmonary disease: the impact of a diagnostic breathing test on vaccination rates PLoS One. 2013;8(6):e67600.
27. Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405-e413.
28. Arinez-Fernandez MC, Carrasco-Garrido P, Garcia-Carballo M, Hernandez-Barrera V, de Miguel AG, Jimenez-Garcia R. Determinants of pneumococcal vaccination among patients with chronic obstructive pulmonary disease in Spain. Hum Vaccin. 2006;2(3):99-104.29. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
30. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.
31. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10(2):1349.
32. Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123-1130.
33. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1-161.
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-1276.
5. Ghattas C, Dai A, Gemmel DJ, Awad MH. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis. 2013;8:545-549.
6. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171.
7. Lopez-Campos JL, Abad Arranz M, Calero-Acuña C, et al. Guideline adherence in outpatient clinics for chronic obstructive pulmonary disease: results from a clinical audit. PLoS One. 2016;11(3):e0151896.
8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645.
9. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: U.S. Department of Veteran Affairs; 2011.
10. Weeks WB, Wallace AE, Wang S, Lee A, Kazis LE. Rural-urban disparities in health-related quality of life within disease categories of Veterans. J Rural Health. 2006;22(3):204-211.
11. Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57(6):851-856.
12. Meit M, Knudson A, Gilbert T, et al; Rural Health Reform Policy Research Center. The 2014 update of the rural-urban chartbook. https://ruralhealth.und .edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published October 2014. Accessed April 18, 2017.
13. Jackson BE, Suzuki S, Coultas D, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563-571.
14. Jackson BE, Suzuki S, Lo K, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105(5):734-739.15. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006-2011: statistical brief #179. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Published September 2014. Accessed May 9, 2017.
16. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(suppl 1):62S-69S.
17. Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19-e29.
18. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155(2):80-86.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155.
20. U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#U20K1F50H0A. Updated October 12, 2016. Accessed May 9, 2017.
21. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38-45.
22. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120-1124.
23. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818-825.
24. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2016. Ann Intern Med. 2016;164(3):184-194.
25. Cimen P, Unlu M, Kirakli C, et al. Should patients with COPD be vaccinated? Respir Care. 2015;60(2):239-243.
26. Mowls DS, Cheruvu VK, Zullo MD. Influenza vaccination in adults with chronic obstructive pulmonary disease: the impact of a diagnostic breathing test on vaccination rates PLoS One. 2013;8(6):e67600.
27. Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405-e413.
28. Arinez-Fernandez MC, Carrasco-Garrido P, Garcia-Carballo M, Hernandez-Barrera V, de Miguel AG, Jimenez-Garcia R. Determinants of pneumococcal vaccination among patients with chronic obstructive pulmonary disease in Spain. Hum Vaccin. 2006;2(3):99-104.29. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
30. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.
31. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10(2):1349.
32. Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123-1130.
33. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331