User login
Continuous Versus Intermittent Furosemide in ADHF
Acute decompensated heart failure (ADHF) is the most common cause of hospitalization among adults in the United States and is associated with high morbidity and mortality.1 The estimated direct and indirect cost of ADHF management in the United States was $40 billion in 2010.1 There are approximately 5.7 million patients with heart failure in the United States with an annual mortality rate of 300,000 deaths per year.2 The Healthcare Cost and Utilization Project reported 1.1 million hospital admissions, an average hospital stay of 5.5 days, and 4% in‐hospital mortality for patients with heart failure in 2004.3
Intravenous administration of loop diuretics is the mainstay of treatment of volume overload in patients hospitalized with ADHF.4 However, when administered as intermittent bolus injections, loop diuretics usually lead to rapid intravascular volume changes,5 significant electrolyte abnormalities,6, 7 renal dysfunction,8, 9 and undesired neurohormonal activity.10, 11 Compared with intermittent bolus injections, continuous infusion of loop diuretics may induce a more sustained and greater diuresis and fewer electrolyte abnormalities.1216 Several studies of limited duration have compared the effectiveness of the 2 routes of intravenous administration of loop diuretics; however, the results of these studies are contradictory.13, 14, 17, 18 In a meta‐analysis, Salvador et al19 compared the effectiveness of continuous infusion and intermittent bolus injections of loop diuretics. The authors reported greater diuresis (measured as 24‐hour urinary output) in patients receiving continuous infusion of loop diuretics. However, the meta‐analysis included studies that examined loop diuretics other than furosemide,20 allowed concomitant use of hypertonic saline infusions,21 and included patients with pulmonary edema from noncardiogenic causes.22
Furosemide is one of the most commonly used loop diuretics.23 The current literature lacks a systematic review and meta‐analysis comparing the effectiveness of continuous infusion and intermittent bolus furosemide therapy among nonsurgical, hemodynamically stable, hospitalized patients with ADHF. In addition, several important randomized trials published in recent years comparing the effectiveness of the 2 routes of intravenous furosemide delivery warrant17, 2427 systematic review, because the last published meta‐analysis (by Salvador et al19) was in 2005.
We therefore conducted a systematic review and meta‐analysis of randomized controlled trials that compared the effects of continuous infusion and intermittent bolus of furosemide in patients hospitalized with ADHF.
METHODS
Study Selection
We searched the PubMed, EMBASE, and The Cochrane Central Register of Controlled Trials electronic databases systematically from their inception through March 2011 using the search terms lasix, furosemide, diuretic, congestive heart failure, infusion, and bolus. The electronic database search was supplemented by hand‐searching bibliographies of the retrieved articles. Two investigators independently reviewed all retrieved articles for their eligibility based on predefined criteria. Disagreement on study selection was resolved by mutual consensus and by the involvement of a third investigator. All selected studies were assessed for content validity.
We included both crossover and parallel‐arm randomized control trials. Studies were included if patients were randomized to intermittent bolus or continuous infusion of furosemide, and data were reported on 24‐hour urinary volume, total body weight loss, 24‐hour urinary sodium excretion, and duration of hospital stay. Randomized control trials that included patients with cardiogenic shock requiring concomitant vasopressor therapy, renal failure with or without hemodialysis, and loop diuretics other than furosemide were excluded. The primary authors of the included studies were contacted if the results of the selected outcomes either were not reported or required further clarification. A flow diagram was produced following guidelines from The Quality of Reporting of Meta‐analyses (QUOROM) group28 to provide information on randomized clinical trial identification for the final inclusion in the meta‐analysis.
Data Extraction
Data on study design, participant characteristics, methods, intervention, and selected outcomes were independently extracted by 2 investigators. Interobserver agreement for full study selection was calculated using an unweighted kappa statistic. A chi‐square test (2) and I2 statistic were used to report the percentage of variability in the effect estimates across studies.
Quality Assessment
The quality of included trials was assessed using a validated scale developed by Jadad et al29 that assigns a score from 0 to 5, with a higher score indicating higher quality. Two investigators independently evaluated studies on 3 parameters: randomization, blinding, and dropouts. The third investigator helped resolve discordant assessments. We assessed publication bias visually by examining the symmetry of funnel plots and statistically using Begg30 and Egger31 tests.
Data Synthesis and Analysis
For the reported outcomes, we recorded the mean difference between the groups and measures of dispersion. If a mean difference was not reported, we calculated point estimates by using the mean difference from baseline for each group. If a mean difference from baseline was not reported, we calculated point estimates using the baseline and final value for each group. If a measure of dispersion was not reported for the between‐group difference, we calculated it by using the sum of the variance for the mean difference from baseline in each group. If no measure of dispersion was reported for the mean difference from baseline for each group, we calculated variance by using the standard deviation of the baseline and final values, and assumed a correlation between the baseline and final values of 0.5.
Urinary volume was measured in milliliters per 24 hours per 100 mg furosemide to compare the diuretic effect between the 2 routes of intravenous administration. Total body weight loss was measured in kilograms. Urinary sodium was measured in millimoles per 24 hours, and duration of hospital stay was measured in days.
Weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated for all prespecified outcomes using Review Manager (RevMan) Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008. We pooled results from individual studies using a random‐effects model. Statistical significance was set at P 0.05 using a 2‐tailed Z‐test. Sensitivity analyses were conducted by omitting one study at a time for all outcomes.
RESULTS
Study Selection
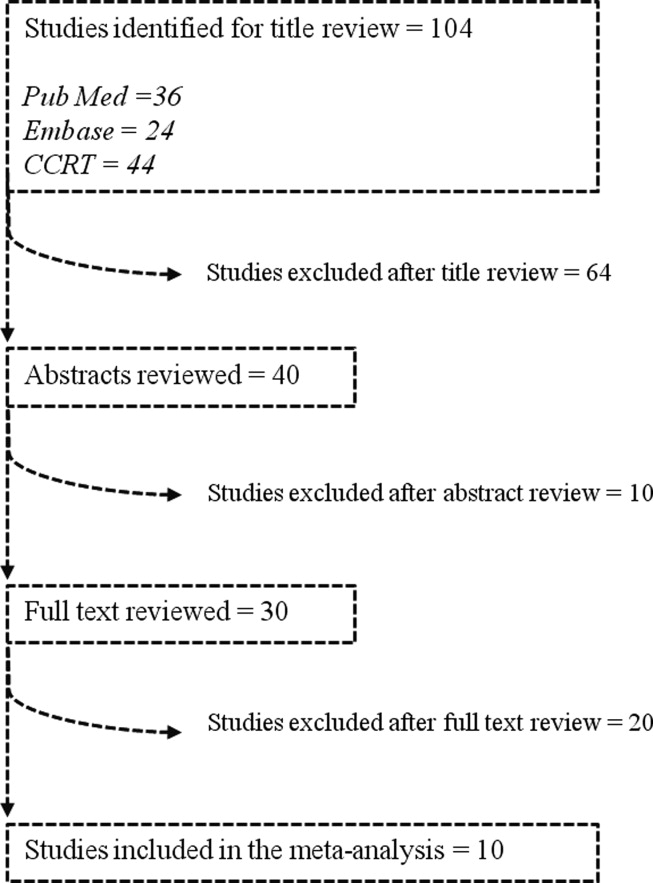
We identified 104 studies using the previously stated search terms. Following QUOROM guidelines, ten randomized clinical trials, enrolling a total of 564 patients, fulfilled the inclusion criteria (Figure 1). The interobserver agreement (unweighted kappa statistic) between investigators for study selection was 0.75.

Study Characteristics
The majority of patients were male (60%) with a mean age of 62.8 years (range 54 ‐ 74.1 years). The duration of follow‐up while on furosemide in both arms ranged from twelve hours24 to six days13 (Table 1). We found significant variability in dose, frequency, and duration of treatment across studies for both routes of intravenous furosemide administration (Table 2). Four of 10 studies were crossover trials13, 14, 18, 32 and the rest were parallel‐arm trials. Randomization to 1 of the 2 treatment groups was reported in all 4 crossover trials.
| Study | Study Design* | Total (N) | Mean Age (years) | Male (n) | Duration on Furosemide (days) | Country of Study | NYHA Class | Jadad Quality Score |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Aaser et al18 | CO | 8 | 54 | 6 | 2 | Norway | III‐IV | 1 |
| Allen et al17 | PA | 41 | 61 | 26 | 2 | USA | NR | 3 |
| Dormans et al13 | CO | 20 | 71 | 13 | 6 | Netherlands | III‐IV | 1 |
| Felker et al27 | PA | 308 | 66 | 226 | 3 | USA | NR | 4 |
| Lahav et al14 | CO | 9 | 74.1 | 5 | 4 | Israel | III‐IV | 1 |
| Mojtahedzadeh et al33 | PA | 22 | NR | NR | 1.5 | Iran | NR | 2 |
| Mojtahedzadeh et al24 | PA | 21 | 56.5 | 11 | 0.5 | Iran | NR | 2 |
| Ostermann et al26 | PA | 59 | 64 | 31 | 2 | UK/Canada | NR | 3 |
| Pivac et al32 | CO | 20 | 62.2 | 9 | 3 | Croatia | III | 1 |
| Thomson et al25 | PA | 56 | 56.4 | 32 | 3.54.6 | USA | III‐IV | 3 |
| Study | Furosemide Dose (Mean SD) | Additional Comments | |
|---|---|---|---|
| Intermittent Bolus | Continuous Infusion | ||
| |||
| Aaser et al18 | 145 80 mg bid | 145 80 mg/24 hr | Furosemide dose was same as usual daily oral dose |
| Allen et al17 | 162 48 mg bid | 162 52 mg/24 hr | Dose was determined by attending physician after enrollement |
| Dormans et al13 | Single bolus of continous dose | 690 mg/8 hr (2502000 mg) | Patients received additional single oral doses of furosemide on first and second day |
| Felker et al27 | 134 53 mg/day | 127 50 mg/day | Treatment was continued for up to 72 hours; at 48 hours, the treating physician had the option of adjusting the diurtetic dose on the basis of clinical response |
| Lahav et al14 | 3040 mg/8 hr | 6080 mg/24 hr | Continuous group received 3040 mg bolus furosemide as loading dose |
| Mojtahedzadeh et al33 | 320 mg/dose | 0.75 mg/kg/hr | All patients received 20 mg of furosemide as initial bolus in both arms |
| Mojtahedzadeh et al24 | 20 mg initial, then doubled every 3 hr* | 0.1 mg/kg/hr (total 250 mg) | Both regimens were titrated for a goal net fluid balance of at least 1 mL/kg/hr |
| Ostermann et al26 | 0.65.14/kg/dose | 0.40.6 mg/kg/hr | Predefined alogrithms aiming for minimum hourly urine output was used in both arms |
| Pivac et al32 | 40 mg bid | 40 mg bid | Goal was to increase urine output to at least 50% from baseline or a minimum of 1 mL/kg/hr |
| Thomson et al25 | 172 97 mg | 197 148 mg/day | The mean duration of study drug administration was shorter by approximately 1 day in the continous group |
Outcomes
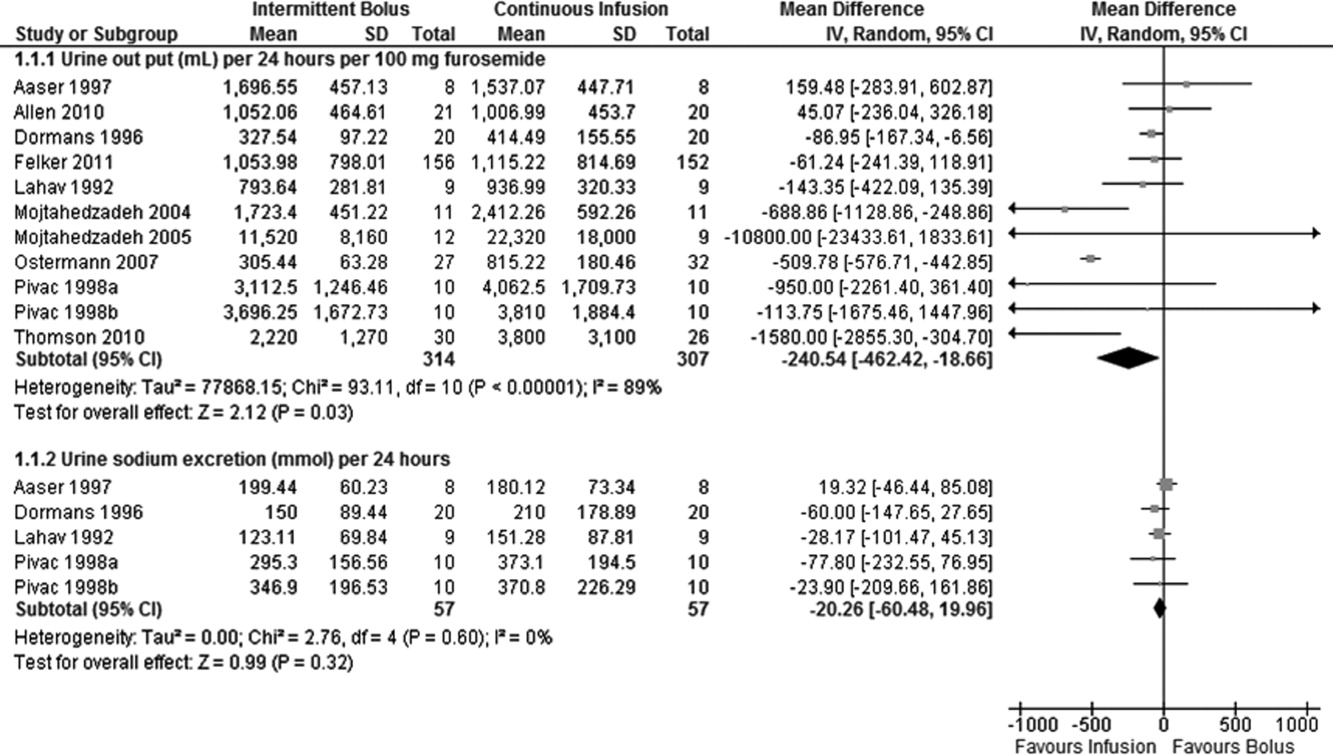
Data on 24‐hour urinary volume were reported in all 10 studies. We found that the continuous infusion of furosemide was associated with a statistically significant increase in 24‐hour urinary output compared with intermittent bolus injections (WMD, 240.54 mL/24 hours/100 mg furosemide; 95% CI, 462.42 to 18.66; P = 0.03). There was evidence of statistically significant heterogeneity between the studies for the outcome of 24‐hour urinary volume (I2 = 89%; 2 = 93.11; P < 0.001) (Figure 2). The magnitude of statistical heterogeneity decreased (I2 = 53%; 2 = 19.11; P = 0.02) but remained significant after removing a study by Ostermann et al.26
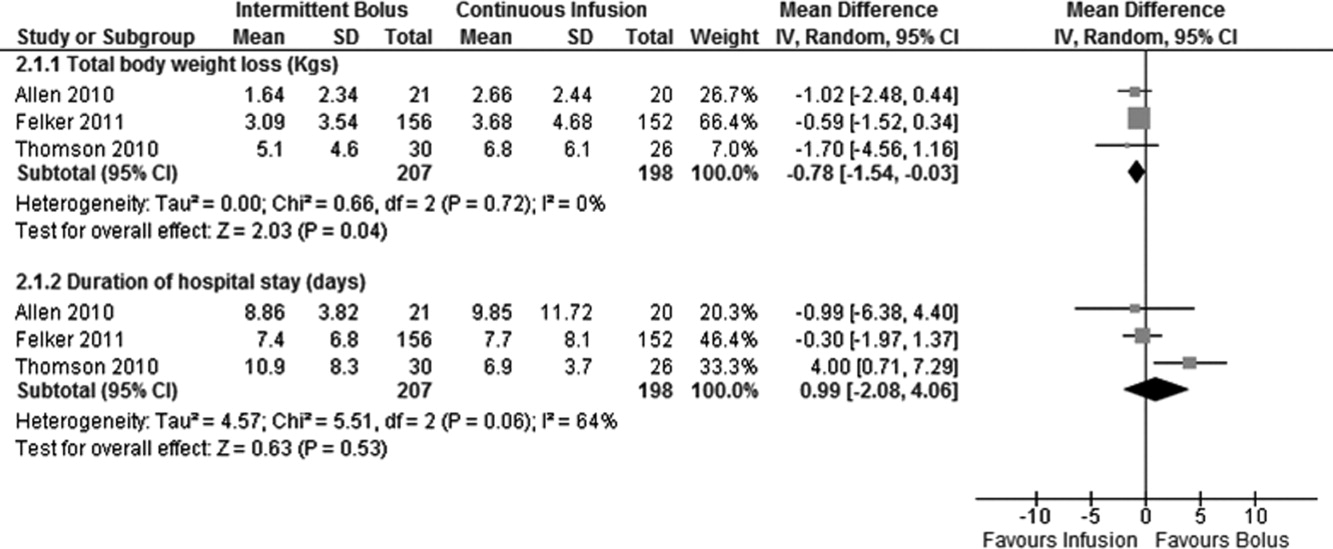
Data on total body weight loss was reported in 3 parallel trials. Patients treated with a continuous infusion of furosemide had statistically greater changes in total body weight (WMD, 0.78 kg; 95% CI, 1.54 to 0.03; P = 0.04) when compared with patients receiving bolus injections of furosemide. Data for total body weight loss were collected at 72 hours of treatment in 2 trials17, 27 and was reported for the duration of the entire study by Thomson et al.25 There was no statistical evidence of heterogeneity between the studies for total body weight loss (I2 = 0 %; 2 = 0.66; P = 0.72) (Figure 3).
Data on 24‐hour urinary sodium excretion was reported for 57 patients in the 4 crossover studies. A continuous infusion of furosemide was associated with a statistically insignificant increase in 24‐hour urinary sodium (WMD, 20.26 mmol/24 hours; 95% CI, 60.48 to 19.96; P = 0.32). There was no statistical evidence of heterogeneity between studies for 24‐hour urinary sodium excretion (I2 = 0%; 2 = 2.76; P = 0.60) (Figure 2).
Duration of hospital stay was reported in 3 parallel trials. Patients receiving intermittent injections of bolus furosemide had longer hospital stays (WMD, 0.99 days; 95% CI, 2.08 to 4.06; P = 0.53), but this difference was not statistically significant. There was no evidence of heterogeneity between the studies for the duration of hospital stay (I2 = 64%; 2 = 5.51; P = 0.06) (Figure 3).
Risk of Bias and Sensitivity Analysis
Individual quality assessment scores based on a scoring system developed by Jadad et al29 for included trials are reported (Table 1). Randomization was reported by all studies, but the explicit methodology of randomization was defined in only 4 studies.17, 2527 Allocation concealment was defined in 1 study.26 Dropouts were reported in 4 studies.2426, 33 Adherence to intervention per study protocol was not reported in any of the selected studies. Three studies mentioned intention to treat.25, 26 Sensitivity analyses demonstrated that the direction of the mean estimates did not change for any of the 4 outcomes when individual studies were excluded.
DISCUSSION
Our meta‐analysis of 10 randomized, controlled clinical trials found that continuous infusion of furosemide results in significantly greater diuresis and reduction in total body weight than intermittent boluses in patients hospitalized with ADHF. No statistical differences were observed in urinary sodium excretion or the duration of hospital stay between the 2 routes of intravenous furosemide administration. The data on greater diuresis from the available clinical trials was widely heterogeneous that may limit the merits of assessment of greater diuresis between the 2 methods of intravenous furosemide administration. In addition, data on clinical outcomes such as rates of rehospitalization, cardiovascular, and all‐cause mortality were not reported in the studies selected for this meta‐analysis.
The mean effective dose of loop diuretics administered as intermittent boluses varies widely5 and quickly dissipates to a level that fails to block Na+ reabsorption in renal tubules.34 Additionally, the effectiveness of loop diuretics is limited by the rebound in sodium reabsorption during periods of subtherapeutic renal tubular concentration because of their short half‐life.4, 6, 35 It is possible that the ineffectiveness of subtherapeutic tubular filtrate levels of loop diuretics toward the end of a dosing interval when administered as a bolus is responsible for their unsustained diuretic effects. Bolus injections of furosemide have been associated with diuretic tolerance, reduced short‐term natriuresis, and a probable rise in plasma aldosterone levels in the settings of salt restriction.36 Data from physiological studies suggest that greater diuresis, which also results in weight loss with continuous infusion of loop diuretics, is due to the minimal variation in the mean effective dose of drug in the renal tubules.1216 By preventing subtherapeutic tubular dose concentrations, continuous infusion may limit rebound resorption helping to improve symptoms of ADHF.4
Our study has several limitations. First, we examined only surrogate endpoints. Second, we included crossover trials13, 14, 18, 32 in the analysis, and the variation in the washout periods of these trials may have affected the reported outcomes. The study by Aaser et al18 lacked a washout period because the authors were concerned for the hemodynamic stability of diuretic‐dependent ADHF patients. Lahav et al14 reported a washout period of 3 hours, while Dormans et al13 and Pivac et al32 did not report the duration of washout periods. Finally, we excluded studies that enrolled postsurgical patients and patients with pulmonary edema from noncardiac causes. As a result, the generalizability of our findings is limited to relatively stable ADHF patients hospitalized because of medical, dietary, or pharmacological noncompliance. We restricted our analysis to studies using furosemide therapy only. By excluding trials using loop diuretics other than furosemide and trials reporting concomitant use of vasopressors or hypertonic saline in the study population, we are confident in the assessment of the isolated effects of furosemide for either route of its intravenous administration in patients hospitalized with ADHF.
The continuous infusion of furosemide has been well tolerated in most instances.13, 2527, 32 Thomson et al25 found no difference on the incidence of significant hemodynamic changes or need for renal replacement therapy between the 2 groups. Similarly, Ostermann et al26 reported no significant differences in heart rate and mean arterial pressures changes from two treatment groups. In addition, Felker et al27 and Pivac et al32 found no differences in the proportion of serious adverse effects between the 2 routes of intravenous furosemide administration.
In the absence of information on clinical endpoints such as rehospitalization, all‐cause mortality, and cardiovascular mortality, this meta‐analysis could not settle the issue to provide definitive recommendations for treatment guidelines to use either route of intravenous furosemide in ADHF patients. However, it is important to note that despite different study populations, our finding of greater diuresis with continuous infusion of furosemide is consistent with results reported by Salvador et al.19 Given the higher prevalence, mortality, and significant cost related with ADHF management in the United States, we support the use of furosemide as a continuous infusion to ensure limited but established benefits, such as greater diuresis and reduction in total body weight,. This approach seems reasonable, especially when the safety profiles between the 2 treatment groups are not different.2527, 32 However, the benefits on surrogate outcomes cannot be overstressed due to lack of information on the cost‐effectiveness of furosemide or other loop diuretics administered as a continuous infusion.
CONCLUSIONS
We report a systematic review and meta‐analysis comparing the effectiveness of 2 routes of intravenous furosemide administration in patients with ADHF. We found that continuous infusion of furosemide results in greater diuresis and greater reduction in total body weight. With the exception of greater diuresis, available data are homogenous for the reported outcomes in this meta‐analysis. Due to lack of information on clinical endpoints and cost‐effectiveness from currently available data, robust recommendations for clinical practice guidelines cannot be made at this time. Randomized controlled trials measuring hard clinical endpoints in larger patient populations may add stronger evidence to settle this issue in future. Further studies comparing cost‐effectiveness related with continuous infusion of furosemide may provide critical information to establish it as the preferred route over intermittent bolus injection in clinical practice.
- ,,, et al.Heart disease and stroke statistics 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2009;119:e21–e181.
- National Heart, Lung, and Blood Institute. What Is Heart Failure? Available at: http://www.nhlbi.nih.gov/health/health‐topics/topics/hf/. Accessed March 6,2011.
- ,,. Hospital Stays for Circulatory Diseases, 2004. Healthcare Cost and Utilization Project Statistical Brief No. 26. Rockville, MD: Agency for Healthcare Research and Quality; February 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb26.jsp. Accessed February 22,2010.
- ,,, et al.2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation.Circulation.2009;119:1977–2016.
- ,,,.Determinants of response to furosemide in normal subjects.Br J Clin Pharmacol.1977;4:121–127.
- .Diuretic therapy.N Engl J Med.1998;339:387–395.
- ,,,,.Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction.Circulation.1999;100:1311–1315.
- ,,, et al.Increased toxicity of high dose furosemide versus low‐dose dopamine in the treatment of refractory congestive heart failure.Clin Pharmacol Ther.1997;62:187–193.
- ,,, et al.Relationship between heart failure treatment and development of worsening renal function among hospitalized patients.Am Heart J.2004;147:331–338.
- ,,,.Haemodynamic and hormone responses to acute and chronic furosemide therapy in congestive heart failure.Clin Sci.1980;59:443–449.
- ,,,,.Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics.Br Heart J.1987;57:17–22.
- ,,.The time course of delivery of furosemide into urine: an independent determinant of overall response.Kidney Int.1982;22:69–74.
- ,,,,,.Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion.J Am Coll Cardiol.1996;28:376–382.
- ,,,.Intermittent administration of furosemide vs continuous infusion preceded by a loading dose for congestive heart failure.Chest.1992;102:725–731.
- ,,,,.Diuresis with continuous infusion of furosemide after cardiac surgery.Am J Surg.1983;146:796.
- ,,,.Continuous infusion of furosemide in refractory edema.BMJ.1978;2:476.
- ,,,,,.Continuous versus bolus dosing of furosemide for patients hospitalized for heart failure.Am J Cardiol.2010;105:1794–1797.
- ,,, et al.Effect of bolus injection versus continuous infusion of furosemide on diuresis and neurohormonal activation in patients with severe congestive heart failure.Scand J Clin Lab Invest.1997;57:361–367.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2005;(3):CD003178.
- ,,, et al.Pharmacodynamics of torsemide administered as an intravenous injection and as a continuous infusion to patients with congestive heart failure.J Clin Pharmacol.1996;36:265–270.
- ,,, et al.Effects of high‐dose furosemide and small‐volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long‐term effects.Am Heart J.2003;145:459–466.
- ,,.Protocol‐ guided diuretic management: comparison of furosemide by continuous infusion and intermittent bolus.Crit Care Med.1997;25:1969–1975.
- Cardiovascular Pharmacology Concepts. Diuretics. Available at: http://www.cvpharmacology.com/diuretic/diuretics.htm. Accessed July 22,2010.
- ,,, et al.The relationship between pharmacokinetics variables and pharmacodynamics profiles of bolus versus continuous infusion of furosemide in critically ill patients.J Infus Nurs.2005;13:127–132.
- ,,,,,.Continuous versus intermittent infusion of furosemide in acute decompensated heart failure.J Card Fail.2010;16:188–193.
- ,,,.Frusemide administration in critically ill patients by continuous compared to bolus therapy.Nephron Clin Pract.2007;107:c70–c76.
- ,,, et al;NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure.N Engl J Med.2011;364:797–805.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses.Lancet.1999;354:1896–1900.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17:1–12.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50:1088–1101.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- ,,, et al.Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure.Int J Clin Pharmacol Res.1998;18:121–128.
- ,,, et al.Comparison of hemodynamic and biochemical effects of furosemide by continuous infusion and intermittent bolus in critically ill patients.Infus Nurs.2004;27:255–261.
- .Diuretic resistance: mechanisms and therapeutic strategies.Cardiology.1994;84(suppl 2):57–67.
- ,.Loop diuretics: from the Na‐K‐2Cl transporter to clinical use.Am J Physiol Renal Physiol.2003;284:F11–F21.
- ,,, et al.Response to furosemide. I. Effects of salt intake and renal compensation.J Lab Clin Med.1983;102:450–458.
Acute decompensated heart failure (ADHF) is the most common cause of hospitalization among adults in the United States and is associated with high morbidity and mortality.1 The estimated direct and indirect cost of ADHF management in the United States was $40 billion in 2010.1 There are approximately 5.7 million patients with heart failure in the United States with an annual mortality rate of 300,000 deaths per year.2 The Healthcare Cost and Utilization Project reported 1.1 million hospital admissions, an average hospital stay of 5.5 days, and 4% in‐hospital mortality for patients with heart failure in 2004.3
Intravenous administration of loop diuretics is the mainstay of treatment of volume overload in patients hospitalized with ADHF.4 However, when administered as intermittent bolus injections, loop diuretics usually lead to rapid intravascular volume changes,5 significant electrolyte abnormalities,6, 7 renal dysfunction,8, 9 and undesired neurohormonal activity.10, 11 Compared with intermittent bolus injections, continuous infusion of loop diuretics may induce a more sustained and greater diuresis and fewer electrolyte abnormalities.1216 Several studies of limited duration have compared the effectiveness of the 2 routes of intravenous administration of loop diuretics; however, the results of these studies are contradictory.13, 14, 17, 18 In a meta‐analysis, Salvador et al19 compared the effectiveness of continuous infusion and intermittent bolus injections of loop diuretics. The authors reported greater diuresis (measured as 24‐hour urinary output) in patients receiving continuous infusion of loop diuretics. However, the meta‐analysis included studies that examined loop diuretics other than furosemide,20 allowed concomitant use of hypertonic saline infusions,21 and included patients with pulmonary edema from noncardiogenic causes.22
Furosemide is one of the most commonly used loop diuretics.23 The current literature lacks a systematic review and meta‐analysis comparing the effectiveness of continuous infusion and intermittent bolus furosemide therapy among nonsurgical, hemodynamically stable, hospitalized patients with ADHF. In addition, several important randomized trials published in recent years comparing the effectiveness of the 2 routes of intravenous furosemide delivery warrant17, 2427 systematic review, because the last published meta‐analysis (by Salvador et al19) was in 2005.
We therefore conducted a systematic review and meta‐analysis of randomized controlled trials that compared the effects of continuous infusion and intermittent bolus of furosemide in patients hospitalized with ADHF.
METHODS
Study Selection
We searched the PubMed, EMBASE, and The Cochrane Central Register of Controlled Trials electronic databases systematically from their inception through March 2011 using the search terms lasix, furosemide, diuretic, congestive heart failure, infusion, and bolus. The electronic database search was supplemented by hand‐searching bibliographies of the retrieved articles. Two investigators independently reviewed all retrieved articles for their eligibility based on predefined criteria. Disagreement on study selection was resolved by mutual consensus and by the involvement of a third investigator. All selected studies were assessed for content validity.
We included both crossover and parallel‐arm randomized control trials. Studies were included if patients were randomized to intermittent bolus or continuous infusion of furosemide, and data were reported on 24‐hour urinary volume, total body weight loss, 24‐hour urinary sodium excretion, and duration of hospital stay. Randomized control trials that included patients with cardiogenic shock requiring concomitant vasopressor therapy, renal failure with or without hemodialysis, and loop diuretics other than furosemide were excluded. The primary authors of the included studies were contacted if the results of the selected outcomes either were not reported or required further clarification. A flow diagram was produced following guidelines from The Quality of Reporting of Meta‐analyses (QUOROM) group28 to provide information on randomized clinical trial identification for the final inclusion in the meta‐analysis.
Data Extraction
Data on study design, participant characteristics, methods, intervention, and selected outcomes were independently extracted by 2 investigators. Interobserver agreement for full study selection was calculated using an unweighted kappa statistic. A chi‐square test (2) and I2 statistic were used to report the percentage of variability in the effect estimates across studies.
Quality Assessment
The quality of included trials was assessed using a validated scale developed by Jadad et al29 that assigns a score from 0 to 5, with a higher score indicating higher quality. Two investigators independently evaluated studies on 3 parameters: randomization, blinding, and dropouts. The third investigator helped resolve discordant assessments. We assessed publication bias visually by examining the symmetry of funnel plots and statistically using Begg30 and Egger31 tests.
Data Synthesis and Analysis
For the reported outcomes, we recorded the mean difference between the groups and measures of dispersion. If a mean difference was not reported, we calculated point estimates by using the mean difference from baseline for each group. If a mean difference from baseline was not reported, we calculated point estimates using the baseline and final value for each group. If a measure of dispersion was not reported for the between‐group difference, we calculated it by using the sum of the variance for the mean difference from baseline in each group. If no measure of dispersion was reported for the mean difference from baseline for each group, we calculated variance by using the standard deviation of the baseline and final values, and assumed a correlation between the baseline and final values of 0.5.
Urinary volume was measured in milliliters per 24 hours per 100 mg furosemide to compare the diuretic effect between the 2 routes of intravenous administration. Total body weight loss was measured in kilograms. Urinary sodium was measured in millimoles per 24 hours, and duration of hospital stay was measured in days.
Weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated for all prespecified outcomes using Review Manager (RevMan) Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008. We pooled results from individual studies using a random‐effects model. Statistical significance was set at P 0.05 using a 2‐tailed Z‐test. Sensitivity analyses were conducted by omitting one study at a time for all outcomes.
RESULTS
Study Selection
We identified 104 studies using the previously stated search terms. Following QUOROM guidelines, ten randomized clinical trials, enrolling a total of 564 patients, fulfilled the inclusion criteria (Figure 1). The interobserver agreement (unweighted kappa statistic) between investigators for study selection was 0.75.

Study Characteristics
The majority of patients were male (60%) with a mean age of 62.8 years (range 54 ‐ 74.1 years). The duration of follow‐up while on furosemide in both arms ranged from twelve hours24 to six days13 (Table 1). We found significant variability in dose, frequency, and duration of treatment across studies for both routes of intravenous furosemide administration (Table 2). Four of 10 studies were crossover trials13, 14, 18, 32 and the rest were parallel‐arm trials. Randomization to 1 of the 2 treatment groups was reported in all 4 crossover trials.
| Study | Study Design* | Total (N) | Mean Age (years) | Male (n) | Duration on Furosemide (days) | Country of Study | NYHA Class | Jadad Quality Score |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Aaser et al18 | CO | 8 | 54 | 6 | 2 | Norway | III‐IV | 1 |
| Allen et al17 | PA | 41 | 61 | 26 | 2 | USA | NR | 3 |
| Dormans et al13 | CO | 20 | 71 | 13 | 6 | Netherlands | III‐IV | 1 |
| Felker et al27 | PA | 308 | 66 | 226 | 3 | USA | NR | 4 |
| Lahav et al14 | CO | 9 | 74.1 | 5 | 4 | Israel | III‐IV | 1 |
| Mojtahedzadeh et al33 | PA | 22 | NR | NR | 1.5 | Iran | NR | 2 |
| Mojtahedzadeh et al24 | PA | 21 | 56.5 | 11 | 0.5 | Iran | NR | 2 |
| Ostermann et al26 | PA | 59 | 64 | 31 | 2 | UK/Canada | NR | 3 |
| Pivac et al32 | CO | 20 | 62.2 | 9 | 3 | Croatia | III | 1 |
| Thomson et al25 | PA | 56 | 56.4 | 32 | 3.54.6 | USA | III‐IV | 3 |
| Study | Furosemide Dose (Mean SD) | Additional Comments | |
|---|---|---|---|
| Intermittent Bolus | Continuous Infusion | ||
| |||
| Aaser et al18 | 145 80 mg bid | 145 80 mg/24 hr | Furosemide dose was same as usual daily oral dose |
| Allen et al17 | 162 48 mg bid | 162 52 mg/24 hr | Dose was determined by attending physician after enrollement |
| Dormans et al13 | Single bolus of continous dose | 690 mg/8 hr (2502000 mg) | Patients received additional single oral doses of furosemide on first and second day |
| Felker et al27 | 134 53 mg/day | 127 50 mg/day | Treatment was continued for up to 72 hours; at 48 hours, the treating physician had the option of adjusting the diurtetic dose on the basis of clinical response |
| Lahav et al14 | 3040 mg/8 hr | 6080 mg/24 hr | Continuous group received 3040 mg bolus furosemide as loading dose |
| Mojtahedzadeh et al33 | 320 mg/dose | 0.75 mg/kg/hr | All patients received 20 mg of furosemide as initial bolus in both arms |
| Mojtahedzadeh et al24 | 20 mg initial, then doubled every 3 hr* | 0.1 mg/kg/hr (total 250 mg) | Both regimens were titrated for a goal net fluid balance of at least 1 mL/kg/hr |
| Ostermann et al26 | 0.65.14/kg/dose | 0.40.6 mg/kg/hr | Predefined alogrithms aiming for minimum hourly urine output was used in both arms |
| Pivac et al32 | 40 mg bid | 40 mg bid | Goal was to increase urine output to at least 50% from baseline or a minimum of 1 mL/kg/hr |
| Thomson et al25 | 172 97 mg | 197 148 mg/day | The mean duration of study drug administration was shorter by approximately 1 day in the continous group |
Outcomes
Data on 24‐hour urinary volume were reported in all 10 studies. We found that the continuous infusion of furosemide was associated with a statistically significant increase in 24‐hour urinary output compared with intermittent bolus injections (WMD, 240.54 mL/24 hours/100 mg furosemide; 95% CI, 462.42 to 18.66; P = 0.03). There was evidence of statistically significant heterogeneity between the studies for the outcome of 24‐hour urinary volume (I2 = 89%; 2 = 93.11; P < 0.001) (Figure 2). The magnitude of statistical heterogeneity decreased (I2 = 53%; 2 = 19.11; P = 0.02) but remained significant after removing a study by Ostermann et al.26

Data on total body weight loss was reported in 3 parallel trials. Patients treated with a continuous infusion of furosemide had statistically greater changes in total body weight (WMD, 0.78 kg; 95% CI, 1.54 to 0.03; P = 0.04) when compared with patients receiving bolus injections of furosemide. Data for total body weight loss were collected at 72 hours of treatment in 2 trials17, 27 and was reported for the duration of the entire study by Thomson et al.25 There was no statistical evidence of heterogeneity between the studies for total body weight loss (I2 = 0 %; 2 = 0.66; P = 0.72) (Figure 3).

Data on 24‐hour urinary sodium excretion was reported for 57 patients in the 4 crossover studies. A continuous infusion of furosemide was associated with a statistically insignificant increase in 24‐hour urinary sodium (WMD, 20.26 mmol/24 hours; 95% CI, 60.48 to 19.96; P = 0.32). There was no statistical evidence of heterogeneity between studies for 24‐hour urinary sodium excretion (I2 = 0%; 2 = 2.76; P = 0.60) (Figure 2).
Duration of hospital stay was reported in 3 parallel trials. Patients receiving intermittent injections of bolus furosemide had longer hospital stays (WMD, 0.99 days; 95% CI, 2.08 to 4.06; P = 0.53), but this difference was not statistically significant. There was no evidence of heterogeneity between the studies for the duration of hospital stay (I2 = 64%; 2 = 5.51; P = 0.06) (Figure 3).
Risk of Bias and Sensitivity Analysis
Individual quality assessment scores based on a scoring system developed by Jadad et al29 for included trials are reported (Table 1). Randomization was reported by all studies, but the explicit methodology of randomization was defined in only 4 studies.17, 2527 Allocation concealment was defined in 1 study.26 Dropouts were reported in 4 studies.2426, 33 Adherence to intervention per study protocol was not reported in any of the selected studies. Three studies mentioned intention to treat.25, 26 Sensitivity analyses demonstrated that the direction of the mean estimates did not change for any of the 4 outcomes when individual studies were excluded.
DISCUSSION
Our meta‐analysis of 10 randomized, controlled clinical trials found that continuous infusion of furosemide results in significantly greater diuresis and reduction in total body weight than intermittent boluses in patients hospitalized with ADHF. No statistical differences were observed in urinary sodium excretion or the duration of hospital stay between the 2 routes of intravenous furosemide administration. The data on greater diuresis from the available clinical trials was widely heterogeneous that may limit the merits of assessment of greater diuresis between the 2 methods of intravenous furosemide administration. In addition, data on clinical outcomes such as rates of rehospitalization, cardiovascular, and all‐cause mortality were not reported in the studies selected for this meta‐analysis.
The mean effective dose of loop diuretics administered as intermittent boluses varies widely5 and quickly dissipates to a level that fails to block Na+ reabsorption in renal tubules.34 Additionally, the effectiveness of loop diuretics is limited by the rebound in sodium reabsorption during periods of subtherapeutic renal tubular concentration because of their short half‐life.4, 6, 35 It is possible that the ineffectiveness of subtherapeutic tubular filtrate levels of loop diuretics toward the end of a dosing interval when administered as a bolus is responsible for their unsustained diuretic effects. Bolus injections of furosemide have been associated with diuretic tolerance, reduced short‐term natriuresis, and a probable rise in plasma aldosterone levels in the settings of salt restriction.36 Data from physiological studies suggest that greater diuresis, which also results in weight loss with continuous infusion of loop diuretics, is due to the minimal variation in the mean effective dose of drug in the renal tubules.1216 By preventing subtherapeutic tubular dose concentrations, continuous infusion may limit rebound resorption helping to improve symptoms of ADHF.4
Our study has several limitations. First, we examined only surrogate endpoints. Second, we included crossover trials13, 14, 18, 32 in the analysis, and the variation in the washout periods of these trials may have affected the reported outcomes. The study by Aaser et al18 lacked a washout period because the authors were concerned for the hemodynamic stability of diuretic‐dependent ADHF patients. Lahav et al14 reported a washout period of 3 hours, while Dormans et al13 and Pivac et al32 did not report the duration of washout periods. Finally, we excluded studies that enrolled postsurgical patients and patients with pulmonary edema from noncardiac causes. As a result, the generalizability of our findings is limited to relatively stable ADHF patients hospitalized because of medical, dietary, or pharmacological noncompliance. We restricted our analysis to studies using furosemide therapy only. By excluding trials using loop diuretics other than furosemide and trials reporting concomitant use of vasopressors or hypertonic saline in the study population, we are confident in the assessment of the isolated effects of furosemide for either route of its intravenous administration in patients hospitalized with ADHF.
The continuous infusion of furosemide has been well tolerated in most instances.13, 2527, 32 Thomson et al25 found no difference on the incidence of significant hemodynamic changes or need for renal replacement therapy between the 2 groups. Similarly, Ostermann et al26 reported no significant differences in heart rate and mean arterial pressures changes from two treatment groups. In addition, Felker et al27 and Pivac et al32 found no differences in the proportion of serious adverse effects between the 2 routes of intravenous furosemide administration.
In the absence of information on clinical endpoints such as rehospitalization, all‐cause mortality, and cardiovascular mortality, this meta‐analysis could not settle the issue to provide definitive recommendations for treatment guidelines to use either route of intravenous furosemide in ADHF patients. However, it is important to note that despite different study populations, our finding of greater diuresis with continuous infusion of furosemide is consistent with results reported by Salvador et al.19 Given the higher prevalence, mortality, and significant cost related with ADHF management in the United States, we support the use of furosemide as a continuous infusion to ensure limited but established benefits, such as greater diuresis and reduction in total body weight,. This approach seems reasonable, especially when the safety profiles between the 2 treatment groups are not different.2527, 32 However, the benefits on surrogate outcomes cannot be overstressed due to lack of information on the cost‐effectiveness of furosemide or other loop diuretics administered as a continuous infusion.
CONCLUSIONS
We report a systematic review and meta‐analysis comparing the effectiveness of 2 routes of intravenous furosemide administration in patients with ADHF. We found that continuous infusion of furosemide results in greater diuresis and greater reduction in total body weight. With the exception of greater diuresis, available data are homogenous for the reported outcomes in this meta‐analysis. Due to lack of information on clinical endpoints and cost‐effectiveness from currently available data, robust recommendations for clinical practice guidelines cannot be made at this time. Randomized controlled trials measuring hard clinical endpoints in larger patient populations may add stronger evidence to settle this issue in future. Further studies comparing cost‐effectiveness related with continuous infusion of furosemide may provide critical information to establish it as the preferred route over intermittent bolus injection in clinical practice.
Acute decompensated heart failure (ADHF) is the most common cause of hospitalization among adults in the United States and is associated with high morbidity and mortality.1 The estimated direct and indirect cost of ADHF management in the United States was $40 billion in 2010.1 There are approximately 5.7 million patients with heart failure in the United States with an annual mortality rate of 300,000 deaths per year.2 The Healthcare Cost and Utilization Project reported 1.1 million hospital admissions, an average hospital stay of 5.5 days, and 4% in‐hospital mortality for patients with heart failure in 2004.3
Intravenous administration of loop diuretics is the mainstay of treatment of volume overload in patients hospitalized with ADHF.4 However, when administered as intermittent bolus injections, loop diuretics usually lead to rapid intravascular volume changes,5 significant electrolyte abnormalities,6, 7 renal dysfunction,8, 9 and undesired neurohormonal activity.10, 11 Compared with intermittent bolus injections, continuous infusion of loop diuretics may induce a more sustained and greater diuresis and fewer electrolyte abnormalities.1216 Several studies of limited duration have compared the effectiveness of the 2 routes of intravenous administration of loop diuretics; however, the results of these studies are contradictory.13, 14, 17, 18 In a meta‐analysis, Salvador et al19 compared the effectiveness of continuous infusion and intermittent bolus injections of loop diuretics. The authors reported greater diuresis (measured as 24‐hour urinary output) in patients receiving continuous infusion of loop diuretics. However, the meta‐analysis included studies that examined loop diuretics other than furosemide,20 allowed concomitant use of hypertonic saline infusions,21 and included patients with pulmonary edema from noncardiogenic causes.22
Furosemide is one of the most commonly used loop diuretics.23 The current literature lacks a systematic review and meta‐analysis comparing the effectiveness of continuous infusion and intermittent bolus furosemide therapy among nonsurgical, hemodynamically stable, hospitalized patients with ADHF. In addition, several important randomized trials published in recent years comparing the effectiveness of the 2 routes of intravenous furosemide delivery warrant17, 2427 systematic review, because the last published meta‐analysis (by Salvador et al19) was in 2005.
We therefore conducted a systematic review and meta‐analysis of randomized controlled trials that compared the effects of continuous infusion and intermittent bolus of furosemide in patients hospitalized with ADHF.
METHODS
Study Selection
We searched the PubMed, EMBASE, and The Cochrane Central Register of Controlled Trials electronic databases systematically from their inception through March 2011 using the search terms lasix, furosemide, diuretic, congestive heart failure, infusion, and bolus. The electronic database search was supplemented by hand‐searching bibliographies of the retrieved articles. Two investigators independently reviewed all retrieved articles for their eligibility based on predefined criteria. Disagreement on study selection was resolved by mutual consensus and by the involvement of a third investigator. All selected studies were assessed for content validity.
We included both crossover and parallel‐arm randomized control trials. Studies were included if patients were randomized to intermittent bolus or continuous infusion of furosemide, and data were reported on 24‐hour urinary volume, total body weight loss, 24‐hour urinary sodium excretion, and duration of hospital stay. Randomized control trials that included patients with cardiogenic shock requiring concomitant vasopressor therapy, renal failure with or without hemodialysis, and loop diuretics other than furosemide were excluded. The primary authors of the included studies were contacted if the results of the selected outcomes either were not reported or required further clarification. A flow diagram was produced following guidelines from The Quality of Reporting of Meta‐analyses (QUOROM) group28 to provide information on randomized clinical trial identification for the final inclusion in the meta‐analysis.
Data Extraction
Data on study design, participant characteristics, methods, intervention, and selected outcomes were independently extracted by 2 investigators. Interobserver agreement for full study selection was calculated using an unweighted kappa statistic. A chi‐square test (2) and I2 statistic were used to report the percentage of variability in the effect estimates across studies.
Quality Assessment
The quality of included trials was assessed using a validated scale developed by Jadad et al29 that assigns a score from 0 to 5, with a higher score indicating higher quality. Two investigators independently evaluated studies on 3 parameters: randomization, blinding, and dropouts. The third investigator helped resolve discordant assessments. We assessed publication bias visually by examining the symmetry of funnel plots and statistically using Begg30 and Egger31 tests.
Data Synthesis and Analysis
For the reported outcomes, we recorded the mean difference between the groups and measures of dispersion. If a mean difference was not reported, we calculated point estimates by using the mean difference from baseline for each group. If a mean difference from baseline was not reported, we calculated point estimates using the baseline and final value for each group. If a measure of dispersion was not reported for the between‐group difference, we calculated it by using the sum of the variance for the mean difference from baseline in each group. If no measure of dispersion was reported for the mean difference from baseline for each group, we calculated variance by using the standard deviation of the baseline and final values, and assumed a correlation between the baseline and final values of 0.5.
Urinary volume was measured in milliliters per 24 hours per 100 mg furosemide to compare the diuretic effect between the 2 routes of intravenous administration. Total body weight loss was measured in kilograms. Urinary sodium was measured in millimoles per 24 hours, and duration of hospital stay was measured in days.
Weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated for all prespecified outcomes using Review Manager (RevMan) Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008. We pooled results from individual studies using a random‐effects model. Statistical significance was set at P 0.05 using a 2‐tailed Z‐test. Sensitivity analyses were conducted by omitting one study at a time for all outcomes.
RESULTS
Study Selection
We identified 104 studies using the previously stated search terms. Following QUOROM guidelines, ten randomized clinical trials, enrolling a total of 564 patients, fulfilled the inclusion criteria (Figure 1). The interobserver agreement (unweighted kappa statistic) between investigators for study selection was 0.75.

Study Characteristics
The majority of patients were male (60%) with a mean age of 62.8 years (range 54 ‐ 74.1 years). The duration of follow‐up while on furosemide in both arms ranged from twelve hours24 to six days13 (Table 1). We found significant variability in dose, frequency, and duration of treatment across studies for both routes of intravenous furosemide administration (Table 2). Four of 10 studies were crossover trials13, 14, 18, 32 and the rest were parallel‐arm trials. Randomization to 1 of the 2 treatment groups was reported in all 4 crossover trials.
| Study | Study Design* | Total (N) | Mean Age (years) | Male (n) | Duration on Furosemide (days) | Country of Study | NYHA Class | Jadad Quality Score |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Aaser et al18 | CO | 8 | 54 | 6 | 2 | Norway | III‐IV | 1 |
| Allen et al17 | PA | 41 | 61 | 26 | 2 | USA | NR | 3 |
| Dormans et al13 | CO | 20 | 71 | 13 | 6 | Netherlands | III‐IV | 1 |
| Felker et al27 | PA | 308 | 66 | 226 | 3 | USA | NR | 4 |
| Lahav et al14 | CO | 9 | 74.1 | 5 | 4 | Israel | III‐IV | 1 |
| Mojtahedzadeh et al33 | PA | 22 | NR | NR | 1.5 | Iran | NR | 2 |
| Mojtahedzadeh et al24 | PA | 21 | 56.5 | 11 | 0.5 | Iran | NR | 2 |
| Ostermann et al26 | PA | 59 | 64 | 31 | 2 | UK/Canada | NR | 3 |
| Pivac et al32 | CO | 20 | 62.2 | 9 | 3 | Croatia | III | 1 |
| Thomson et al25 | PA | 56 | 56.4 | 32 | 3.54.6 | USA | III‐IV | 3 |
| Study | Furosemide Dose (Mean SD) | Additional Comments | |
|---|---|---|---|
| Intermittent Bolus | Continuous Infusion | ||
| |||
| Aaser et al18 | 145 80 mg bid | 145 80 mg/24 hr | Furosemide dose was same as usual daily oral dose |
| Allen et al17 | 162 48 mg bid | 162 52 mg/24 hr | Dose was determined by attending physician after enrollement |
| Dormans et al13 | Single bolus of continous dose | 690 mg/8 hr (2502000 mg) | Patients received additional single oral doses of furosemide on first and second day |
| Felker et al27 | 134 53 mg/day | 127 50 mg/day | Treatment was continued for up to 72 hours; at 48 hours, the treating physician had the option of adjusting the diurtetic dose on the basis of clinical response |
| Lahav et al14 | 3040 mg/8 hr | 6080 mg/24 hr | Continuous group received 3040 mg bolus furosemide as loading dose |
| Mojtahedzadeh et al33 | 320 mg/dose | 0.75 mg/kg/hr | All patients received 20 mg of furosemide as initial bolus in both arms |
| Mojtahedzadeh et al24 | 20 mg initial, then doubled every 3 hr* | 0.1 mg/kg/hr (total 250 mg) | Both regimens were titrated for a goal net fluid balance of at least 1 mL/kg/hr |
| Ostermann et al26 | 0.65.14/kg/dose | 0.40.6 mg/kg/hr | Predefined alogrithms aiming for minimum hourly urine output was used in both arms |
| Pivac et al32 | 40 mg bid | 40 mg bid | Goal was to increase urine output to at least 50% from baseline or a minimum of 1 mL/kg/hr |
| Thomson et al25 | 172 97 mg | 197 148 mg/day | The mean duration of study drug administration was shorter by approximately 1 day in the continous group |
Outcomes
Data on 24‐hour urinary volume were reported in all 10 studies. We found that the continuous infusion of furosemide was associated with a statistically significant increase in 24‐hour urinary output compared with intermittent bolus injections (WMD, 240.54 mL/24 hours/100 mg furosemide; 95% CI, 462.42 to 18.66; P = 0.03). There was evidence of statistically significant heterogeneity between the studies for the outcome of 24‐hour urinary volume (I2 = 89%; 2 = 93.11; P < 0.001) (Figure 2). The magnitude of statistical heterogeneity decreased (I2 = 53%; 2 = 19.11; P = 0.02) but remained significant after removing a study by Ostermann et al.26

Data on total body weight loss was reported in 3 parallel trials. Patients treated with a continuous infusion of furosemide had statistically greater changes in total body weight (WMD, 0.78 kg; 95% CI, 1.54 to 0.03; P = 0.04) when compared with patients receiving bolus injections of furosemide. Data for total body weight loss were collected at 72 hours of treatment in 2 trials17, 27 and was reported for the duration of the entire study by Thomson et al.25 There was no statistical evidence of heterogeneity between the studies for total body weight loss (I2 = 0 %; 2 = 0.66; P = 0.72) (Figure 3).

Data on 24‐hour urinary sodium excretion was reported for 57 patients in the 4 crossover studies. A continuous infusion of furosemide was associated with a statistically insignificant increase in 24‐hour urinary sodium (WMD, 20.26 mmol/24 hours; 95% CI, 60.48 to 19.96; P = 0.32). There was no statistical evidence of heterogeneity between studies for 24‐hour urinary sodium excretion (I2 = 0%; 2 = 2.76; P = 0.60) (Figure 2).
Duration of hospital stay was reported in 3 parallel trials. Patients receiving intermittent injections of bolus furosemide had longer hospital stays (WMD, 0.99 days; 95% CI, 2.08 to 4.06; P = 0.53), but this difference was not statistically significant. There was no evidence of heterogeneity between the studies for the duration of hospital stay (I2 = 64%; 2 = 5.51; P = 0.06) (Figure 3).
Risk of Bias and Sensitivity Analysis
Individual quality assessment scores based on a scoring system developed by Jadad et al29 for included trials are reported (Table 1). Randomization was reported by all studies, but the explicit methodology of randomization was defined in only 4 studies.17, 2527 Allocation concealment was defined in 1 study.26 Dropouts were reported in 4 studies.2426, 33 Adherence to intervention per study protocol was not reported in any of the selected studies. Three studies mentioned intention to treat.25, 26 Sensitivity analyses demonstrated that the direction of the mean estimates did not change for any of the 4 outcomes when individual studies were excluded.
DISCUSSION
Our meta‐analysis of 10 randomized, controlled clinical trials found that continuous infusion of furosemide results in significantly greater diuresis and reduction in total body weight than intermittent boluses in patients hospitalized with ADHF. No statistical differences were observed in urinary sodium excretion or the duration of hospital stay between the 2 routes of intravenous furosemide administration. The data on greater diuresis from the available clinical trials was widely heterogeneous that may limit the merits of assessment of greater diuresis between the 2 methods of intravenous furosemide administration. In addition, data on clinical outcomes such as rates of rehospitalization, cardiovascular, and all‐cause mortality were not reported in the studies selected for this meta‐analysis.
The mean effective dose of loop diuretics administered as intermittent boluses varies widely5 and quickly dissipates to a level that fails to block Na+ reabsorption in renal tubules.34 Additionally, the effectiveness of loop diuretics is limited by the rebound in sodium reabsorption during periods of subtherapeutic renal tubular concentration because of their short half‐life.4, 6, 35 It is possible that the ineffectiveness of subtherapeutic tubular filtrate levels of loop diuretics toward the end of a dosing interval when administered as a bolus is responsible for their unsustained diuretic effects. Bolus injections of furosemide have been associated with diuretic tolerance, reduced short‐term natriuresis, and a probable rise in plasma aldosterone levels in the settings of salt restriction.36 Data from physiological studies suggest that greater diuresis, which also results in weight loss with continuous infusion of loop diuretics, is due to the minimal variation in the mean effective dose of drug in the renal tubules.1216 By preventing subtherapeutic tubular dose concentrations, continuous infusion may limit rebound resorption helping to improve symptoms of ADHF.4
Our study has several limitations. First, we examined only surrogate endpoints. Second, we included crossover trials13, 14, 18, 32 in the analysis, and the variation in the washout periods of these trials may have affected the reported outcomes. The study by Aaser et al18 lacked a washout period because the authors were concerned for the hemodynamic stability of diuretic‐dependent ADHF patients. Lahav et al14 reported a washout period of 3 hours, while Dormans et al13 and Pivac et al32 did not report the duration of washout periods. Finally, we excluded studies that enrolled postsurgical patients and patients with pulmonary edema from noncardiac causes. As a result, the generalizability of our findings is limited to relatively stable ADHF patients hospitalized because of medical, dietary, or pharmacological noncompliance. We restricted our analysis to studies using furosemide therapy only. By excluding trials using loop diuretics other than furosemide and trials reporting concomitant use of vasopressors or hypertonic saline in the study population, we are confident in the assessment of the isolated effects of furosemide for either route of its intravenous administration in patients hospitalized with ADHF.
The continuous infusion of furosemide has been well tolerated in most instances.13, 2527, 32 Thomson et al25 found no difference on the incidence of significant hemodynamic changes or need for renal replacement therapy between the 2 groups. Similarly, Ostermann et al26 reported no significant differences in heart rate and mean arterial pressures changes from two treatment groups. In addition, Felker et al27 and Pivac et al32 found no differences in the proportion of serious adverse effects between the 2 routes of intravenous furosemide administration.
In the absence of information on clinical endpoints such as rehospitalization, all‐cause mortality, and cardiovascular mortality, this meta‐analysis could not settle the issue to provide definitive recommendations for treatment guidelines to use either route of intravenous furosemide in ADHF patients. However, it is important to note that despite different study populations, our finding of greater diuresis with continuous infusion of furosemide is consistent with results reported by Salvador et al.19 Given the higher prevalence, mortality, and significant cost related with ADHF management in the United States, we support the use of furosemide as a continuous infusion to ensure limited but established benefits, such as greater diuresis and reduction in total body weight,. This approach seems reasonable, especially when the safety profiles between the 2 treatment groups are not different.2527, 32 However, the benefits on surrogate outcomes cannot be overstressed due to lack of information on the cost‐effectiveness of furosemide or other loop diuretics administered as a continuous infusion.
CONCLUSIONS
We report a systematic review and meta‐analysis comparing the effectiveness of 2 routes of intravenous furosemide administration in patients with ADHF. We found that continuous infusion of furosemide results in greater diuresis and greater reduction in total body weight. With the exception of greater diuresis, available data are homogenous for the reported outcomes in this meta‐analysis. Due to lack of information on clinical endpoints and cost‐effectiveness from currently available data, robust recommendations for clinical practice guidelines cannot be made at this time. Randomized controlled trials measuring hard clinical endpoints in larger patient populations may add stronger evidence to settle this issue in future. Further studies comparing cost‐effectiveness related with continuous infusion of furosemide may provide critical information to establish it as the preferred route over intermittent bolus injection in clinical practice.
- ,,, et al.Heart disease and stroke statistics 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2009;119:e21–e181.
- National Heart, Lung, and Blood Institute. What Is Heart Failure? Available at: http://www.nhlbi.nih.gov/health/health‐topics/topics/hf/. Accessed March 6,2011.
- ,,. Hospital Stays for Circulatory Diseases, 2004. Healthcare Cost and Utilization Project Statistical Brief No. 26. Rockville, MD: Agency for Healthcare Research and Quality; February 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb26.jsp. Accessed February 22,2010.
- ,,, et al.2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation.Circulation.2009;119:1977–2016.
- ,,,.Determinants of response to furosemide in normal subjects.Br J Clin Pharmacol.1977;4:121–127.
- .Diuretic therapy.N Engl J Med.1998;339:387–395.
- ,,,,.Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction.Circulation.1999;100:1311–1315.
- ,,, et al.Increased toxicity of high dose furosemide versus low‐dose dopamine in the treatment of refractory congestive heart failure.Clin Pharmacol Ther.1997;62:187–193.
- ,,, et al.Relationship between heart failure treatment and development of worsening renal function among hospitalized patients.Am Heart J.2004;147:331–338.
- ,,,.Haemodynamic and hormone responses to acute and chronic furosemide therapy in congestive heart failure.Clin Sci.1980;59:443–449.
- ,,,,.Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics.Br Heart J.1987;57:17–22.
- ,,.The time course of delivery of furosemide into urine: an independent determinant of overall response.Kidney Int.1982;22:69–74.
- ,,,,,.Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion.J Am Coll Cardiol.1996;28:376–382.
- ,,,.Intermittent administration of furosemide vs continuous infusion preceded by a loading dose for congestive heart failure.Chest.1992;102:725–731.
- ,,,,.Diuresis with continuous infusion of furosemide after cardiac surgery.Am J Surg.1983;146:796.
- ,,,.Continuous infusion of furosemide in refractory edema.BMJ.1978;2:476.
- ,,,,,.Continuous versus bolus dosing of furosemide for patients hospitalized for heart failure.Am J Cardiol.2010;105:1794–1797.
- ,,, et al.Effect of bolus injection versus continuous infusion of furosemide on diuresis and neurohormonal activation in patients with severe congestive heart failure.Scand J Clin Lab Invest.1997;57:361–367.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2005;(3):CD003178.
- ,,, et al.Pharmacodynamics of torsemide administered as an intravenous injection and as a continuous infusion to patients with congestive heart failure.J Clin Pharmacol.1996;36:265–270.
- ,,, et al.Effects of high‐dose furosemide and small‐volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long‐term effects.Am Heart J.2003;145:459–466.
- ,,.Protocol‐ guided diuretic management: comparison of furosemide by continuous infusion and intermittent bolus.Crit Care Med.1997;25:1969–1975.
- Cardiovascular Pharmacology Concepts. Diuretics. Available at: http://www.cvpharmacology.com/diuretic/diuretics.htm. Accessed July 22,2010.
- ,,, et al.The relationship between pharmacokinetics variables and pharmacodynamics profiles of bolus versus continuous infusion of furosemide in critically ill patients.J Infus Nurs.2005;13:127–132.
- ,,,,,.Continuous versus intermittent infusion of furosemide in acute decompensated heart failure.J Card Fail.2010;16:188–193.
- ,,,.Frusemide administration in critically ill patients by continuous compared to bolus therapy.Nephron Clin Pract.2007;107:c70–c76.
- ,,, et al;NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure.N Engl J Med.2011;364:797–805.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses.Lancet.1999;354:1896–1900.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17:1–12.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50:1088–1101.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- ,,, et al.Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure.Int J Clin Pharmacol Res.1998;18:121–128.
- ,,, et al.Comparison of hemodynamic and biochemical effects of furosemide by continuous infusion and intermittent bolus in critically ill patients.Infus Nurs.2004;27:255–261.
- .Diuretic resistance: mechanisms and therapeutic strategies.Cardiology.1994;84(suppl 2):57–67.
- ,.Loop diuretics: from the Na‐K‐2Cl transporter to clinical use.Am J Physiol Renal Physiol.2003;284:F11–F21.
- ,,, et al.Response to furosemide. I. Effects of salt intake and renal compensation.J Lab Clin Med.1983;102:450–458.
- ,,, et al.Heart disease and stroke statistics 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2009;119:e21–e181.
- National Heart, Lung, and Blood Institute. What Is Heart Failure? Available at: http://www.nhlbi.nih.gov/health/health‐topics/topics/hf/. Accessed March 6,2011.
- ,,. Hospital Stays for Circulatory Diseases, 2004. Healthcare Cost and Utilization Project Statistical Brief No. 26. Rockville, MD: Agency for Healthcare Research and Quality; February 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb26.jsp. Accessed February 22,2010.
- ,,, et al.2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation.Circulation.2009;119:1977–2016.
- ,,,.Determinants of response to furosemide in normal subjects.Br J Clin Pharmacol.1977;4:121–127.
- .Diuretic therapy.N Engl J Med.1998;339:387–395.
- ,,,,.Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction.Circulation.1999;100:1311–1315.
- ,,, et al.Increased toxicity of high dose furosemide versus low‐dose dopamine in the treatment of refractory congestive heart failure.Clin Pharmacol Ther.1997;62:187–193.
- ,,, et al.Relationship between heart failure treatment and development of worsening renal function among hospitalized patients.Am Heart J.2004;147:331–338.
- ,,,.Haemodynamic and hormone responses to acute and chronic furosemide therapy in congestive heart failure.Clin Sci.1980;59:443–449.
- ,,,,.Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics.Br Heart J.1987;57:17–22.
- ,,.The time course of delivery of furosemide into urine: an independent determinant of overall response.Kidney Int.1982;22:69–74.
- ,,,,,.Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion.J Am Coll Cardiol.1996;28:376–382.
- ,,,.Intermittent administration of furosemide vs continuous infusion preceded by a loading dose for congestive heart failure.Chest.1992;102:725–731.
- ,,,,.Diuresis with continuous infusion of furosemide after cardiac surgery.Am J Surg.1983;146:796.
- ,,,.Continuous infusion of furosemide in refractory edema.BMJ.1978;2:476.
- ,,,,,.Continuous versus bolus dosing of furosemide for patients hospitalized for heart failure.Am J Cardiol.2010;105:1794–1797.
- ,,, et al.Effect of bolus injection versus continuous infusion of furosemide on diuresis and neurohormonal activation in patients with severe congestive heart failure.Scand J Clin Lab Invest.1997;57:361–367.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2005;(3):CD003178.
- ,,, et al.Pharmacodynamics of torsemide administered as an intravenous injection and as a continuous infusion to patients with congestive heart failure.J Clin Pharmacol.1996;36:265–270.
- ,,, et al.Effects of high‐dose furosemide and small‐volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long‐term effects.Am Heart J.2003;145:459–466.
- ,,.Protocol‐ guided diuretic management: comparison of furosemide by continuous infusion and intermittent bolus.Crit Care Med.1997;25:1969–1975.
- Cardiovascular Pharmacology Concepts. Diuretics. Available at: http://www.cvpharmacology.com/diuretic/diuretics.htm. Accessed July 22,2010.
- ,,, et al.The relationship between pharmacokinetics variables and pharmacodynamics profiles of bolus versus continuous infusion of furosemide in critically ill patients.J Infus Nurs.2005;13:127–132.
- ,,,,,.Continuous versus intermittent infusion of furosemide in acute decompensated heart failure.J Card Fail.2010;16:188–193.
- ,,,.Frusemide administration in critically ill patients by continuous compared to bolus therapy.Nephron Clin Pract.2007;107:c70–c76.
- ,,, et al;NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure.N Engl J Med.2011;364:797–805.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses.Lancet.1999;354:1896–1900.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17:1–12.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50:1088–1101.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- ,,, et al.Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure.Int J Clin Pharmacol Res.1998;18:121–128.
- ,,, et al.Comparison of hemodynamic and biochemical effects of furosemide by continuous infusion and intermittent bolus in critically ill patients.Infus Nurs.2004;27:255–261.
- .Diuretic resistance: mechanisms and therapeutic strategies.Cardiology.1994;84(suppl 2):57–67.
- ,.Loop diuretics: from the Na‐K‐2Cl transporter to clinical use.Am J Physiol Renal Physiol.2003;284:F11–F21.
- ,,, et al.Response to furosemide. I. Effects of salt intake and renal compensation.J Lab Clin Med.1983;102:450–458.