User login
Multiple Keratoacanthomas Occurring in Surgical Margins and De Novo Treated With Intralesional Methotrexate
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
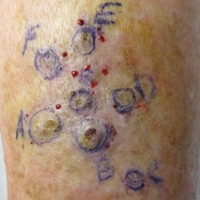
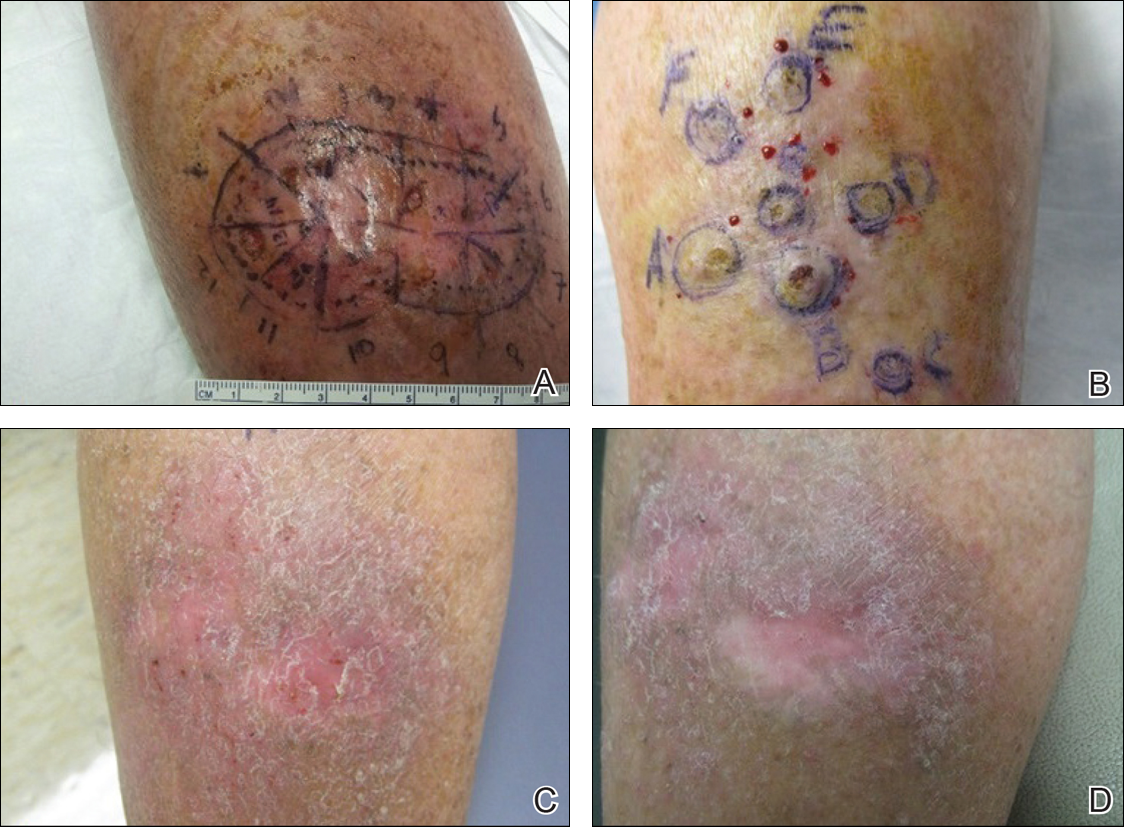
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.
Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Practice Points
- Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas but also may develop in areas of trauma including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.
- Intralesional methotrexate is a potential alternative to surgical treatment of KAs as a less invasive and less costly treatment modality with decreased morbidity for multiple KAs.
- Isotopic response refers to the occurrence of a new skin disorder arising at the site of another unrelated and already healed skin disease. Isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of injuries.