User login
Nonscarring Alopecia Associated With Vitamin D Deficiency
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
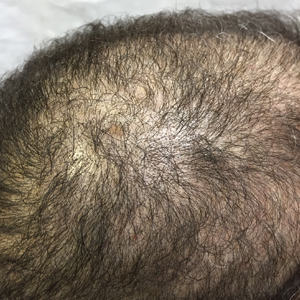
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.
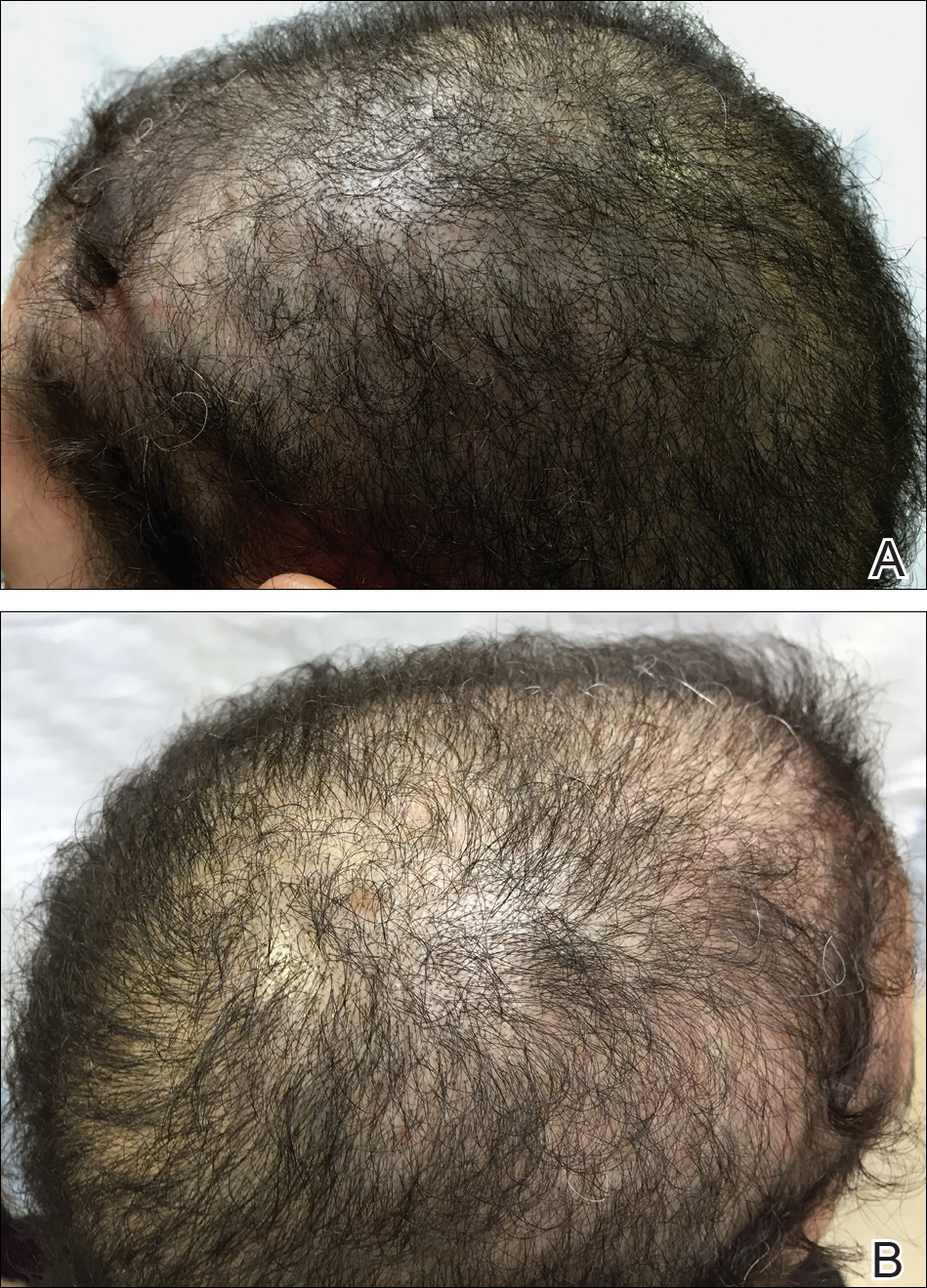
On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
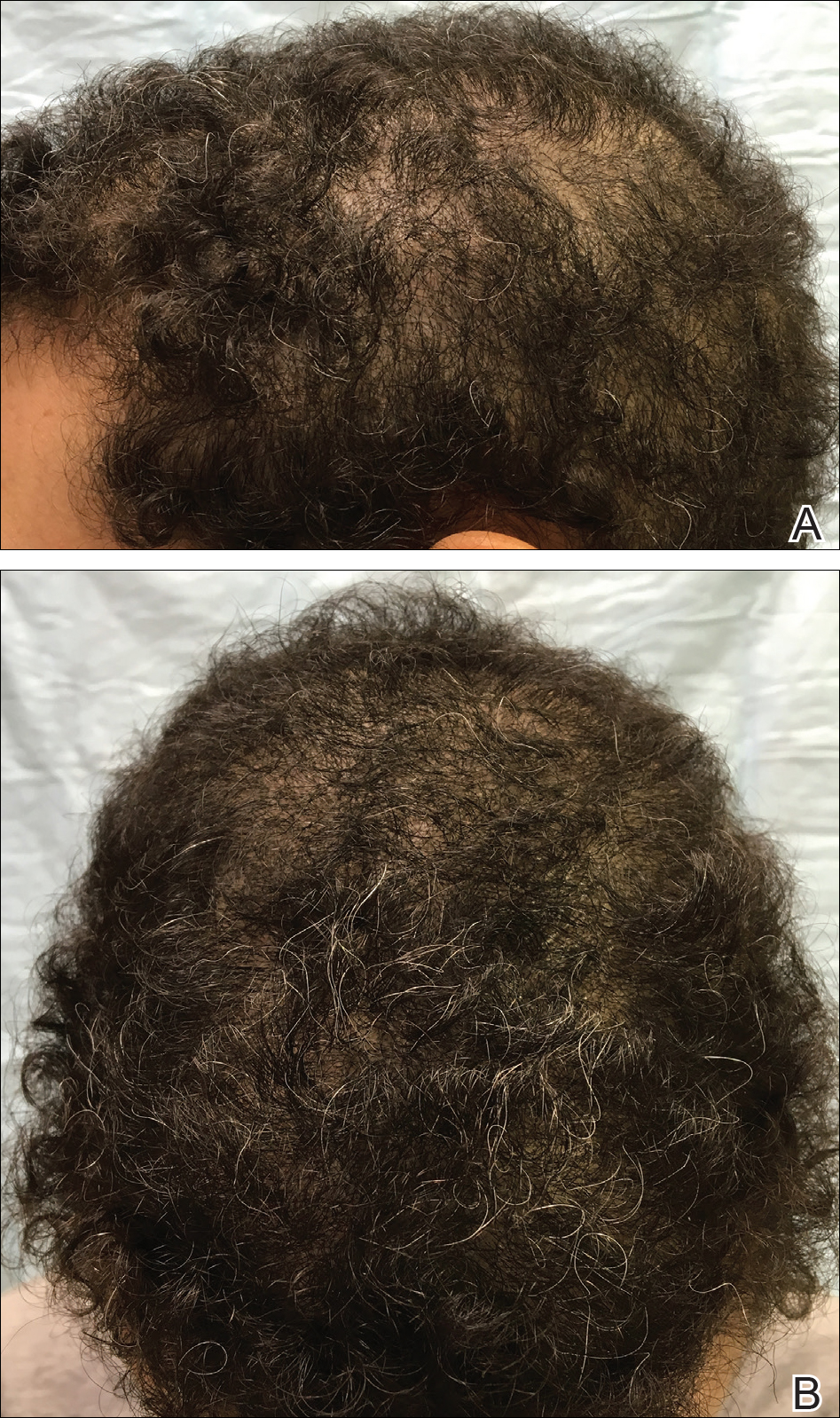
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.
Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Practice Points
- The evaluation of vitamin D levels is important in the management of nonscarring alopecia.
- Vitamin D deficiency can present as nonscarring alopecia not associated with alopecia areata, androgenetic alopecia, or telogen effluvium.