User login
Exploring options for POP treatment: Patient selection, surgical approaches, and ways to manage risks
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.
Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
Public speaking fundamentals. Presentation follow-up: What to do after the last slide is shown

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Public speaking fundamentals. The program: Key elements in capturing and holding audience attention
In the first part of this article series (“Preparation: Tips that lead to a solid, engaging presentation,” OBG Manag. 2016;28[7]:31–36.), we offered tips on preparing for a group presentation. In this article, part 2, we discuss the presentation itself and what you can do to capture and hold your audience’s attention.
How to connect with the audience
Let’s assume the meeting host has just introduced you to the audience using, as we suggested in the previous article, an autobiographical profile you provided. You now have the audience’s undivided attention. What you do and say in the next 30 to 60 seconds will set the stage for your program. Following the requisite “thank you” to the host and meeting sponsor, use this time to establish your expertise as a spokesperson on the chosen topic. Or, if the introductory remarks made your expertise plain, you may choose to connect with the audience on an informal, personal level. If you are from out of town, for instance, you could remark on an interesting aspect of the city or region you are visiting that you learned on the Internet before arriving.
Underscore the topic’s importance. On the other hand, you might want to begin with an insightful statistic germane to your talk. For example, a talk on breast cancer might begin with, “According to the American Cancer Society, there are nearly 250,000 new cases of breast cancer each year, and breast cancer accounts for more than 40,000 deaths per year. That means more women die from breast cancer than die in auto accidents each year. So this emphasizes the importance of appropriately screening women for breast cancer annually after age 40.”
An opening story about a patient can be powerful. Better yet, a personal experience reflecting your topic is a great way to connect with your audience members and get their attention. For example, one of us (NHB) gives talks on practice management and practice efficiency. I might talk about when I was called from an exam room 3 times to answer “emergency” phone calls from a patient who wanted only to request her medical records. To ensure that this embarrassment would never happen again, I put in place a system that I then describe for the audience.
Alternatively, an opening that addresses the audience’s unspoken question, “What’s in this for me?” is sure to grab their attention. For instance, a talk on office productivity might begin by promising to share a way to increase annual collections by $250,000 per physician through scheduling adjustments that can increase the number of examined patients by one per hour.
Steer clear of these openings. In general, avoid “I’m delighted to be here” and other clichés. One exception would be if you can make that cliché humorous. For example, if a speaker from the deep South is visiting the northern part of the country in summer, she might say, “Most speakers say they’re delighted to be here, and you may well question their sincerity. However, I’m from New Orleans where the temperature is approaching 105 degrees with 95% humidity. You know I’m really delighted to be here!”
Importantly, avoid starting with an apology. Do not mention problems with the audiovisual equipment or why you arrived late. The audience does not care, and you will immediately lose their attention. They want to be educated and entertained. There is no better way to do this than by offering a compelling and captivating opening that begins the moment after you are introduced.
Finally, avoid use of the “royal I,” as in “I am here to talk about XYZ.” It places you in a position superior to the audience, and that is a turnoff. Instead, you could say to the audience, “The reason you are here is to learn about XYZ.” This places the audience on an equal level with you, and they know there will be something in the presentation for them.
Housekeeping notes
The audience will appreciate knowing how long you plan to speak and whether you will take questions during or after the presentation. Based on our experience, if there are fewer than 20 attendees, we often encourage questions during the program instead of waiting until the end. This makes the program more conversational and usually generates more questions. With a dinner presentation, we prefer to speak while the audience is eating. We usually start after the waiters have taken the orders and the attendees have had their appetizers. We might say we will finish the program by the time they are ready for dessert. We also mention that we will distribute a handout after the presentation so they do not have to worry about following the handout, taking notes, and watching the speaker while trying to eat.
The main body of the program
As for structuring your talk, we suggest you follow this time-honored advice often attributed to Aristotle: Tell the audience what you are going to say, say it, and tell them what you said.
So we begin a presentation by stating the objectives of our program, usually limited to 3 and no more than 4. For example, a talk on hormone therapy (HT) for treating vasomotor symptoms of menopause might mention 1) the history of HT use, 2) which women are appropriate candidates for HT, and 3) how to monitor women who receive HT.
Enhance the talk’s relevance. We like to begin a clinical program with a case scenario wherein we describe how one of our patients had the specific problem and how we used a particular drug, treatment, or device to manage the case. We try to select a patient similar to ones who would be seen by members of the audience.
Simplify as much as possible. We then present the slides exactly as they have been provided by the pharmaceutical company. Most company slides contain too many words as well as diagrams that are too complex for the audience to grasp easily. We try to find one salient point on each slide and focus attention on that single word, phrase, or sentence. We can do this in a small audience by walking over to the screen and pointing it out, or we can use the laser pointer from a distance.
Change things up to keep the message fresh. Let’s be honest, most medical talks are dry and boring. Try to inject some energy and enthusiasm in the middle of the presentation. Every few minutes we tell a story or ask the audience a question. For example, during a program on practice management, one of us (NHB) will relate a story about an unhappy patient and then ask a physician in the audience how he or she might handle the disgruntled patient. This is a nice break from the main content of the presentation, re-engaging the audience in an interactive exchange.
Should you use humor?
Although many physicians attempt to use humor during a presentation, few are talented at stand-up comedy. However, used judiciously humor, like seasoning in fine cuisine, can do great things for a presentation. It can break the ice, drive home a point, and enhance your likeability. It can, though, also backfire. One of us (NHB) once gave a talk to a large audience of pharmaceutical representatives. As part of my wrap-up I displayed a slide from the cover of Economics that showed 2 camels in the mating position. My closing line was that reps need to “hump to it” and get involved with their physicians and be value-added in their product detailing. Afterward, the meeting planner told me that he would never hire me again. He said I had a great program, great material, and a good connection with the audience. But my closing was over the top. I learned my lesson. Never use material that has the potential to offend. If you want to use humor, the self-deprecatingkind is always safest.
Try using visual aids
Our observation is that few physician speakers use visual aids other than their slides. We have learned that audience attention will stay focused on you if you make use of visual aids. For example, if we are speaking to a lay audience about urinary incontinence, we might use a balloon to demonstrate the bladder and the urethra.
Studies have shown that there are more nerve endings from the eye to the brain than from the ear to the brain. Humans purportedly receive 25 times as much stimulus from visual cues than from auditory ones. To paraphrase an old proverb, “One seeing is better than 100 times hearing about”!
A few suggestions regarding the use of visual aids:
- Keep the visual aid out of sight until you are ready to use it. You do not want the audience staring at it when they should be focusing on you or your slide material. We usually keep our visual aids under the table that supports the computer and projector.
- Make certain the visual aid is large enough to be seen by everyone in the audience.
- Do not hand out the aid to the audience during your program. Doing so will divert their attention from you and your material.
- When you have finished using the aid, put it away.
Closing out the program
After we have covered the program’s 3 objectives, we let the audience know we are approaching the end of the presentation. For a dinner program, we try to time the ending just as plates are being cleared and before dessert is served. We then restate the 3 objectives as they might pertain to the attendees’ patients and practices. At this time, we take questions from the audience, even if some were asked during the presentation. We repeat each question when it is asked so that everyone can hear it. (This also gives us a few seconds to think about it and frame our answer.) If it appears that many questions will be asked, we assure everyone that we plan to finish on time and will remain after the program is over to answer additional questions.
Tips on fielding questions. When responding to a question, direct your attention initially to the person who asked it. After that, spend about 20% of the time focused on that person and 80% of the time on the rest of the audience. If you focus only on the questioner, it becomes a one-on-one conversation. You want to end your response with your eyes on the group and not on the questioner. Looking at the group will also act as a bridge to the next question. Although we used to reply to an inquiry with, “That’s a great question,” we now suggest avoiding this comment. Why? Because it is unlikely that you’ll keep using that line, and the next questioner who does not receive the same compliment might feel slighted.
Wrap up. When you announce, “I would like to conclude my program with…,” this is the magical time when you hold the complete attention of the audience. Often, the speaker’s last words are the ones the audience remembers the longest. So this is the time to offer your take-home message. For example, a talk on how to motivate your staff might conclude, “Remember, your staff members are the people that patients encounter first and the ones they see last as they leave the office. Every patient can have a positive experience with you and your practice if you ensure that your personnel are highly motivated. This happens in part by your effort to recognize their accomplishments.” Then hold up your hands and spread out your arms as you end with “Thank you.” The audience likely will applaud and, if your speech is truly exceptional, you might receive a gratifying standing ovation!
Be seated
Renowned for his speeches, Franklin Delano Roosevelt summarized the art of effective speaking when he said, “Be sincere. Be brief. Be seated.” When your time is up, turn the program back over to the meeting host and take a seat.
In the final article in this public speaking series, we will discuss the follow-up steps to take once the program is over, including the call to action or what you want the audience to do after you have left the podium or the speaking venue.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In the first part of this article series (“Preparation: Tips that lead to a solid, engaging presentation,” OBG Manag. 2016;28[7]:31–36.), we offered tips on preparing for a group presentation. In this article, part 2, we discuss the presentation itself and what you can do to capture and hold your audience’s attention.
How to connect with the audience
Let’s assume the meeting host has just introduced you to the audience using, as we suggested in the previous article, an autobiographical profile you provided. You now have the audience’s undivided attention. What you do and say in the next 30 to 60 seconds will set the stage for your program. Following the requisite “thank you” to the host and meeting sponsor, use this time to establish your expertise as a spokesperson on the chosen topic. Or, if the introductory remarks made your expertise plain, you may choose to connect with the audience on an informal, personal level. If you are from out of town, for instance, you could remark on an interesting aspect of the city or region you are visiting that you learned on the Internet before arriving.
Underscore the topic’s importance. On the other hand, you might want to begin with an insightful statistic germane to your talk. For example, a talk on breast cancer might begin with, “According to the American Cancer Society, there are nearly 250,000 new cases of breast cancer each year, and breast cancer accounts for more than 40,000 deaths per year. That means more women die from breast cancer than die in auto accidents each year. So this emphasizes the importance of appropriately screening women for breast cancer annually after age 40.”
An opening story about a patient can be powerful. Better yet, a personal experience reflecting your topic is a great way to connect with your audience members and get their attention. For example, one of us (NHB) gives talks on practice management and practice efficiency. I might talk about when I was called from an exam room 3 times to answer “emergency” phone calls from a patient who wanted only to request her medical records. To ensure that this embarrassment would never happen again, I put in place a system that I then describe for the audience.
Alternatively, an opening that addresses the audience’s unspoken question, “What’s in this for me?” is sure to grab their attention. For instance, a talk on office productivity might begin by promising to share a way to increase annual collections by $250,000 per physician through scheduling adjustments that can increase the number of examined patients by one per hour.
Steer clear of these openings. In general, avoid “I’m delighted to be here” and other clichés. One exception would be if you can make that cliché humorous. For example, if a speaker from the deep South is visiting the northern part of the country in summer, she might say, “Most speakers say they’re delighted to be here, and you may well question their sincerity. However, I’m from New Orleans where the temperature is approaching 105 degrees with 95% humidity. You know I’m really delighted to be here!”
Importantly, avoid starting with an apology. Do not mention problems with the audiovisual equipment or why you arrived late. The audience does not care, and you will immediately lose their attention. They want to be educated and entertained. There is no better way to do this than by offering a compelling and captivating opening that begins the moment after you are introduced.
Finally, avoid use of the “royal I,” as in “I am here to talk about XYZ.” It places you in a position superior to the audience, and that is a turnoff. Instead, you could say to the audience, “The reason you are here is to learn about XYZ.” This places the audience on an equal level with you, and they know there will be something in the presentation for them.
Housekeeping notes
The audience will appreciate knowing how long you plan to speak and whether you will take questions during or after the presentation. Based on our experience, if there are fewer than 20 attendees, we often encourage questions during the program instead of waiting until the end. This makes the program more conversational and usually generates more questions. With a dinner presentation, we prefer to speak while the audience is eating. We usually start after the waiters have taken the orders and the attendees have had their appetizers. We might say we will finish the program by the time they are ready for dessert. We also mention that we will distribute a handout after the presentation so they do not have to worry about following the handout, taking notes, and watching the speaker while trying to eat.
The main body of the program
As for structuring your talk, we suggest you follow this time-honored advice often attributed to Aristotle: Tell the audience what you are going to say, say it, and tell them what you said.
So we begin a presentation by stating the objectives of our program, usually limited to 3 and no more than 4. For example, a talk on hormone therapy (HT) for treating vasomotor symptoms of menopause might mention 1) the history of HT use, 2) which women are appropriate candidates for HT, and 3) how to monitor women who receive HT.
Enhance the talk’s relevance. We like to begin a clinical program with a case scenario wherein we describe how one of our patients had the specific problem and how we used a particular drug, treatment, or device to manage the case. We try to select a patient similar to ones who would be seen by members of the audience.
Simplify as much as possible. We then present the slides exactly as they have been provided by the pharmaceutical company. Most company slides contain too many words as well as diagrams that are too complex for the audience to grasp easily. We try to find one salient point on each slide and focus attention on that single word, phrase, or sentence. We can do this in a small audience by walking over to the screen and pointing it out, or we can use the laser pointer from a distance.
Change things up to keep the message fresh. Let’s be honest, most medical talks are dry and boring. Try to inject some energy and enthusiasm in the middle of the presentation. Every few minutes we tell a story or ask the audience a question. For example, during a program on practice management, one of us (NHB) will relate a story about an unhappy patient and then ask a physician in the audience how he or she might handle the disgruntled patient. This is a nice break from the main content of the presentation, re-engaging the audience in an interactive exchange.
Should you use humor?
Although many physicians attempt to use humor during a presentation, few are talented at stand-up comedy. However, used judiciously humor, like seasoning in fine cuisine, can do great things for a presentation. It can break the ice, drive home a point, and enhance your likeability. It can, though, also backfire. One of us (NHB) once gave a talk to a large audience of pharmaceutical representatives. As part of my wrap-up I displayed a slide from the cover of Economics that showed 2 camels in the mating position. My closing line was that reps need to “hump to it” and get involved with their physicians and be value-added in their product detailing. Afterward, the meeting planner told me that he would never hire me again. He said I had a great program, great material, and a good connection with the audience. But my closing was over the top. I learned my lesson. Never use material that has the potential to offend. If you want to use humor, the self-deprecatingkind is always safest.
Try using visual aids
Our observation is that few physician speakers use visual aids other than their slides. We have learned that audience attention will stay focused on you if you make use of visual aids. For example, if we are speaking to a lay audience about urinary incontinence, we might use a balloon to demonstrate the bladder and the urethra.
Studies have shown that there are more nerve endings from the eye to the brain than from the ear to the brain. Humans purportedly receive 25 times as much stimulus from visual cues than from auditory ones. To paraphrase an old proverb, “One seeing is better than 100 times hearing about”!
A few suggestions regarding the use of visual aids:
- Keep the visual aid out of sight until you are ready to use it. You do not want the audience staring at it when they should be focusing on you or your slide material. We usually keep our visual aids under the table that supports the computer and projector.
- Make certain the visual aid is large enough to be seen by everyone in the audience.
- Do not hand out the aid to the audience during your program. Doing so will divert their attention from you and your material.
- When you have finished using the aid, put it away.
Closing out the program
After we have covered the program’s 3 objectives, we let the audience know we are approaching the end of the presentation. For a dinner program, we try to time the ending just as plates are being cleared and before dessert is served. We then restate the 3 objectives as they might pertain to the attendees’ patients and practices. At this time, we take questions from the audience, even if some were asked during the presentation. We repeat each question when it is asked so that everyone can hear it. (This also gives us a few seconds to think about it and frame our answer.) If it appears that many questions will be asked, we assure everyone that we plan to finish on time and will remain after the program is over to answer additional questions.
Tips on fielding questions. When responding to a question, direct your attention initially to the person who asked it. After that, spend about 20% of the time focused on that person and 80% of the time on the rest of the audience. If you focus only on the questioner, it becomes a one-on-one conversation. You want to end your response with your eyes on the group and not on the questioner. Looking at the group will also act as a bridge to the next question. Although we used to reply to an inquiry with, “That’s a great question,” we now suggest avoiding this comment. Why? Because it is unlikely that you’ll keep using that line, and the next questioner who does not receive the same compliment might feel slighted.
Wrap up. When you announce, “I would like to conclude my program with…,” this is the magical time when you hold the complete attention of the audience. Often, the speaker’s last words are the ones the audience remembers the longest. So this is the time to offer your take-home message. For example, a talk on how to motivate your staff might conclude, “Remember, your staff members are the people that patients encounter first and the ones they see last as they leave the office. Every patient can have a positive experience with you and your practice if you ensure that your personnel are highly motivated. This happens in part by your effort to recognize their accomplishments.” Then hold up your hands and spread out your arms as you end with “Thank you.” The audience likely will applaud and, if your speech is truly exceptional, you might receive a gratifying standing ovation!
Be seated
Renowned for his speeches, Franklin Delano Roosevelt summarized the art of effective speaking when he said, “Be sincere. Be brief. Be seated.” When your time is up, turn the program back over to the meeting host and take a seat.
In the final article in this public speaking series, we will discuss the follow-up steps to take once the program is over, including the call to action or what you want the audience to do after you have left the podium or the speaking venue.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In the first part of this article series (“Preparation: Tips that lead to a solid, engaging presentation,” OBG Manag. 2016;28[7]:31–36.), we offered tips on preparing for a group presentation. In this article, part 2, we discuss the presentation itself and what you can do to capture and hold your audience’s attention.
How to connect with the audience
Let’s assume the meeting host has just introduced you to the audience using, as we suggested in the previous article, an autobiographical profile you provided. You now have the audience’s undivided attention. What you do and say in the next 30 to 60 seconds will set the stage for your program. Following the requisite “thank you” to the host and meeting sponsor, use this time to establish your expertise as a spokesperson on the chosen topic. Or, if the introductory remarks made your expertise plain, you may choose to connect with the audience on an informal, personal level. If you are from out of town, for instance, you could remark on an interesting aspect of the city or region you are visiting that you learned on the Internet before arriving.
Underscore the topic’s importance. On the other hand, you might want to begin with an insightful statistic germane to your talk. For example, a talk on breast cancer might begin with, “According to the American Cancer Society, there are nearly 250,000 new cases of breast cancer each year, and breast cancer accounts for more than 40,000 deaths per year. That means more women die from breast cancer than die in auto accidents each year. So this emphasizes the importance of appropriately screening women for breast cancer annually after age 40.”
An opening story about a patient can be powerful. Better yet, a personal experience reflecting your topic is a great way to connect with your audience members and get their attention. For example, one of us (NHB) gives talks on practice management and practice efficiency. I might talk about when I was called from an exam room 3 times to answer “emergency” phone calls from a patient who wanted only to request her medical records. To ensure that this embarrassment would never happen again, I put in place a system that I then describe for the audience.
Alternatively, an opening that addresses the audience’s unspoken question, “What’s in this for me?” is sure to grab their attention. For instance, a talk on office productivity might begin by promising to share a way to increase annual collections by $250,000 per physician through scheduling adjustments that can increase the number of examined patients by one per hour.
Steer clear of these openings. In general, avoid “I’m delighted to be here” and other clichés. One exception would be if you can make that cliché humorous. For example, if a speaker from the deep South is visiting the northern part of the country in summer, she might say, “Most speakers say they’re delighted to be here, and you may well question their sincerity. However, I’m from New Orleans where the temperature is approaching 105 degrees with 95% humidity. You know I’m really delighted to be here!”
Importantly, avoid starting with an apology. Do not mention problems with the audiovisual equipment or why you arrived late. The audience does not care, and you will immediately lose their attention. They want to be educated and entertained. There is no better way to do this than by offering a compelling and captivating opening that begins the moment after you are introduced.
Finally, avoid use of the “royal I,” as in “I am here to talk about XYZ.” It places you in a position superior to the audience, and that is a turnoff. Instead, you could say to the audience, “The reason you are here is to learn about XYZ.” This places the audience on an equal level with you, and they know there will be something in the presentation for them.
Housekeeping notes
The audience will appreciate knowing how long you plan to speak and whether you will take questions during or after the presentation. Based on our experience, if there are fewer than 20 attendees, we often encourage questions during the program instead of waiting until the end. This makes the program more conversational and usually generates more questions. With a dinner presentation, we prefer to speak while the audience is eating. We usually start after the waiters have taken the orders and the attendees have had their appetizers. We might say we will finish the program by the time they are ready for dessert. We also mention that we will distribute a handout after the presentation so they do not have to worry about following the handout, taking notes, and watching the speaker while trying to eat.
The main body of the program
As for structuring your talk, we suggest you follow this time-honored advice often attributed to Aristotle: Tell the audience what you are going to say, say it, and tell them what you said.
So we begin a presentation by stating the objectives of our program, usually limited to 3 and no more than 4. For example, a talk on hormone therapy (HT) for treating vasomotor symptoms of menopause might mention 1) the history of HT use, 2) which women are appropriate candidates for HT, and 3) how to monitor women who receive HT.
Enhance the talk’s relevance. We like to begin a clinical program with a case scenario wherein we describe how one of our patients had the specific problem and how we used a particular drug, treatment, or device to manage the case. We try to select a patient similar to ones who would be seen by members of the audience.
Simplify as much as possible. We then present the slides exactly as they have been provided by the pharmaceutical company. Most company slides contain too many words as well as diagrams that are too complex for the audience to grasp easily. We try to find one salient point on each slide and focus attention on that single word, phrase, or sentence. We can do this in a small audience by walking over to the screen and pointing it out, or we can use the laser pointer from a distance.
Change things up to keep the message fresh. Let’s be honest, most medical talks are dry and boring. Try to inject some energy and enthusiasm in the middle of the presentation. Every few minutes we tell a story or ask the audience a question. For example, during a program on practice management, one of us (NHB) will relate a story about an unhappy patient and then ask a physician in the audience how he or she might handle the disgruntled patient. This is a nice break from the main content of the presentation, re-engaging the audience in an interactive exchange.
Should you use humor?
Although many physicians attempt to use humor during a presentation, few are talented at stand-up comedy. However, used judiciously humor, like seasoning in fine cuisine, can do great things for a presentation. It can break the ice, drive home a point, and enhance your likeability. It can, though, also backfire. One of us (NHB) once gave a talk to a large audience of pharmaceutical representatives. As part of my wrap-up I displayed a slide from the cover of Economics that showed 2 camels in the mating position. My closing line was that reps need to “hump to it” and get involved with their physicians and be value-added in their product detailing. Afterward, the meeting planner told me that he would never hire me again. He said I had a great program, great material, and a good connection with the audience. But my closing was over the top. I learned my lesson. Never use material that has the potential to offend. If you want to use humor, the self-deprecatingkind is always safest.
Try using visual aids
Our observation is that few physician speakers use visual aids other than their slides. We have learned that audience attention will stay focused on you if you make use of visual aids. For example, if we are speaking to a lay audience about urinary incontinence, we might use a balloon to demonstrate the bladder and the urethra.
Studies have shown that there are more nerve endings from the eye to the brain than from the ear to the brain. Humans purportedly receive 25 times as much stimulus from visual cues than from auditory ones. To paraphrase an old proverb, “One seeing is better than 100 times hearing about”!
A few suggestions regarding the use of visual aids:
- Keep the visual aid out of sight until you are ready to use it. You do not want the audience staring at it when they should be focusing on you or your slide material. We usually keep our visual aids under the table that supports the computer and projector.
- Make certain the visual aid is large enough to be seen by everyone in the audience.
- Do not hand out the aid to the audience during your program. Doing so will divert their attention from you and your material.
- When you have finished using the aid, put it away.
Closing out the program
After we have covered the program’s 3 objectives, we let the audience know we are approaching the end of the presentation. For a dinner program, we try to time the ending just as plates are being cleared and before dessert is served. We then restate the 3 objectives as they might pertain to the attendees’ patients and practices. At this time, we take questions from the audience, even if some were asked during the presentation. We repeat each question when it is asked so that everyone can hear it. (This also gives us a few seconds to think about it and frame our answer.) If it appears that many questions will be asked, we assure everyone that we plan to finish on time and will remain after the program is over to answer additional questions.
Tips on fielding questions. When responding to a question, direct your attention initially to the person who asked it. After that, spend about 20% of the time focused on that person and 80% of the time on the rest of the audience. If you focus only on the questioner, it becomes a one-on-one conversation. You want to end your response with your eyes on the group and not on the questioner. Looking at the group will also act as a bridge to the next question. Although we used to reply to an inquiry with, “That’s a great question,” we now suggest avoiding this comment. Why? Because it is unlikely that you’ll keep using that line, and the next questioner who does not receive the same compliment might feel slighted.
Wrap up. When you announce, “I would like to conclude my program with…,” this is the magical time when you hold the complete attention of the audience. Often, the speaker’s last words are the ones the audience remembers the longest. So this is the time to offer your take-home message. For example, a talk on how to motivate your staff might conclude, “Remember, your staff members are the people that patients encounter first and the ones they see last as they leave the office. Every patient can have a positive experience with you and your practice if you ensure that your personnel are highly motivated. This happens in part by your effort to recognize their accomplishments.” Then hold up your hands and spread out your arms as you end with “Thank you.” The audience likely will applaud and, if your speech is truly exceptional, you might receive a gratifying standing ovation!
Be seated
Renowned for his speeches, Franklin Delano Roosevelt summarized the art of effective speaking when he said, “Be sincere. Be brief. Be seated.” When your time is up, turn the program back over to the meeting host and take a seat.
In the final article in this public speaking series, we will discuss the follow-up steps to take once the program is over, including the call to action or what you want the audience to do after you have left the podium or the speaking venue.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Public speaking fundamentals. Preparation: Tips that lead to a solid, engaging presentation
Public speaking is one of the best ways to market and promote your skills as a physician. It is an ethical way of communicating and showcasing your areas of interest and expertise to professional or lay audiences. Most physicians and health care professionals take pride in their ability to communicate. After all, that is how we take a history, discuss our findings with patients, and educate individuals on restoring or maintaining their health. Public speaking, though, for the most part is a learned skill. Except for presentations to faculty at bedside or at grand rounds, we have received little training in public speaking.
Few of us are naturally comfortable in front of a live audience or a TV or video camera. But with a little practice and diligent preparation, we can become good or even excellent, confident public speakers. This article—the first in a series of 3—provides you with preparatory tips and techniques to enhance your public speaking skills.
First, know your audience
Whether you are presenting to a group of 20 or 200, you can do certain things in advance to ensure that your presentation achieves the desired response. Most important: Know your audience. Don’t assume the audience is like you. To connect with them, you need to understand why your topic is important to them. What do they expect to learn from the presentation? Each attendee will be asking, “What’s in it for me?”
To keep listeners interested and engaged, you also must know their level of knowledge about the topic. If you are speaking to a group of residents about pelvic organ prolapse, you would use different language and content than if you were speaking to practicing primary care doctors; and these elements would be different again if you were speaking to a group of practicing urogynecologists. It’s insulting to recite basic information to highly knowledgeable physicians, or to present sophisticated technical content and complicated slides to novice physicians or lay people.
When presenting in a foreign country, learn how the culture of the audience differs from yours. How do they dress? What style of humor do they favor? How do they typically communicate? What gestures are appropriate or inappropriate? Are there religious influences to consider?
Practical steps. Before the meeting or event, speak to the organizer or meeting planner and find out the audience’s level of knowledge on the topic. Ask about audience expectations as well as demographics (such as age and background). If you are speaking at an industry event, research the event’s website and familiarize yourself with the mission of the event and who are the typical attendees. If you are presenting to a corporation, learn as much as you can about it by visiting its website, reading news reports, and reviewing associated blogs.
In addition to knowing the needs of the audience, ask the meeting planner about the goals and objectives for the program to make certain you can deliver on the requests.
Know your talk stem to stern
Review your slide material thoroughly. Understand each slide in the presentation and be comfortable with its content.
Avoid reading from slides. Reciting content that viewers can read for themselves breeds boredom and makes them lose interest. Further, when you are looking at the slides, you are not making eye contact with the audience and risk losing their attention. Good speakers are so comfortable with their slides that they can discuss each one without having to look at it.
Rehearse. The best way to achieve the foregoing is to rehearse. Your audience will be able to tell if you took the slide deck directly from a CD and loaded it into a computer and are giving the talk for the first time. You’ll need to know how long the program is to last and how long you are to speak. We suggest you practice with a timer to be certain you do not exceed the allotted time. Rehearse your talk aloud several times with all the props and audiovisual equipment you plan to use. This practice will help to curb filler words such as “ah” and “um.” It is also helpful to practice slide transitions, pauses, and even your breathing.
Prepare for the unexpected, too. Dinner meetings, for instance, may not start on time due to office or hospital delays for attending physicians, possibly resulting in a need to shorten your presentation.
Ask about the meeting agenda. If a meal is to be served, will you be speaking beforehand? This is the least favorable time slot, as you are holding people hostage before they can eat. Our preference is to speak after the appetizer is served and the orders have been taken by the wait staff. This way, attendees are not starving and they have something to drink. You can assure the waiters they won’t be disturbing you, and you can ask them to avoid walking in front of the projector. Ideally, you should end your presentation before dessert arrives and use the remaining time to field questions.
We suggest that you prepare a handout to be distributed at the end of the program, not before. You want your audience focused on you and your slides as you are speaking. Tell the audience you will be providing a handout of your presentation, which will minimize note-taking during your talk.
Your speech opening
The first and last 30 seconds of any speech probably have the most impact.1 Give extra thought, time, and effort to your opening and closing remarks. Do not open with “Good evening, it is a pleasure to be here tonight.” That wastes precious seconds.
While opening a speech with a joke or funny story is the conventional wisdom, ask yourself1:
- Is my selection appropriate to the occasion and for this audience?
- Is it in good taste?
- Does it relate to me (my service) or to the event or the group? Does it support my topic or its key points?
A humorous story or inspirational vignette that relates to your topic or audience can grab the audience’s attention. If you feel that demands more presentation skill than you possess at the outset of your public-speaking career, give the audience what you know and what they most want to hear. You know the questions that you have heard most at cocktail receptions or professional society meetings. So, put the answers to those questions in your speech.
For example. A scientist working with a major corporation was preparing a speech for a lay audience. Since most of the audience did not know what scientists do, he offered the following analogy: “Being a scientist is like doing a jigsaw puzzle in a snowstorm at night...you don’t have all the pieces...and you don’t have the picture on the front of the box to work from.” You can say more with less.1
Your closing
The closing is an important aspect of your speech. Summarize the key elements to your presentation. If you are going to take questions, a good approach is to say, “Before my closing remarks, are there any questions?” Following the questions, finish with a takeaway message that ties into your theme.1
Prepare an autobiographical introduction
We suggest that you write your own introduction and e-mail it to the person who will be introducing you. Let them know it is a suggestion that they are welcome to modify. We have found that most emcees or meeting planners are delighted to have the introduction and will use it just as you have written it. Also bring hard copy with you; many emcees will have forgotten to download what you sent. The figure shows an example of the introduction that one of us (NHB) uses, and you are welcome to modify it for your own use.
Ask about and confirm audiovisual support
Ask the meeting planner ahead of time if they will be providing the computer, projector, and screen. And if, for instance, they will provide a projector but not a computer, make sure the computer you will bring is compatible with their projector. Also, you will probably not require a microphone for a small group, but if you are speaking in a loud restaurant, a portable microphone-speaker system may be helpful.
Arrive early at the program venue to make sure the computers, projector, screen placement, and seating arrangement are all in order. Nothing can sidetrack a speaker (even a seasoned one) like a problem with the computer or equipment setup—for example, your flash drive requiring a USB port cannot connect to the sponsor’s computer, or your program created on a Mac does not run on the sponsor’s PC.
Show time: Getting ready
Another benefit in arriving early, besides being able to check on the equipment, is the chance to greet the audience members as they enter. It is easier to speak to a group of friends than strangers. And if you can remember their names, you can call on them and ask their opinion or how they might manage a patient who has the condition you are discussing. You also could suggest to the meeting planner that name tags for attendees would be helpful.
Warming up. A public speaker, like an athlete, needs to warm up physically before the event. If the facility has an anteroom available, use it for the following exercises suggested by public speaking coach Patricia Fripp1:
- Stand on one leg and shake the other (remove high heels first). When you place your raised foot back on the floor, it will feel lighter than the other one. Repeat the exercise using the other leg. Imagine your energy going down through the floor and up out of your head. While this sounds quite comical, it is not. It is a practical technique used by actors.
- Shake your hands vigorously. Hold them above your head, bending your wrists and elbows, then return your arms to your sides. This will make your hand movements more natural.
- Warm up your facial muscles by chewing in a highly exaggerated way. Do shoulder and neck rolls. Imagine you are at eye level with a clock. As you look at 12 o’clock, pull as much of your face up to the 12 as you can; move your eyes to 3 and repeat, then down to 6, and finally over to 9.
Not only do these exercises warm you up but they also relax you. The exaggerated movements help your movements to flow more naturally.1
This is just the start
Thorough preparation is key to making a solid presentation. But other factors are important too. Your goal is for the audience to take action or to implement suggestions from your presentation. In part 2 of this series, we will share tips on elements of the presentation itself that will encourage audience engagement and message retention. We will discuss how to make your message “stick” and how to make a dynamic, effective presentation that holds your audience’s attention for your entire talk.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
- Fripp P. Add credibility to your business reputation through public speaking. Patricia Fripp website. http://www.fripp.com/add-credibility-to-your-business-reputation-through-public-speaking/. Accessed June 15, 2016.
Public speaking is one of the best ways to market and promote your skills as a physician. It is an ethical way of communicating and showcasing your areas of interest and expertise to professional or lay audiences. Most physicians and health care professionals take pride in their ability to communicate. After all, that is how we take a history, discuss our findings with patients, and educate individuals on restoring or maintaining their health. Public speaking, though, for the most part is a learned skill. Except for presentations to faculty at bedside or at grand rounds, we have received little training in public speaking.
Few of us are naturally comfortable in front of a live audience or a TV or video camera. But with a little practice and diligent preparation, we can become good or even excellent, confident public speakers. This article—the first in a series of 3—provides you with preparatory tips and techniques to enhance your public speaking skills.
First, know your audience
Whether you are presenting to a group of 20 or 200, you can do certain things in advance to ensure that your presentation achieves the desired response. Most important: Know your audience. Don’t assume the audience is like you. To connect with them, you need to understand why your topic is important to them. What do they expect to learn from the presentation? Each attendee will be asking, “What’s in it for me?”
To keep listeners interested and engaged, you also must know their level of knowledge about the topic. If you are speaking to a group of residents about pelvic organ prolapse, you would use different language and content than if you were speaking to practicing primary care doctors; and these elements would be different again if you were speaking to a group of practicing urogynecologists. It’s insulting to recite basic information to highly knowledgeable physicians, or to present sophisticated technical content and complicated slides to novice physicians or lay people.
When presenting in a foreign country, learn how the culture of the audience differs from yours. How do they dress? What style of humor do they favor? How do they typically communicate? What gestures are appropriate or inappropriate? Are there religious influences to consider?
Practical steps. Before the meeting or event, speak to the organizer or meeting planner and find out the audience’s level of knowledge on the topic. Ask about audience expectations as well as demographics (such as age and background). If you are speaking at an industry event, research the event’s website and familiarize yourself with the mission of the event and who are the typical attendees. If you are presenting to a corporation, learn as much as you can about it by visiting its website, reading news reports, and reviewing associated blogs.
In addition to knowing the needs of the audience, ask the meeting planner about the goals and objectives for the program to make certain you can deliver on the requests.
Know your talk stem to stern
Review your slide material thoroughly. Understand each slide in the presentation and be comfortable with its content.
Avoid reading from slides. Reciting content that viewers can read for themselves breeds boredom and makes them lose interest. Further, when you are looking at the slides, you are not making eye contact with the audience and risk losing their attention. Good speakers are so comfortable with their slides that they can discuss each one without having to look at it.
Rehearse. The best way to achieve the foregoing is to rehearse. Your audience will be able to tell if you took the slide deck directly from a CD and loaded it into a computer and are giving the talk for the first time. You’ll need to know how long the program is to last and how long you are to speak. We suggest you practice with a timer to be certain you do not exceed the allotted time. Rehearse your talk aloud several times with all the props and audiovisual equipment you plan to use. This practice will help to curb filler words such as “ah” and “um.” It is also helpful to practice slide transitions, pauses, and even your breathing.
Prepare for the unexpected, too. Dinner meetings, for instance, may not start on time due to office or hospital delays for attending physicians, possibly resulting in a need to shorten your presentation.
Ask about the meeting agenda. If a meal is to be served, will you be speaking beforehand? This is the least favorable time slot, as you are holding people hostage before they can eat. Our preference is to speak after the appetizer is served and the orders have been taken by the wait staff. This way, attendees are not starving and they have something to drink. You can assure the waiters they won’t be disturbing you, and you can ask them to avoid walking in front of the projector. Ideally, you should end your presentation before dessert arrives and use the remaining time to field questions.
We suggest that you prepare a handout to be distributed at the end of the program, not before. You want your audience focused on you and your slides as you are speaking. Tell the audience you will be providing a handout of your presentation, which will minimize note-taking during your talk.
Your speech opening
The first and last 30 seconds of any speech probably have the most impact.1 Give extra thought, time, and effort to your opening and closing remarks. Do not open with “Good evening, it is a pleasure to be here tonight.” That wastes precious seconds.
While opening a speech with a joke or funny story is the conventional wisdom, ask yourself1:
- Is my selection appropriate to the occasion and for this audience?
- Is it in good taste?
- Does it relate to me (my service) or to the event or the group? Does it support my topic or its key points?
A humorous story or inspirational vignette that relates to your topic or audience can grab the audience’s attention. If you feel that demands more presentation skill than you possess at the outset of your public-speaking career, give the audience what you know and what they most want to hear. You know the questions that you have heard most at cocktail receptions or professional society meetings. So, put the answers to those questions in your speech.
For example. A scientist working with a major corporation was preparing a speech for a lay audience. Since most of the audience did not know what scientists do, he offered the following analogy: “Being a scientist is like doing a jigsaw puzzle in a snowstorm at night...you don’t have all the pieces...and you don’t have the picture on the front of the box to work from.” You can say more with less.1
Your closing
The closing is an important aspect of your speech. Summarize the key elements to your presentation. If you are going to take questions, a good approach is to say, “Before my closing remarks, are there any questions?” Following the questions, finish with a takeaway message that ties into your theme.1
Prepare an autobiographical introduction
We suggest that you write your own introduction and e-mail it to the person who will be introducing you. Let them know it is a suggestion that they are welcome to modify. We have found that most emcees or meeting planners are delighted to have the introduction and will use it just as you have written it. Also bring hard copy with you; many emcees will have forgotten to download what you sent. The figure shows an example of the introduction that one of us (NHB) uses, and you are welcome to modify it for your own use.
Ask about and confirm audiovisual support
Ask the meeting planner ahead of time if they will be providing the computer, projector, and screen. And if, for instance, they will provide a projector but not a computer, make sure the computer you will bring is compatible with their projector. Also, you will probably not require a microphone for a small group, but if you are speaking in a loud restaurant, a portable microphone-speaker system may be helpful.
Arrive early at the program venue to make sure the computers, projector, screen placement, and seating arrangement are all in order. Nothing can sidetrack a speaker (even a seasoned one) like a problem with the computer or equipment setup—for example, your flash drive requiring a USB port cannot connect to the sponsor’s computer, or your program created on a Mac does not run on the sponsor’s PC.
Show time: Getting ready
Another benefit in arriving early, besides being able to check on the equipment, is the chance to greet the audience members as they enter. It is easier to speak to a group of friends than strangers. And if you can remember their names, you can call on them and ask their opinion or how they might manage a patient who has the condition you are discussing. You also could suggest to the meeting planner that name tags for attendees would be helpful.
Warming up. A public speaker, like an athlete, needs to warm up physically before the event. If the facility has an anteroom available, use it for the following exercises suggested by public speaking coach Patricia Fripp1:
- Stand on one leg and shake the other (remove high heels first). When you place your raised foot back on the floor, it will feel lighter than the other one. Repeat the exercise using the other leg. Imagine your energy going down through the floor and up out of your head. While this sounds quite comical, it is not. It is a practical technique used by actors.
- Shake your hands vigorously. Hold them above your head, bending your wrists and elbows, then return your arms to your sides. This will make your hand movements more natural.
- Warm up your facial muscles by chewing in a highly exaggerated way. Do shoulder and neck rolls. Imagine you are at eye level with a clock. As you look at 12 o’clock, pull as much of your face up to the 12 as you can; move your eyes to 3 and repeat, then down to 6, and finally over to 9.
Not only do these exercises warm you up but they also relax you. The exaggerated movements help your movements to flow more naturally.1
This is just the start
Thorough preparation is key to making a solid presentation. But other factors are important too. Your goal is for the audience to take action or to implement suggestions from your presentation. In part 2 of this series, we will share tips on elements of the presentation itself that will encourage audience engagement and message retention. We will discuss how to make your message “stick” and how to make a dynamic, effective presentation that holds your audience’s attention for your entire talk.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Public speaking is one of the best ways to market and promote your skills as a physician. It is an ethical way of communicating and showcasing your areas of interest and expertise to professional or lay audiences. Most physicians and health care professionals take pride in their ability to communicate. After all, that is how we take a history, discuss our findings with patients, and educate individuals on restoring or maintaining their health. Public speaking, though, for the most part is a learned skill. Except for presentations to faculty at bedside or at grand rounds, we have received little training in public speaking.
Few of us are naturally comfortable in front of a live audience or a TV or video camera. But with a little practice and diligent preparation, we can become good or even excellent, confident public speakers. This article—the first in a series of 3—provides you with preparatory tips and techniques to enhance your public speaking skills.
First, know your audience
Whether you are presenting to a group of 20 or 200, you can do certain things in advance to ensure that your presentation achieves the desired response. Most important: Know your audience. Don’t assume the audience is like you. To connect with them, you need to understand why your topic is important to them. What do they expect to learn from the presentation? Each attendee will be asking, “What’s in it for me?”
To keep listeners interested and engaged, you also must know their level of knowledge about the topic. If you are speaking to a group of residents about pelvic organ prolapse, you would use different language and content than if you were speaking to practicing primary care doctors; and these elements would be different again if you were speaking to a group of practicing urogynecologists. It’s insulting to recite basic information to highly knowledgeable physicians, or to present sophisticated technical content and complicated slides to novice physicians or lay people.
When presenting in a foreign country, learn how the culture of the audience differs from yours. How do they dress? What style of humor do they favor? How do they typically communicate? What gestures are appropriate or inappropriate? Are there religious influences to consider?
Practical steps. Before the meeting or event, speak to the organizer or meeting planner and find out the audience’s level of knowledge on the topic. Ask about audience expectations as well as demographics (such as age and background). If you are speaking at an industry event, research the event’s website and familiarize yourself with the mission of the event and who are the typical attendees. If you are presenting to a corporation, learn as much as you can about it by visiting its website, reading news reports, and reviewing associated blogs.
In addition to knowing the needs of the audience, ask the meeting planner about the goals and objectives for the program to make certain you can deliver on the requests.
Know your talk stem to stern
Review your slide material thoroughly. Understand each slide in the presentation and be comfortable with its content.
Avoid reading from slides. Reciting content that viewers can read for themselves breeds boredom and makes them lose interest. Further, when you are looking at the slides, you are not making eye contact with the audience and risk losing their attention. Good speakers are so comfortable with their slides that they can discuss each one without having to look at it.
Rehearse. The best way to achieve the foregoing is to rehearse. Your audience will be able to tell if you took the slide deck directly from a CD and loaded it into a computer and are giving the talk for the first time. You’ll need to know how long the program is to last and how long you are to speak. We suggest you practice with a timer to be certain you do not exceed the allotted time. Rehearse your talk aloud several times with all the props and audiovisual equipment you plan to use. This practice will help to curb filler words such as “ah” and “um.” It is also helpful to practice slide transitions, pauses, and even your breathing.
Prepare for the unexpected, too. Dinner meetings, for instance, may not start on time due to office or hospital delays for attending physicians, possibly resulting in a need to shorten your presentation.
Ask about the meeting agenda. If a meal is to be served, will you be speaking beforehand? This is the least favorable time slot, as you are holding people hostage before they can eat. Our preference is to speak after the appetizer is served and the orders have been taken by the wait staff. This way, attendees are not starving and they have something to drink. You can assure the waiters they won’t be disturbing you, and you can ask them to avoid walking in front of the projector. Ideally, you should end your presentation before dessert arrives and use the remaining time to field questions.
We suggest that you prepare a handout to be distributed at the end of the program, not before. You want your audience focused on you and your slides as you are speaking. Tell the audience you will be providing a handout of your presentation, which will minimize note-taking during your talk.
Your speech opening
The first and last 30 seconds of any speech probably have the most impact.1 Give extra thought, time, and effort to your opening and closing remarks. Do not open with “Good evening, it is a pleasure to be here tonight.” That wastes precious seconds.
While opening a speech with a joke or funny story is the conventional wisdom, ask yourself1:
- Is my selection appropriate to the occasion and for this audience?
- Is it in good taste?
- Does it relate to me (my service) or to the event or the group? Does it support my topic or its key points?
A humorous story or inspirational vignette that relates to your topic or audience can grab the audience’s attention. If you feel that demands more presentation skill than you possess at the outset of your public-speaking career, give the audience what you know and what they most want to hear. You know the questions that you have heard most at cocktail receptions or professional society meetings. So, put the answers to those questions in your speech.
For example. A scientist working with a major corporation was preparing a speech for a lay audience. Since most of the audience did not know what scientists do, he offered the following analogy: “Being a scientist is like doing a jigsaw puzzle in a snowstorm at night...you don’t have all the pieces...and you don’t have the picture on the front of the box to work from.” You can say more with less.1
Your closing
The closing is an important aspect of your speech. Summarize the key elements to your presentation. If you are going to take questions, a good approach is to say, “Before my closing remarks, are there any questions?” Following the questions, finish with a takeaway message that ties into your theme.1
Prepare an autobiographical introduction
We suggest that you write your own introduction and e-mail it to the person who will be introducing you. Let them know it is a suggestion that they are welcome to modify. We have found that most emcees or meeting planners are delighted to have the introduction and will use it just as you have written it. Also bring hard copy with you; many emcees will have forgotten to download what you sent. The figure shows an example of the introduction that one of us (NHB) uses, and you are welcome to modify it for your own use.
Ask about and confirm audiovisual support
Ask the meeting planner ahead of time if they will be providing the computer, projector, and screen. And if, for instance, they will provide a projector but not a computer, make sure the computer you will bring is compatible with their projector. Also, you will probably not require a microphone for a small group, but if you are speaking in a loud restaurant, a portable microphone-speaker system may be helpful.
Arrive early at the program venue to make sure the computers, projector, screen placement, and seating arrangement are all in order. Nothing can sidetrack a speaker (even a seasoned one) like a problem with the computer or equipment setup—for example, your flash drive requiring a USB port cannot connect to the sponsor’s computer, or your program created on a Mac does not run on the sponsor’s PC.
Show time: Getting ready
Another benefit in arriving early, besides being able to check on the equipment, is the chance to greet the audience members as they enter. It is easier to speak to a group of friends than strangers. And if you can remember their names, you can call on them and ask their opinion or how they might manage a patient who has the condition you are discussing. You also could suggest to the meeting planner that name tags for attendees would be helpful.
Warming up. A public speaker, like an athlete, needs to warm up physically before the event. If the facility has an anteroom available, use it for the following exercises suggested by public speaking coach Patricia Fripp1:
- Stand on one leg and shake the other (remove high heels first). When you place your raised foot back on the floor, it will feel lighter than the other one. Repeat the exercise using the other leg. Imagine your energy going down through the floor and up out of your head. While this sounds quite comical, it is not. It is a practical technique used by actors.
- Shake your hands vigorously. Hold them above your head, bending your wrists and elbows, then return your arms to your sides. This will make your hand movements more natural.
- Warm up your facial muscles by chewing in a highly exaggerated way. Do shoulder and neck rolls. Imagine you are at eye level with a clock. As you look at 12 o’clock, pull as much of your face up to the 12 as you can; move your eyes to 3 and repeat, then down to 6, and finally over to 9.
Not only do these exercises warm you up but they also relax you. The exaggerated movements help your movements to flow more naturally.1
This is just the start
Thorough preparation is key to making a solid presentation. But other factors are important too. Your goal is for the audience to take action or to implement suggestions from your presentation. In part 2 of this series, we will share tips on elements of the presentation itself that will encourage audience engagement and message retention. We will discuss how to make your message “stick” and how to make a dynamic, effective presentation that holds your audience’s attention for your entire talk.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
- Fripp P. Add credibility to your business reputation through public speaking. Patricia Fripp website. http://www.fripp.com/add-credibility-to-your-business-reputation-through-public-speaking/. Accessed June 15, 2016.
Reference
- Fripp P. Add credibility to your business reputation through public speaking. Patricia Fripp website. http://www.fripp.com/add-credibility-to-your-business-reputation-through-public-speaking/. Accessed June 15, 2016.
In this Article
- Preparing a presentation
- Your speech opening
- AV equipment and support
Using videos to educate your ObGyn patients
Patient barriers to optimal health-care outcomes are well documented. According to a 2003 estimate from the National Center for Education Statistics, 9 in 10 individuals do not know how to adequately access information readily available for their own health care.1 A December 7, 2013, report in Modern Healthcare stated, “When patients are in doctors’ offices, they (might) hear 50% of what’s being said and maybe their relative hears another 30%, but they walk away without 20%.”2
In addition, patients often do not fill or refill their prescriptions. More than 31% of about 37,000 prescriptions written in a primary care setting for nearly 16,000 patients were not filled.3 Reasons may be poor health literacy, a medication’s expense, or disappointment with lack of drug efficacy. In a 2010 Commonwealth Fund survey, 23.1% of US patients reported not filling a drug prescription in the previous 12 months due to cost,4 and in 2012, 27% did not follow through with recommended testing or treatment.5
On the physician side, the advent of managed care, electronic health records, and requirements to document extraneous information have shortened “face time” with patients. This means less time to educate patients about their conditions and treatments. And patients who have insufficient information may have trouble adhering with recommendations and experience unsatisfactory outcomes.
Using focused patient-education videos can help you circumvent in-office time constraints and inform patients of their conditions and your recommendations, thereby increasing practice efficiency and improving patient outcomes. There are certain considerations you should keep in mind when implementing and executing videos for patients.
Planning your video
With videos, you can convey to patients the exact message you want them to receive. This is far more effective and more appreciatedthan videos distributed by pharmaceutical companies and vendors of equipment used in your office or hospital. If you do not have the time to create patient videos, purchasing professionally created videos could be worth the cost; however, those created by physicians are far better and can be a source of enhanced communication when patients see their own physician on the screen discussing the condition, procedure, or medications prescribed.
We suggest selecting topics you regularly discuss with patients. If the topic of prolapse arises several times a day or week, a video presentation about it would be appropriate. Other topics of interest to gynecology patients are shown in the TABLE. The topics included are those that many of our colleagues find that they discuss with patients frequently and are in need of an instructional video.
Example video topics for patient viewing
• Evaluation of urinary incontinence
• Recurrent urinary tract infection
• Infertility evaluation
• Options for hysterectomy
• Management of menometrorrhagia
• Contraception options (including bilateral tubal ligation)
• Pros and cons of hormone replacement therapy
• Breast self examination
One of us (NB) likes to select topics that are receiving lots of publicity. For example, when flibanserin was approved by the US Food and Drug Administration in 2015 and patients were asking about it, we created a video with a handout that summarized the drug’s actions and its adverse effects and that emphasized the precaution about using flibanserin in conjunction with alcohol.
Production elements
The script.
- Define the problem/condition
- Offer how the problem is evaluated
- Discuss treatment options
- Go over risks and complications
- Include a summary.
Embedding details of these bullet points into a PowerPoint presentation can serve as your teleprompter. Each video might end with the statement, “I hope you have found this video on <name of topic> informative. If you open the door at the end of the video, I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have.” We refer to this as the “sandwich technique,” in which the physician interacts with the patient first and performs the examination, invites the patient to watch the video, and returns to the room to conclude the patient visit.
The recording device. Recording can be accomplished easily with technology available in nearly every ObGyn office. You can use a video camera, the webcam on your computer, or a smart phone (probably the easiest choice). The quality of video created with the Apple, Samsung, or Motorola devices is excellent. The only other piece of equipment we recommend is a flexible tripod to hold the phone. Several such tripod stands are available for purchase, but the type with a flexible stand can be beneficial (FIGURE 1). These are available for purchase on Amazon.
| FIGURE 1 Our recommended tripod stand | ||
| ||
The TriFlex Mini Phone Tripod Stand, available for purchase at retailers and at Amazon (http://www.amazon.com/dp/B017NA7V1U?psc=1). | ||
Putting it all together. With the smartphone in the tripod attached to the computer and the PowerPoint program serving as your notes, you are ready to create a video. We suggest limiting the recording to 5 to 7 minutes, the attention span of most patients. Those who want to produce a more professional looking video can use the editing programs iMovie on the Mac or Movie Maker on the PC.
Videos can be uploaded to your website, your EMR, or onto separate computers in each of your examination rooms. Depending on where you upload your videos (your own website or YouTube), patients can access them from home. An advantage of your own website and YouTube is that the videos can be viewed again and by patients’ significant others (which patients often inquire about the ability to do).
Other considerations
Videos that are conversational in nature, using the pronouns “I” and “we” and using such language as “my opinion” and “our patients” may hold the attention of viewers more than didactic “talking head” videos. In addition, creating videos on controversial topics that patients are interested in and need more information about can benefit patients and your practice.
Creating videos in other languages for your patients is an option as well. If you speak the language, then create your video in both English and the other language. Or you can create the script and ask a patient who speaks the non-English language to assist with the video production or voiceover. Also, there are other language videos for patients on YouTube. An excellent example of a Spanish-language gynecologic video on the pelvic examination is available (https://www.youtube.com/watch?v=IKsGYc-dCSI). It is easy to create a link from your website to a YouTube video. This requires requesting permission from the creator of the video. (We do not recommend showcasing another physician on your website.)
Example Patient education videos
Examples of videos on stress urinary incontinence and treatment with a midurethral sling can be viewed at: https://www.youtube.com/watch?v=BFZj8x3-oCA and https://www.youtube.com/watch?v=-gnOqkXiye0.
Dr. Neil Baum is the author of Social Media for the Healthcare Professional (Greenbranch Publishing, 2012).
Advantages of creating videos
When patients are watching the video, you can conduct visits with other patients and even perform brief office procedures. You can anticipate an up to 15% to 20% improvement in office efficiency by using educational videos. And patients will appreciate the information and the written summary accompanying each video.
Videos and medical-legal protection
Documentation is necessary to protect yourself from litigation. Record the viewing of a video in a patient’s chart, as well as the receipt of pertinent written information. We suggest you also note that all of the patient’s questions were answered before the patient left the office. To confirm that the patient understood the condition, procedure, or surgery, you can ask the patient to fill out a true/false questionnaire after watching the video and also include it in the chart. A questionnaire I (NB) use after the patient watches a video on stress incontinence is shown in FIGURE 2.
A statement to accompany the questionnaire is also a good idea. Example: “<name of patient> watched a video on the treatment of stress incontinence. The video discussed the procedure and its risks and complications, and alternate treatments, including the option to have no treatment. She agrees to proceeding with a midurethral sling using synthetic mesh and understands the risks and complications associated with the use of mesh.”
An additional helpful option is to end your videos with a comment that addresses the statement and consent form you will ask the patient to sign. For instance, “I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have. I also will ask you to sign a procedure or operative consent form as well as sign a statement that says you have watched the video, understand the content, and have had your questions answered.”
We believe that this makes the video an excellent medical-legal protection tool for the physician and that the video enhances the informed consent process.
Bottom line
We are challenged today to provide quality care in an efficient and cost-effective manner. This is a concern for every ObGyn practice regardless of its size or location or whether it is a solo or group practice or academic or private. We can improve our efficiency and our productivity, maintain quality of care, improve patient adherence, and even improve outcomes using patient videos. So get ready for lights, camera, and action!
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, US Dept of Education, 2006.
- 1NCES publication 2006-483.2. Modern Healthcare. Providers help patients address emotion, money, health literacy. Available at: http://www.modernhealthcare.com/article/20131207/MAGAZINE/312079983. Accessed April 15, 2016.
- Tamblyn R, Eguale T, Huang A, Winsdale N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Issue Brief (Commonw Fund). 2010;89:1012.
- Collins SR, Robertson R, Garber T, et al. Insuring the future. Current trends in health coverage and the effects of implementing the Affordable Care Act. Available at: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2013/Apr/1681_Collins_insuring_future_biennial_survey_2012_FINAL.pdf. Accessed April 15, 2016.
Patient barriers to optimal health-care outcomes are well documented. According to a 2003 estimate from the National Center for Education Statistics, 9 in 10 individuals do not know how to adequately access information readily available for their own health care.1 A December 7, 2013, report in Modern Healthcare stated, “When patients are in doctors’ offices, they (might) hear 50% of what’s being said and maybe their relative hears another 30%, but they walk away without 20%.”2
In addition, patients often do not fill or refill their prescriptions. More than 31% of about 37,000 prescriptions written in a primary care setting for nearly 16,000 patients were not filled.3 Reasons may be poor health literacy, a medication’s expense, or disappointment with lack of drug efficacy. In a 2010 Commonwealth Fund survey, 23.1% of US patients reported not filling a drug prescription in the previous 12 months due to cost,4 and in 2012, 27% did not follow through with recommended testing or treatment.5
On the physician side, the advent of managed care, electronic health records, and requirements to document extraneous information have shortened “face time” with patients. This means less time to educate patients about their conditions and treatments. And patients who have insufficient information may have trouble adhering with recommendations and experience unsatisfactory outcomes.
Using focused patient-education videos can help you circumvent in-office time constraints and inform patients of their conditions and your recommendations, thereby increasing practice efficiency and improving patient outcomes. There are certain considerations you should keep in mind when implementing and executing videos for patients.
Planning your video
With videos, you can convey to patients the exact message you want them to receive. This is far more effective and more appreciatedthan videos distributed by pharmaceutical companies and vendors of equipment used in your office or hospital. If you do not have the time to create patient videos, purchasing professionally created videos could be worth the cost; however, those created by physicians are far better and can be a source of enhanced communication when patients see their own physician on the screen discussing the condition, procedure, or medications prescribed.
We suggest selecting topics you regularly discuss with patients. If the topic of prolapse arises several times a day or week, a video presentation about it would be appropriate. Other topics of interest to gynecology patients are shown in the TABLE. The topics included are those that many of our colleagues find that they discuss with patients frequently and are in need of an instructional video.
Example video topics for patient viewing
• Evaluation of urinary incontinence
• Recurrent urinary tract infection
• Infertility evaluation
• Options for hysterectomy
• Management of menometrorrhagia
• Contraception options (including bilateral tubal ligation)
• Pros and cons of hormone replacement therapy
• Breast self examination
One of us (NB) likes to select topics that are receiving lots of publicity. For example, when flibanserin was approved by the US Food and Drug Administration in 2015 and patients were asking about it, we created a video with a handout that summarized the drug’s actions and its adverse effects and that emphasized the precaution about using flibanserin in conjunction with alcohol.
Production elements
The script.
- Define the problem/condition
- Offer how the problem is evaluated
- Discuss treatment options
- Go over risks and complications
- Include a summary.
Embedding details of these bullet points into a PowerPoint presentation can serve as your teleprompter. Each video might end with the statement, “I hope you have found this video on <name of topic> informative. If you open the door at the end of the video, I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have.” We refer to this as the “sandwich technique,” in which the physician interacts with the patient first and performs the examination, invites the patient to watch the video, and returns to the room to conclude the patient visit.
The recording device. Recording can be accomplished easily with technology available in nearly every ObGyn office. You can use a video camera, the webcam on your computer, or a smart phone (probably the easiest choice). The quality of video created with the Apple, Samsung, or Motorola devices is excellent. The only other piece of equipment we recommend is a flexible tripod to hold the phone. Several such tripod stands are available for purchase, but the type with a flexible stand can be beneficial (FIGURE 1). These are available for purchase on Amazon.
| FIGURE 1 Our recommended tripod stand | ||
| ||
The TriFlex Mini Phone Tripod Stand, available for purchase at retailers and at Amazon (http://www.amazon.com/dp/B017NA7V1U?psc=1). | ||
Putting it all together. With the smartphone in the tripod attached to the computer and the PowerPoint program serving as your notes, you are ready to create a video. We suggest limiting the recording to 5 to 7 minutes, the attention span of most patients. Those who want to produce a more professional looking video can use the editing programs iMovie on the Mac or Movie Maker on the PC.
Videos can be uploaded to your website, your EMR, or onto separate computers in each of your examination rooms. Depending on where you upload your videos (your own website or YouTube), patients can access them from home. An advantage of your own website and YouTube is that the videos can be viewed again and by patients’ significant others (which patients often inquire about the ability to do).
Other considerations
Videos that are conversational in nature, using the pronouns “I” and “we” and using such language as “my opinion” and “our patients” may hold the attention of viewers more than didactic “talking head” videos. In addition, creating videos on controversial topics that patients are interested in and need more information about can benefit patients and your practice.
Creating videos in other languages for your patients is an option as well. If you speak the language, then create your video in both English and the other language. Or you can create the script and ask a patient who speaks the non-English language to assist with the video production or voiceover. Also, there are other language videos for patients on YouTube. An excellent example of a Spanish-language gynecologic video on the pelvic examination is available (https://www.youtube.com/watch?v=IKsGYc-dCSI). It is easy to create a link from your website to a YouTube video. This requires requesting permission from the creator of the video. (We do not recommend showcasing another physician on your website.)
Example Patient education videos
Examples of videos on stress urinary incontinence and treatment with a midurethral sling can be viewed at: https://www.youtube.com/watch?v=BFZj8x3-oCA and https://www.youtube.com/watch?v=-gnOqkXiye0.
Dr. Neil Baum is the author of Social Media for the Healthcare Professional (Greenbranch Publishing, 2012).
Advantages of creating videos
When patients are watching the video, you can conduct visits with other patients and even perform brief office procedures. You can anticipate an up to 15% to 20% improvement in office efficiency by using educational videos. And patients will appreciate the information and the written summary accompanying each video.
Videos and medical-legal protection
Documentation is necessary to protect yourself from litigation. Record the viewing of a video in a patient’s chart, as well as the receipt of pertinent written information. We suggest you also note that all of the patient’s questions were answered before the patient left the office. To confirm that the patient understood the condition, procedure, or surgery, you can ask the patient to fill out a true/false questionnaire after watching the video and also include it in the chart. A questionnaire I (NB) use after the patient watches a video on stress incontinence is shown in FIGURE 2.
A statement to accompany the questionnaire is also a good idea. Example: “<name of patient> watched a video on the treatment of stress incontinence. The video discussed the procedure and its risks and complications, and alternate treatments, including the option to have no treatment. She agrees to proceeding with a midurethral sling using synthetic mesh and understands the risks and complications associated with the use of mesh.”
An additional helpful option is to end your videos with a comment that addresses the statement and consent form you will ask the patient to sign. For instance, “I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have. I also will ask you to sign a procedure or operative consent form as well as sign a statement that says you have watched the video, understand the content, and have had your questions answered.”
We believe that this makes the video an excellent medical-legal protection tool for the physician and that the video enhances the informed consent process.
Bottom line
We are challenged today to provide quality care in an efficient and cost-effective manner. This is a concern for every ObGyn practice regardless of its size or location or whether it is a solo or group practice or academic or private. We can improve our efficiency and our productivity, maintain quality of care, improve patient adherence, and even improve outcomes using patient videos. So get ready for lights, camera, and action!
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Patient barriers to optimal health-care outcomes are well documented. According to a 2003 estimate from the National Center for Education Statistics, 9 in 10 individuals do not know how to adequately access information readily available for their own health care.1 A December 7, 2013, report in Modern Healthcare stated, “When patients are in doctors’ offices, they (might) hear 50% of what’s being said and maybe their relative hears another 30%, but they walk away without 20%.”2
In addition, patients often do not fill or refill their prescriptions. More than 31% of about 37,000 prescriptions written in a primary care setting for nearly 16,000 patients were not filled.3 Reasons may be poor health literacy, a medication’s expense, or disappointment with lack of drug efficacy. In a 2010 Commonwealth Fund survey, 23.1% of US patients reported not filling a drug prescription in the previous 12 months due to cost,4 and in 2012, 27% did not follow through with recommended testing or treatment.5
On the physician side, the advent of managed care, electronic health records, and requirements to document extraneous information have shortened “face time” with patients. This means less time to educate patients about their conditions and treatments. And patients who have insufficient information may have trouble adhering with recommendations and experience unsatisfactory outcomes.
Using focused patient-education videos can help you circumvent in-office time constraints and inform patients of their conditions and your recommendations, thereby increasing practice efficiency and improving patient outcomes. There are certain considerations you should keep in mind when implementing and executing videos for patients.
Planning your video
With videos, you can convey to patients the exact message you want them to receive. This is far more effective and more appreciatedthan videos distributed by pharmaceutical companies and vendors of equipment used in your office or hospital. If you do not have the time to create patient videos, purchasing professionally created videos could be worth the cost; however, those created by physicians are far better and can be a source of enhanced communication when patients see their own physician on the screen discussing the condition, procedure, or medications prescribed.
We suggest selecting topics you regularly discuss with patients. If the topic of prolapse arises several times a day or week, a video presentation about it would be appropriate. Other topics of interest to gynecology patients are shown in the TABLE. The topics included are those that many of our colleagues find that they discuss with patients frequently and are in need of an instructional video.
Example video topics for patient viewing
• Evaluation of urinary incontinence
• Recurrent urinary tract infection
• Infertility evaluation
• Options for hysterectomy
• Management of menometrorrhagia
• Contraception options (including bilateral tubal ligation)
• Pros and cons of hormone replacement therapy
• Breast self examination
One of us (NB) likes to select topics that are receiving lots of publicity. For example, when flibanserin was approved by the US Food and Drug Administration in 2015 and patients were asking about it, we created a video with a handout that summarized the drug’s actions and its adverse effects and that emphasized the precaution about using flibanserin in conjunction with alcohol.
Production elements
The script.
- Define the problem/condition
- Offer how the problem is evaluated
- Discuss treatment options
- Go over risks and complications
- Include a summary.
Embedding details of these bullet points into a PowerPoint presentation can serve as your teleprompter. Each video might end with the statement, “I hope you have found this video on <name of topic> informative. If you open the door at the end of the video, I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have.” We refer to this as the “sandwich technique,” in which the physician interacts with the patient first and performs the examination, invites the patient to watch the video, and returns to the room to conclude the patient visit.
The recording device. Recording can be accomplished easily with technology available in nearly every ObGyn office. You can use a video camera, the webcam on your computer, or a smart phone (probably the easiest choice). The quality of video created with the Apple, Samsung, or Motorola devices is excellent. The only other piece of equipment we recommend is a flexible tripod to hold the phone. Several such tripod stands are available for purchase, but the type with a flexible stand can be beneficial (FIGURE 1). These are available for purchase on Amazon.
| FIGURE 1 Our recommended tripod stand | ||
| ||
The TriFlex Mini Phone Tripod Stand, available for purchase at retailers and at Amazon (http://www.amazon.com/dp/B017NA7V1U?psc=1). | ||
Putting it all together. With the smartphone in the tripod attached to the computer and the PowerPoint program serving as your notes, you are ready to create a video. We suggest limiting the recording to 5 to 7 minutes, the attention span of most patients. Those who want to produce a more professional looking video can use the editing programs iMovie on the Mac or Movie Maker on the PC.
Videos can be uploaded to your website, your EMR, or onto separate computers in each of your examination rooms. Depending on where you upload your videos (your own website or YouTube), patients can access them from home. An advantage of your own website and YouTube is that the videos can be viewed again and by patients’ significant others (which patients often inquire about the ability to do).
Other considerations
Videos that are conversational in nature, using the pronouns “I” and “we” and using such language as “my opinion” and “our patients” may hold the attention of viewers more than didactic “talking head” videos. In addition, creating videos on controversial topics that patients are interested in and need more information about can benefit patients and your practice.
Creating videos in other languages for your patients is an option as well. If you speak the language, then create your video in both English and the other language. Or you can create the script and ask a patient who speaks the non-English language to assist with the video production or voiceover. Also, there are other language videos for patients on YouTube. An excellent example of a Spanish-language gynecologic video on the pelvic examination is available (https://www.youtube.com/watch?v=IKsGYc-dCSI). It is easy to create a link from your website to a YouTube video. This requires requesting permission from the creator of the video. (We do not recommend showcasing another physician on your website.)
Example Patient education videos
Examples of videos on stress urinary incontinence and treatment with a midurethral sling can be viewed at: https://www.youtube.com/watch?v=BFZj8x3-oCA and https://www.youtube.com/watch?v=-gnOqkXiye0.
Dr. Neil Baum is the author of Social Media for the Healthcare Professional (Greenbranch Publishing, 2012).
Advantages of creating videos
When patients are watching the video, you can conduct visits with other patients and even perform brief office procedures. You can anticipate an up to 15% to 20% improvement in office efficiency by using educational videos. And patients will appreciate the information and the written summary accompanying each video.
Videos and medical-legal protection
Documentation is necessary to protect yourself from litigation. Record the viewing of a video in a patient’s chart, as well as the receipt of pertinent written information. We suggest you also note that all of the patient’s questions were answered before the patient left the office. To confirm that the patient understood the condition, procedure, or surgery, you can ask the patient to fill out a true/false questionnaire after watching the video and also include it in the chart. A questionnaire I (NB) use after the patient watches a video on stress incontinence is shown in FIGURE 2.
A statement to accompany the questionnaire is also a good idea. Example: “<name of patient> watched a video on the treatment of stress incontinence. The video discussed the procedure and its risks and complications, and alternate treatments, including the option to have no treatment. She agrees to proceeding with a midurethral sling using synthetic mesh and understands the risks and complications associated with the use of mesh.”
An additional helpful option is to end your videos with a comment that addresses the statement and consent form you will ask the patient to sign. For instance, “I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have. I also will ask you to sign a procedure or operative consent form as well as sign a statement that says you have watched the video, understand the content, and have had your questions answered.”
We believe that this makes the video an excellent medical-legal protection tool for the physician and that the video enhances the informed consent process.
Bottom line
We are challenged today to provide quality care in an efficient and cost-effective manner. This is a concern for every ObGyn practice regardless of its size or location or whether it is a solo or group practice or academic or private. We can improve our efficiency and our productivity, maintain quality of care, improve patient adherence, and even improve outcomes using patient videos. So get ready for lights, camera, and action!
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, US Dept of Education, 2006.
- 1NCES publication 2006-483.2. Modern Healthcare. Providers help patients address emotion, money, health literacy. Available at: http://www.modernhealthcare.com/article/20131207/MAGAZINE/312079983. Accessed April 15, 2016.
- Tamblyn R, Eguale T, Huang A, Winsdale N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Issue Brief (Commonw Fund). 2010;89:1012.
- Collins SR, Robertson R, Garber T, et al. Insuring the future. Current trends in health coverage and the effects of implementing the Affordable Care Act. Available at: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2013/Apr/1681_Collins_insuring_future_biennial_survey_2012_FINAL.pdf. Accessed April 15, 2016.
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, US Dept of Education, 2006.
- 1NCES publication 2006-483.2. Modern Healthcare. Providers help patients address emotion, money, health literacy. Available at: http://www.modernhealthcare.com/article/20131207/MAGAZINE/312079983. Accessed April 15, 2016.
- Tamblyn R, Eguale T, Huang A, Winsdale N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Issue Brief (Commonw Fund). 2010;89:1012.
- Collins SR, Robertson R, Garber T, et al. Insuring the future. Current trends in health coverage and the effects of implementing the Affordable Care Act. Available at: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2013/Apr/1681_Collins_insuring_future_biennial_survey_2012_FINAL.pdf. Accessed April 15, 2016.
In this article
• Videos and medical-legal protection
• Patient questionnaire post-video viewing
Fecal incontinence: New therapies, age-old problem
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
ROUNDTABLE PART 2 OF 2: Using mesh to repair prolapse: Averting, managing complications
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.
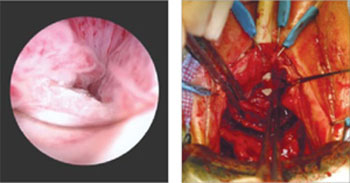
FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.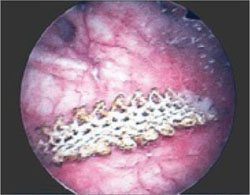
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.

FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.

FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
ROUNDTABLE: PART 1 OF 2: Using mesh to repair prolapse calls for more than a kit—it takes skill
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.
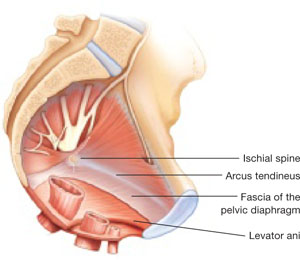
FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 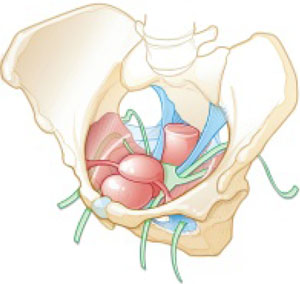
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.
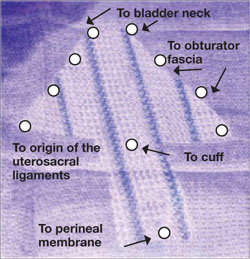
FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
Repair of a constricted or shortened vagina: What works?
To watch a demonstration of the surgical treatment of vaginal stenosis using bilateral groin flaps and a demonstration of the takedown of iatrogenic vaginal constriction, visit the Video Library.
Patients who want to be sexually active but suffer iatrogenic vaginal constriction or shortening, or both, are a surgical challenge. Their condition may require any of a variety of nonsurgical and surgical procedures to restore the ability to have gratifying sexual intercourse, and they may need considerable preoperative and postoperative counseling and management.
What is the basis of this problem?
The cause of vaginal shortening or constriction is most often surgical. Rarely is systemic disease or a localized condition, such as urogenital atrophy, responsible.
Prolapse procedures. Most procedures that result in vaginal shortening or constriction are ones performed to correct pelvic organ prolapse (POP), notably:
- posterior colpoperineorrhaphy with levatorplasty
- hysterectomy, whether abdominal or vaginal, during which too much of the upper vagina is taken with the cervix
- anterior and posterior colporrhaphy in which vaginal plication and trimming are performed overzealously.
Radiation therapy to the pelvis can result in vaginal shortening, constriction, and obliteration.
How do you avoid creating these problems?
Techniques to avoid vaginal shortening and constriction during vaginal reconstructive surgery include appropriate use of levatorplasty during posterior colpoperineorrhaphy. Although levatorplasty is, at times, the only way to decrease the size of a large vaginal hiatus, it should be used only in the distal third of the vagina. Levatorplasty above this area often creates vaginal constriction that results in postoperative dyspareunia.
Also, avoid 1) overzealous trimming during anterior and posterior colporrhaphy and 2) removing too much vagina at vaginal or abdominal hysterectomy.
Last, it is important that a patient who has undergone vaginal reconstructive surgery have a vaginal exam within 2 weeks after surgery. This will ensure that the vaginal incisions do not fuse, thus creating vaginal scarring, closure, and constriction.
How is correction approached?
Various modifications of a McIndoe procedure have been described for vaginal agenesis, but surgical correction of iatrogenic vaginal shortening or constriction is not well described; few case series exist in the literature. Consensus is lacking on what the minimal length of a vagina must be to preserve normal sexual function, and no standard exists in regards to either normal vaginal caliber or the relationship of the perineum and perineal body to the distal posterior vagina.
That being said, we have recognized the following correlates of a successful return to sexual function after surgery for vaginal constriction or shortening:
- The vagina should, at minimum, be 7 cm long to have the potential for normal function
- The vaginal opening should easily admit two fingers during examination
- The relationship of the posterior vagina and the perineum should be a perpendicular one, in which a built-up perineum attaches to the posterior vagina at the posterior fourchette at a 90° angle
- There should be no buildup of perineal skin above and beyond the posterior fourchette.
Is surgery the first intervention?
No. The patient should first undergo an attempt at nonsurgical management. This usually involves:
- vaginal estrogen cream in a postmenopausal patient
- appropriate utilization of a vaginal dilator.
What are options for surgery?
If nonsurgical management of vaginal constriction or shortening is unsuccessful or unsatisfactory, next choose an operation based on the needs of the individual patient. Some procedures involve placement of a skin graft or, possibly, other biologic material in the vagina to close over defects after constriction has been taken down or the vagina has been appropriately opened up. (It is fortunate that the vagina heals well by secondary intention; often, simply taking down the constriction and allowing the vagina to heal by secondary intention is successful.)
It is important to cut through the constriction and completely separate the tissue during the takedown of vaginal constriction. At this point, you need to decide whether to allow the separated vagina to heal by secondary intention or to cover the defect with a skin graft or other biologic material.
Whichever course you choose, keep the vagina open during the immediate postoperative period. Doing so may require placement of a vaginal stent, numerous postoperative exams, use of a vaginal dilator, or a combination of these measures.
When constriction rings are present in the face of ample vaginal length, you can perform a Z-plasty, in which the lines of a letter Z are incised transversely or longitudinally across the constricted region and the two flaps that have been created from the Z are transposed. This maneuver releases constriction well.
When constriction extends distally, the procedure used is, basically, a reverse perineoplasty: Cut the constriction band longitudinally, undermine the vagina, and then sew it back transversely. This relieves the distal band.
In a severe case of vaginal constriction, thigh flaps that are left on their vascular pedicle can be brought into the vagina to fill the gap created by cutting through the constriction. Initial incisions are made laterally in the vagina (unilaterally or bilaterally, depending on the degree of constriction) and extended to the perineum/vulva. Measurements are made to determine the length and width of flap(s) needed. The flaps are then mobilized, rotated into the defect(s), and sutured into place. This technique significantly increases the diameter of the vagina and can add length, if needed.
What about correcting shortening?
An iatrogenically shortened vagina presents the most challenging of cases. The vagina must be opened up at the cuff; ideally, this produces adequate length without having to enter the peritoneum.
Dr. Karram is a course director and Dr. Gebhart is on the faculty of the 10th Annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS), to be held December 6-8, 2007, in Las Vegas (www.pags-cme.org).
To watch a demonstration of the surgical treatment of vaginal stenosis using bilateral groin flaps and a demonstration of the takedown of iatrogenic vaginal constriction, visit the Video Library.
Patients who want to be sexually active but suffer iatrogenic vaginal constriction or shortening, or both, are a surgical challenge. Their condition may require any of a variety of nonsurgical and surgical procedures to restore the ability to have gratifying sexual intercourse, and they may need considerable preoperative and postoperative counseling and management.
What is the basis of this problem?
The cause of vaginal shortening or constriction is most often surgical. Rarely is systemic disease or a localized condition, such as urogenital atrophy, responsible.
Prolapse procedures. Most procedures that result in vaginal shortening or constriction are ones performed to correct pelvic organ prolapse (POP), notably:
- posterior colpoperineorrhaphy with levatorplasty
- hysterectomy, whether abdominal or vaginal, during which too much of the upper vagina is taken with the cervix
- anterior and posterior colporrhaphy in which vaginal plication and trimming are performed overzealously.
Radiation therapy to the pelvis can result in vaginal shortening, constriction, and obliteration.
How do you avoid creating these problems?
Techniques to avoid vaginal shortening and constriction during vaginal reconstructive surgery include appropriate use of levatorplasty during posterior colpoperineorrhaphy. Although levatorplasty is, at times, the only way to decrease the size of a large vaginal hiatus, it should be used only in the distal third of the vagina. Levatorplasty above this area often creates vaginal constriction that results in postoperative dyspareunia.
Also, avoid 1) overzealous trimming during anterior and posterior colporrhaphy and 2) removing too much vagina at vaginal or abdominal hysterectomy.
Last, it is important that a patient who has undergone vaginal reconstructive surgery have a vaginal exam within 2 weeks after surgery. This will ensure that the vaginal incisions do not fuse, thus creating vaginal scarring, closure, and constriction.
How is correction approached?
Various modifications of a McIndoe procedure have been described for vaginal agenesis, but surgical correction of iatrogenic vaginal shortening or constriction is not well described; few case series exist in the literature. Consensus is lacking on what the minimal length of a vagina must be to preserve normal sexual function, and no standard exists in regards to either normal vaginal caliber or the relationship of the perineum and perineal body to the distal posterior vagina.
That being said, we have recognized the following correlates of a successful return to sexual function after surgery for vaginal constriction or shortening:
- The vagina should, at minimum, be 7 cm long to have the potential for normal function
- The vaginal opening should easily admit two fingers during examination
- The relationship of the posterior vagina and the perineum should be a perpendicular one, in which a built-up perineum attaches to the posterior vagina at the posterior fourchette at a 90° angle
- There should be no buildup of perineal skin above and beyond the posterior fourchette.
Is surgery the first intervention?
No. The patient should first undergo an attempt at nonsurgical management. This usually involves:
- vaginal estrogen cream in a postmenopausal patient
- appropriate utilization of a vaginal dilator.
What are options for surgery?
If nonsurgical management of vaginal constriction or shortening is unsuccessful or unsatisfactory, next choose an operation based on the needs of the individual patient. Some procedures involve placement of a skin graft or, possibly, other biologic material in the vagina to close over defects after constriction has been taken down or the vagina has been appropriately opened up. (It is fortunate that the vagina heals well by secondary intention; often, simply taking down the constriction and allowing the vagina to heal by secondary intention is successful.)
It is important to cut through the constriction and completely separate the tissue during the takedown of vaginal constriction. At this point, you need to decide whether to allow the separated vagina to heal by secondary intention or to cover the defect with a skin graft or other biologic material.
Whichever course you choose, keep the vagina open during the immediate postoperative period. Doing so may require placement of a vaginal stent, numerous postoperative exams, use of a vaginal dilator, or a combination of these measures.
When constriction rings are present in the face of ample vaginal length, you can perform a Z-plasty, in which the lines of a letter Z are incised transversely or longitudinally across the constricted region and the two flaps that have been created from the Z are transposed. This maneuver releases constriction well.
When constriction extends distally, the procedure used is, basically, a reverse perineoplasty: Cut the constriction band longitudinally, undermine the vagina, and then sew it back transversely. This relieves the distal band.
In a severe case of vaginal constriction, thigh flaps that are left on their vascular pedicle can be brought into the vagina to fill the gap created by cutting through the constriction. Initial incisions are made laterally in the vagina (unilaterally or bilaterally, depending on the degree of constriction) and extended to the perineum/vulva. Measurements are made to determine the length and width of flap(s) needed. The flaps are then mobilized, rotated into the defect(s), and sutured into place. This technique significantly increases the diameter of the vagina and can add length, if needed.
What about correcting shortening?
An iatrogenically shortened vagina presents the most challenging of cases. The vagina must be opened up at the cuff; ideally, this produces adequate length without having to enter the peritoneum.
To watch a demonstration of the surgical treatment of vaginal stenosis using bilateral groin flaps and a demonstration of the takedown of iatrogenic vaginal constriction, visit the Video Library.
Patients who want to be sexually active but suffer iatrogenic vaginal constriction or shortening, or both, are a surgical challenge. Their condition may require any of a variety of nonsurgical and surgical procedures to restore the ability to have gratifying sexual intercourse, and they may need considerable preoperative and postoperative counseling and management.
What is the basis of this problem?
The cause of vaginal shortening or constriction is most often surgical. Rarely is systemic disease or a localized condition, such as urogenital atrophy, responsible.
Prolapse procedures. Most procedures that result in vaginal shortening or constriction are ones performed to correct pelvic organ prolapse (POP), notably:
- posterior colpoperineorrhaphy with levatorplasty
- hysterectomy, whether abdominal or vaginal, during which too much of the upper vagina is taken with the cervix
- anterior and posterior colporrhaphy in which vaginal plication and trimming are performed overzealously.
Radiation therapy to the pelvis can result in vaginal shortening, constriction, and obliteration.
How do you avoid creating these problems?
Techniques to avoid vaginal shortening and constriction during vaginal reconstructive surgery include appropriate use of levatorplasty during posterior colpoperineorrhaphy. Although levatorplasty is, at times, the only way to decrease the size of a large vaginal hiatus, it should be used only in the distal third of the vagina. Levatorplasty above this area often creates vaginal constriction that results in postoperative dyspareunia.
Also, avoid 1) overzealous trimming during anterior and posterior colporrhaphy and 2) removing too much vagina at vaginal or abdominal hysterectomy.
Last, it is important that a patient who has undergone vaginal reconstructive surgery have a vaginal exam within 2 weeks after surgery. This will ensure that the vaginal incisions do not fuse, thus creating vaginal scarring, closure, and constriction.
How is correction approached?
Various modifications of a McIndoe procedure have been described for vaginal agenesis, but surgical correction of iatrogenic vaginal shortening or constriction is not well described; few case series exist in the literature. Consensus is lacking on what the minimal length of a vagina must be to preserve normal sexual function, and no standard exists in regards to either normal vaginal caliber or the relationship of the perineum and perineal body to the distal posterior vagina.
That being said, we have recognized the following correlates of a successful return to sexual function after surgery for vaginal constriction or shortening:
- The vagina should, at minimum, be 7 cm long to have the potential for normal function
- The vaginal opening should easily admit two fingers during examination
- The relationship of the posterior vagina and the perineum should be a perpendicular one, in which a built-up perineum attaches to the posterior vagina at the posterior fourchette at a 90° angle
- There should be no buildup of perineal skin above and beyond the posterior fourchette.
Is surgery the first intervention?
No. The patient should first undergo an attempt at nonsurgical management. This usually involves:
- vaginal estrogen cream in a postmenopausal patient
- appropriate utilization of a vaginal dilator.
What are options for surgery?
If nonsurgical management of vaginal constriction or shortening is unsuccessful or unsatisfactory, next choose an operation based on the needs of the individual patient. Some procedures involve placement of a skin graft or, possibly, other biologic material in the vagina to close over defects after constriction has been taken down or the vagina has been appropriately opened up. (It is fortunate that the vagina heals well by secondary intention; often, simply taking down the constriction and allowing the vagina to heal by secondary intention is successful.)
It is important to cut through the constriction and completely separate the tissue during the takedown of vaginal constriction. At this point, you need to decide whether to allow the separated vagina to heal by secondary intention or to cover the defect with a skin graft or other biologic material.
Whichever course you choose, keep the vagina open during the immediate postoperative period. Doing so may require placement of a vaginal stent, numerous postoperative exams, use of a vaginal dilator, or a combination of these measures.
When constriction rings are present in the face of ample vaginal length, you can perform a Z-plasty, in which the lines of a letter Z are incised transversely or longitudinally across the constricted region and the two flaps that have been created from the Z are transposed. This maneuver releases constriction well.
When constriction extends distally, the procedure used is, basically, a reverse perineoplasty: Cut the constriction band longitudinally, undermine the vagina, and then sew it back transversely. This relieves the distal band.
In a severe case of vaginal constriction, thigh flaps that are left on their vascular pedicle can be brought into the vagina to fill the gap created by cutting through the constriction. Initial incisions are made laterally in the vagina (unilaterally or bilaterally, depending on the degree of constriction) and extended to the perineum/vulva. Measurements are made to determine the length and width of flap(s) needed. The flaps are then mobilized, rotated into the defect(s), and sutured into place. This technique significantly increases the diameter of the vagina and can add length, if needed.
What about correcting shortening?
An iatrogenically shortened vagina presents the most challenging of cases. The vagina must be opened up at the cuff; ideally, this produces adequate length without having to enter the peritoneum.
Dr. Karram is a course director and Dr. Gebhart is on the faculty of the 10th Annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS), to be held December 6-8, 2007, in Las Vegas (www.pags-cme.org).
Dr. Karram is a course director and Dr. Gebhart is on the faculty of the 10th Annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS), to be held December 6-8, 2007, in Las Vegas (www.pags-cme.org).
Treating the range of lower-tract symptoms in prolapse
To watch a demonstration of how pelvic organ prolapse is repaired, visit the Video Library.
Lower urinary tract symptoms are common in women who have pelvic organ prolapse (POP). For some, these symptoms resolve or improve after surgery for prolapse; for others, symptoms remain unchanged or become worse. These clinical pearls can help you decide how to counsel, evaluate, and treat patients who have POP and coexisting lower-tract symptoms.
How are POP and lower-tract symptoms related?
Lower-tract symptoms that result from, or coexist with, POP include urinary incontinence (stress, urge, mixed), irritative symptoms (frequency, urgency, nocturia), and difficulty voiding (hesitancy, weak or intermittent stream). Prolapse can produce lower-tract symptoms by:
- causing urethral obstruction
- dissipating the effects of abdominal pressure during Valsalva voiding, which makes voiding more difficult
- masking sphincteric incontinence.
Is prolapse causing symptoms or masking stress incontinence?
Some clues to answering this question can be obtained from the history:
- If the patient says that she voids better when the prolapse is reduced, prolapse is probably causing urethral obstruction
- If the patient says that she experienced stress incontinence previously but that it has subsided and she now only has difficulty voiding, she probably has occult stress incontinence and, possibly, urethral obstruction.
How is treatment established for lower-tract symptoms?
Prolapse is graded by any of several classifications that are based on the severity and extent of the condition.
Mild degrees of prolapse rarely, if ever, cause urethral obstruction or mask stress incontinence; you can manage lower-tract symptoms in these patients as if they did not have prolapse. Stress incontinence, which is common among these women, can be corrected either in isolation or in conjunction with repair of the prolapse, if such repair is indicated.
More advanced degrees of prolapse, defined as prolapse that extends to or beyond the hymen, are commonly associated with urethral obstruction or occult sphincteric incontinence, or both. This makes it important to diagnose these conditions (by means described earlier) before you intervene surgically to repair the prolapse.
Can surgery for POP affect lower-tract symptoms?
Paradoxically, surgical treatment of prolapse can treat lower-tract symptoms successfully in some patients but cause them in others. How can this be?
- Surgery works when prolapse has caused obstruction and the obstruction is relieved when you resupport the pelvic floor
- Surgery can cause symptoms when occult stress incontinence goes unrecognized and is unmasked after repair of prolapse without concomitant anti-incontinence surgery
- De novo irritative symptoms and OAB can arise secondary to placement of a sling.
Treat all prolapse surgery patients with prophylactic anti-incontinence surgery?
Some experts recommend that practice. But anti-incontinence surgery carries its own risk of complications, so we believe that the need for anti-incontinence surgery should be individualized—based on symptoms, anatomy, the results of diagnostic testing, and the patient’s quality-of-life priorities. Of course, when there is pre-existing or occult stress incontinence, you should routinely consider concomitant anti-incontinence surgery.
When is POP surgery effective for OAB symptoms?
The literature is scant on this question. We believe that, in patients who have an advanced degree of prolapse (especially when urethral obstruction has been documented), symptoms of OAB subside most of the time after effective prolapse surgery.
We do not recommend surgery for mild degrees of prolapse or when there is no pre-existing obstruction.
Drs. Blaivas and Karram co-chair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007, in Las Vegas (www.urogyn-cme.org).
To watch a demonstration of how pelvic organ prolapse is repaired, visit the Video Library.
Lower urinary tract symptoms are common in women who have pelvic organ prolapse (POP). For some, these symptoms resolve or improve after surgery for prolapse; for others, symptoms remain unchanged or become worse. These clinical pearls can help you decide how to counsel, evaluate, and treat patients who have POP and coexisting lower-tract symptoms.
How are POP and lower-tract symptoms related?
Lower-tract symptoms that result from, or coexist with, POP include urinary incontinence (stress, urge, mixed), irritative symptoms (frequency, urgency, nocturia), and difficulty voiding (hesitancy, weak or intermittent stream). Prolapse can produce lower-tract symptoms by:
- causing urethral obstruction
- dissipating the effects of abdominal pressure during Valsalva voiding, which makes voiding more difficult
- masking sphincteric incontinence.
Is prolapse causing symptoms or masking stress incontinence?
Some clues to answering this question can be obtained from the history:
- If the patient says that she voids better when the prolapse is reduced, prolapse is probably causing urethral obstruction
- If the patient says that she experienced stress incontinence previously but that it has subsided and she now only has difficulty voiding, she probably has occult stress incontinence and, possibly, urethral obstruction.
How is treatment established for lower-tract symptoms?
Prolapse is graded by any of several classifications that are based on the severity and extent of the condition.
Mild degrees of prolapse rarely, if ever, cause urethral obstruction or mask stress incontinence; you can manage lower-tract symptoms in these patients as if they did not have prolapse. Stress incontinence, which is common among these women, can be corrected either in isolation or in conjunction with repair of the prolapse, if such repair is indicated.
More advanced degrees of prolapse, defined as prolapse that extends to or beyond the hymen, are commonly associated with urethral obstruction or occult sphincteric incontinence, or both. This makes it important to diagnose these conditions (by means described earlier) before you intervene surgically to repair the prolapse.
Can surgery for POP affect lower-tract symptoms?
Paradoxically, surgical treatment of prolapse can treat lower-tract symptoms successfully in some patients but cause them in others. How can this be?
- Surgery works when prolapse has caused obstruction and the obstruction is relieved when you resupport the pelvic floor
- Surgery can cause symptoms when occult stress incontinence goes unrecognized and is unmasked after repair of prolapse without concomitant anti-incontinence surgery
- De novo irritative symptoms and OAB can arise secondary to placement of a sling.
Treat all prolapse surgery patients with prophylactic anti-incontinence surgery?
Some experts recommend that practice. But anti-incontinence surgery carries its own risk of complications, so we believe that the need for anti-incontinence surgery should be individualized—based on symptoms, anatomy, the results of diagnostic testing, and the patient’s quality-of-life priorities. Of course, when there is pre-existing or occult stress incontinence, you should routinely consider concomitant anti-incontinence surgery.
When is POP surgery effective for OAB symptoms?
The literature is scant on this question. We believe that, in patients who have an advanced degree of prolapse (especially when urethral obstruction has been documented), symptoms of OAB subside most of the time after effective prolapse surgery.
We do not recommend surgery for mild degrees of prolapse or when there is no pre-existing obstruction.
To watch a demonstration of how pelvic organ prolapse is repaired, visit the Video Library.
Lower urinary tract symptoms are common in women who have pelvic organ prolapse (POP). For some, these symptoms resolve or improve after surgery for prolapse; for others, symptoms remain unchanged or become worse. These clinical pearls can help you decide how to counsel, evaluate, and treat patients who have POP and coexisting lower-tract symptoms.
How are POP and lower-tract symptoms related?
Lower-tract symptoms that result from, or coexist with, POP include urinary incontinence (stress, urge, mixed), irritative symptoms (frequency, urgency, nocturia), and difficulty voiding (hesitancy, weak or intermittent stream). Prolapse can produce lower-tract symptoms by:
- causing urethral obstruction
- dissipating the effects of abdominal pressure during Valsalva voiding, which makes voiding more difficult
- masking sphincteric incontinence.
Is prolapse causing symptoms or masking stress incontinence?
Some clues to answering this question can be obtained from the history:
- If the patient says that she voids better when the prolapse is reduced, prolapse is probably causing urethral obstruction
- If the patient says that she experienced stress incontinence previously but that it has subsided and she now only has difficulty voiding, she probably has occult stress incontinence and, possibly, urethral obstruction.
How is treatment established for lower-tract symptoms?
Prolapse is graded by any of several classifications that are based on the severity and extent of the condition.
Mild degrees of prolapse rarely, if ever, cause urethral obstruction or mask stress incontinence; you can manage lower-tract symptoms in these patients as if they did not have prolapse. Stress incontinence, which is common among these women, can be corrected either in isolation or in conjunction with repair of the prolapse, if such repair is indicated.
More advanced degrees of prolapse, defined as prolapse that extends to or beyond the hymen, are commonly associated with urethral obstruction or occult sphincteric incontinence, or both. This makes it important to diagnose these conditions (by means described earlier) before you intervene surgically to repair the prolapse.
Can surgery for POP affect lower-tract symptoms?
Paradoxically, surgical treatment of prolapse can treat lower-tract symptoms successfully in some patients but cause them in others. How can this be?
- Surgery works when prolapse has caused obstruction and the obstruction is relieved when you resupport the pelvic floor
- Surgery can cause symptoms when occult stress incontinence goes unrecognized and is unmasked after repair of prolapse without concomitant anti-incontinence surgery
- De novo irritative symptoms and OAB can arise secondary to placement of a sling.
Treat all prolapse surgery patients with prophylactic anti-incontinence surgery?
Some experts recommend that practice. But anti-incontinence surgery carries its own risk of complications, so we believe that the need for anti-incontinence surgery should be individualized—based on symptoms, anatomy, the results of diagnostic testing, and the patient’s quality-of-life priorities. Of course, when there is pre-existing or occult stress incontinence, you should routinely consider concomitant anti-incontinence surgery.
When is POP surgery effective for OAB symptoms?
The literature is scant on this question. We believe that, in patients who have an advanced degree of prolapse (especially when urethral obstruction has been documented), symptoms of OAB subside most of the time after effective prolapse surgery.
We do not recommend surgery for mild degrees of prolapse or when there is no pre-existing obstruction.
Drs. Blaivas and Karram co-chair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007, in Las Vegas (www.urogyn-cme.org).
Drs. Blaivas and Karram co-chair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007, in Las Vegas (www.urogyn-cme.org).