User login
Autonomic function and prognosis
First-year medical students are well aware that the autonomic nervous system regulates heart rate and blood pressure along with respiratory and digestive functions. The past 10 to 20 years have seen increased appreciation of the medical relevance of the the autonomic nervous system beyond first-year physiology examinations; even mild disturbances of autonomic nervous system function predict materially worse prognosis.1–3 Researchers have focused on the use of readily available measures, such as heart rate,4 heart rate variability,5 and heart rate recovery,6 to link autonomic nervous system dysfunction with mortality and morbidity.1 In addition, epidemiologists have exploited these tools to identify correlates of autonomic nervous system dysfunction at patient and environmental levels.7 Although it is not yet known how best to incorporate autonomic nervous system measures into routine clinical care, there is increasing excitement about the insights that this work has revealed.
MEASURES OF AUTONOMIC NERVOUS SYSTEM FUNCTION
Although many measures of autonomic nervous system function have been described, three relatively straightforward approaches are based on heart rate.1
Resting heart rate is the simplest to obtain, as it does not require any special technology. People with high levels of parasympathetic nervous system tone have lower resting heart rates, as is typically seen in world-class athletes. Conversely, conditions characterized by increased levels of sympathetic tone manifest as sinus tachycardia; classic examples include congestive heart failure, anemia, and hypovolemia.
Heart rate recovery. Heart rate variability measures require continuous Holter monitoring as well as sophisticated software. The numerous types of heart rate variability measures are not intuitive for most clinicians. Exercise heart rate recovery is an arguably more straightforward method of assessing parasympathetic tone.1 During a graded exercise test, heart rate increases as a result of withdrawal of parasympathetic tone and increased sympathetic tone. During the first 30 seconds after exercise, heart rate decreases quickly, mainly because of rapid reactivation of the parasympathetic nervous system.10
AUTONOMIC NERVOUS SYSTEM FUNCTION AND MORTALITY
Resting heart rate
There is a remarkably strong association between heart rate and survival, an association that transcends species.4 Small mammals that have rapid heart rates have short life expectancies. Larger mammals that have slower heart rates have correspondingly higher life expectancies. Among nearly all mammals, life expectancy is close to 1 billion heartbeats.
Investigators have been able to increase survival in animal models by deliberate slowing of heart rate. An experiment performed in mice more than 30 years ago showed that life expectancy increases with low-dose digoxin, a parasympathomimetic agent.11 More recently, a mouse model has been used to show that ivabradine, a sinus node ion channel blocking agent that specifically reduces heart rate without affecting vascular tone, inhibits development of atherosclerosis in genetically susceptible knockout mice.12
There is an extensive epidemiological literature linking heart rate to mortality in large human populations. 4,13 As heart rate increases to 75 to 80 beats per minute, there are marked increases in total mortality and mortality due to coronary heart disease. As is well known, administration of beta-blockers reduces mortality in survivors of myocardial infarction. What is particularly remarkable is that the magnitude of reduction in mortality with beta-blocker therapy is directly proportional to the magnitude of heart rate decrease.14 In a recent analysis of hypertensive patients enrolled in a large-scale randomized trial, a strong association was noted between mortality and increasing heart rate at the time of randomization as well as after treatment with either verapamil or a beta-blocker.15
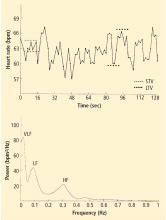
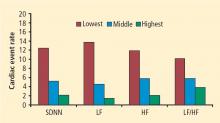
Heart rate variability
Heart rate recovery
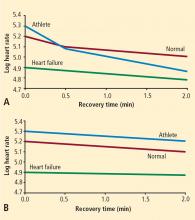
In 1999, Cole and colleagues reported on the association between heart rate recovery during the first minute after exercise and all-cause mortality in approximately 2,400 patients who were candidates for first-time coronary angiography.6 An abnormal heart rate recovery was defined as a reduction from the peak heart rate of 12 beats per minute or less, which corresponded to the lowest quartile. Thus, a patient achieving a peak heart rate of 160 beats per minute would be considered to have an abnormal heart rate recovery if 1 minute later the heart rate was 148 beats per minute or higher. Patients who had an abnormal heart rate recovery had a nearly fourfold increased risk of all-cause death; even after adjusting for numerous confounders, including exercise capacity, there was still a twofold independent increased risk of death. This initial observation has since been confirmed in other cohorts.19,20 The link between heart rate recovery, mortality, and cardiovascular prognosis appears to be independent of symptom status,21 type of recovery protocol,22 left ventricular ejection fraction,22 and angiographic severity of coronary artery disease.23
The mechanism by which an abnormal heart rate recovery predicts increased mortality is unclear. Given that heart rate recovery is thought to reflect parasympathetic nervous system function, and given that increased parasympathetic tone is believed to have antiarrhythmic effects, one might hypothesize that lower heart rate recovery would predict sudden cardiac death. In 2005, investigators from the Paris Civil Service Study reported on the association of exercise heart rate recovery and type of mortality; low heart rate recovery was strongly predictive of sudden cardiac death but not of non-sudden cardiac myocardial infarction death.20 A separate study from the Cleveland Clinic showed that among more than 29,000 patients, frequent ventricular ectopy during early recovery was strongly predictive of death, whereas frequent ventricular ectopy during exercise was not.24 These two studies together suggest that the link between heart rate recovery and mortality may be a reflection of the antiarrhythmic properties of the parasympathetic nervous system.
It is well known that there is an exceptionally powerful link between functional capacity and cardiovascular risk.25,26 People who are in excellent physical shape have high levels of parasympathetic tone. Among patients with suspected coronary artery disease, there is a strong dose-response relationship between heart rate recovery and physical fitness.6 While the link between functional capacity and prognosis is complex, it is conceivable that parasympathetic protection against arrhythmias and shear-induced plaque rupture may play a role.
DETERMINANTS OF AUTONOMIC NERVOUS SYSTEM FUNCTION
There is an extensive literature documenting a number of determinants of autonomic tone.3,7 On a patient level, decreased levels of parasympathetic tone or increased levels of sympathetic tone have been linked to obesity, insulin resistance, diabetes, hypertension, hypercholesterolemia, depression, anxiety, heart failure, and peripheral vascular disease.3
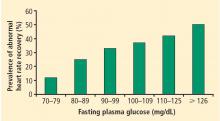
Perturbations of autonomic nervous system function have also been associated with environmental exposures. People who have lower levels of education,29 live in neighborhoods characterized by lower socio-economic status,30 or are exposed to small-particulate air pollution31 have been shown to manifest abnormal heart rate recovery or decreased heart rate variability.
CONCLUSIONS
Autonomic nervous system function can be measured in the clinic by recording resting heart rate, heart rate variability, or exercise heart rate recovery.1 All three of these measures are strong predictors of cardiovascular risk and all-cause mortality in both primary and secondary prevention settings. A number of determinants of autonomic nervous system function have been identified, including patient-level factors like obesity, diabetes, and heart failure as well as environmental correlates like smoking, social stress, and air pollution. It is not yet known, however, how best to take advantage of the associations between abnormal autonomic nervous system function and poor prognosis to improve patient outcomes. Future research will be needed to identify strategies of favorably modulating autonomic function that improve outcomes in the clinic and among large populations.
- Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol 2008; 51:1725–1733.
- Katz A, Liberty IF, Porath A, Ovsyshcher I, Prystowsky EN. A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. Am Heart J 1999; 138(1 Pt 1):32–38.
- Curtis BM, O’Keefe JH. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 2002; 77:45–54.
- Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol 1997; 30:1104–1106.
- Huikuri HV, Makikallio T, Airaksinen KE, Mitrani R, Castellanos A, Myerburg RJ. Measurement of heart rate variability: a clinical tool or a research toy? J Am Coll Cardiol 1999; 34:1878–1883.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007; 74:224–242.
- van Ravenswaaij-Arts CM, Kollee LA, Hopman JC, Stoelinga GB, van Geijn HP. Heart rate variability. Ann Intern Med 1993; 118:436–447.
- Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol 2002; 84:1–14.
- Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994; 24:1529–1535.
- Coburn AF, Grey RM, Rivera SM. Observations on the relation of heart rate, life span, weight and mineralization in the digoxintreated A-J mouse. Johns Hopkins Med J 1971; 128:169–193.
- Custodis F, Baumhakel M, Schlimmer N, et al. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 2008; 117:2377–2387.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. The multifactor primary prevention trial in GÖteborg, Sweden. Eur Heart J 1986; 7:279–288.
- Reil JC, Bohm M. The role of heart rate in the development of cardiovascular disease. Clin Res Cardiol 2007; 96:585–592.
- Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J 2008; 29:1327–1334.
- Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996; 94:2850–2855.
- Makikallio TH, Hoiber S, Kober L, et al. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol 1999; 83:836–839.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998; 351:478–484.
- Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 2001; 38:1980–1987.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 2001; 104:1911–1916.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Lauer MS, Pothier CE, Magid DJ, Smith SS, Kattan MW. An externally validated model for predicting long-term survival after exercise treadmill testing in patients with suspected coronary artery disease and a normal electrocardiogram. Ann Intern Med 2007; 147:821–828.
- Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes 2002; 51:803–807.
- Shishehbor MH, Baker DW, Blackstone EH, Lauer MS. Association of educational status with heart rate recovery: a population-based propensity analysis. Am J Med 2002; 113:643–649.
- Shishehbor MH, Litaker D, Pothier CE, Lauer MS. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA 2006; 295:784–792.
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 2001; 104:986–991.
First-year medical students are well aware that the autonomic nervous system regulates heart rate and blood pressure along with respiratory and digestive functions. The past 10 to 20 years have seen increased appreciation of the medical relevance of the the autonomic nervous system beyond first-year physiology examinations; even mild disturbances of autonomic nervous system function predict materially worse prognosis.1–3 Researchers have focused on the use of readily available measures, such as heart rate,4 heart rate variability,5 and heart rate recovery,6 to link autonomic nervous system dysfunction with mortality and morbidity.1 In addition, epidemiologists have exploited these tools to identify correlates of autonomic nervous system dysfunction at patient and environmental levels.7 Although it is not yet known how best to incorporate autonomic nervous system measures into routine clinical care, there is increasing excitement about the insights that this work has revealed.
MEASURES OF AUTONOMIC NERVOUS SYSTEM FUNCTION
Although many measures of autonomic nervous system function have been described, three relatively straightforward approaches are based on heart rate.1
Resting heart rate is the simplest to obtain, as it does not require any special technology. People with high levels of parasympathetic nervous system tone have lower resting heart rates, as is typically seen in world-class athletes. Conversely, conditions characterized by increased levels of sympathetic tone manifest as sinus tachycardia; classic examples include congestive heart failure, anemia, and hypovolemia.
Heart rate recovery. Heart rate variability measures require continuous Holter monitoring as well as sophisticated software. The numerous types of heart rate variability measures are not intuitive for most clinicians. Exercise heart rate recovery is an arguably more straightforward method of assessing parasympathetic tone.1 During a graded exercise test, heart rate increases as a result of withdrawal of parasympathetic tone and increased sympathetic tone. During the first 30 seconds after exercise, heart rate decreases quickly, mainly because of rapid reactivation of the parasympathetic nervous system.10
AUTONOMIC NERVOUS SYSTEM FUNCTION AND MORTALITY
Resting heart rate
There is a remarkably strong association between heart rate and survival, an association that transcends species.4 Small mammals that have rapid heart rates have short life expectancies. Larger mammals that have slower heart rates have correspondingly higher life expectancies. Among nearly all mammals, life expectancy is close to 1 billion heartbeats.
Investigators have been able to increase survival in animal models by deliberate slowing of heart rate. An experiment performed in mice more than 30 years ago showed that life expectancy increases with low-dose digoxin, a parasympathomimetic agent.11 More recently, a mouse model has been used to show that ivabradine, a sinus node ion channel blocking agent that specifically reduces heart rate without affecting vascular tone, inhibits development of atherosclerosis in genetically susceptible knockout mice.12
There is an extensive epidemiological literature linking heart rate to mortality in large human populations. 4,13 As heart rate increases to 75 to 80 beats per minute, there are marked increases in total mortality and mortality due to coronary heart disease. As is well known, administration of beta-blockers reduces mortality in survivors of myocardial infarction. What is particularly remarkable is that the magnitude of reduction in mortality with beta-blocker therapy is directly proportional to the magnitude of heart rate decrease.14 In a recent analysis of hypertensive patients enrolled in a large-scale randomized trial, a strong association was noted between mortality and increasing heart rate at the time of randomization as well as after treatment with either verapamil or a beta-blocker.15
Heart rate variability
Heart rate recovery
In 1999, Cole and colleagues reported on the association between heart rate recovery during the first minute after exercise and all-cause mortality in approximately 2,400 patients who were candidates for first-time coronary angiography.6 An abnormal heart rate recovery was defined as a reduction from the peak heart rate of 12 beats per minute or less, which corresponded to the lowest quartile. Thus, a patient achieving a peak heart rate of 160 beats per minute would be considered to have an abnormal heart rate recovery if 1 minute later the heart rate was 148 beats per minute or higher. Patients who had an abnormal heart rate recovery had a nearly fourfold increased risk of all-cause death; even after adjusting for numerous confounders, including exercise capacity, there was still a twofold independent increased risk of death. This initial observation has since been confirmed in other cohorts.19,20 The link between heart rate recovery, mortality, and cardiovascular prognosis appears to be independent of symptom status,21 type of recovery protocol,22 left ventricular ejection fraction,22 and angiographic severity of coronary artery disease.23
The mechanism by which an abnormal heart rate recovery predicts increased mortality is unclear. Given that heart rate recovery is thought to reflect parasympathetic nervous system function, and given that increased parasympathetic tone is believed to have antiarrhythmic effects, one might hypothesize that lower heart rate recovery would predict sudden cardiac death. In 2005, investigators from the Paris Civil Service Study reported on the association of exercise heart rate recovery and type of mortality; low heart rate recovery was strongly predictive of sudden cardiac death but not of non-sudden cardiac myocardial infarction death.20 A separate study from the Cleveland Clinic showed that among more than 29,000 patients, frequent ventricular ectopy during early recovery was strongly predictive of death, whereas frequent ventricular ectopy during exercise was not.24 These two studies together suggest that the link between heart rate recovery and mortality may be a reflection of the antiarrhythmic properties of the parasympathetic nervous system.
It is well known that there is an exceptionally powerful link between functional capacity and cardiovascular risk.25,26 People who are in excellent physical shape have high levels of parasympathetic tone. Among patients with suspected coronary artery disease, there is a strong dose-response relationship between heart rate recovery and physical fitness.6 While the link between functional capacity and prognosis is complex, it is conceivable that parasympathetic protection against arrhythmias and shear-induced plaque rupture may play a role.
DETERMINANTS OF AUTONOMIC NERVOUS SYSTEM FUNCTION
There is an extensive literature documenting a number of determinants of autonomic tone.3,7 On a patient level, decreased levels of parasympathetic tone or increased levels of sympathetic tone have been linked to obesity, insulin resistance, diabetes, hypertension, hypercholesterolemia, depression, anxiety, heart failure, and peripheral vascular disease.3
Perturbations of autonomic nervous system function have also been associated with environmental exposures. People who have lower levels of education,29 live in neighborhoods characterized by lower socio-economic status,30 or are exposed to small-particulate air pollution31 have been shown to manifest abnormal heart rate recovery or decreased heart rate variability.
CONCLUSIONS
Autonomic nervous system function can be measured in the clinic by recording resting heart rate, heart rate variability, or exercise heart rate recovery.1 All three of these measures are strong predictors of cardiovascular risk and all-cause mortality in both primary and secondary prevention settings. A number of determinants of autonomic nervous system function have been identified, including patient-level factors like obesity, diabetes, and heart failure as well as environmental correlates like smoking, social stress, and air pollution. It is not yet known, however, how best to take advantage of the associations between abnormal autonomic nervous system function and poor prognosis to improve patient outcomes. Future research will be needed to identify strategies of favorably modulating autonomic function that improve outcomes in the clinic and among large populations.
First-year medical students are well aware that the autonomic nervous system regulates heart rate and blood pressure along with respiratory and digestive functions. The past 10 to 20 years have seen increased appreciation of the medical relevance of the the autonomic nervous system beyond first-year physiology examinations; even mild disturbances of autonomic nervous system function predict materially worse prognosis.1–3 Researchers have focused on the use of readily available measures, such as heart rate,4 heart rate variability,5 and heart rate recovery,6 to link autonomic nervous system dysfunction with mortality and morbidity.1 In addition, epidemiologists have exploited these tools to identify correlates of autonomic nervous system dysfunction at patient and environmental levels.7 Although it is not yet known how best to incorporate autonomic nervous system measures into routine clinical care, there is increasing excitement about the insights that this work has revealed.
MEASURES OF AUTONOMIC NERVOUS SYSTEM FUNCTION
Although many measures of autonomic nervous system function have been described, three relatively straightforward approaches are based on heart rate.1
Resting heart rate is the simplest to obtain, as it does not require any special technology. People with high levels of parasympathetic nervous system tone have lower resting heart rates, as is typically seen in world-class athletes. Conversely, conditions characterized by increased levels of sympathetic tone manifest as sinus tachycardia; classic examples include congestive heart failure, anemia, and hypovolemia.
Heart rate recovery. Heart rate variability measures require continuous Holter monitoring as well as sophisticated software. The numerous types of heart rate variability measures are not intuitive for most clinicians. Exercise heart rate recovery is an arguably more straightforward method of assessing parasympathetic tone.1 During a graded exercise test, heart rate increases as a result of withdrawal of parasympathetic tone and increased sympathetic tone. During the first 30 seconds after exercise, heart rate decreases quickly, mainly because of rapid reactivation of the parasympathetic nervous system.10
AUTONOMIC NERVOUS SYSTEM FUNCTION AND MORTALITY
Resting heart rate
There is a remarkably strong association between heart rate and survival, an association that transcends species.4 Small mammals that have rapid heart rates have short life expectancies. Larger mammals that have slower heart rates have correspondingly higher life expectancies. Among nearly all mammals, life expectancy is close to 1 billion heartbeats.
Investigators have been able to increase survival in animal models by deliberate slowing of heart rate. An experiment performed in mice more than 30 years ago showed that life expectancy increases with low-dose digoxin, a parasympathomimetic agent.11 More recently, a mouse model has been used to show that ivabradine, a sinus node ion channel blocking agent that specifically reduces heart rate without affecting vascular tone, inhibits development of atherosclerosis in genetically susceptible knockout mice.12
There is an extensive epidemiological literature linking heart rate to mortality in large human populations. 4,13 As heart rate increases to 75 to 80 beats per minute, there are marked increases in total mortality and mortality due to coronary heart disease. As is well known, administration of beta-blockers reduces mortality in survivors of myocardial infarction. What is particularly remarkable is that the magnitude of reduction in mortality with beta-blocker therapy is directly proportional to the magnitude of heart rate decrease.14 In a recent analysis of hypertensive patients enrolled in a large-scale randomized trial, a strong association was noted between mortality and increasing heart rate at the time of randomization as well as after treatment with either verapamil or a beta-blocker.15
Heart rate variability
Heart rate recovery
In 1999, Cole and colleagues reported on the association between heart rate recovery during the first minute after exercise and all-cause mortality in approximately 2,400 patients who were candidates for first-time coronary angiography.6 An abnormal heart rate recovery was defined as a reduction from the peak heart rate of 12 beats per minute or less, which corresponded to the lowest quartile. Thus, a patient achieving a peak heart rate of 160 beats per minute would be considered to have an abnormal heart rate recovery if 1 minute later the heart rate was 148 beats per minute or higher. Patients who had an abnormal heart rate recovery had a nearly fourfold increased risk of all-cause death; even after adjusting for numerous confounders, including exercise capacity, there was still a twofold independent increased risk of death. This initial observation has since been confirmed in other cohorts.19,20 The link between heart rate recovery, mortality, and cardiovascular prognosis appears to be independent of symptom status,21 type of recovery protocol,22 left ventricular ejection fraction,22 and angiographic severity of coronary artery disease.23
The mechanism by which an abnormal heart rate recovery predicts increased mortality is unclear. Given that heart rate recovery is thought to reflect parasympathetic nervous system function, and given that increased parasympathetic tone is believed to have antiarrhythmic effects, one might hypothesize that lower heart rate recovery would predict sudden cardiac death. In 2005, investigators from the Paris Civil Service Study reported on the association of exercise heart rate recovery and type of mortality; low heart rate recovery was strongly predictive of sudden cardiac death but not of non-sudden cardiac myocardial infarction death.20 A separate study from the Cleveland Clinic showed that among more than 29,000 patients, frequent ventricular ectopy during early recovery was strongly predictive of death, whereas frequent ventricular ectopy during exercise was not.24 These two studies together suggest that the link between heart rate recovery and mortality may be a reflection of the antiarrhythmic properties of the parasympathetic nervous system.
It is well known that there is an exceptionally powerful link between functional capacity and cardiovascular risk.25,26 People who are in excellent physical shape have high levels of parasympathetic tone. Among patients with suspected coronary artery disease, there is a strong dose-response relationship between heart rate recovery and physical fitness.6 While the link between functional capacity and prognosis is complex, it is conceivable that parasympathetic protection against arrhythmias and shear-induced plaque rupture may play a role.
DETERMINANTS OF AUTONOMIC NERVOUS SYSTEM FUNCTION
There is an extensive literature documenting a number of determinants of autonomic tone.3,7 On a patient level, decreased levels of parasympathetic tone or increased levels of sympathetic tone have been linked to obesity, insulin resistance, diabetes, hypertension, hypercholesterolemia, depression, anxiety, heart failure, and peripheral vascular disease.3
Perturbations of autonomic nervous system function have also been associated with environmental exposures. People who have lower levels of education,29 live in neighborhoods characterized by lower socio-economic status,30 or are exposed to small-particulate air pollution31 have been shown to manifest abnormal heart rate recovery or decreased heart rate variability.
CONCLUSIONS
Autonomic nervous system function can be measured in the clinic by recording resting heart rate, heart rate variability, or exercise heart rate recovery.1 All three of these measures are strong predictors of cardiovascular risk and all-cause mortality in both primary and secondary prevention settings. A number of determinants of autonomic nervous system function have been identified, including patient-level factors like obesity, diabetes, and heart failure as well as environmental correlates like smoking, social stress, and air pollution. It is not yet known, however, how best to take advantage of the associations between abnormal autonomic nervous system function and poor prognosis to improve patient outcomes. Future research will be needed to identify strategies of favorably modulating autonomic function that improve outcomes in the clinic and among large populations.
- Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol 2008; 51:1725–1733.
- Katz A, Liberty IF, Porath A, Ovsyshcher I, Prystowsky EN. A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. Am Heart J 1999; 138(1 Pt 1):32–38.
- Curtis BM, O’Keefe JH. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 2002; 77:45–54.
- Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol 1997; 30:1104–1106.
- Huikuri HV, Makikallio T, Airaksinen KE, Mitrani R, Castellanos A, Myerburg RJ. Measurement of heart rate variability: a clinical tool or a research toy? J Am Coll Cardiol 1999; 34:1878–1883.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007; 74:224–242.
- van Ravenswaaij-Arts CM, Kollee LA, Hopman JC, Stoelinga GB, van Geijn HP. Heart rate variability. Ann Intern Med 1993; 118:436–447.
- Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol 2002; 84:1–14.
- Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994; 24:1529–1535.
- Coburn AF, Grey RM, Rivera SM. Observations on the relation of heart rate, life span, weight and mineralization in the digoxintreated A-J mouse. Johns Hopkins Med J 1971; 128:169–193.
- Custodis F, Baumhakel M, Schlimmer N, et al. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 2008; 117:2377–2387.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. The multifactor primary prevention trial in GÖteborg, Sweden. Eur Heart J 1986; 7:279–288.
- Reil JC, Bohm M. The role of heart rate in the development of cardiovascular disease. Clin Res Cardiol 2007; 96:585–592.
- Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J 2008; 29:1327–1334.
- Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996; 94:2850–2855.
- Makikallio TH, Hoiber S, Kober L, et al. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol 1999; 83:836–839.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998; 351:478–484.
- Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 2001; 38:1980–1987.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 2001; 104:1911–1916.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Lauer MS, Pothier CE, Magid DJ, Smith SS, Kattan MW. An externally validated model for predicting long-term survival after exercise treadmill testing in patients with suspected coronary artery disease and a normal electrocardiogram. Ann Intern Med 2007; 147:821–828.
- Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes 2002; 51:803–807.
- Shishehbor MH, Baker DW, Blackstone EH, Lauer MS. Association of educational status with heart rate recovery: a population-based propensity analysis. Am J Med 2002; 113:643–649.
- Shishehbor MH, Litaker D, Pothier CE, Lauer MS. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA 2006; 295:784–792.
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 2001; 104:986–991.
- Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol 2008; 51:1725–1733.
- Katz A, Liberty IF, Porath A, Ovsyshcher I, Prystowsky EN. A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. Am Heart J 1999; 138(1 Pt 1):32–38.
- Curtis BM, O’Keefe JH. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 2002; 77:45–54.
- Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol 1997; 30:1104–1106.
- Huikuri HV, Makikallio T, Airaksinen KE, Mitrani R, Castellanos A, Myerburg RJ. Measurement of heart rate variability: a clinical tool or a research toy? J Am Coll Cardiol 1999; 34:1878–1883.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007; 74:224–242.
- van Ravenswaaij-Arts CM, Kollee LA, Hopman JC, Stoelinga GB, van Geijn HP. Heart rate variability. Ann Intern Med 1993; 118:436–447.
- Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol 2002; 84:1–14.
- Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994; 24:1529–1535.
- Coburn AF, Grey RM, Rivera SM. Observations on the relation of heart rate, life span, weight and mineralization in the digoxintreated A-J mouse. Johns Hopkins Med J 1971; 128:169–193.
- Custodis F, Baumhakel M, Schlimmer N, et al. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 2008; 117:2377–2387.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. The multifactor primary prevention trial in GÖteborg, Sweden. Eur Heart J 1986; 7:279–288.
- Reil JC, Bohm M. The role of heart rate in the development of cardiovascular disease. Clin Res Cardiol 2007; 96:585–592.
- Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J 2008; 29:1327–1334.
- Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996; 94:2850–2855.
- Makikallio TH, Hoiber S, Kober L, et al. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol 1999; 83:836–839.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998; 351:478–484.
- Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 2001; 38:1980–1987.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 2001; 104:1911–1916.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Lauer MS, Pothier CE, Magid DJ, Smith SS, Kattan MW. An externally validated model for predicting long-term survival after exercise treadmill testing in patients with suspected coronary artery disease and a normal electrocardiogram. Ann Intern Med 2007; 147:821–828.
- Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes 2002; 51:803–807.
- Shishehbor MH, Baker DW, Blackstone EH, Lauer MS. Association of educational status with heart rate recovery: a population-based propensity analysis. Am J Med 2002; 113:643–649.
- Shishehbor MH, Litaker D, Pothier CE, Lauer MS. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA 2006; 295:784–792.
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 2001; 104:986–991.