User login
Innovative Therapies for Severe Asthma
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
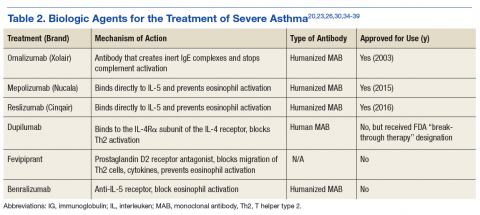
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
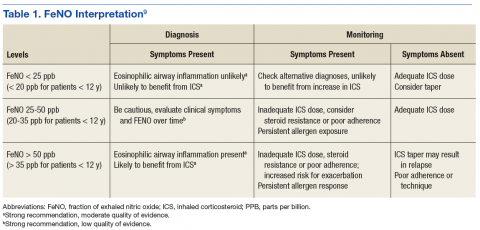
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
One Hundred Case Series of Vocal Cord Dysfunction in a Military Treatment Facility
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.