User login
The benefits of a standardized approach to opioid prescribing
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
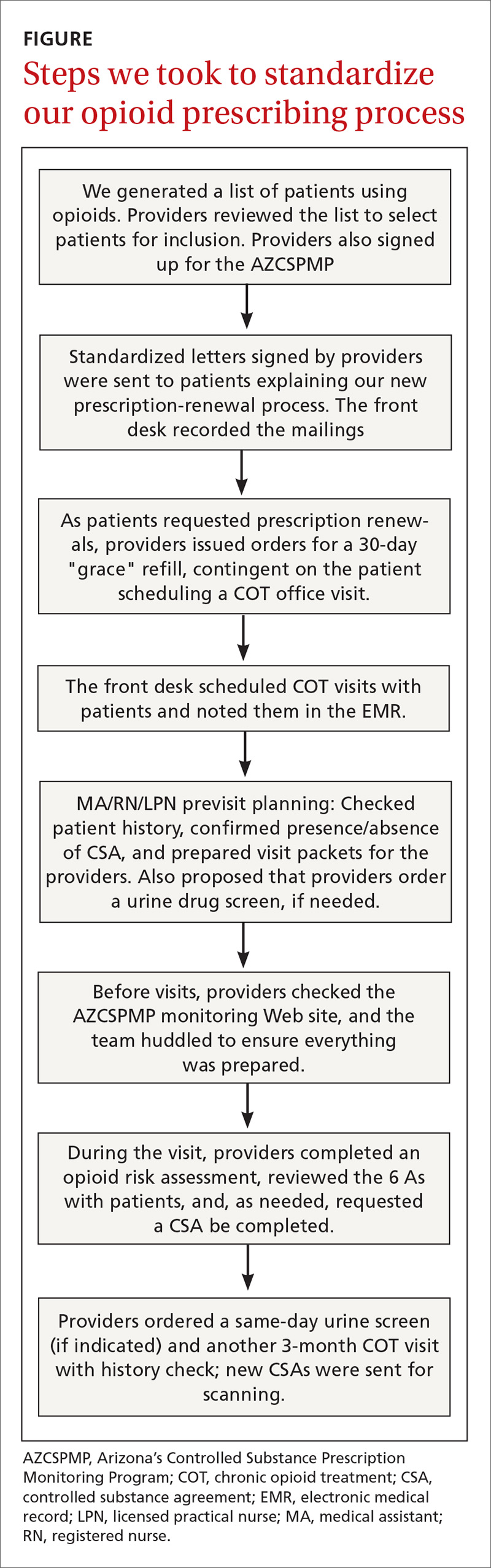
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.
RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.
Continue to: We excluded 33 patients...
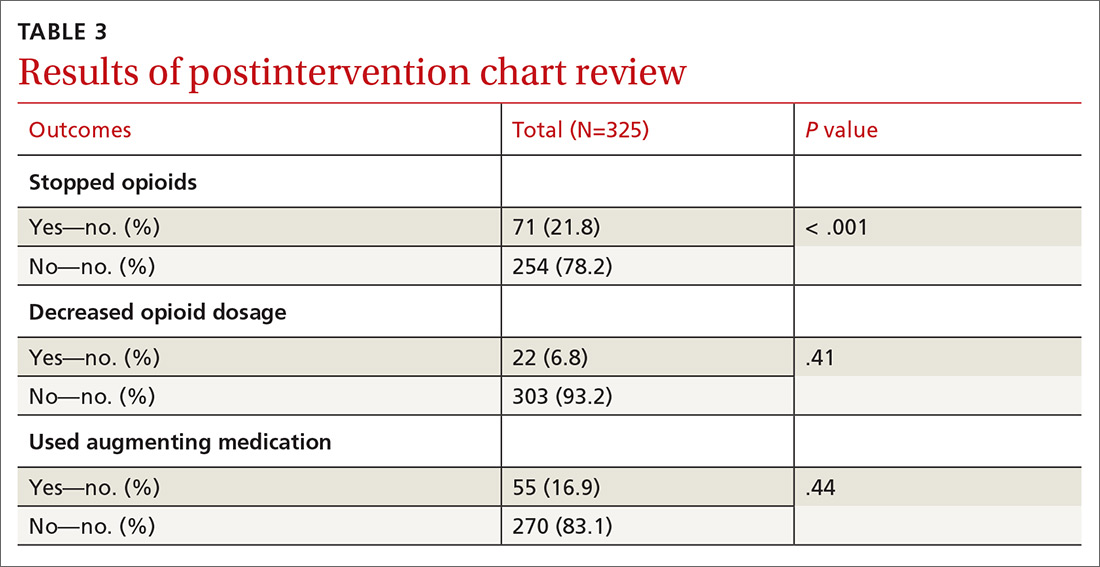
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
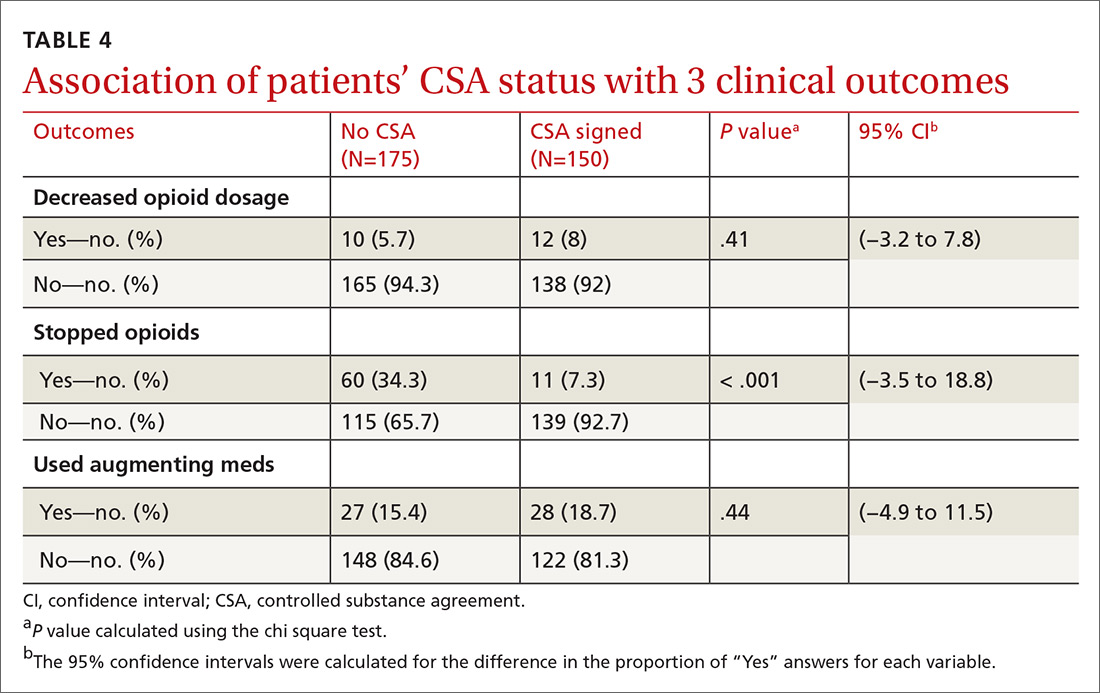
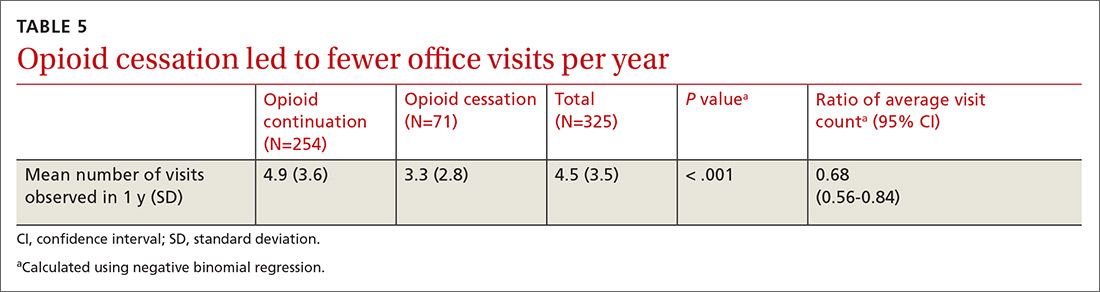
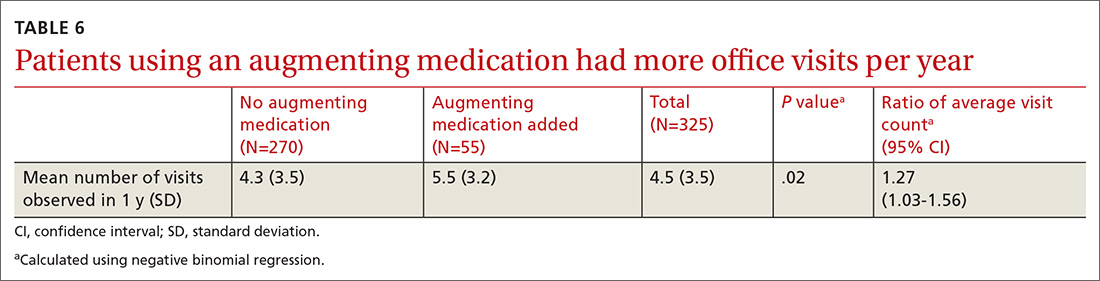
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; patchett.david@mayo.edu
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.
RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.
Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; patchett.david@mayo.edu
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.
RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.
Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; patchett.david@mayo.edu
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.