User login
Complex Malignancies: A Diagnostic and Therapeutic Trilemma
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
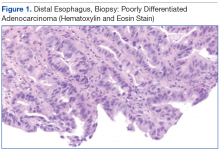
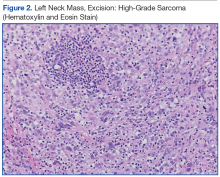
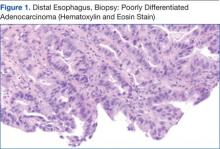
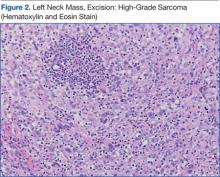
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of highgrade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
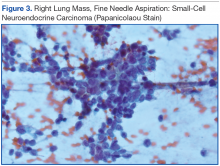
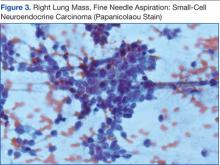
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of highgrade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of highgrade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
Complex Malignancies: A Diagnostic and Therapeutic Trilemma
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of high-grade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of high-grade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of high-grade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.