User login
Painful Indurated Plaque on the Groin
The Diagnosis: Cutaneous Metastasis
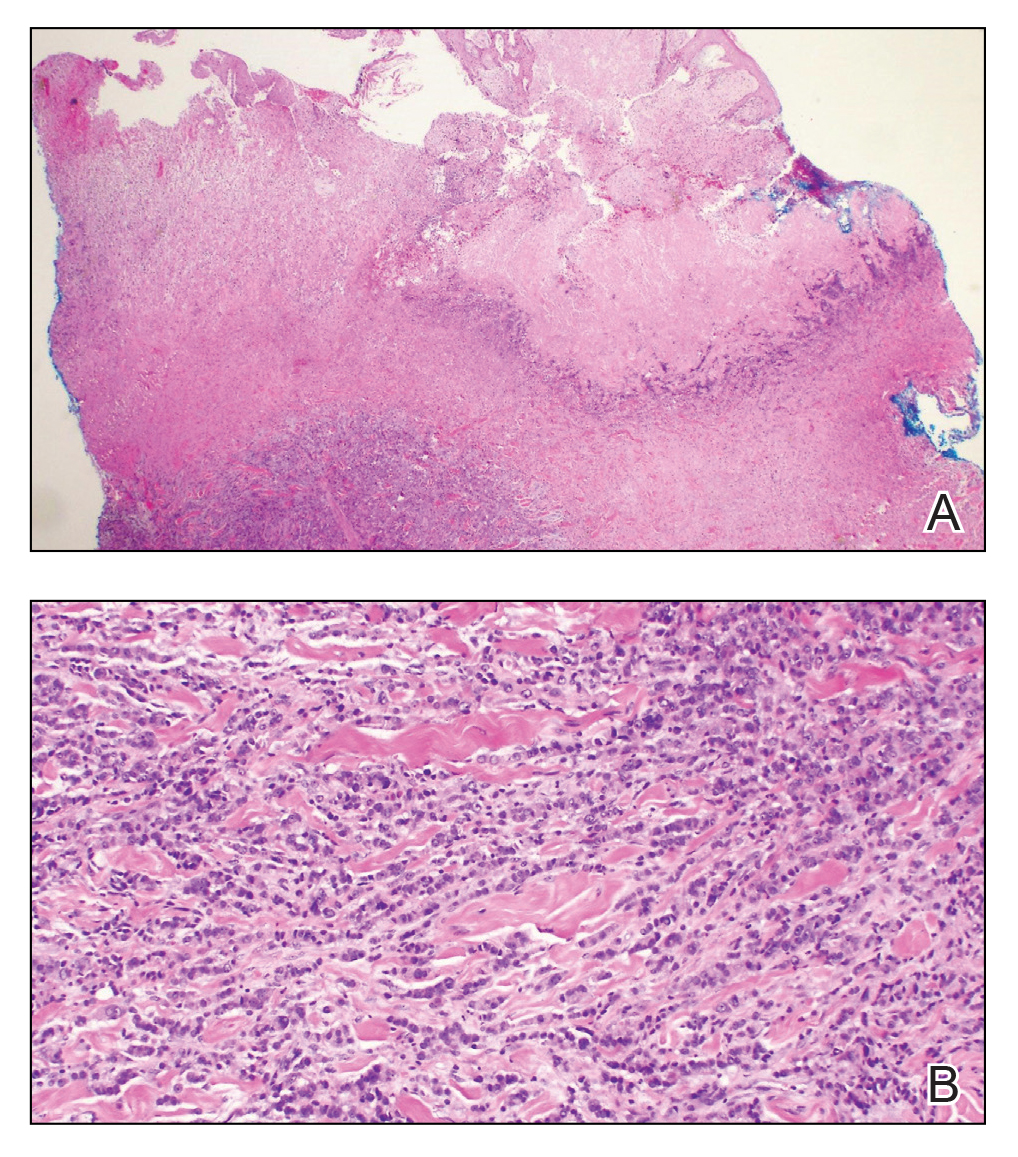
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.
Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
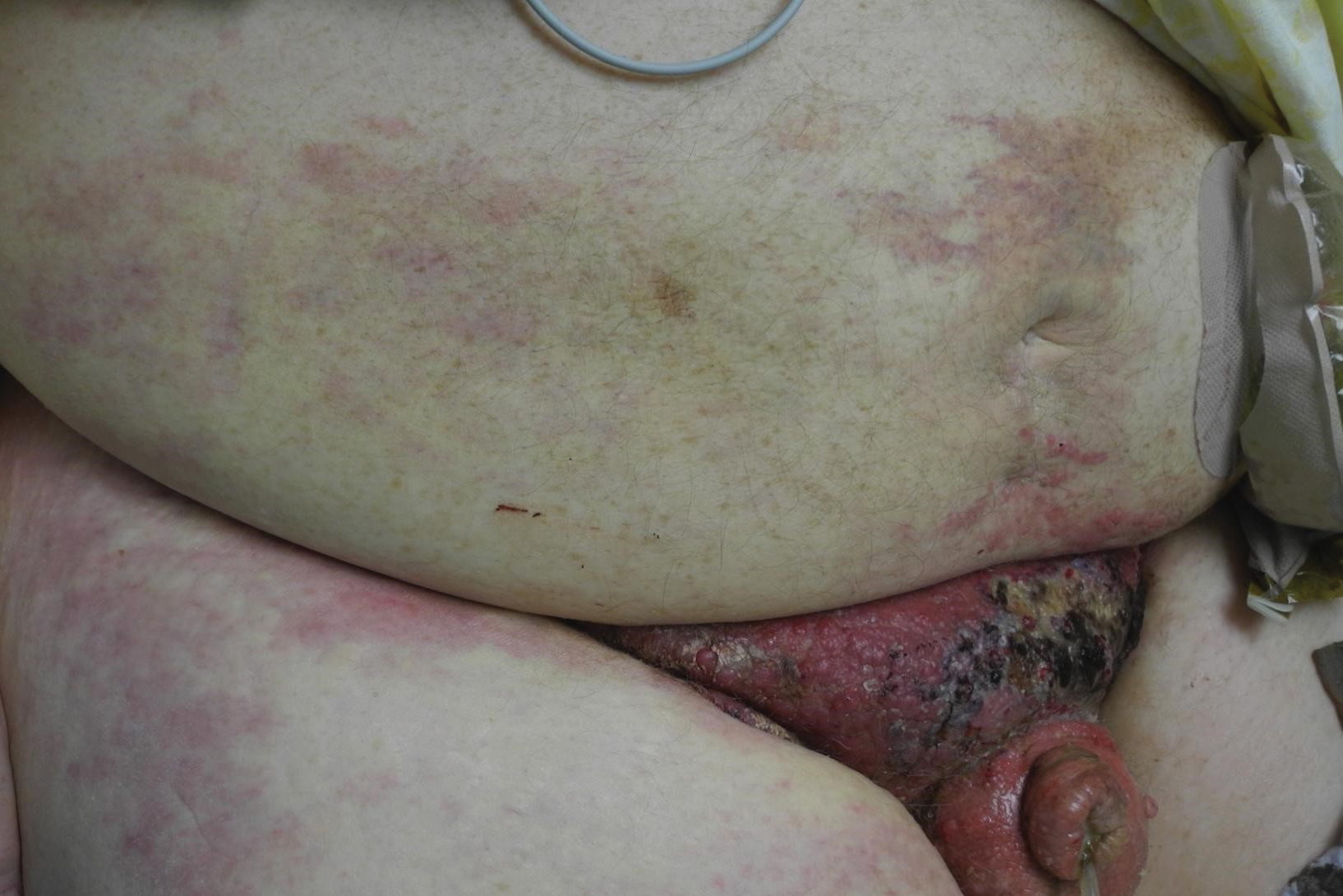
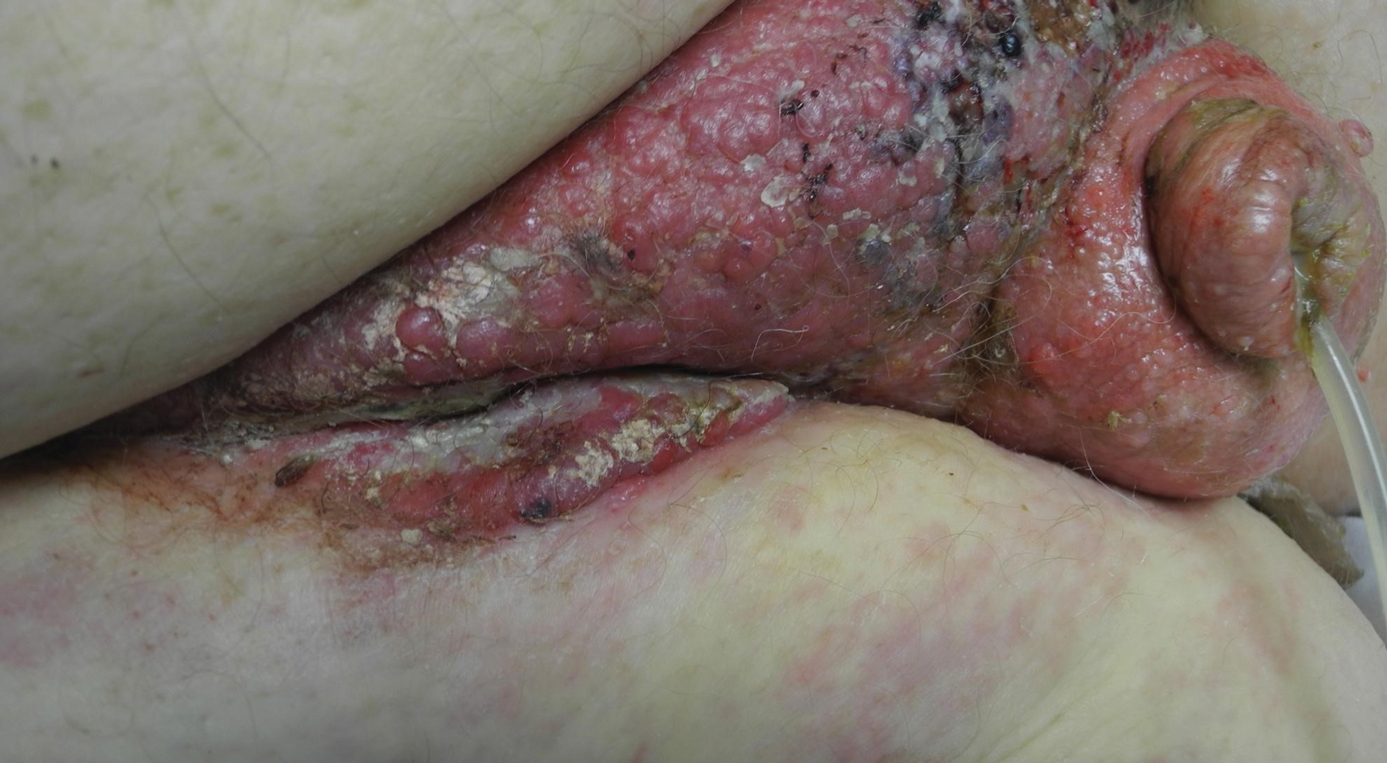
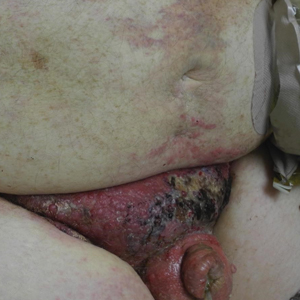
A 67-year-old man presented with a chronic lesion on the groin of 6 weeks' duration. The patient had a history of type 2 diabetes mellitus and colonic adenocarcinoma diagnosed 4 years prior that was treated with a colectomy, radiation therapy, and chemotherapy. Six weeks prior to the current presentation, the patient first sought treatment of swelling, redness, pain, and a bumpy texture on the groin. He was unsuccessfully managed by several physicians including at a long-term care facility where he was admitted and treated for presumed cellulitis. Attempted treatments included a topical antifungal, fluconazole, ciprofloxacin, metronidazole, cefepime, clindamycin, daptomycin, and vancomycin. The affected area continued to worsen along with the patient's overall health. He was transferred to the hospital for more advanced care and was evaluated by inpatient dermatology. Physical examination revealed firm, pink to red-brown, ulcerating papulonodules that coalesced into a large indurated plaque over the pubis, scrotum, penis, and inguinal folds (top). There also were red-violet, indurated plaques on the lower abdomen and bilateral proximal thighs (bottom). Punch biopsies were taken from the indurated area on the left side of the pubis--one for histopathologic evaluation and the other for bacterial, fungal, atypical mycobacterial, and Nocardia tissue cultures.