User login
Characterizing Opioid Response in Older Veterans in the Post-Acute Setting
Older adults admitted to post-acute settings frequently have complex rehabilitation needs and multimorbidity, which predisposes them to pain management challenges.1,2 The prevalence of pain in post-acute and long-term care is as high as 65%, and opioid use is common among this population with 1 in 7 residents receiving long-term opioids.3,4
Opioids that do not adequately control pain represent a missed opportunity for deprescribing. There is limited evidence regarding efficacy of long-term opioid use (> 90 days) for improving pain and physical functioning.5 In addition, long-term opioid use carries significant risks, including overdose-related death, dependence, and increased emergency department visits.5 These risks are likely to be pronounced among veterans receiving post-acute care (PAC) who are older, have comorbid psychiatric disorders, are prescribed several centrally acting medications, and experience substance use disorder (SUD).6
Older adults are at increased risk for opioid toxicity because of reduced drug clearance and smaller therapeutic window.5 Centers for Disease Control and Prevention (CDC) guidelines recommend frequently assessing patients for benefit in terms of sustained improvement in pain as well as physical function.5 If pain and functional improvements are minimal, opioid use and nonopioid pain management strategies should be considered. Some patients will struggle with this approach. Directly asking patients about the effectiveness of opioids is challenging. Opioid users with chronic pain frequently report problems with opioids even as they describe them as indispensable for pain management.7,8
Earlier studies have assessed patient perspectives regarding opioid difficulties as well as their helpfulness, which could introduce recall bias. Patient-level factors that contribute to a global sense of distress, in addition to the presence of painful physical conditions, also could contribute to patients requesting opioids without experiencing adequate pain relief. One study in veterans residing in PAC facilities found that individuals with depression, posttraumatic stress disorder (PTSD), and SUD were more likely to report pain and receive scheduled analgesics; this effect persisted in individuals with PTSD even after adjusting for demographic and functional status variables.9 The study looked only at analgesics as a class and did not examine opioids specifically. It is possible that distressed individuals, such as those with uncontrolled depression, PTSD, and SUD, might be more likely to report high pain levels and receive opioids with inadequate benefit and increased risk. Identifying the primary condition causing distress and targeting treatment to that condition (ie, depression) is preferable to escalating opioids in an attempt to treat pain in the context of nonresponse. Assessing an individual’s aggregate response to opioids rather than relying on a single self-report is a useful addition to current pain management strategies.
The goal of this study was to pilot a method of identifying opioid-nonresponsive pain using administrative data, measure its prevalence in a PAC population of veterans, and explore clinical and demographic correlates with particular attention to variates that could indicate high levels of psychological and physical distress. Identifying pain that is poorly responsive to opioids would give clinicians the opportunity to avoid or minimize opioid use and prioritize treatments that are likely to improve the resident’s pain, quality of life, and physical function while minimizing recall bias. We hypothesized that pain that responds poorly to opioids would be prevalent among veterans residing in a PAC unit. We considered that veterans with pain poorly responsive to opioids would be more likely to have factors that would place them at increased risk of adverse effects, such as comorbid psychiatric conditions, history of SUD, and multimorbidity, providing further rationale for clinical equipoise in that population.6
Methods
This was a small, retrospective cross-sectional study using administrative data and chart review. The study included veterans who were administered opioids while residing in a single US Department of Veterans Affairs (VA) community living center PAC (CLC-PAC) unit during at least 1 of 4 nonconsecutive, random days in 2016 and 2017. The study was approved by the institutional review board of the Ann Arbor VA Health System (#2017-1034) as part of a larger project involving models of care in vulnerable older veterans.
Inclusion criteria were the presence of at least moderate pain (≥ 4 on a 0 to 10 scale); receiving ≥ 2 opioids ordered as needed over the prespecified 24-hour observation period; and having ≥ 2 pre-and postopioid administration pain scores during the observation period. Veterans who did not meet these criteria were excluded. At the time of initial sample selection, we did not capture information related to coprescribed analgesics, including a standing order of opioids. To obtain the sample, we initially characterized all veterans on the 4 days residing in the CLC-PAC unit as those reporting at least moderate pain (≥ 4) and those who reported no or mild pain (< 4). The cut point of 4 of 10 is consistent with moderate pain based on earlier work showing higher likelihood of pain that interferes with physical function.10 We then restricted the sample to veterans who received ≥ 2 opioids ordered as needed for pain and had ≥ 2 pre- and postopioid administration numeric pain rating scores during the 24-hour observation period. This methodology was chosen to enrich our sample for those who received opioids regularly for ongoing pain. Opioids were defined as full µ-opioid receptor agonists and included hydrocodone, oxycodone, morphine, hydromorphone, fentanyl, tramadol, and methadone.
Medication administration data were obtained from the VA corporate data warehouse, which houses all barcode medication administration data collected at the point of care. The dataset includes pain scores gathered by nursing staff before and after administering an as-needed analgesic. The corporate data warehouse records data/time of pain scores and the analgesic name, dosage, formulation, and date/time of administration. Using a standardized assessment form developed iteratively, we calculated opioid dosage in oral morphine equivalents (OME) for comparison.11,12 All abstracted data were reexamined for accuracy. Data initially were collected in an anonymized, blinded fashion. Participants were then unblinded for chart review. Initial data was captured in resident-days instead of unique residents because an individual resident might have been admitted on several observation days. We were primarily interested in how pain responded to opioids administered in response to resident request; therefore, we did not examine response to opioids that were continuously ordered (ie, scheduled). We did consider scheduled opioids when calculating total daily opioid dosage during the chart review.
Outcome of Interest
The primary outcome of interest was an individual’s response to as-needed opioids, which we defined as change in the pain score after opioid administration. The pre-opioid pain score was the score that immediately preceded administration of an as-needed opioid. The postopioid administration pain score was the first score after opioid administration if obtained within 3 hours of administration. Scores collected > 3 hours after opioid administration were excluded because they no longer accurately reflected the impact of the opioid due to the short half-lives. Observations were excluded if an opioid was administered without a recorded pain score; this occurred once for 6 individuals. Observations also were excluded if an opioid was administered but the data were captured on the following day (outside of the 24-hour window); this occurred once for 3 individuals.
We calculated a ∆ score by subtracting the postopioid pain rating score from the pre-opioid score. Individual ∆ scores were then averaged over the 24-hour period (range, 2-5 opioid doses). For example, if an individual reported a pre-opioid pain score of 10, and a postopioid pain score of 2, the ∆ was recorded as 8. If the individual’s next pre-opioid score was 10, and post-opioid score was 6, the ∆ was recorded as 4. ∆ scores over the 24-hour period were averaged together to determine that individual’s response to as-needed opioids. In the previous example, the mean ∆ score is 6. Lower mean ∆ scores reflect decreased responsiveness to opioids’ analgesic effect.
Demographic and clinical data were obtained from electronic health record review using a standardized assessment form. These data included information about medical and psychiatric comorbidities, specialist consultations, and CLC-PAC unit admission indications and diagnoses. Medications of interest were categorized as antidepressants, antipsychotics, benzodiazepines, muscle relaxants, hypnotics, stimulants, antiepileptic drugs/mood stabilizers (including gabapentin and pregabalin), and all adjuvant analgesics. Adjuvant analgesics were defined as medications administered for pain as documented by chart notes or those ordered as needed for pain, and analyzed as a composite variable. Antidepressants with analgesic properties (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) were considered adjuvant analgesics. Psychiatric information collected included presence of mood, anxiety, and psychotic disorders, and PTSD. SUD information was collected separately from other psychiatric disorders.
Analyses
The study population was described using tabulations for categorical data and means and standard deviations for continuous data. Responsiveness to opioids was analyzed as a continuous variable. Those with higher mean ∆ scores were considered to have pain relatively more responsive to opioids, while lower mean ∆ scores indicated pain less responsive to opioids. We constructed linear regression models controlling for average pre-opioid pain rating scores to explore associations between opioid responsiveness and variables of interest. All analyses were completed using Stata version 15. This study was not adequately powered to detect differences across the spectrum of opioid responsiveness, although the authors have reported differences in this article.
Results
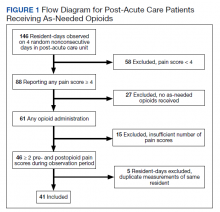
Over the 4-day observational period there were 146 resident-days. Of these, 88 (60.3%) reported at least 1 pain score of ≥ 4. Of those, 61 (41.8%) received ≥ 1 as-needed opioid for pain. We identified 46 resident-days meeting study criteria of ≥ 2 pre- and postanalgesic scores. We identified 41 unique individuals (Figure 1). Two individuals were admitted to the CLC-PAC unit on 2 of the 4 observation days, and 1 individual was admitted to the CLC-PAC unit on 3 of the 4 observation days. For individuals admitted several days, we included data only from the initial observation day.
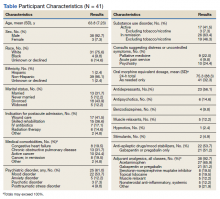
Response to opioids varied greatly in this sample. The mean (SD) ∆ pain score was 3.4 (1.6) and ranged from 0.5 to 6.3. Using linear regression, we found no relationship between admission indication, medical comorbidities (including active cancer), and opioid responsiveness (Table).
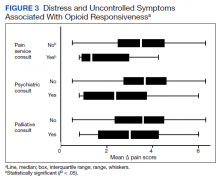
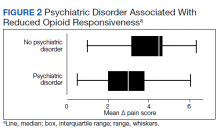
Psychiatric disorders were highly prevalent, with 25 individuals (61.0%) having ≥ 1 any psychiatric diagnosis identified on chart review. The presence of any psychiatric diagnosis was significantly associated with reduced responsiveness to opioids (β = −1.08; 95% CI, −2.04 to −0.13; P = .03). SUDs also were common, with 17 individuals (41.5%) having an active SUD; most were tobacco/nicotine. Twenty-six veterans (63.4%) had documentation of SUD in remission with 19 (46.3%) for substances other than tobacco/nicotine. There was no indication that any veteran in the sample was prescribed medication for opioid use disorder (OUD) at the time of observation. There was no relationship between opioid responsiveness and SUDs, neither active or in remission. Consults to other services that suggested distress or difficult-to-control symptoms also were frequent. Consults to the pain service were significantly associated with reduced responsiveness to opioids (β = −1.75; 95% CI, −3.33 to −0.17; P = .03). Association between psychiatry consultation and reduced opioid responsiveness trended toward significance (β = −0.95; 95% CI, −2.06 to 0.17; P = .09) (Figures 2 and 3). There was no significant association with palliative medicine consultation and opioid responsiveness.
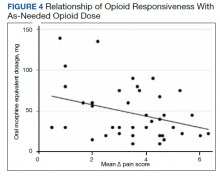
A poorer response to opioids was associated with a significantly higher as-needed opioid dosage (β = −0.02; 95% CI, −0.04 to −0.01; P = .002) as well as a trend toward higher total opioid dosage (β = −0.005; 95% CI, −0.01 to 0.0003; P = .06) (Figure 4). Thirty-eight (92.7%) participants received nonopioid adjuvant analgesics for pain. More than half (56.1%) received antidepressants or gabapentinoids (51.2%), although we did not assess whether they were prescribed for pain or another indication. We did not identify a relationship between any specific psychoactive drug class and opioid responsiveness in this sample.
Discussion
This exploratory study used readily available administrative data in a CLC-PAC unit to assess responsiveness to opioids via a numeric mean ∆ score, with higher values indicating more pain relief in response to opioids. We then constructed linear regression models to characterize the relationship between the mean ∆ score and factors known to be associated with difficult-to-control pain and psychosocial distress. As expected, opioid responsiveness was highly variable among residents; some residents experienced essentially no reduction in pain, on average, despite receiving opioids. Psychiatric comorbidity, higher dosage in OMEs, and the presence of a pain service consult significantly correlated with poorer response to opioids. To our knowledge, this is the first study to quantify opioid responsiveness and describe the relationship with clinical correlates in the understudied PAC population.
Earlier research has demonstrated a relationship between the presence of psychiatric disorders and increased likelihood of receiving any analgesics among veterans residing in PAC.9 Our study adds to the literature by quantifying opioid response using readily available administrative data and examining associations with psychiatric diagnoses. These findings highlight the possibility that attempting to treat high levels of pain by escalating the opioid dosage in patients with a comorbid psychiatric diagnosis should be re-addressed, particularly if there is no meaningful pain reduction at lower opioid dosages. Our sample had a variety of admission diagnoses and medical comorbidities, however, we did not identify a relationship with opioid responsiveness, including an active cancer diagnosis. Although SUDs were highly prevalent in our sample, there was no relationship with opioid responsiveness. This suggests that lack of response to opioids is not merely a matter of drug tolerance or an indication of drug-seeking behavior.
Factors Impacting Response
Many factors could affect whether an individual obtains an adequate analgesic response to opioids or other pain medications, including variations in genes encoding opioid receptors and hepatic enzymes involved in drug metabolism and an individual’s opioid exposure history.13 The phenomenon of requiring more drug to produce the same relief after repeated exposures (ie, tolerance) is well known.14 Opioid-induced hyperalgesia is a phenomenon whereby a patient’s overall pain increases while receiving opioids, but each opioid dose might be perceived as beneficial.15 Increasingly, psychosocial distress is an important factor in opioid response. Adverse selection is the process culminating in those with psychosocial distress and/or SUDs being prescribed more opioids for longer durations.16 Our data suggests that this process could play a role in PAC settings. In addition, exaggerating pain to obtain additional opioids for nonmedical purposes, such as euphoria or relaxation, also is possible.17
When clinically assessing an individual whose pain is not well controlled despite escalating opioid dosages, prescribers must consider which of these factors likely is predominant. However, the first step of determining who has a poor opioid response is not straightforward. Directly asking patients is challenging; many individuals perceive opioids to be helpful while simultaneously reporting inadequately controlled pain.7,8 The primary value of this study is the possibility of providing prescribers a quick, simple method of assessing a patient’s response to opioids. Using this method, individuals who are responding poorly to opioids, including those who might exaggerate pain for secondary gain, could be identified. Health care professionals could consider revisiting pain management strategies, assess for the presence of OUD, or evaluate other contributors to inadequately controlled pain. Although we only collected data regarding response to opioids in this study, any pain medication administered as needed (ie, nonsteroidal anti-inflammatory drugs, acetaminophen) could be analyzed using this methodology, allowing identification of other helpful pain management strategies. We began the validation process with extensive chart review, but further validation is required before this method can be applied to routine clinical practice.
Patients who report uncontrolled pain despite receiving opioids are a clinically challenging population. The traditional strategy has been to escalate opioids, which is recommended by the World Health Organization stepladder approach for patients with cancer pain and limited life expectancy.18 Applying this approach to a general population of patients with chronic pain is ineffective and dangerous.19 The CDC and the VA/US Department of Defense (VA/DoD) guidelines both recommend carefully reassessing risks and benefits at total daily dosages > 50 OME and avoid increasing dosages to > 90 OME daily in most circumstances.5,20 Our finding that participants taking higher dosages of opioids were not more likely to have better control over their pain supports this recommendation.
Limitations
This study has several limitations, the most significant is its small sample size because of the exploratory nature of the project. Results are based on a small pilot sample enriched to include individuals with at least moderate pain who receive opioids frequently at 1 VA CLC-PAC unit; therefore, the results might not be representative of all veterans or a more general population. Our small sample size limits power to detect small differences. Data collected should be used to inform formal power calculations before subsequent larger studies to select adequate sample size. Validation studies, including samples from the same population using different dates, which reproduce findings are an important step. Moreover, we only had data on a single dimension of pain (intensity/severity), as measured by the pain scale, which nursing staff used to make a real-time clinical decision of whether to administer an as-needed opioid. Future studies should consider using pain measures that provide multidimensional assessment (ie, severity, functional interference) and/or were developed specifically for veterans, such as the Defense and Veterans Pain Rating Scale.21
Our study was cross-sectional in nature and addressed a single 24-hour period of data per participant. The years of data collection (2016 and 2017) followed a decline in overall opioid prescribing that has continued, likely influenced by CDC and VA/DoD guidelines.22 It is unclear whether our observations are an accurate reflection of individuals’ response over time or whether prescribing practices in PAC have shifted.
We did not consider the type of pain being treated or explore clinicians’ reasons for prescribing opioids, therefore limiting our ability to know whether opioids were indicated. Information regarding OUD and other SUDs was limited to what was documented in the chart during the CLC-PAC unit admission. We did not have information on length of exposure to opioids. It is possible that opioid tolerance could play a role in reducing opioid responsiveness. However, simple tolerance would not be expected to explain robust correlations with psychiatric comorbidities. Also, simple tolerance would be expected to be overcome with higher opioid dosages, whereas our study demonstrates less responsiveness. These data suggests that some individuals’ pain might be poorly opioid responsive, and psychiatric factors could increase this risk. We used a novel data source in combination with chart review; to our knowledge, barcode medication administration data have not been used in this manner previously. Future work needs to validate this method, using larger sample sizes and several clinical sites. Finally, we used regression models that controlled for average pre-opioid pain rating scores, which is only 1 covariate important for examining effects. Larger studies with adequate power should control for multiple covariates known to be associated with pain and opioid response.
Conclusions
Opioid responsiveness is important clinically yet challenging to assess. This pilot study identifies a way of classifying pain as relatively opioid nonresponsive using administrative data but requires further validation before considering scaling for more general use. The possibility that a substantial percentage of residents in a CLC-PAC unit could be receiving increasing dosages of opioids without adequate benefit justifies the need for more research and underscores the need for prescribers to assess individuals frequently for ongoing benefit of opioids regardless of diagnosis or mechanism of pain.
Acknowledgments
The authors thank Andrzej Galecki, Corey Powell, and the University of Michigan Consulting for Statistics, Computing and Analytics Research Center for assistance with statistical analysis.
1. Marshall TL, Reinhardt JP. Pain management in the last 6 months of life: predictors of opioid and non-opioid use. J Am Med Dir Assoc. 2019;20(6):789-790. doi:10.1016/j.jamda.2019.02.026
2. Tait RC, Chibnall JT. Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 2002;3(3):231-239. doi:10.1046/j.1526-4637.2002.02031.x
3. Pimentel CB, Briesacher BA, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63(4):633-641. doi:10.1111/jgs.13345
4. Hunnicutt JN, Tjia J, Lapane KL. Hospice use and pain management in elderly nursing home residents with cancer. J Pain Symptom Manage. 2017;53(3):561-570. doi:10.1016/j.jpainsymman.2016.10.369
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi:10.15585/mmwr.rr6501e1
6. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
7. Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297-306. doi:10.1093/pm/pnw263
8. Sullivan M, Von Korff M, Banta-Green C. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345-353. doi:10.1016/j.pain.2010.02.037
9. Brennan PL, Greenbaum MA, Lemke S, Schutte KK. Mental health disorder, pain, and pain treatment among long-term care residents: evidence from the Minimum Data Set 3.0. Aging Ment Health. 2019;23(9):1146-1155. doi:10.1080/13607863.2018.1481922
10. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
11. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2017. Accessed December 15, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
12. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. NDARC Technical Report No. 329. National Drug and Alcohol Research Centre; 2014. Accessed December 15, 2021. http://www.drugsandalcohol.ie/22703/1/NDARC Comparing opioids.pdf
13. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237-248.
14. Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991;14(6):465-488. doi:10.1097/00002826-199112000-00001
15. Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122(6):e114-e126. doi:10.1016/j.bja.2018.09.019
16. Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99-104. doi:10.1016/j.genhosppsych.2013.10.003
17. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 2019;170:51-58. Accessed December 15, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
18. World Health Organization. WHO’s cancer pain ladder for adults. Accessed September 21, 2018. www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf
19. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. doi:10.1136/bmj.i20
20. The Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. US Dept of Veterans Affairs and Dept of Defense; 2017. Accessed December 15, 2021. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
21. Defense & Veterans Pain Rating Scale (DVPRS). Defense & Veterans Center for Integrative Pain Management. Accessed July 21, 2021. https://www.dvcipm.org/clinical-resources/defense-veterans-pain-rating-scale-dvprs/
22. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi:10.15585/mmwr.mm6626a4
Older adults admitted to post-acute settings frequently have complex rehabilitation needs and multimorbidity, which predisposes them to pain management challenges.1,2 The prevalence of pain in post-acute and long-term care is as high as 65%, and opioid use is common among this population with 1 in 7 residents receiving long-term opioids.3,4
Opioids that do not adequately control pain represent a missed opportunity for deprescribing. There is limited evidence regarding efficacy of long-term opioid use (> 90 days) for improving pain and physical functioning.5 In addition, long-term opioid use carries significant risks, including overdose-related death, dependence, and increased emergency department visits.5 These risks are likely to be pronounced among veterans receiving post-acute care (PAC) who are older, have comorbid psychiatric disorders, are prescribed several centrally acting medications, and experience substance use disorder (SUD).6
Older adults are at increased risk for opioid toxicity because of reduced drug clearance and smaller therapeutic window.5 Centers for Disease Control and Prevention (CDC) guidelines recommend frequently assessing patients for benefit in terms of sustained improvement in pain as well as physical function.5 If pain and functional improvements are minimal, opioid use and nonopioid pain management strategies should be considered. Some patients will struggle with this approach. Directly asking patients about the effectiveness of opioids is challenging. Opioid users with chronic pain frequently report problems with opioids even as they describe them as indispensable for pain management.7,8
Earlier studies have assessed patient perspectives regarding opioid difficulties as well as their helpfulness, which could introduce recall bias. Patient-level factors that contribute to a global sense of distress, in addition to the presence of painful physical conditions, also could contribute to patients requesting opioids without experiencing adequate pain relief. One study in veterans residing in PAC facilities found that individuals with depression, posttraumatic stress disorder (PTSD), and SUD were more likely to report pain and receive scheduled analgesics; this effect persisted in individuals with PTSD even after adjusting for demographic and functional status variables.9 The study looked only at analgesics as a class and did not examine opioids specifically. It is possible that distressed individuals, such as those with uncontrolled depression, PTSD, and SUD, might be more likely to report high pain levels and receive opioids with inadequate benefit and increased risk. Identifying the primary condition causing distress and targeting treatment to that condition (ie, depression) is preferable to escalating opioids in an attempt to treat pain in the context of nonresponse. Assessing an individual’s aggregate response to opioids rather than relying on a single self-report is a useful addition to current pain management strategies.
The goal of this study was to pilot a method of identifying opioid-nonresponsive pain using administrative data, measure its prevalence in a PAC population of veterans, and explore clinical and demographic correlates with particular attention to variates that could indicate high levels of psychological and physical distress. Identifying pain that is poorly responsive to opioids would give clinicians the opportunity to avoid or minimize opioid use and prioritize treatments that are likely to improve the resident’s pain, quality of life, and physical function while minimizing recall bias. We hypothesized that pain that responds poorly to opioids would be prevalent among veterans residing in a PAC unit. We considered that veterans with pain poorly responsive to opioids would be more likely to have factors that would place them at increased risk of adverse effects, such as comorbid psychiatric conditions, history of SUD, and multimorbidity, providing further rationale for clinical equipoise in that population.6
Methods
This was a small, retrospective cross-sectional study using administrative data and chart review. The study included veterans who were administered opioids while residing in a single US Department of Veterans Affairs (VA) community living center PAC (CLC-PAC) unit during at least 1 of 4 nonconsecutive, random days in 2016 and 2017. The study was approved by the institutional review board of the Ann Arbor VA Health System (#2017-1034) as part of a larger project involving models of care in vulnerable older veterans.
Inclusion criteria were the presence of at least moderate pain (≥ 4 on a 0 to 10 scale); receiving ≥ 2 opioids ordered as needed over the prespecified 24-hour observation period; and having ≥ 2 pre-and postopioid administration pain scores during the observation period. Veterans who did not meet these criteria were excluded. At the time of initial sample selection, we did not capture information related to coprescribed analgesics, including a standing order of opioids. To obtain the sample, we initially characterized all veterans on the 4 days residing in the CLC-PAC unit as those reporting at least moderate pain (≥ 4) and those who reported no or mild pain (< 4). The cut point of 4 of 10 is consistent with moderate pain based on earlier work showing higher likelihood of pain that interferes with physical function.10 We then restricted the sample to veterans who received ≥ 2 opioids ordered as needed for pain and had ≥ 2 pre- and postopioid administration numeric pain rating scores during the 24-hour observation period. This methodology was chosen to enrich our sample for those who received opioids regularly for ongoing pain. Opioids were defined as full µ-opioid receptor agonists and included hydrocodone, oxycodone, morphine, hydromorphone, fentanyl, tramadol, and methadone.
Medication administration data were obtained from the VA corporate data warehouse, which houses all barcode medication administration data collected at the point of care. The dataset includes pain scores gathered by nursing staff before and after administering an as-needed analgesic. The corporate data warehouse records data/time of pain scores and the analgesic name, dosage, formulation, and date/time of administration. Using a standardized assessment form developed iteratively, we calculated opioid dosage in oral morphine equivalents (OME) for comparison.11,12 All abstracted data were reexamined for accuracy. Data initially were collected in an anonymized, blinded fashion. Participants were then unblinded for chart review. Initial data was captured in resident-days instead of unique residents because an individual resident might have been admitted on several observation days. We were primarily interested in how pain responded to opioids administered in response to resident request; therefore, we did not examine response to opioids that were continuously ordered (ie, scheduled). We did consider scheduled opioids when calculating total daily opioid dosage during the chart review.
Outcome of Interest
The primary outcome of interest was an individual’s response to as-needed opioids, which we defined as change in the pain score after opioid administration. The pre-opioid pain score was the score that immediately preceded administration of an as-needed opioid. The postopioid administration pain score was the first score after opioid administration if obtained within 3 hours of administration. Scores collected > 3 hours after opioid administration were excluded because they no longer accurately reflected the impact of the opioid due to the short half-lives. Observations were excluded if an opioid was administered without a recorded pain score; this occurred once for 6 individuals. Observations also were excluded if an opioid was administered but the data were captured on the following day (outside of the 24-hour window); this occurred once for 3 individuals.
We calculated a ∆ score by subtracting the postopioid pain rating score from the pre-opioid score. Individual ∆ scores were then averaged over the 24-hour period (range, 2-5 opioid doses). For example, if an individual reported a pre-opioid pain score of 10, and a postopioid pain score of 2, the ∆ was recorded as 8. If the individual’s next pre-opioid score was 10, and post-opioid score was 6, the ∆ was recorded as 4. ∆ scores over the 24-hour period were averaged together to determine that individual’s response to as-needed opioids. In the previous example, the mean ∆ score is 6. Lower mean ∆ scores reflect decreased responsiveness to opioids’ analgesic effect.
Demographic and clinical data were obtained from electronic health record review using a standardized assessment form. These data included information about medical and psychiatric comorbidities, specialist consultations, and CLC-PAC unit admission indications and diagnoses. Medications of interest were categorized as antidepressants, antipsychotics, benzodiazepines, muscle relaxants, hypnotics, stimulants, antiepileptic drugs/mood stabilizers (including gabapentin and pregabalin), and all adjuvant analgesics. Adjuvant analgesics were defined as medications administered for pain as documented by chart notes or those ordered as needed for pain, and analyzed as a composite variable. Antidepressants with analgesic properties (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) were considered adjuvant analgesics. Psychiatric information collected included presence of mood, anxiety, and psychotic disorders, and PTSD. SUD information was collected separately from other psychiatric disorders.
Analyses
The study population was described using tabulations for categorical data and means and standard deviations for continuous data. Responsiveness to opioids was analyzed as a continuous variable. Those with higher mean ∆ scores were considered to have pain relatively more responsive to opioids, while lower mean ∆ scores indicated pain less responsive to opioids. We constructed linear regression models controlling for average pre-opioid pain rating scores to explore associations between opioid responsiveness and variables of interest. All analyses were completed using Stata version 15. This study was not adequately powered to detect differences across the spectrum of opioid responsiveness, although the authors have reported differences in this article.
Results
Over the 4-day observational period there were 146 resident-days. Of these, 88 (60.3%) reported at least 1 pain score of ≥ 4. Of those, 61 (41.8%) received ≥ 1 as-needed opioid for pain. We identified 46 resident-days meeting study criteria of ≥ 2 pre- and postanalgesic scores. We identified 41 unique individuals (Figure 1). Two individuals were admitted to the CLC-PAC unit on 2 of the 4 observation days, and 1 individual was admitted to the CLC-PAC unit on 3 of the 4 observation days. For individuals admitted several days, we included data only from the initial observation day.
Response to opioids varied greatly in this sample. The mean (SD) ∆ pain score was 3.4 (1.6) and ranged from 0.5 to 6.3. Using linear regression, we found no relationship between admission indication, medical comorbidities (including active cancer), and opioid responsiveness (Table).
Psychiatric disorders were highly prevalent, with 25 individuals (61.0%) having ≥ 1 any psychiatric diagnosis identified on chart review. The presence of any psychiatric diagnosis was significantly associated with reduced responsiveness to opioids (β = −1.08; 95% CI, −2.04 to −0.13; P = .03). SUDs also were common, with 17 individuals (41.5%) having an active SUD; most were tobacco/nicotine. Twenty-six veterans (63.4%) had documentation of SUD in remission with 19 (46.3%) for substances other than tobacco/nicotine. There was no indication that any veteran in the sample was prescribed medication for opioid use disorder (OUD) at the time of observation. There was no relationship between opioid responsiveness and SUDs, neither active or in remission. Consults to other services that suggested distress or difficult-to-control symptoms also were frequent. Consults to the pain service were significantly associated with reduced responsiveness to opioids (β = −1.75; 95% CI, −3.33 to −0.17; P = .03). Association between psychiatry consultation and reduced opioid responsiveness trended toward significance (β = −0.95; 95% CI, −2.06 to 0.17; P = .09) (Figures 2 and 3). There was no significant association with palliative medicine consultation and opioid responsiveness.
A poorer response to opioids was associated with a significantly higher as-needed opioid dosage (β = −0.02; 95% CI, −0.04 to −0.01; P = .002) as well as a trend toward higher total opioid dosage (β = −0.005; 95% CI, −0.01 to 0.0003; P = .06) (Figure 4). Thirty-eight (92.7%) participants received nonopioid adjuvant analgesics for pain. More than half (56.1%) received antidepressants or gabapentinoids (51.2%), although we did not assess whether they were prescribed for pain or another indication. We did not identify a relationship between any specific psychoactive drug class and opioid responsiveness in this sample.
Discussion
This exploratory study used readily available administrative data in a CLC-PAC unit to assess responsiveness to opioids via a numeric mean ∆ score, with higher values indicating more pain relief in response to opioids. We then constructed linear regression models to characterize the relationship between the mean ∆ score and factors known to be associated with difficult-to-control pain and psychosocial distress. As expected, opioid responsiveness was highly variable among residents; some residents experienced essentially no reduction in pain, on average, despite receiving opioids. Psychiatric comorbidity, higher dosage in OMEs, and the presence of a pain service consult significantly correlated with poorer response to opioids. To our knowledge, this is the first study to quantify opioid responsiveness and describe the relationship with clinical correlates in the understudied PAC population.
Earlier research has demonstrated a relationship between the presence of psychiatric disorders and increased likelihood of receiving any analgesics among veterans residing in PAC.9 Our study adds to the literature by quantifying opioid response using readily available administrative data and examining associations with psychiatric diagnoses. These findings highlight the possibility that attempting to treat high levels of pain by escalating the opioid dosage in patients with a comorbid psychiatric diagnosis should be re-addressed, particularly if there is no meaningful pain reduction at lower opioid dosages. Our sample had a variety of admission diagnoses and medical comorbidities, however, we did not identify a relationship with opioid responsiveness, including an active cancer diagnosis. Although SUDs were highly prevalent in our sample, there was no relationship with opioid responsiveness. This suggests that lack of response to opioids is not merely a matter of drug tolerance or an indication of drug-seeking behavior.
Factors Impacting Response
Many factors could affect whether an individual obtains an adequate analgesic response to opioids or other pain medications, including variations in genes encoding opioid receptors and hepatic enzymes involved in drug metabolism and an individual’s opioid exposure history.13 The phenomenon of requiring more drug to produce the same relief after repeated exposures (ie, tolerance) is well known.14 Opioid-induced hyperalgesia is a phenomenon whereby a patient’s overall pain increases while receiving opioids, but each opioid dose might be perceived as beneficial.15 Increasingly, psychosocial distress is an important factor in opioid response. Adverse selection is the process culminating in those with psychosocial distress and/or SUDs being prescribed more opioids for longer durations.16 Our data suggests that this process could play a role in PAC settings. In addition, exaggerating pain to obtain additional opioids for nonmedical purposes, such as euphoria or relaxation, also is possible.17
When clinically assessing an individual whose pain is not well controlled despite escalating opioid dosages, prescribers must consider which of these factors likely is predominant. However, the first step of determining who has a poor opioid response is not straightforward. Directly asking patients is challenging; many individuals perceive opioids to be helpful while simultaneously reporting inadequately controlled pain.7,8 The primary value of this study is the possibility of providing prescribers a quick, simple method of assessing a patient’s response to opioids. Using this method, individuals who are responding poorly to opioids, including those who might exaggerate pain for secondary gain, could be identified. Health care professionals could consider revisiting pain management strategies, assess for the presence of OUD, or evaluate other contributors to inadequately controlled pain. Although we only collected data regarding response to opioids in this study, any pain medication administered as needed (ie, nonsteroidal anti-inflammatory drugs, acetaminophen) could be analyzed using this methodology, allowing identification of other helpful pain management strategies. We began the validation process with extensive chart review, but further validation is required before this method can be applied to routine clinical practice.
Patients who report uncontrolled pain despite receiving opioids are a clinically challenging population. The traditional strategy has been to escalate opioids, which is recommended by the World Health Organization stepladder approach for patients with cancer pain and limited life expectancy.18 Applying this approach to a general population of patients with chronic pain is ineffective and dangerous.19 The CDC and the VA/US Department of Defense (VA/DoD) guidelines both recommend carefully reassessing risks and benefits at total daily dosages > 50 OME and avoid increasing dosages to > 90 OME daily in most circumstances.5,20 Our finding that participants taking higher dosages of opioids were not more likely to have better control over their pain supports this recommendation.
Limitations
This study has several limitations, the most significant is its small sample size because of the exploratory nature of the project. Results are based on a small pilot sample enriched to include individuals with at least moderate pain who receive opioids frequently at 1 VA CLC-PAC unit; therefore, the results might not be representative of all veterans or a more general population. Our small sample size limits power to detect small differences. Data collected should be used to inform formal power calculations before subsequent larger studies to select adequate sample size. Validation studies, including samples from the same population using different dates, which reproduce findings are an important step. Moreover, we only had data on a single dimension of pain (intensity/severity), as measured by the pain scale, which nursing staff used to make a real-time clinical decision of whether to administer an as-needed opioid. Future studies should consider using pain measures that provide multidimensional assessment (ie, severity, functional interference) and/or were developed specifically for veterans, such as the Defense and Veterans Pain Rating Scale.21
Our study was cross-sectional in nature and addressed a single 24-hour period of data per participant. The years of data collection (2016 and 2017) followed a decline in overall opioid prescribing that has continued, likely influenced by CDC and VA/DoD guidelines.22 It is unclear whether our observations are an accurate reflection of individuals’ response over time or whether prescribing practices in PAC have shifted.
We did not consider the type of pain being treated or explore clinicians’ reasons for prescribing opioids, therefore limiting our ability to know whether opioids were indicated. Information regarding OUD and other SUDs was limited to what was documented in the chart during the CLC-PAC unit admission. We did not have information on length of exposure to opioids. It is possible that opioid tolerance could play a role in reducing opioid responsiveness. However, simple tolerance would not be expected to explain robust correlations with psychiatric comorbidities. Also, simple tolerance would be expected to be overcome with higher opioid dosages, whereas our study demonstrates less responsiveness. These data suggests that some individuals’ pain might be poorly opioid responsive, and psychiatric factors could increase this risk. We used a novel data source in combination with chart review; to our knowledge, barcode medication administration data have not been used in this manner previously. Future work needs to validate this method, using larger sample sizes and several clinical sites. Finally, we used regression models that controlled for average pre-opioid pain rating scores, which is only 1 covariate important for examining effects. Larger studies with adequate power should control for multiple covariates known to be associated with pain and opioid response.
Conclusions
Opioid responsiveness is important clinically yet challenging to assess. This pilot study identifies a way of classifying pain as relatively opioid nonresponsive using administrative data but requires further validation before considering scaling for more general use. The possibility that a substantial percentage of residents in a CLC-PAC unit could be receiving increasing dosages of opioids without adequate benefit justifies the need for more research and underscores the need for prescribers to assess individuals frequently for ongoing benefit of opioids regardless of diagnosis or mechanism of pain.
Acknowledgments
The authors thank Andrzej Galecki, Corey Powell, and the University of Michigan Consulting for Statistics, Computing and Analytics Research Center for assistance with statistical analysis.
Older adults admitted to post-acute settings frequently have complex rehabilitation needs and multimorbidity, which predisposes them to pain management challenges.1,2 The prevalence of pain in post-acute and long-term care is as high as 65%, and opioid use is common among this population with 1 in 7 residents receiving long-term opioids.3,4
Opioids that do not adequately control pain represent a missed opportunity for deprescribing. There is limited evidence regarding efficacy of long-term opioid use (> 90 days) for improving pain and physical functioning.5 In addition, long-term opioid use carries significant risks, including overdose-related death, dependence, and increased emergency department visits.5 These risks are likely to be pronounced among veterans receiving post-acute care (PAC) who are older, have comorbid psychiatric disorders, are prescribed several centrally acting medications, and experience substance use disorder (SUD).6
Older adults are at increased risk for opioid toxicity because of reduced drug clearance and smaller therapeutic window.5 Centers for Disease Control and Prevention (CDC) guidelines recommend frequently assessing patients for benefit in terms of sustained improvement in pain as well as physical function.5 If pain and functional improvements are minimal, opioid use and nonopioid pain management strategies should be considered. Some patients will struggle with this approach. Directly asking patients about the effectiveness of opioids is challenging. Opioid users with chronic pain frequently report problems with opioids even as they describe them as indispensable for pain management.7,8
Earlier studies have assessed patient perspectives regarding opioid difficulties as well as their helpfulness, which could introduce recall bias. Patient-level factors that contribute to a global sense of distress, in addition to the presence of painful physical conditions, also could contribute to patients requesting opioids without experiencing adequate pain relief. One study in veterans residing in PAC facilities found that individuals with depression, posttraumatic stress disorder (PTSD), and SUD were more likely to report pain and receive scheduled analgesics; this effect persisted in individuals with PTSD even after adjusting for demographic and functional status variables.9 The study looked only at analgesics as a class and did not examine opioids specifically. It is possible that distressed individuals, such as those with uncontrolled depression, PTSD, and SUD, might be more likely to report high pain levels and receive opioids with inadequate benefit and increased risk. Identifying the primary condition causing distress and targeting treatment to that condition (ie, depression) is preferable to escalating opioids in an attempt to treat pain in the context of nonresponse. Assessing an individual’s aggregate response to opioids rather than relying on a single self-report is a useful addition to current pain management strategies.
The goal of this study was to pilot a method of identifying opioid-nonresponsive pain using administrative data, measure its prevalence in a PAC population of veterans, and explore clinical and demographic correlates with particular attention to variates that could indicate high levels of psychological and physical distress. Identifying pain that is poorly responsive to opioids would give clinicians the opportunity to avoid or minimize opioid use and prioritize treatments that are likely to improve the resident’s pain, quality of life, and physical function while minimizing recall bias. We hypothesized that pain that responds poorly to opioids would be prevalent among veterans residing in a PAC unit. We considered that veterans with pain poorly responsive to opioids would be more likely to have factors that would place them at increased risk of adverse effects, such as comorbid psychiatric conditions, history of SUD, and multimorbidity, providing further rationale for clinical equipoise in that population.6
Methods
This was a small, retrospective cross-sectional study using administrative data and chart review. The study included veterans who were administered opioids while residing in a single US Department of Veterans Affairs (VA) community living center PAC (CLC-PAC) unit during at least 1 of 4 nonconsecutive, random days in 2016 and 2017. The study was approved by the institutional review board of the Ann Arbor VA Health System (#2017-1034) as part of a larger project involving models of care in vulnerable older veterans.
Inclusion criteria were the presence of at least moderate pain (≥ 4 on a 0 to 10 scale); receiving ≥ 2 opioids ordered as needed over the prespecified 24-hour observation period; and having ≥ 2 pre-and postopioid administration pain scores during the observation period. Veterans who did not meet these criteria were excluded. At the time of initial sample selection, we did not capture information related to coprescribed analgesics, including a standing order of opioids. To obtain the sample, we initially characterized all veterans on the 4 days residing in the CLC-PAC unit as those reporting at least moderate pain (≥ 4) and those who reported no or mild pain (< 4). The cut point of 4 of 10 is consistent with moderate pain based on earlier work showing higher likelihood of pain that interferes with physical function.10 We then restricted the sample to veterans who received ≥ 2 opioids ordered as needed for pain and had ≥ 2 pre- and postopioid administration numeric pain rating scores during the 24-hour observation period. This methodology was chosen to enrich our sample for those who received opioids regularly for ongoing pain. Opioids were defined as full µ-opioid receptor agonists and included hydrocodone, oxycodone, morphine, hydromorphone, fentanyl, tramadol, and methadone.
Medication administration data were obtained from the VA corporate data warehouse, which houses all barcode medication administration data collected at the point of care. The dataset includes pain scores gathered by nursing staff before and after administering an as-needed analgesic. The corporate data warehouse records data/time of pain scores and the analgesic name, dosage, formulation, and date/time of administration. Using a standardized assessment form developed iteratively, we calculated opioid dosage in oral morphine equivalents (OME) for comparison.11,12 All abstracted data were reexamined for accuracy. Data initially were collected in an anonymized, blinded fashion. Participants were then unblinded for chart review. Initial data was captured in resident-days instead of unique residents because an individual resident might have been admitted on several observation days. We were primarily interested in how pain responded to opioids administered in response to resident request; therefore, we did not examine response to opioids that were continuously ordered (ie, scheduled). We did consider scheduled opioids when calculating total daily opioid dosage during the chart review.
Outcome of Interest
The primary outcome of interest was an individual’s response to as-needed opioids, which we defined as change in the pain score after opioid administration. The pre-opioid pain score was the score that immediately preceded administration of an as-needed opioid. The postopioid administration pain score was the first score after opioid administration if obtained within 3 hours of administration. Scores collected > 3 hours after opioid administration were excluded because they no longer accurately reflected the impact of the opioid due to the short half-lives. Observations were excluded if an opioid was administered without a recorded pain score; this occurred once for 6 individuals. Observations also were excluded if an opioid was administered but the data were captured on the following day (outside of the 24-hour window); this occurred once for 3 individuals.
We calculated a ∆ score by subtracting the postopioid pain rating score from the pre-opioid score. Individual ∆ scores were then averaged over the 24-hour period (range, 2-5 opioid doses). For example, if an individual reported a pre-opioid pain score of 10, and a postopioid pain score of 2, the ∆ was recorded as 8. If the individual’s next pre-opioid score was 10, and post-opioid score was 6, the ∆ was recorded as 4. ∆ scores over the 24-hour period were averaged together to determine that individual’s response to as-needed opioids. In the previous example, the mean ∆ score is 6. Lower mean ∆ scores reflect decreased responsiveness to opioids’ analgesic effect.
Demographic and clinical data were obtained from electronic health record review using a standardized assessment form. These data included information about medical and psychiatric comorbidities, specialist consultations, and CLC-PAC unit admission indications and diagnoses. Medications of interest were categorized as antidepressants, antipsychotics, benzodiazepines, muscle relaxants, hypnotics, stimulants, antiepileptic drugs/mood stabilizers (including gabapentin and pregabalin), and all adjuvant analgesics. Adjuvant analgesics were defined as medications administered for pain as documented by chart notes or those ordered as needed for pain, and analyzed as a composite variable. Antidepressants with analgesic properties (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) were considered adjuvant analgesics. Psychiatric information collected included presence of mood, anxiety, and psychotic disorders, and PTSD. SUD information was collected separately from other psychiatric disorders.
Analyses
The study population was described using tabulations for categorical data and means and standard deviations for continuous data. Responsiveness to opioids was analyzed as a continuous variable. Those with higher mean ∆ scores were considered to have pain relatively more responsive to opioids, while lower mean ∆ scores indicated pain less responsive to opioids. We constructed linear regression models controlling for average pre-opioid pain rating scores to explore associations between opioid responsiveness and variables of interest. All analyses were completed using Stata version 15. This study was not adequately powered to detect differences across the spectrum of opioid responsiveness, although the authors have reported differences in this article.
Results
Over the 4-day observational period there were 146 resident-days. Of these, 88 (60.3%) reported at least 1 pain score of ≥ 4. Of those, 61 (41.8%) received ≥ 1 as-needed opioid for pain. We identified 46 resident-days meeting study criteria of ≥ 2 pre- and postanalgesic scores. We identified 41 unique individuals (Figure 1). Two individuals were admitted to the CLC-PAC unit on 2 of the 4 observation days, and 1 individual was admitted to the CLC-PAC unit on 3 of the 4 observation days. For individuals admitted several days, we included data only from the initial observation day.
Response to opioids varied greatly in this sample. The mean (SD) ∆ pain score was 3.4 (1.6) and ranged from 0.5 to 6.3. Using linear regression, we found no relationship between admission indication, medical comorbidities (including active cancer), and opioid responsiveness (Table).
Psychiatric disorders were highly prevalent, with 25 individuals (61.0%) having ≥ 1 any psychiatric diagnosis identified on chart review. The presence of any psychiatric diagnosis was significantly associated with reduced responsiveness to opioids (β = −1.08; 95% CI, −2.04 to −0.13; P = .03). SUDs also were common, with 17 individuals (41.5%) having an active SUD; most were tobacco/nicotine. Twenty-six veterans (63.4%) had documentation of SUD in remission with 19 (46.3%) for substances other than tobacco/nicotine. There was no indication that any veteran in the sample was prescribed medication for opioid use disorder (OUD) at the time of observation. There was no relationship between opioid responsiveness and SUDs, neither active or in remission. Consults to other services that suggested distress or difficult-to-control symptoms also were frequent. Consults to the pain service were significantly associated with reduced responsiveness to opioids (β = −1.75; 95% CI, −3.33 to −0.17; P = .03). Association between psychiatry consultation and reduced opioid responsiveness trended toward significance (β = −0.95; 95% CI, −2.06 to 0.17; P = .09) (Figures 2 and 3). There was no significant association with palliative medicine consultation and opioid responsiveness.
A poorer response to opioids was associated with a significantly higher as-needed opioid dosage (β = −0.02; 95% CI, −0.04 to −0.01; P = .002) as well as a trend toward higher total opioid dosage (β = −0.005; 95% CI, −0.01 to 0.0003; P = .06) (Figure 4). Thirty-eight (92.7%) participants received nonopioid adjuvant analgesics for pain. More than half (56.1%) received antidepressants or gabapentinoids (51.2%), although we did not assess whether they were prescribed for pain or another indication. We did not identify a relationship between any specific psychoactive drug class and opioid responsiveness in this sample.
Discussion
This exploratory study used readily available administrative data in a CLC-PAC unit to assess responsiveness to opioids via a numeric mean ∆ score, with higher values indicating more pain relief in response to opioids. We then constructed linear regression models to characterize the relationship between the mean ∆ score and factors known to be associated with difficult-to-control pain and psychosocial distress. As expected, opioid responsiveness was highly variable among residents; some residents experienced essentially no reduction in pain, on average, despite receiving opioids. Psychiatric comorbidity, higher dosage in OMEs, and the presence of a pain service consult significantly correlated with poorer response to opioids. To our knowledge, this is the first study to quantify opioid responsiveness and describe the relationship with clinical correlates in the understudied PAC population.
Earlier research has demonstrated a relationship between the presence of psychiatric disorders and increased likelihood of receiving any analgesics among veterans residing in PAC.9 Our study adds to the literature by quantifying opioid response using readily available administrative data and examining associations with psychiatric diagnoses. These findings highlight the possibility that attempting to treat high levels of pain by escalating the opioid dosage in patients with a comorbid psychiatric diagnosis should be re-addressed, particularly if there is no meaningful pain reduction at lower opioid dosages. Our sample had a variety of admission diagnoses and medical comorbidities, however, we did not identify a relationship with opioid responsiveness, including an active cancer diagnosis. Although SUDs were highly prevalent in our sample, there was no relationship with opioid responsiveness. This suggests that lack of response to opioids is not merely a matter of drug tolerance or an indication of drug-seeking behavior.
Factors Impacting Response
Many factors could affect whether an individual obtains an adequate analgesic response to opioids or other pain medications, including variations in genes encoding opioid receptors and hepatic enzymes involved in drug metabolism and an individual’s opioid exposure history.13 The phenomenon of requiring more drug to produce the same relief after repeated exposures (ie, tolerance) is well known.14 Opioid-induced hyperalgesia is a phenomenon whereby a patient’s overall pain increases while receiving opioids, but each opioid dose might be perceived as beneficial.15 Increasingly, psychosocial distress is an important factor in opioid response. Adverse selection is the process culminating in those with psychosocial distress and/or SUDs being prescribed more opioids for longer durations.16 Our data suggests that this process could play a role in PAC settings. In addition, exaggerating pain to obtain additional opioids for nonmedical purposes, such as euphoria or relaxation, also is possible.17
When clinically assessing an individual whose pain is not well controlled despite escalating opioid dosages, prescribers must consider which of these factors likely is predominant. However, the first step of determining who has a poor opioid response is not straightforward. Directly asking patients is challenging; many individuals perceive opioids to be helpful while simultaneously reporting inadequately controlled pain.7,8 The primary value of this study is the possibility of providing prescribers a quick, simple method of assessing a patient’s response to opioids. Using this method, individuals who are responding poorly to opioids, including those who might exaggerate pain for secondary gain, could be identified. Health care professionals could consider revisiting pain management strategies, assess for the presence of OUD, or evaluate other contributors to inadequately controlled pain. Although we only collected data regarding response to opioids in this study, any pain medication administered as needed (ie, nonsteroidal anti-inflammatory drugs, acetaminophen) could be analyzed using this methodology, allowing identification of other helpful pain management strategies. We began the validation process with extensive chart review, but further validation is required before this method can be applied to routine clinical practice.
Patients who report uncontrolled pain despite receiving opioids are a clinically challenging population. The traditional strategy has been to escalate opioids, which is recommended by the World Health Organization stepladder approach for patients with cancer pain and limited life expectancy.18 Applying this approach to a general population of patients with chronic pain is ineffective and dangerous.19 The CDC and the VA/US Department of Defense (VA/DoD) guidelines both recommend carefully reassessing risks and benefits at total daily dosages > 50 OME and avoid increasing dosages to > 90 OME daily in most circumstances.5,20 Our finding that participants taking higher dosages of opioids were not more likely to have better control over their pain supports this recommendation.
Limitations
This study has several limitations, the most significant is its small sample size because of the exploratory nature of the project. Results are based on a small pilot sample enriched to include individuals with at least moderate pain who receive opioids frequently at 1 VA CLC-PAC unit; therefore, the results might not be representative of all veterans or a more general population. Our small sample size limits power to detect small differences. Data collected should be used to inform formal power calculations before subsequent larger studies to select adequate sample size. Validation studies, including samples from the same population using different dates, which reproduce findings are an important step. Moreover, we only had data on a single dimension of pain (intensity/severity), as measured by the pain scale, which nursing staff used to make a real-time clinical decision of whether to administer an as-needed opioid. Future studies should consider using pain measures that provide multidimensional assessment (ie, severity, functional interference) and/or were developed specifically for veterans, such as the Defense and Veterans Pain Rating Scale.21
Our study was cross-sectional in nature and addressed a single 24-hour period of data per participant. The years of data collection (2016 and 2017) followed a decline in overall opioid prescribing that has continued, likely influenced by CDC and VA/DoD guidelines.22 It is unclear whether our observations are an accurate reflection of individuals’ response over time or whether prescribing practices in PAC have shifted.
We did not consider the type of pain being treated or explore clinicians’ reasons for prescribing opioids, therefore limiting our ability to know whether opioids were indicated. Information regarding OUD and other SUDs was limited to what was documented in the chart during the CLC-PAC unit admission. We did not have information on length of exposure to opioids. It is possible that opioid tolerance could play a role in reducing opioid responsiveness. However, simple tolerance would not be expected to explain robust correlations with psychiatric comorbidities. Also, simple tolerance would be expected to be overcome with higher opioid dosages, whereas our study demonstrates less responsiveness. These data suggests that some individuals’ pain might be poorly opioid responsive, and psychiatric factors could increase this risk. We used a novel data source in combination with chart review; to our knowledge, barcode medication administration data have not been used in this manner previously. Future work needs to validate this method, using larger sample sizes and several clinical sites. Finally, we used regression models that controlled for average pre-opioid pain rating scores, which is only 1 covariate important for examining effects. Larger studies with adequate power should control for multiple covariates known to be associated with pain and opioid response.
Conclusions
Opioid responsiveness is important clinically yet challenging to assess. This pilot study identifies a way of classifying pain as relatively opioid nonresponsive using administrative data but requires further validation before considering scaling for more general use. The possibility that a substantial percentage of residents in a CLC-PAC unit could be receiving increasing dosages of opioids without adequate benefit justifies the need for more research and underscores the need for prescribers to assess individuals frequently for ongoing benefit of opioids regardless of diagnosis or mechanism of pain.
Acknowledgments
The authors thank Andrzej Galecki, Corey Powell, and the University of Michigan Consulting for Statistics, Computing and Analytics Research Center for assistance with statistical analysis.
1. Marshall TL, Reinhardt JP. Pain management in the last 6 months of life: predictors of opioid and non-opioid use. J Am Med Dir Assoc. 2019;20(6):789-790. doi:10.1016/j.jamda.2019.02.026
2. Tait RC, Chibnall JT. Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 2002;3(3):231-239. doi:10.1046/j.1526-4637.2002.02031.x
3. Pimentel CB, Briesacher BA, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63(4):633-641. doi:10.1111/jgs.13345
4. Hunnicutt JN, Tjia J, Lapane KL. Hospice use and pain management in elderly nursing home residents with cancer. J Pain Symptom Manage. 2017;53(3):561-570. doi:10.1016/j.jpainsymman.2016.10.369
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi:10.15585/mmwr.rr6501e1
6. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
7. Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297-306. doi:10.1093/pm/pnw263
8. Sullivan M, Von Korff M, Banta-Green C. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345-353. doi:10.1016/j.pain.2010.02.037
9. Brennan PL, Greenbaum MA, Lemke S, Schutte KK. Mental health disorder, pain, and pain treatment among long-term care residents: evidence from the Minimum Data Set 3.0. Aging Ment Health. 2019;23(9):1146-1155. doi:10.1080/13607863.2018.1481922
10. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
11. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2017. Accessed December 15, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
12. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. NDARC Technical Report No. 329. National Drug and Alcohol Research Centre; 2014. Accessed December 15, 2021. http://www.drugsandalcohol.ie/22703/1/NDARC Comparing opioids.pdf
13. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237-248.
14. Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991;14(6):465-488. doi:10.1097/00002826-199112000-00001
15. Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122(6):e114-e126. doi:10.1016/j.bja.2018.09.019
16. Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99-104. doi:10.1016/j.genhosppsych.2013.10.003
17. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 2019;170:51-58. Accessed December 15, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
18. World Health Organization. WHO’s cancer pain ladder for adults. Accessed September 21, 2018. www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf
19. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. doi:10.1136/bmj.i20
20. The Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. US Dept of Veterans Affairs and Dept of Defense; 2017. Accessed December 15, 2021. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
21. Defense & Veterans Pain Rating Scale (DVPRS). Defense & Veterans Center for Integrative Pain Management. Accessed July 21, 2021. https://www.dvcipm.org/clinical-resources/defense-veterans-pain-rating-scale-dvprs/
22. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi:10.15585/mmwr.mm6626a4
1. Marshall TL, Reinhardt JP. Pain management in the last 6 months of life: predictors of opioid and non-opioid use. J Am Med Dir Assoc. 2019;20(6):789-790. doi:10.1016/j.jamda.2019.02.026
2. Tait RC, Chibnall JT. Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 2002;3(3):231-239. doi:10.1046/j.1526-4637.2002.02031.x
3. Pimentel CB, Briesacher BA, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63(4):633-641. doi:10.1111/jgs.13345
4. Hunnicutt JN, Tjia J, Lapane KL. Hospice use and pain management in elderly nursing home residents with cancer. J Pain Symptom Manage. 2017;53(3):561-570. doi:10.1016/j.jpainsymman.2016.10.369
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi:10.15585/mmwr.rr6501e1
6. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
7. Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297-306. doi:10.1093/pm/pnw263
8. Sullivan M, Von Korff M, Banta-Green C. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345-353. doi:10.1016/j.pain.2010.02.037
9. Brennan PL, Greenbaum MA, Lemke S, Schutte KK. Mental health disorder, pain, and pain treatment among long-term care residents: evidence from the Minimum Data Set 3.0. Aging Ment Health. 2019;23(9):1146-1155. doi:10.1080/13607863.2018.1481922
10. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
11. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2017. Accessed December 15, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
12. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. NDARC Technical Report No. 329. National Drug and Alcohol Research Centre; 2014. Accessed December 15, 2021. http://www.drugsandalcohol.ie/22703/1/NDARC Comparing opioids.pdf
13. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237-248.
14. Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991;14(6):465-488. doi:10.1097/00002826-199112000-00001
15. Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122(6):e114-e126. doi:10.1016/j.bja.2018.09.019
16. Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99-104. doi:10.1016/j.genhosppsych.2013.10.003
17. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 2019;170:51-58. Accessed December 15, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
18. World Health Organization. WHO’s cancer pain ladder for adults. Accessed September 21, 2018. www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf
19. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. doi:10.1136/bmj.i20
20. The Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. US Dept of Veterans Affairs and Dept of Defense; 2017. Accessed December 15, 2021. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
21. Defense & Veterans Pain Rating Scale (DVPRS). Defense & Veterans Center for Integrative Pain Management. Accessed July 21, 2021. https://www.dvcipm.org/clinical-resources/defense-veterans-pain-rating-scale-dvprs/
22. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi:10.15585/mmwr.mm6626a4
End‐of‐Life Discussions
Most patients prefer to die at home, pain free and without the use of life‐sustaining treatments[1, 2] yet the majority of patients with serious illness die in the hospital.[3, 4, 5] When hospitalized patient die, the care is frequently focused on life‐sustaining treatments[6]; pain, dyspnea, and agitation levels are higher when compared with patients who die in non‐hospital settings.[7, 8, 9] End‐of‐life discussions and their products (ie, advanced directives) can clarify treatment options with patients and family,[10] and help ensure that patients receive care consistent with their beliefs.[11, 12, 13] End‐of‐life discussions are associated with a decrease use of life‐sustaining treatments, improved quality of life, and reduced costs of care.[14, 15] For the majority of patients dying of cancer, the first end‐of‐life discussion takes place in the hospital setting.[16]
Conducting end‐of‐life conversations in the hospital setting can be challenging. Patients are acutely ill and nearly 40% are incapable of making their own medical decisions.[17] In order to participate in an end‐of‐life discussion, a physician must determine that a patient meets the 4 criteria of decisional capacity as outlined by Appelbaum and Grisso:[18] Does the patient (1) communicate a clear and consistent choice; (2) understand the relevant information surrounding that decision; (3) appreciate the consequences of that decision; and (4) communicate reasoning for that decision?[19] In practice, however, clinicians inaccurately assign capacity up to 25% of the time.[17] When a physician determines, accurately or inaccurately, that the patient does not meet this standard for decisional capacity, discussions must be held instead with a surrogate decision‐maker. Surrogate decision‐making can make communication with the physician more difficult,[20] delay important medical decisions,[21] and be stressful on the decision‐maker.[22] To our knowledge, no studies have examined patient and surrogate participation in end‐of‐life discussions at the time of terminal hospitalization and its association with end‐of‐life treatments received.
Our goals were to examine physician assessment of decisional capacity and the prevalence of end‐of‐life discussions during the terminal hospitalization of patients with advanced cancer. Our research questions were: (1) What proportion of patients were assessed to have decisional capacity by the clinical team at the time of their terminal hospital admission? (2) What proportion of these patients had a documented discussion about end‐of‐life care with the clinical team, and for what proportion was the conversation held instead by the patient's surrogate decision‐maker because the patient was considered to have lost decisional capacity? (3) Was patient participation in a discussion about end‐of‐life care associated with life‐sustaining and palliative treatments received?
METHODS
Design
This is a retrospective cohort study of consecutive adult patients with advanced cancer who died at the University of Michigan Hospital, Ann Arbor, MI, from January 1, 2004 through December 31, 2007. This study was exempted from review by the University of Michigan (UM) Institutional Review Board because decedent data were used.
Sample
The UM Cancer Registry is a database of all cancer patients treated at the UM Comprehensive Cancer Center. We used the Registry to identify patients who met the following criteria: (1) age 18 years at time of cancer diagnosis; (2) estimated probability of 5‐year survival 20% at time of diagnosis, as predicted by the SEER Cancer Statistics Review[23]; (3) the entirety of cancer treatment was received at University of Michigan Health System (UMHS); and (4) the patient died while admitted to the University Hospital between January 1, 2004 and December 31, 2007.
UMHS is a healthcare system and academic medical center consisting of hospitals, health centers, and clinics, including the University of Michigan Hospital and Comprehensive Cancer Center. Over 4000 cancer patients are admitted to University Hospital and 80,000 outpatient visits occur to the Cancer Center every year. It serves as a major cancer referral center for the patients of Michigan and the greater Midwest. The University of Michigan implemented a palliative care consult service that was started in 2005, during the time period of our study.
Data Collection
Data were abstracted by review of the medical record by an internist (M.Z.) using a comprehensive chart abstraction instrument based on a previously validated tool.[24] Demographic data abstracted from the medical record included age, religious affiliation, race, ethnicity, and marital status. The original abstraction tool, which included 83 items, was reduced to include 47 items focusing on information related to advanced care planning, hospital course, and end‐of‐life discussions. Specific items included: reasons for admission, primary hospital service, occurrence and timing of end‐of‐life decision‐making, whether patient or family preferences were elicited through an end‐of‐life discussion, clinician's assessment of patient's decisional capacity at time of admission and end‐of‐life conversations, whether a comfort care plan was made, and whether palliative morphine was used.
The chart abstraction tool was pilot tested on the medical records of 10 patients, who were not included in the study, and refined to improve completeness of data collection. A copy of the abstraction tool is available upon request.
Definition of Key Variables
Decisional Capacity Assessment
Decisional capacity assessment is a reflection of the clinical team's assessment of the patient's decisional capacity on admission, and was determined through examination of the medical record in the first 24 hours of admission. Positive decision‐making capacity assessment was assumed if the clinician assessment of decisional capacity was documented in the mental status exam, or if the clinician documented conversations between clinician and patient in the history that suggested intact decision‐making capacity (ie, clinician documented terms such as patient stated or patient described and then described a coherent or sensible statement which implied patient capacity and intact mental status), or if the clinician's documentation of the assessment and plan stated or suggested decisions were being made by the patient. Other supportive information from the record was used to corroborate the evidence used to determine the clinician's assessment of the patient's decisional capacity, including whether the patient signed consent forms.
End‐of‐Life Discussions
The presence of an end‐of‐life discussion was presumed when the clinician documented a discussion with the patient, discussion with family, or family meeting concerning treatment preferences, or when the clinician quoted the patient's preferences in a fashion that documents a face‐to‐face discussion or directly described the elicitation of preferences from the patient or family.
Living Will
A living will was identified as present if the document was scanned into the patient's medical record, or if the chart indicated that the patient or family stated that the patient had completed a living will.
Health Care Proxy
Health Care Proxy or Durable Power of Attorney for Healthcare was identified as present if the document was scanned into the patient's medical record, or if the chart indicated that the patient or family stated that the patient had completed such a document.
Do Not Resuscitate (DNR) on Admission
DNR status was identified as present if the patient had documents with established DNR orders, or if a physician explicitly documented code status as DNR in the admission note or other documents placed in the record within the first 24 hours of admission.
Intensive Care Unit (ICU)Treatment
Patients were defined as having received ICU treatment if they were admitted directly or subsequently transferred to the ICU during the hospital course.
Comfort Care
Comfort care was defined as present only if the phrases comfort care, palliative care, or supportive care, were documented.
Palliative Opioid Therapy
Treatments with morphine or other opioids were recorded as palliative only if it was explicitly stated that these medications were used in the context of palliative or end‐of‐life care.
Data Analysis
We report the proportion of patients who were documented to lack decisional capacity at the time of hospital admission. We compared patient characteristics for those with and without documentation of decisional capacity on admission, and patients with decisional capacity on admission who did and did not participate in discussions about end‐of‐life care using chi‐square tests for categorical data, t tests for normally distributed continuous variables, and MannWhitney U tests for non‐normally distributed continuous variables. We examined whether documentation of a discussion about end‐of‐life care was associated with life‐sustaining and palliative treatments received using chi‐square for categorical treatments, and MannWhitney U tests for days from admission to initiation of comfort care. We used P < 0.05 to signify statistical significance.
RESULTS
Characteristics of Population and Decisional Capacity on Admission
The characteristics of the 145 patients who met entry criteria are summarized in Table 1. The most common types of cancers were lung cancer and leukemia/lymphoma. Of the 145 patients, the medical team's assessment of the patient's decisional capacity on admission could be established for 142 patients. As documented within the first 24 hours of admission, 27 patients (19%) were considered not to have decisional capacity, and 115 patients (79%) were considered to have decisional capacity. Both of these groups had similar age and gender distributions. There were no significant differences in the distribution of cancer type between those with and without decisional capacity. In both groups, the majority of the cancer diagnoses were made prior to admission. There was no difference in DNR orders established prior to or on admission between the groups (Table 1).
| Decision‐Making Capacity on Admissiona | |||
|---|---|---|---|
| No (N = 27) | Yes (N = 115) | ||
| N (%) | N (%) | P Value | |
| |||
| Age >65 | 15 (55.6) | 60 (52.2) | 0.75 |
| Gender (male) | 14 (51.9) | 68 (59.1) | 0.46 |
| Cancer type | |||
| Lung | 12 (44.4) | 42 (36.5) | 0.67 |
| Bone marrow | 5 (18.5) | 35 (30.4) | |
| Liver | 3 (11.1) | 6 (5.2) | |
| Pancreas | 3 (11.1) | 7 (6.10) | |
| Esophagus | 1 (3.7) | 8 (7.0) | |
| Colon | 1 (3.7) | 6 (5.2) | |
| Otherb | 2 (7.4) | 11 (9.6) | |
| Prior to admission | |||
| Cancer diagnosis known | 23 (85.2) | 91 (79.1) | 0.48 |
| Living will completed | 6 (22.2) | 26 (22.6 | 0.97 |
| Health care proxy established | 3 (11.1) | 26 (22.6) | 0.18 |
| DNR established | 8 (29.6) | 27 (23.5) | 0.79 |
End‐of‐Life Discussion in Patients With Decisional Capacity on Admission
Of the 115 patients assessed to have intact decisional capacity on admission, 56 (48.7%) participated in an end‐of‐life discussion with the medical team during their terminal hospitalization. For the remaining 59 patients who did not participate in an end‐of‐life discussion during the terminal hospital course, 46 (40.0%) had documentation suggesting they lost decisional capacity prior to a conversation and that the end‐of‐life discussions were held instead with the patient's surrogate decision‐maker, and 13 (11.3%) had no evidence of any end‐of‐life discussion (with the patient or surrogate).
When comparing those patients who participated in an end‐of‐life discussion with those patients whose surrogate participated in the discussion, there were no significant differences in gender and age distributions (Table 2). There was a significant difference in the type of cancers between the 2 groups. Among patients who participated in their end‐of‐life discussions, bone marrow cancer was proportionately more prevalent (17.9% vs 2.2%; P <0.01) and lung cancer was less prevalent (16.1% vs 41.3%; P <0.01), when compared to those who required surrogate participation. Timing of cancer diagnosis and prevalence of advance directives were similar between the 2 groups. The proportion of patients who had an established DNR order prior to admission was higher among those who did participate in end‐of‐life discussions when compared to those who had surrogate participation in these discussions (30.4% vs 17.4%; P < 0.04).
| Patients With Documented Decision‐Making Capacity on Admission (N = 115) | ||||
|---|---|---|---|---|
| N =13 | N = 102 | |||
| End‐of‐Life discussion not documented | End‐of‐Life discussion with surrogate (N = 46) | End‐of‐Life discussion with patient (N = 56) | ||
| N (%) | N (%) | N (%) | P Value | |
| ||||
| Age 65 | 9 (69.2) | 18 (39.1) | 27 (48.2) | 0.36 |
| Gender (male) | 10 (76.9) | 27 (60.0) | 31 (55.4) | 0.64 |
| Cancer type | ||||
| Lung | 3 (23.1) | 19 (41.3) | 9 (16.1) | |
| Bone marrow | 7 (53.9) | 1 (2.2) | 10 (17.9) | |
| Liver | 1 (7.7) | 3 (6.5) | 2 (3.6) | |
| Pancreas | 1 (7.7) | 2 (4.4) | 4 (7.1) | <0.01 |
| Esophagus | 0 | 6 (13.0) | 2 (3.6) | |
| Colon | 1 (7.7) | 1 (2.2) | 3 (5.4) | |
| Othera | 0 | 1 (2.2) | 10 (17.9) | |
| Prior to Admission | ||||
| Cancer diagnosis known | 10 (76.9) | 37 (80.4) | 43 (76.8) | 0.66 |
| Living will completed | 4 (30.8) | 10 (21.7) | 12 (21.4) | 0.97 |
| Health care proxy established | ||||
| 3 (23.1) | 13 (28.3) | 10 (17.9) | 0.21 | |
| DNR established | 2 (15.4) | 8 (17.4) | 17 (30.4) | 0.04 |
Life‐Prolonging and Palliative Care Treatments Received and Participation in End‐of‐Life Discussions
Life‐prolonging treatments were more likely to be used for patients whose end‐of‐life discussions were held by patient's surrogate decision‐maker, in comparison to those patients who participated in the discussions themselves. Patients who had conversations held by surrogates were more likely to receive ventilator support (56.5% vs 23.2%, P < 0.01), chemotherapy (39.1% vs 5.4%, P < 0.01), artificial nutrition or hydration (45.7% vs 25.0%, P = 0.03), and antibiotics (97.8% vs 78.6%, P < 0.01), when compared to patients who participated in their own end‐of‐life discussion. Intensive care treatment rates also differed significantly between the 2 groups; 56.5% of those who did not participate in end‐of‐life discussions, and only 23.2% of those patients who did participate, were admitted or transferred to the intensive care unit (P < 0.01). There was no significant difference in the proportion of patients who received cardiopulmonary resuscitation (CPR) (15.2% vs 7.1%, P = 0.56) (Table 3). Patients who lost decisional capacity and required a surrogate decision‐maker to participate in their end‐of‐life discussions had longer length of stay (15.8 vs 10.3 days, P = 0.03) and length of time to end‐of‐life discussions (14.0 vs 6.1 days, P < 0.01) (Table 3).
| Patients With Documented Decision‐Making Capacity on Admission (N = 115) | |||
|---|---|---|---|
| End‐of‐Life Discussion With Surrogate (N =46) | End‐of‐Life Discussion With Patient (N = 56) | ||
| N (%) | N (%) | P Value | |
| |||
| Life‐Prolonging Treatment | |||
| Ventilatory support | 26 (56.5) | 13 (23.2) | <0.01 |
| Chemotherapy | 18 (39.1) | 3 (5.4) | <0.01 |
| Artificial nutrition or hydration | 21 (45.7) | 14 (25.0) | 0.03 |
| Antibiotics | 45 (97.8) | 44 (78.6) | <0.01 |
| Cardiopulmonary resuscitation | 7 (15.2) | 4 (7.1) | 0.19 |
| ICU treatment (admit or transfer) | 26 (56.5) | 13 (23.2) | <0.01 |
| Palliative Treatments | |||
| Comfort care | 39 (84.8) | 45 (80.4) | 0.56 |
| Palliative morphine drip | 24 (52.2) | 33 (57.1) | 0.62 |
| Mean (95% CI) [Range] | Mean (95% CI) [Range] | ||
| Timing of Advanced Care Planning | |||
| Length of hospitalization | 15.8 d (11.420.2) [157] | 10.3 d (146) [146] | 0.03 |
| Time to end‐of‐life discussion | 14.0 d (9.918.1) [055] | 6.1 d (3.88.4) [046] | <0.01 |
| Time to comfort care | 23.5 d (4.342.8) [1374] | 9.2 d (6.312.1) [046] | 0.12 |
A comparison of the proportion of patients receiving palliative treatments, such as palliative comfort care orders and morphine infusions, revealed no significant differences between those with and without a discussion about end‐of‐life care (Table 3). Furthermore, while the time interval from hospital admission to initiation of comfort care was shorter for those who did participate in end‐of‐life discussions compared with those had surrogate participation (9.3 vs 23.5 days, P = 0.13 for equality), this difference was not statistically significant (Table 3).
Since a higher proportion of those who participated in end‐of‐life discussions during the hospitalization were admitted with a DNR order established prior to or on admission, we also examined the use of life‐prolonging treatments in the subgroup of patients who did not have a DNR order prior to admission. The difference in life‐prolonging treatments between those who did and did not participate in end‐of‐life discussions was preserved among those patients who did not have DNR status on admission (data not shown).
DISCUSSION
In this retrospective study of 145 terminal cancer patients who died in the hospital, we found that half of the patients had documentation suggesting that they lost decisional capacity during hospitalization and did not participate in end‐of‐life discussion with their healthcare providers. Among cancer patients in our study, 19% were determined not to have decisional capacity on admission, and another 32% were determined to lose decisional capacity during the hospital course. When patients did participate in documented discussions about end‐of‐life care, they were less likely to receive intensive life‐sustaining treatments, less likely to be admitted or transferred to the ICU, and more likely to avoid prolonged hospitalization. The finding that the majority of cancer patients are assessed to have intact decisional capacity upon admission to their terminal hospitalization, but less than half of them participate in their own end‐of‐life conversation, suggests that there is an important lost opportunity to provide quality advanced care planning to hospitalized patients.
Advanced care planning is the act of defining a competent patient's wishes regarding their future healthcare in the event of loss of decisional capacity. For many of the patients who were determined to lose decisional capacity during their hospitalization in our study, a surrogate decision‐maker was involved in a subsequent end‐of‐life discussion. This represents a missed opportunity in 2 ways. First, because surrogates may incorrectly predict patients' end‐of‐life treatment preferences in approximately one‐third of the cases,[25] patients may receive care that is inconsistent with their beliefs. Second, reliance on surrogate decision‐making may result in a greater burden on family members. Surrogate decision‐making places a large burden on surrogates, and can lead to emotional and psychological stress that can last well beyond the death of the loved one.[16, 26]
Our results reinforce the growing body of evidence suggesting that communication with the dying patient, both before and during the terminal hospitalization, promotes end‐of‐life care that involves less invasive life‐prolonging treatments,[10, 11, 12, 13, 14, 15] which is a consistently stated goal of most patients at the end of life.[1, 2] Our findings are consistent with a recent randomized trial of hospitalized patients over the age of 80 which showed that advance care planning discussions were associated with decreased use of intensive life‐sustaining treatments, increased patient and family satisfaction with care, as well as decreased psychological symptoms among family members.[27] In addition, studies examining patients with advanced cancer have shown that discussions about end‐of‐life care in the outpatient setting resulted in less intensive life‐sustaining treatments.[14, 15] Nearly 20% of our cohort was determined by the clinical team to lack decisional capacity at the time of their terminal hospitalization, highlighting the importance of end‐of‐life discussion prior to hospitalization. Our results also extend these recent findings to the inpatient setting. Seventy‐nine percent of patients in our study were determined to have decisional capacity at the time of their terminal hospitalization, and end‐of‐life discussion conducted with these patients was associated with decreased use of life‐sustaining treatments.
Seriously ill patients, particularly in hospital settings, have high symptom burden and subsequent poor quality of life.[6] These patients and families often report inadequate pain and symptom relief.[8, 28, 29] It is therefore reassuring that we found no difference in the proportion of patients who received comfort care and palliative morphine infusions according to whether they or a surrogate participated in the end‐of‐life discussions. In fact, the majority of patients were made DNR and received some form of comfort measure prior to death, although these comfort measures were frequently initiated only hours prior to death.
While our findings are consistent with research that has demonstrated that end‐of‐life discussions with patients are associated with a decrease in life‐prolonging treatments, it is important to note that our observational study cannot establish a causal relationship between the end‐of‐life discussion and the subsequent use of life‐sustaining treatments. It is possible that patients who have discussions about end‐of‐life care inherently prefer to have less intensive life‐sustaining treatment at the end of life. Physicians may be more apt to engage in these types of discussions with patients who express interest in limited intervention. Interestingly, patients with a DNR order at the time of admission were more likely to participate in end‐of‐life care with their provider during their terminal hospitalization, which supports this alternate explanation. However, when we excluded all patients with a DNR on admission, our findings persisted. Regardless of the explanation for the association between end‐of‐life discussions and life‐sustaining treatments, our study identifies a cohort of hospitalized patients who could benefit from improved end‐of‐life communication, and a clinical setting where opportunities remain to improve the quality of advanced care planning.
Discussions about end‐of‐life care with patients result in earlier transition to care focused on palliation.[14] Although we examined the timing to initiation of comfort care between those who participated and those who did not participate in end‐of‐life discussions, we were not able to demonstrate a statistically significant difference. However, our cohort may not be suitable for an examination of this type of intervention, as early discussions about end‐of‐life care may have lead to early referral to hospice, and therefore death outside the hospital setting, making the patients ineligible for our study.
There are several additional limitations to our study. First, our study is subject to the potential biases inherent in retrospective chart review. For example, since we identified patients who died in the hospital, our study does not generalize to patients with advanced cancer who survive the hospitalization or who were discharged to die at home.[30] Furthermore, our use of the medical record to assess documentation of communication about end‐of‐life care and decisional capacity relies on the accuracy of clinician documentation. There may have been communication about end‐of‐life care that was not documented in the medical record, resulting in underestimation of the effects of end‐of‐life discussions. In clinical practice, the assessment of decisional capacity can be challenging.[19, 31, 32] Although clinicians are often accurate in identifying patients with capacity, in nearly one‐quarter of all assessments, they mistakenly assign capacity to patients who lack decision‐making capacity.[17] In our study, we examine clinician assessment of the patient's decisional capacity, but cannot assess either the accuracy of their clinical assessment or the accuracy of their documentation of this assessment. A second limitation is that the chart abstraction was conducted by a single reviewer without inter‐rater assessment, although the abstraction tool was modified from a well‐established tool.[24] A third limitation is that all of the patients were from a single academic center and the results may not generalize to other regions. Fourth, we did not assess for the potential effect of individual clinicians. Since most patients were cared for by multiple physicians, our study is not capable of assessing a clinician‐level effect. Finally, our study was not able to address whether the care received by patients was in accord with their informed preferences.
We have shown that opportunities exist for advanced care planning at the time of the terminal hospitalization of patients with advanced cancer. The majority of these patients have documentation of decisional capacity at the time of admission, suggesting that opportunities exist to conduct end‐of‐life discussion while the patient retains decisional capacity. Furthermore, we found that patients who participate in these discussions about end‐of‐life care with their clinicians have an associated decrease in the use of life‐sustaining treatments, which is consistent with prior studies.[11, 13, 14, 15, 27] Improving communication about end‐of‐life care for patients hospitalized with advanced cancer may represent an important opportunity to improve the concordance between patients' wishes for care at the end‐of‐life and the care that these patients actually receive. Such communication may also decrease the burden on family members who are frequently asked to play the role of surrogate decision‐maker without an opportunity to discuss these issues with the patient.
Acknowledgments
The authors acknowledge Melissa Braxton for her assistance with the preparation of this manuscript.
Disclosures: A portion of this data was presented at the annual meeting of the Society of Hospital Medicine, 2011, in Grapevine, TX. This work was supported by the National Cancer Institute at the National Institutes of Health (KO5 CA 104699 to J.G.E.) and the National Heart, Lung, and Blood Institute (K24HL68593 to J.R.C.). Dr Silveira's salary was supported by core funds from the Ann Arbor VA HSRD Center of Excellence. The authors have no relevant financial interest in the subject matter contained within this manuscript.
- , , , . Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076–2080.
- , , , . Dying trajectory in the last year of life: does cancer trajectory fit other diseases?J Palliat Med. 2001;4(4):457–464.
- , , , . Trends and Variation in End‐of‐Life Care for Medicare Beneficiaries with Severe Chronic Illness.The Dartmouth Institute for Health Policy 32(3):638–643.
- , , , , . The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment. J Pain Symptom Manage. 1999;17(4):248–255.
- , , , . Family reports of pain in dying hospitalized patients: a structured telephone survey. West J Med. 2000;172(6):374–377.
- , , , et al. Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;126(2):97–106.
- , , , , . Death in the hospital. Arch Intern Med. 1998;158(14):1570–1572.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291(1):88–93.
- , , , , . Association between advance directives and quality of end‐of‐life care: a national study. J Am Geriatr Soc. 2007;55(2):189–194.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Health care costs in the last week of life: associations with end‐of‐life conversations. Arch Intern Med. 2009;169(5):480–488.
- , , , et al. End‐of‐life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012;156(3):204–210.
- , , , et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: cross‐sectional study. Lancet. 2004;364(9443):1421–1427.
- , . Assessing patients' capacities to consent to treatment. N Engl J Med 1998;319:1635–1638.
- . Clinical practice. Assessment of patients' competence to consent to treatment. N Engl J Med. 2007;357(18):1834–1840.
- , , , . The physician‐surrogate relationship. Arch Intern Med. 2007;167(11):1117–1121.
- , , , , . Physicians' experience with surrogate decision making for hospitalized adults. J Gen Intern Med. 2009;24(9):1023–1028.
- , , , . The surrogate's experience in authorizing a do not resuscitate order. Palliat Support Care. 2008;6(1):13–19.
- , , , et al. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975–2004. Bethesda, MD:National Cancer Institute. Based on November 2006 SEER data submission, posted to the SEER Web site,2007. Available at: http://seer.cancer.gov/csr/1975_2004/. Accessed on November 1, 2007.
- , , , , , . End‐of‐life decision‐making in the hospital: current practice and future prospects. J Pain Symptom Manage. 1999;17(1):6–15.
- , , . The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166(5):493–497.
- , . Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–346.
- , , , . The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
- , , , et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 suppl):S146–S153.
- , , , . The use of life‐sustaining treatments in hospitalized persons aged 80 and older. J Am Geriatr Soc. 2002;50(5):930–934.
- , , . Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770.
- , , , et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34.
- , , . The MacCAT‐T: a clinical tool to assess patients' capacities to make treatment decisions. Psychiatr Serv. 1997;48(11):1415–1419.
Most patients prefer to die at home, pain free and without the use of life‐sustaining treatments[1, 2] yet the majority of patients with serious illness die in the hospital.[3, 4, 5] When hospitalized patient die, the care is frequently focused on life‐sustaining treatments[6]; pain, dyspnea, and agitation levels are higher when compared with patients who die in non‐hospital settings.[7, 8, 9] End‐of‐life discussions and their products (ie, advanced directives) can clarify treatment options with patients and family,[10] and help ensure that patients receive care consistent with their beliefs.[11, 12, 13] End‐of‐life discussions are associated with a decrease use of life‐sustaining treatments, improved quality of life, and reduced costs of care.[14, 15] For the majority of patients dying of cancer, the first end‐of‐life discussion takes place in the hospital setting.[16]
Conducting end‐of‐life conversations in the hospital setting can be challenging. Patients are acutely ill and nearly 40% are incapable of making their own medical decisions.[17] In order to participate in an end‐of‐life discussion, a physician must determine that a patient meets the 4 criteria of decisional capacity as outlined by Appelbaum and Grisso:[18] Does the patient (1) communicate a clear and consistent choice; (2) understand the relevant information surrounding that decision; (3) appreciate the consequences of that decision; and (4) communicate reasoning for that decision?[19] In practice, however, clinicians inaccurately assign capacity up to 25% of the time.[17] When a physician determines, accurately or inaccurately, that the patient does not meet this standard for decisional capacity, discussions must be held instead with a surrogate decision‐maker. Surrogate decision‐making can make communication with the physician more difficult,[20] delay important medical decisions,[21] and be stressful on the decision‐maker.[22] To our knowledge, no studies have examined patient and surrogate participation in end‐of‐life discussions at the time of terminal hospitalization and its association with end‐of‐life treatments received.
Our goals were to examine physician assessment of decisional capacity and the prevalence of end‐of‐life discussions during the terminal hospitalization of patients with advanced cancer. Our research questions were: (1) What proportion of patients were assessed to have decisional capacity by the clinical team at the time of their terminal hospital admission? (2) What proportion of these patients had a documented discussion about end‐of‐life care with the clinical team, and for what proportion was the conversation held instead by the patient's surrogate decision‐maker because the patient was considered to have lost decisional capacity? (3) Was patient participation in a discussion about end‐of‐life care associated with life‐sustaining and palliative treatments received?
METHODS
Design
This is a retrospective cohort study of consecutive adult patients with advanced cancer who died at the University of Michigan Hospital, Ann Arbor, MI, from January 1, 2004 through December 31, 2007. This study was exempted from review by the University of Michigan (UM) Institutional Review Board because decedent data were used.
Sample
The UM Cancer Registry is a database of all cancer patients treated at the UM Comprehensive Cancer Center. We used the Registry to identify patients who met the following criteria: (1) age 18 years at time of cancer diagnosis; (2) estimated probability of 5‐year survival 20% at time of diagnosis, as predicted by the SEER Cancer Statistics Review[23]; (3) the entirety of cancer treatment was received at University of Michigan Health System (UMHS); and (4) the patient died while admitted to the University Hospital between January 1, 2004 and December 31, 2007.
UMHS is a healthcare system and academic medical center consisting of hospitals, health centers, and clinics, including the University of Michigan Hospital and Comprehensive Cancer Center. Over 4000 cancer patients are admitted to University Hospital and 80,000 outpatient visits occur to the Cancer Center every year. It serves as a major cancer referral center for the patients of Michigan and the greater Midwest. The University of Michigan implemented a palliative care consult service that was started in 2005, during the time period of our study.
Data Collection
Data were abstracted by review of the medical record by an internist (M.Z.) using a comprehensive chart abstraction instrument based on a previously validated tool.[24] Demographic data abstracted from the medical record included age, religious affiliation, race, ethnicity, and marital status. The original abstraction tool, which included 83 items, was reduced to include 47 items focusing on information related to advanced care planning, hospital course, and end‐of‐life discussions. Specific items included: reasons for admission, primary hospital service, occurrence and timing of end‐of‐life decision‐making, whether patient or family preferences were elicited through an end‐of‐life discussion, clinician's assessment of patient's decisional capacity at time of admission and end‐of‐life conversations, whether a comfort care plan was made, and whether palliative morphine was used.
The chart abstraction tool was pilot tested on the medical records of 10 patients, who were not included in the study, and refined to improve completeness of data collection. A copy of the abstraction tool is available upon request.
Definition of Key Variables
Decisional Capacity Assessment
Decisional capacity assessment is a reflection of the clinical team's assessment of the patient's decisional capacity on admission, and was determined through examination of the medical record in the first 24 hours of admission. Positive decision‐making capacity assessment was assumed if the clinician assessment of decisional capacity was documented in the mental status exam, or if the clinician documented conversations between clinician and patient in the history that suggested intact decision‐making capacity (ie, clinician documented terms such as patient stated or patient described and then described a coherent or sensible statement which implied patient capacity and intact mental status), or if the clinician's documentation of the assessment and plan stated or suggested decisions were being made by the patient. Other supportive information from the record was used to corroborate the evidence used to determine the clinician's assessment of the patient's decisional capacity, including whether the patient signed consent forms.
End‐of‐Life Discussions
The presence of an end‐of‐life discussion was presumed when the clinician documented a discussion with the patient, discussion with family, or family meeting concerning treatment preferences, or when the clinician quoted the patient's preferences in a fashion that documents a face‐to‐face discussion or directly described the elicitation of preferences from the patient or family.
Living Will
A living will was identified as present if the document was scanned into the patient's medical record, or if the chart indicated that the patient or family stated that the patient had completed a living will.
Health Care Proxy
Health Care Proxy or Durable Power of Attorney for Healthcare was identified as present if the document was scanned into the patient's medical record, or if the chart indicated that the patient or family stated that the patient had completed such a document.
Do Not Resuscitate (DNR) on Admission
DNR status was identified as present if the patient had documents with established DNR orders, or if a physician explicitly documented code status as DNR in the admission note or other documents placed in the record within the first 24 hours of admission.
Intensive Care Unit (ICU)Treatment
Patients were defined as having received ICU treatment if they were admitted directly or subsequently transferred to the ICU during the hospital course.
Comfort Care
Comfort care was defined as present only if the phrases comfort care, palliative care, or supportive care, were documented.
Palliative Opioid Therapy
Treatments with morphine or other opioids were recorded as palliative only if it was explicitly stated that these medications were used in the context of palliative or end‐of‐life care.
Data Analysis
We report the proportion of patients who were documented to lack decisional capacity at the time of hospital admission. We compared patient characteristics for those with and without documentation of decisional capacity on admission, and patients with decisional capacity on admission who did and did not participate in discussions about end‐of‐life care using chi‐square tests for categorical data, t tests for normally distributed continuous variables, and MannWhitney U tests for non‐normally distributed continuous variables. We examined whether documentation of a discussion about end‐of‐life care was associated with life‐sustaining and palliative treatments received using chi‐square for categorical treatments, and MannWhitney U tests for days from admission to initiation of comfort care. We used P < 0.05 to signify statistical significance.
RESULTS
Characteristics of Population and Decisional Capacity on Admission
The characteristics of the 145 patients who met entry criteria are summarized in Table 1. The most common types of cancers were lung cancer and leukemia/lymphoma. Of the 145 patients, the medical team's assessment of the patient's decisional capacity on admission could be established for 142 patients. As documented within the first 24 hours of admission, 27 patients (19%) were considered not to have decisional capacity, and 115 patients (79%) were considered to have decisional capacity. Both of these groups had similar age and gender distributions. There were no significant differences in the distribution of cancer type between those with and without decisional capacity. In both groups, the majority of the cancer diagnoses were made prior to admission. There was no difference in DNR orders established prior to or on admission between the groups (Table 1).
| Decision‐Making Capacity on Admissiona | |||
|---|---|---|---|
| No (N = 27) | Yes (N = 115) | ||
| N (%) | N (%) | P Value | |
| |||
| Age >65 | 15 (55.6) | 60 (52.2) | 0.75 |
| Gender (male) | 14 (51.9) | 68 (59.1) | 0.46 |
| Cancer type | |||
| Lung | 12 (44.4) | 42 (36.5) | 0.67 |
| Bone marrow | 5 (18.5) | 35 (30.4) | |
| Liver | 3 (11.1) | 6 (5.2) | |
| Pancreas | 3 (11.1) | 7 (6.10) | |
| Esophagus | 1 (3.7) | 8 (7.0) | |
| Colon | 1 (3.7) | 6 (5.2) | |
| Otherb | 2 (7.4) | 11 (9.6) | |
| Prior to admission | |||
| Cancer diagnosis known | 23 (85.2) | 91 (79.1) | 0.48 |
| Living will completed | 6 (22.2) | 26 (22.6 | 0.97 |
| Health care proxy established | 3 (11.1) | 26 (22.6) | 0.18 |
| DNR established | 8 (29.6) | 27 (23.5) | 0.79 |
End‐of‐Life Discussion in Patients With Decisional Capacity on Admission
Of the 115 patients assessed to have intact decisional capacity on admission, 56 (48.7%) participated in an end‐of‐life discussion with the medical team during their terminal hospitalization. For the remaining 59 patients who did not participate in an end‐of‐life discussion during the terminal hospital course, 46 (40.0%) had documentation suggesting they lost decisional capacity prior to a conversation and that the end‐of‐life discussions were held instead with the patient's surrogate decision‐maker, and 13 (11.3%) had no evidence of any end‐of‐life discussion (with the patient or surrogate).
When comparing those patients who participated in an end‐of‐life discussion with those patients whose surrogate participated in the discussion, there were no significant differences in gender and age distributions (Table 2). There was a significant difference in the type of cancers between the 2 groups. Among patients who participated in their end‐of‐life discussions, bone marrow cancer was proportionately more prevalent (17.9% vs 2.2%; P <0.01) and lung cancer was less prevalent (16.1% vs 41.3%; P <0.01), when compared to those who required surrogate participation. Timing of cancer diagnosis and prevalence of advance directives were similar between the 2 groups. The proportion of patients who had an established DNR order prior to admission was higher among those who did participate in end‐of‐life discussions when compared to those who had surrogate participation in these discussions (30.4% vs 17.4%; P < 0.04).
| Patients With Documented Decision‐Making Capacity on Admission (N = 115) | ||||
|---|---|---|---|---|
| N =13 | N = 102 | |||
| End‐of‐Life discussion not documented | End‐of‐Life discussion with surrogate (N = 46) | End‐of‐Life discussion with patient (N = 56) | ||
| N (%) | N (%) | N (%) | P Value | |
| ||||
| Age 65 | 9 (69.2) | 18 (39.1) | 27 (48.2) | 0.36 |
| Gender (male) | 10 (76.9) | 27 (60.0) | 31 (55.4) | 0.64 |
| Cancer type | ||||
| Lung | 3 (23.1) | 19 (41.3) | 9 (16.1) | |
| Bone marrow | 7 (53.9) | 1 (2.2) | 10 (17.9) | |
| Liver | 1 (7.7) | 3 (6.5) | 2 (3.6) | |
| Pancreas | 1 (7.7) | 2 (4.4) | 4 (7.1) | <0.01 |
| Esophagus | 0 | 6 (13.0) | 2 (3.6) | |
| Colon | 1 (7.7) | 1 (2.2) | 3 (5.4) | |
| Othera | 0 | 1 (2.2) | 10 (17.9) | |
| Prior to Admission | ||||
| Cancer diagnosis known | 10 (76.9) | 37 (80.4) | 43 (76.8) | 0.66 |
| Living will completed | 4 (30.8) | 10 (21.7) | 12 (21.4) | 0.97 |
| Health care proxy established | ||||
| 3 (23.1) | 13 (28.3) | 10 (17.9) | 0.21 | |
| DNR established | 2 (15.4) | 8 (17.4) | 17 (30.4) | 0.04 |
Life‐Prolonging and Palliative Care Treatments Received and Participation in End‐of‐Life Discussions
Life‐prolonging treatments were more likely to be used for patients whose end‐of‐life discussions were held by patient's surrogate decision‐maker, in comparison to those patients who participated in the discussions themselves. Patients who had conversations held by surrogates were more likely to receive ventilator support (56.5% vs 23.2%, P < 0.01), chemotherapy (39.1% vs 5.4%, P < 0.01), artificial nutrition or hydration (45.7% vs 25.0%, P = 0.03), and antibiotics (97.8% vs 78.6%, P < 0.01), when compared to patients who participated in their own end‐of‐life discussion. Intensive care treatment rates also differed significantly between the 2 groups; 56.5% of those who did not participate in end‐of‐life discussions, and only 23.2% of those patients who did participate, were admitted or transferred to the intensive care unit (P < 0.01). There was no significant difference in the proportion of patients who received cardiopulmonary resuscitation (CPR) (15.2% vs 7.1%, P = 0.56) (Table 3). Patients who lost decisional capacity and required a surrogate decision‐maker to participate in their end‐of‐life discussions had longer length of stay (15.8 vs 10.3 days, P = 0.03) and length of time to end‐of‐life discussions (14.0 vs 6.1 days, P < 0.01) (Table 3).
| Patients With Documented Decision‐Making Capacity on Admission (N = 115) | |||
|---|---|---|---|
| End‐of‐Life Discussion With Surrogate (N =46) | End‐of‐Life Discussion With Patient (N = 56) | ||
| N (%) | N (%) | P Value | |
| |||
| Life‐Prolonging Treatment | |||
| Ventilatory support | 26 (56.5) | 13 (23.2) | <0.01 |
| Chemotherapy | 18 (39.1) | 3 (5.4) | <0.01 |
| Artificial nutrition or hydration | 21 (45.7) | 14 (25.0) | 0.03 |
| Antibiotics | 45 (97.8) | 44 (78.6) | <0.01 |
| Cardiopulmonary resuscitation | 7 (15.2) | 4 (7.1) | 0.19 |
| ICU treatment (admit or transfer) | 26 (56.5) | 13 (23.2) | <0.01 |
| Palliative Treatments | |||
| Comfort care | 39 (84.8) | 45 (80.4) | 0.56 |
| Palliative morphine drip | 24 (52.2) | 33 (57.1) | 0.62 |
| Mean (95% CI) [Range] | Mean (95% CI) [Range] | ||
| Timing of Advanced Care Planning | |||
| Length of hospitalization | 15.8 d (11.420.2) [157] | 10.3 d (146) [146] | 0.03 |
| Time to end‐of‐life discussion | 14.0 d (9.918.1) [055] | 6.1 d (3.88.4) [046] | <0.01 |
| Time to comfort care | 23.5 d (4.342.8) [1374] | 9.2 d (6.312.1) [046] | 0.12 |
A comparison of the proportion of patients receiving palliative treatments, such as palliative comfort care orders and morphine infusions, revealed no significant differences between those with and without a discussion about end‐of‐life care (Table 3). Furthermore, while the time interval from hospital admission to initiation of comfort care was shorter for those who did participate in end‐of‐life discussions compared with those had surrogate participation (9.3 vs 23.5 days, P = 0.13 for equality), this difference was not statistically significant (Table 3).
Since a higher proportion of those who participated in end‐of‐life discussions during the hospitalization were admitted with a DNR order established prior to or on admission, we also examined the use of life‐prolonging treatments in the subgroup of patients who did not have a DNR order prior to admission. The difference in life‐prolonging treatments between those who did and did not participate in end‐of‐life discussions was preserved among those patients who did not have DNR status on admission (data not shown).
DISCUSSION
In this retrospective study of 145 terminal cancer patients who died in the hospital, we found that half of the patients had documentation suggesting that they lost decisional capacity during hospitalization and did not participate in end‐of‐life discussion with their healthcare providers. Among cancer patients in our study, 19% were determined not to have decisional capacity on admission, and another 32% were determined to lose decisional capacity during the hospital course. When patients did participate in documented discussions about end‐of‐life care, they were less likely to receive intensive life‐sustaining treatments, less likely to be admitted or transferred to the ICU, and more likely to avoid prolonged hospitalization. The finding that the majority of cancer patients are assessed to have intact decisional capacity upon admission to their terminal hospitalization, but less than half of them participate in their own end‐of‐life conversation, suggests that there is an important lost opportunity to provide quality advanced care planning to hospitalized patients.
Advanced care planning is the act of defining a competent patient's wishes regarding their future healthcare in the event of loss of decisional capacity. For many of the patients who were determined to lose decisional capacity during their hospitalization in our study, a surrogate decision‐maker was involved in a subsequent end‐of‐life discussion. This represents a missed opportunity in 2 ways. First, because surrogates may incorrectly predict patients' end‐of‐life treatment preferences in approximately one‐third of the cases,[25] patients may receive care that is inconsistent with their beliefs. Second, reliance on surrogate decision‐making may result in a greater burden on family members. Surrogate decision‐making places a large burden on surrogates, and can lead to emotional and psychological stress that can last well beyond the death of the loved one.[16, 26]
Our results reinforce the growing body of evidence suggesting that communication with the dying patient, both before and during the terminal hospitalization, promotes end‐of‐life care that involves less invasive life‐prolonging treatments,[10, 11, 12, 13, 14, 15] which is a consistently stated goal of most patients at the end of life.[1, 2] Our findings are consistent with a recent randomized trial of hospitalized patients over the age of 80 which showed that advance care planning discussions were associated with decreased use of intensive life‐sustaining treatments, increased patient and family satisfaction with care, as well as decreased psychological symptoms among family members.[27] In addition, studies examining patients with advanced cancer have shown that discussions about end‐of‐life care in the outpatient setting resulted in less intensive life‐sustaining treatments.[14, 15] Nearly 20% of our cohort was determined by the clinical team to lack decisional capacity at the time of their terminal hospitalization, highlighting the importance of end‐of‐life discussion prior to hospitalization. Our results also extend these recent findings to the inpatient setting. Seventy‐nine percent of patients in our study were determined to have decisional capacity at the time of their terminal hospitalization, and end‐of‐life discussion conducted with these patients was associated with decreased use of life‐sustaining treatments.
Seriously ill patients, particularly in hospital settings, have high symptom burden and subsequent poor quality of life.[6] These patients and families often report inadequate pain and symptom relief.[8, 28, 29] It is therefore reassuring that we found no difference in the proportion of patients who received comfort care and palliative morphine infusions according to whether they or a surrogate participated in the end‐of‐life discussions. In fact, the majority of patients were made DNR and received some form of comfort measure prior to death, although these comfort measures were frequently initiated only hours prior to death.
While our findings are consistent with research that has demonstrated that end‐of‐life discussions with patients are associated with a decrease in life‐prolonging treatments, it is important to note that our observational study cannot establish a causal relationship between the end‐of‐life discussion and the subsequent use of life‐sustaining treatments. It is possible that patients who have discussions about end‐of‐life care inherently prefer to have less intensive life‐sustaining treatment at the end of life. Physicians may be more apt to engage in these types of discussions with patients who express interest in limited intervention. Interestingly, patients with a DNR order at the time of admission were more likely to participate in end‐of‐life care with their provider during their terminal hospitalization, which supports this alternate explanation. However, when we excluded all patients with a DNR on admission, our findings persisted. Regardless of the explanation for the association between end‐of‐life discussions and life‐sustaining treatments, our study identifies a cohort of hospitalized patients who could benefit from improved end‐of‐life communication, and a clinical setting where opportunities remain to improve the quality of advanced care planning.
Discussions about end‐of‐life care with patients result in earlier transition to care focused on palliation.[14] Although we examined the timing to initiation of comfort care between those who participated and those who did not participate in end‐of‐life discussions, we were not able to demonstrate a statistically significant difference. However, our cohort may not be suitable for an examination of this type of intervention, as early discussions about end‐of‐life care may have lead to early referral to hospice, and therefore death outside the hospital setting, making the patients ineligible for our study.
There are several additional limitations to our study. First, our study is subject to the potential biases inherent in retrospective chart review. For example, since we identified patients who died in the hospital, our study does not generalize to patients with advanced cancer who survive the hospitalization or who were discharged to die at home.[30] Furthermore, our use of the medical record to assess documentation of communication about end‐of‐life care and decisional capacity relies on the accuracy of clinician documentation. There may have been communication about end‐of‐life care that was not documented in the medical record, resulting in underestimation of the effects of end‐of‐life discussions. In clinical practice, the assessment of decisional capacity can be challenging.[19, 31, 32] Although clinicians are often accurate in identifying patients with capacity, in nearly one‐quarter of all assessments, they mistakenly assign capacity to patients who lack decision‐making capacity.[17] In our study, we examine clinician assessment of the patient's decisional capacity, but cannot assess either the accuracy of their clinical assessment or the accuracy of their documentation of this assessment. A second limitation is that the chart abstraction was conducted by a single reviewer without inter‐rater assessment, although the abstraction tool was modified from a well‐established tool.[24] A third limitation is that all of the patients were from a single academic center and the results may not generalize to other regions. Fourth, we did not assess for the potential effect of individual clinicians. Since most patients were cared for by multiple physicians, our study is not capable of assessing a clinician‐level effect. Finally, our study was not able to address whether the care received by patients was in accord with their informed preferences.
We have shown that opportunities exist for advanced care planning at the time of the terminal hospitalization of patients with advanced cancer. The majority of these patients have documentation of decisional capacity at the time of admission, suggesting that opportunities exist to conduct end‐of‐life discussion while the patient retains decisional capacity. Furthermore, we found that patients who participate in these discussions about end‐of‐life care with their clinicians have an associated decrease in the use of life‐sustaining treatments, which is consistent with prior studies.[11, 13, 14, 15, 27] Improving communication about end‐of‐life care for patients hospitalized with advanced cancer may represent an important opportunity to improve the concordance between patients' wishes for care at the end‐of‐life and the care that these patients actually receive. Such communication may also decrease the burden on family members who are frequently asked to play the role of surrogate decision‐maker without an opportunity to discuss these issues with the patient.
Acknowledgments
The authors acknowledge Melissa Braxton for her assistance with the preparation of this manuscript.
Disclosures: A portion of this data was presented at the annual meeting of the Society of Hospital Medicine, 2011, in Grapevine, TX. This work was supported by the National Cancer Institute at the National Institutes of Health (KO5 CA 104699 to J.G.E.) and the National Heart, Lung, and Blood Institute (K24HL68593 to J.R.C.). Dr Silveira's salary was supported by core funds from the Ann Arbor VA HSRD Center of Excellence. The authors have no relevant financial interest in the subject matter contained within this manuscript.
Most patients prefer to die at home, pain free and without the use of life‐sustaining treatments[1, 2] yet the majority of patients with serious illness die in the hospital.[3, 4, 5] When hospitalized patient die, the care is frequently focused on life‐sustaining treatments[6]; pain, dyspnea, and agitation levels are higher when compared with patients who die in non‐hospital settings.[7, 8, 9] End‐of‐life discussions and their products (ie, advanced directives) can clarify treatment options with patients and family,[10] and help ensure that patients receive care consistent with their beliefs.[11, 12, 13] End‐of‐life discussions are associated with a decrease use of life‐sustaining treatments, improved quality of life, and reduced costs of care.[14, 15] For the majority of patients dying of cancer, the first end‐of‐life discussion takes place in the hospital setting.[16]
Conducting end‐of‐life conversations in the hospital setting can be challenging. Patients are acutely ill and nearly 40% are incapable of making their own medical decisions.[17] In order to participate in an end‐of‐life discussion, a physician must determine that a patient meets the 4 criteria of decisional capacity as outlined by Appelbaum and Grisso:[18] Does the patient (1) communicate a clear and consistent choice; (2) understand the relevant information surrounding that decision; (3) appreciate the consequences of that decision; and (4) communicate reasoning for that decision?[19] In practice, however, clinicians inaccurately assign capacity up to 25% of the time.[17] When a physician determines, accurately or inaccurately, that the patient does not meet this standard for decisional capacity, discussions must be held instead with a surrogate decision‐maker. Surrogate decision‐making can make communication with the physician more difficult,[20] delay important medical decisions,[21] and be stressful on the decision‐maker.[22] To our knowledge, no studies have examined patient and surrogate participation in end‐of‐life discussions at the time of terminal hospitalization and its association with end‐of‐life treatments received.
Our goals were to examine physician assessment of decisional capacity and the prevalence of end‐of‐life discussions during the terminal hospitalization of patients with advanced cancer. Our research questions were: (1) What proportion of patients were assessed to have decisional capacity by the clinical team at the time of their terminal hospital admission? (2) What proportion of these patients had a documented discussion about end‐of‐life care with the clinical team, and for what proportion was the conversation held instead by the patient's surrogate decision‐maker because the patient was considered to have lost decisional capacity? (3) Was patient participation in a discussion about end‐of‐life care associated with life‐sustaining and palliative treatments received?
METHODS
Design
This is a retrospective cohort study of consecutive adult patients with advanced cancer who died at the University of Michigan Hospital, Ann Arbor, MI, from January 1, 2004 through December 31, 2007. This study was exempted from review by the University of Michigan (UM) Institutional Review Board because decedent data were used.
Sample
The UM Cancer Registry is a database of all cancer patients treated at the UM Comprehensive Cancer Center. We used the Registry to identify patients who met the following criteria: (1) age 18 years at time of cancer diagnosis; (2) estimated probability of 5‐year survival 20% at time of diagnosis, as predicted by the SEER Cancer Statistics Review[23]; (3) the entirety of cancer treatment was received at University of Michigan Health System (UMHS); and (4) the patient died while admitted to the University Hospital between January 1, 2004 and December 31, 2007.
UMHS is a healthcare system and academic medical center consisting of hospitals, health centers, and clinics, including the University of Michigan Hospital and Comprehensive Cancer Center. Over 4000 cancer patients are admitted to University Hospital and 80,000 outpatient visits occur to the Cancer Center every year. It serves as a major cancer referral center for the patients of Michigan and the greater Midwest. The University of Michigan implemented a palliative care consult service that was started in 2005, during the time period of our study.
Data Collection
Data were abstracted by review of the medical record by an internist (M.Z.) using a comprehensive chart abstraction instrument based on a previously validated tool.[24] Demographic data abstracted from the medical record included age, religious affiliation, race, ethnicity, and marital status. The original abstraction tool, which included 83 items, was reduced to include 47 items focusing on information related to advanced care planning, hospital course, and end‐of‐life discussions. Specific items included: reasons for admission, primary hospital service, occurrence and timing of end‐of‐life decision‐making, whether patient or family preferences were elicited through an end‐of‐life discussion, clinician's assessment of patient's decisional capacity at time of admission and end‐of‐life conversations, whether a comfort care plan was made, and whether palliative morphine was used.
The chart abstraction tool was pilot tested on the medical records of 10 patients, who were not included in the study, and refined to improve completeness of data collection. A copy of the abstraction tool is available upon request.
Definition of Key Variables
Decisional Capacity Assessment
Decisional capacity assessment is a reflection of the clinical team's assessment of the patient's decisional capacity on admission, and was determined through examination of the medical record in the first 24 hours of admission. Positive decision‐making capacity assessment was assumed if the clinician assessment of decisional capacity was documented in the mental status exam, or if the clinician documented conversations between clinician and patient in the history that suggested intact decision‐making capacity (ie, clinician documented terms such as patient stated or patient described and then described a coherent or sensible statement which implied patient capacity and intact mental status), or if the clinician's documentation of the assessment and plan stated or suggested decisions were being made by the patient. Other supportive information from the record was used to corroborate the evidence used to determine the clinician's assessment of the patient's decisional capacity, including whether the patient signed consent forms.
End‐of‐Life Discussions
The presence of an end‐of‐life discussion was presumed when the clinician documented a discussion with the patient, discussion with family, or family meeting concerning treatment preferences, or when the clinician quoted the patient's preferences in a fashion that documents a face‐to‐face discussion or directly described the elicitation of preferences from the patient or family.
Living Will
A living will was identified as present if the document was scanned into the patient's medical record, or if the chart indicated that the patient or family stated that the patient had completed a living will.
Health Care Proxy
Health Care Proxy or Durable Power of Attorney for Healthcare was identified as present if the document was scanned into the patient's medical record, or if the chart indicated that the patient or family stated that the patient had completed such a document.
Do Not Resuscitate (DNR) on Admission
DNR status was identified as present if the patient had documents with established DNR orders, or if a physician explicitly documented code status as DNR in the admission note or other documents placed in the record within the first 24 hours of admission.
Intensive Care Unit (ICU)Treatment
Patients were defined as having received ICU treatment if they were admitted directly or subsequently transferred to the ICU during the hospital course.
Comfort Care
Comfort care was defined as present only if the phrases comfort care, palliative care, or supportive care, were documented.
Palliative Opioid Therapy
Treatments with morphine or other opioids were recorded as palliative only if it was explicitly stated that these medications were used in the context of palliative or end‐of‐life care.
Data Analysis
We report the proportion of patients who were documented to lack decisional capacity at the time of hospital admission. We compared patient characteristics for those with and without documentation of decisional capacity on admission, and patients with decisional capacity on admission who did and did not participate in discussions about end‐of‐life care using chi‐square tests for categorical data, t tests for normally distributed continuous variables, and MannWhitney U tests for non‐normally distributed continuous variables. We examined whether documentation of a discussion about end‐of‐life care was associated with life‐sustaining and palliative treatments received using chi‐square for categorical treatments, and MannWhitney U tests for days from admission to initiation of comfort care. We used P < 0.05 to signify statistical significance.
RESULTS
Characteristics of Population and Decisional Capacity on Admission
The characteristics of the 145 patients who met entry criteria are summarized in Table 1. The most common types of cancers were lung cancer and leukemia/lymphoma. Of the 145 patients, the medical team's assessment of the patient's decisional capacity on admission could be established for 142 patients. As documented within the first 24 hours of admission, 27 patients (19%) were considered not to have decisional capacity, and 115 patients (79%) were considered to have decisional capacity. Both of these groups had similar age and gender distributions. There were no significant differences in the distribution of cancer type between those with and without decisional capacity. In both groups, the majority of the cancer diagnoses were made prior to admission. There was no difference in DNR orders established prior to or on admission between the groups (Table 1).
| Decision‐Making Capacity on Admissiona | |||
|---|---|---|---|
| No (N = 27) | Yes (N = 115) | ||
| N (%) | N (%) | P Value | |
| |||
| Age >65 | 15 (55.6) | 60 (52.2) | 0.75 |
| Gender (male) | 14 (51.9) | 68 (59.1) | 0.46 |
| Cancer type | |||
| Lung | 12 (44.4) | 42 (36.5) | 0.67 |
| Bone marrow | 5 (18.5) | 35 (30.4) | |
| Liver | 3 (11.1) | 6 (5.2) | |
| Pancreas | 3 (11.1) | 7 (6.10) | |
| Esophagus | 1 (3.7) | 8 (7.0) | |
| Colon | 1 (3.7) | 6 (5.2) | |
| Otherb | 2 (7.4) | 11 (9.6) | |
| Prior to admission | |||
| Cancer diagnosis known | 23 (85.2) | 91 (79.1) | 0.48 |
| Living will completed | 6 (22.2) | 26 (22.6 | 0.97 |
| Health care proxy established | 3 (11.1) | 26 (22.6) | 0.18 |
| DNR established | 8 (29.6) | 27 (23.5) | 0.79 |
End‐of‐Life Discussion in Patients With Decisional Capacity on Admission
Of the 115 patients assessed to have intact decisional capacity on admission, 56 (48.7%) participated in an end‐of‐life discussion with the medical team during their terminal hospitalization. For the remaining 59 patients who did not participate in an end‐of‐life discussion during the terminal hospital course, 46 (40.0%) had documentation suggesting they lost decisional capacity prior to a conversation and that the end‐of‐life discussions were held instead with the patient's surrogate decision‐maker, and 13 (11.3%) had no evidence of any end‐of‐life discussion (with the patient or surrogate).
When comparing those patients who participated in an end‐of‐life discussion with those patients whose surrogate participated in the discussion, there were no significant differences in gender and age distributions (Table 2). There was a significant difference in the type of cancers between the 2 groups. Among patients who participated in their end‐of‐life discussions, bone marrow cancer was proportionately more prevalent (17.9% vs 2.2%; P <0.01) and lung cancer was less prevalent (16.1% vs 41.3%; P <0.01), when compared to those who required surrogate participation. Timing of cancer diagnosis and prevalence of advance directives were similar between the 2 groups. The proportion of patients who had an established DNR order prior to admission was higher among those who did participate in end‐of‐life discussions when compared to those who had surrogate participation in these discussions (30.4% vs 17.4%; P < 0.04).
| Patients With Documented Decision‐Making Capacity on Admission (N = 115) | ||||
|---|---|---|---|---|
| N =13 | N = 102 | |||
| End‐of‐Life discussion not documented | End‐of‐Life discussion with surrogate (N = 46) | End‐of‐Life discussion with patient (N = 56) | ||
| N (%) | N (%) | N (%) | P Value | |
| ||||
| Age 65 | 9 (69.2) | 18 (39.1) | 27 (48.2) | 0.36 |
| Gender (male) | 10 (76.9) | 27 (60.0) | 31 (55.4) | 0.64 |
| Cancer type | ||||
| Lung | 3 (23.1) | 19 (41.3) | 9 (16.1) | |
| Bone marrow | 7 (53.9) | 1 (2.2) | 10 (17.9) | |
| Liver | 1 (7.7) | 3 (6.5) | 2 (3.6) | |
| Pancreas | 1 (7.7) | 2 (4.4) | 4 (7.1) | <0.01 |
| Esophagus | 0 | 6 (13.0) | 2 (3.6) | |
| Colon | 1 (7.7) | 1 (2.2) | 3 (5.4) | |
| Othera | 0 | 1 (2.2) | 10 (17.9) | |
| Prior to Admission | ||||
| Cancer diagnosis known | 10 (76.9) | 37 (80.4) | 43 (76.8) | 0.66 |
| Living will completed | 4 (30.8) | 10 (21.7) | 12 (21.4) | 0.97 |
| Health care proxy established | ||||
| 3 (23.1) | 13 (28.3) | 10 (17.9) | 0.21 | |
| DNR established | 2 (15.4) | 8 (17.4) | 17 (30.4) | 0.04 |
Life‐Prolonging and Palliative Care Treatments Received and Participation in End‐of‐Life Discussions
Life‐prolonging treatments were more likely to be used for patients whose end‐of‐life discussions were held by patient's surrogate decision‐maker, in comparison to those patients who participated in the discussions themselves. Patients who had conversations held by surrogates were more likely to receive ventilator support (56.5% vs 23.2%, P < 0.01), chemotherapy (39.1% vs 5.4%, P < 0.01), artificial nutrition or hydration (45.7% vs 25.0%, P = 0.03), and antibiotics (97.8% vs 78.6%, P < 0.01), when compared to patients who participated in their own end‐of‐life discussion. Intensive care treatment rates also differed significantly between the 2 groups; 56.5% of those who did not participate in end‐of‐life discussions, and only 23.2% of those patients who did participate, were admitted or transferred to the intensive care unit (P < 0.01). There was no significant difference in the proportion of patients who received cardiopulmonary resuscitation (CPR) (15.2% vs 7.1%, P = 0.56) (Table 3). Patients who lost decisional capacity and required a surrogate decision‐maker to participate in their end‐of‐life discussions had longer length of stay (15.8 vs 10.3 days, P = 0.03) and length of time to end‐of‐life discussions (14.0 vs 6.1 days, P < 0.01) (Table 3).
| Patients With Documented Decision‐Making Capacity on Admission (N = 115) | |||
|---|---|---|---|
| End‐of‐Life Discussion With Surrogate (N =46) | End‐of‐Life Discussion With Patient (N = 56) | ||
| N (%) | N (%) | P Value | |
| |||
| Life‐Prolonging Treatment | |||
| Ventilatory support | 26 (56.5) | 13 (23.2) | <0.01 |
| Chemotherapy | 18 (39.1) | 3 (5.4) | <0.01 |
| Artificial nutrition or hydration | 21 (45.7) | 14 (25.0) | 0.03 |
| Antibiotics | 45 (97.8) | 44 (78.6) | <0.01 |
| Cardiopulmonary resuscitation | 7 (15.2) | 4 (7.1) | 0.19 |
| ICU treatment (admit or transfer) | 26 (56.5) | 13 (23.2) | <0.01 |
| Palliative Treatments | |||
| Comfort care | 39 (84.8) | 45 (80.4) | 0.56 |
| Palliative morphine drip | 24 (52.2) | 33 (57.1) | 0.62 |
| Mean (95% CI) [Range] | Mean (95% CI) [Range] | ||
| Timing of Advanced Care Planning | |||
| Length of hospitalization | 15.8 d (11.420.2) [157] | 10.3 d (146) [146] | 0.03 |
| Time to end‐of‐life discussion | 14.0 d (9.918.1) [055] | 6.1 d (3.88.4) [046] | <0.01 |
| Time to comfort care | 23.5 d (4.342.8) [1374] | 9.2 d (6.312.1) [046] | 0.12 |
A comparison of the proportion of patients receiving palliative treatments, such as palliative comfort care orders and morphine infusions, revealed no significant differences between those with and without a discussion about end‐of‐life care (Table 3). Furthermore, while the time interval from hospital admission to initiation of comfort care was shorter for those who did participate in end‐of‐life discussions compared with those had surrogate participation (9.3 vs 23.5 days, P = 0.13 for equality), this difference was not statistically significant (Table 3).
Since a higher proportion of those who participated in end‐of‐life discussions during the hospitalization were admitted with a DNR order established prior to or on admission, we also examined the use of life‐prolonging treatments in the subgroup of patients who did not have a DNR order prior to admission. The difference in life‐prolonging treatments between those who did and did not participate in end‐of‐life discussions was preserved among those patients who did not have DNR status on admission (data not shown).
DISCUSSION
In this retrospective study of 145 terminal cancer patients who died in the hospital, we found that half of the patients had documentation suggesting that they lost decisional capacity during hospitalization and did not participate in end‐of‐life discussion with their healthcare providers. Among cancer patients in our study, 19% were determined not to have decisional capacity on admission, and another 32% were determined to lose decisional capacity during the hospital course. When patients did participate in documented discussions about end‐of‐life care, they were less likely to receive intensive life‐sustaining treatments, less likely to be admitted or transferred to the ICU, and more likely to avoid prolonged hospitalization. The finding that the majority of cancer patients are assessed to have intact decisional capacity upon admission to their terminal hospitalization, but less than half of them participate in their own end‐of‐life conversation, suggests that there is an important lost opportunity to provide quality advanced care planning to hospitalized patients.
Advanced care planning is the act of defining a competent patient's wishes regarding their future healthcare in the event of loss of decisional capacity. For many of the patients who were determined to lose decisional capacity during their hospitalization in our study, a surrogate decision‐maker was involved in a subsequent end‐of‐life discussion. This represents a missed opportunity in 2 ways. First, because surrogates may incorrectly predict patients' end‐of‐life treatment preferences in approximately one‐third of the cases,[25] patients may receive care that is inconsistent with their beliefs. Second, reliance on surrogate decision‐making may result in a greater burden on family members. Surrogate decision‐making places a large burden on surrogates, and can lead to emotional and psychological stress that can last well beyond the death of the loved one.[16, 26]
Our results reinforce the growing body of evidence suggesting that communication with the dying patient, both before and during the terminal hospitalization, promotes end‐of‐life care that involves less invasive life‐prolonging treatments,[10, 11, 12, 13, 14, 15] which is a consistently stated goal of most patients at the end of life.[1, 2] Our findings are consistent with a recent randomized trial of hospitalized patients over the age of 80 which showed that advance care planning discussions were associated with decreased use of intensive life‐sustaining treatments, increased patient and family satisfaction with care, as well as decreased psychological symptoms among family members.[27] In addition, studies examining patients with advanced cancer have shown that discussions about end‐of‐life care in the outpatient setting resulted in less intensive life‐sustaining treatments.[14, 15] Nearly 20% of our cohort was determined by the clinical team to lack decisional capacity at the time of their terminal hospitalization, highlighting the importance of end‐of‐life discussion prior to hospitalization. Our results also extend these recent findings to the inpatient setting. Seventy‐nine percent of patients in our study were determined to have decisional capacity at the time of their terminal hospitalization, and end‐of‐life discussion conducted with these patients was associated with decreased use of life‐sustaining treatments.
Seriously ill patients, particularly in hospital settings, have high symptom burden and subsequent poor quality of life.[6] These patients and families often report inadequate pain and symptom relief.[8, 28, 29] It is therefore reassuring that we found no difference in the proportion of patients who received comfort care and palliative morphine infusions according to whether they or a surrogate participated in the end‐of‐life discussions. In fact, the majority of patients were made DNR and received some form of comfort measure prior to death, although these comfort measures were frequently initiated only hours prior to death.
While our findings are consistent with research that has demonstrated that end‐of‐life discussions with patients are associated with a decrease in life‐prolonging treatments, it is important to note that our observational study cannot establish a causal relationship between the end‐of‐life discussion and the subsequent use of life‐sustaining treatments. It is possible that patients who have discussions about end‐of‐life care inherently prefer to have less intensive life‐sustaining treatment at the end of life. Physicians may be more apt to engage in these types of discussions with patients who express interest in limited intervention. Interestingly, patients with a DNR order at the time of admission were more likely to participate in end‐of‐life care with their provider during their terminal hospitalization, which supports this alternate explanation. However, when we excluded all patients with a DNR on admission, our findings persisted. Regardless of the explanation for the association between end‐of‐life discussions and life‐sustaining treatments, our study identifies a cohort of hospitalized patients who could benefit from improved end‐of‐life communication, and a clinical setting where opportunities remain to improve the quality of advanced care planning.
Discussions about end‐of‐life care with patients result in earlier transition to care focused on palliation.[14] Although we examined the timing to initiation of comfort care between those who participated and those who did not participate in end‐of‐life discussions, we were not able to demonstrate a statistically significant difference. However, our cohort may not be suitable for an examination of this type of intervention, as early discussions about end‐of‐life care may have lead to early referral to hospice, and therefore death outside the hospital setting, making the patients ineligible for our study.
There are several additional limitations to our study. First, our study is subject to the potential biases inherent in retrospective chart review. For example, since we identified patients who died in the hospital, our study does not generalize to patients with advanced cancer who survive the hospitalization or who were discharged to die at home.[30] Furthermore, our use of the medical record to assess documentation of communication about end‐of‐life care and decisional capacity relies on the accuracy of clinician documentation. There may have been communication about end‐of‐life care that was not documented in the medical record, resulting in underestimation of the effects of end‐of‐life discussions. In clinical practice, the assessment of decisional capacity can be challenging.[19, 31, 32] Although clinicians are often accurate in identifying patients with capacity, in nearly one‐quarter of all assessments, they mistakenly assign capacity to patients who lack decision‐making capacity.[17] In our study, we examine clinician assessment of the patient's decisional capacity, but cannot assess either the accuracy of their clinical assessment or the accuracy of their documentation of this assessment. A second limitation is that the chart abstraction was conducted by a single reviewer without inter‐rater assessment, although the abstraction tool was modified from a well‐established tool.[24] A third limitation is that all of the patients were from a single academic center and the results may not generalize to other regions. Fourth, we did not assess for the potential effect of individual clinicians. Since most patients were cared for by multiple physicians, our study is not capable of assessing a clinician‐level effect. Finally, our study was not able to address whether the care received by patients was in accord with their informed preferences.
We have shown that opportunities exist for advanced care planning at the time of the terminal hospitalization of patients with advanced cancer. The majority of these patients have documentation of decisional capacity at the time of admission, suggesting that opportunities exist to conduct end‐of‐life discussion while the patient retains decisional capacity. Furthermore, we found that patients who participate in these discussions about end‐of‐life care with their clinicians have an associated decrease in the use of life‐sustaining treatments, which is consistent with prior studies.[11, 13, 14, 15, 27] Improving communication about end‐of‐life care for patients hospitalized with advanced cancer may represent an important opportunity to improve the concordance between patients' wishes for care at the end‐of‐life and the care that these patients actually receive. Such communication may also decrease the burden on family members who are frequently asked to play the role of surrogate decision‐maker without an opportunity to discuss these issues with the patient.
Acknowledgments
The authors acknowledge Melissa Braxton for her assistance with the preparation of this manuscript.
Disclosures: A portion of this data was presented at the annual meeting of the Society of Hospital Medicine, 2011, in Grapevine, TX. This work was supported by the National Cancer Institute at the National Institutes of Health (KO5 CA 104699 to J.G.E.) and the National Heart, Lung, and Blood Institute (K24HL68593 to J.R.C.). Dr Silveira's salary was supported by core funds from the Ann Arbor VA HSRD Center of Excellence. The authors have no relevant financial interest in the subject matter contained within this manuscript.
- , , , . Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076–2080.
- , , , . Dying trajectory in the last year of life: does cancer trajectory fit other diseases?J Palliat Med. 2001;4(4):457–464.
- , , , . Trends and Variation in End‐of‐Life Care for Medicare Beneficiaries with Severe Chronic Illness.The Dartmouth Institute for Health Policy 32(3):638–643.
- , , , , . The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment. J Pain Symptom Manage. 1999;17(4):248–255.
- , , , . Family reports of pain in dying hospitalized patients: a structured telephone survey. West J Med. 2000;172(6):374–377.
- , , , et al. Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;126(2):97–106.
- , , , , . Death in the hospital. Arch Intern Med. 1998;158(14):1570–1572.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291(1):88–93.
- , , , , . Association between advance directives and quality of end‐of‐life care: a national study. J Am Geriatr Soc. 2007;55(2):189–194.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Health care costs in the last week of life: associations with end‐of‐life conversations. Arch Intern Med. 2009;169(5):480–488.
- , , , et al. End‐of‐life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012;156(3):204–210.
- , , , et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: cross‐sectional study. Lancet. 2004;364(9443):1421–1427.
- , . Assessing patients' capacities to consent to treatment. N Engl J Med 1998;319:1635–1638.
- . Clinical practice. Assessment of patients' competence to consent to treatment. N Engl J Med. 2007;357(18):1834–1840.
- , , , . The physician‐surrogate relationship. Arch Intern Med. 2007;167(11):1117–1121.
- , , , , . Physicians' experience with surrogate decision making for hospitalized adults. J Gen Intern Med. 2009;24(9):1023–1028.
- , , , . The surrogate's experience in authorizing a do not resuscitate order. Palliat Support Care. 2008;6(1):13–19.
- , , , et al. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975–2004. Bethesda, MD:National Cancer Institute. Based on November 2006 SEER data submission, posted to the SEER Web site,2007. Available at: http://seer.cancer.gov/csr/1975_2004/. Accessed on November 1, 2007.
- , , , , , . End‐of‐life decision‐making in the hospital: current practice and future prospects. J Pain Symptom Manage. 1999;17(1):6–15.
- , , . The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166(5):493–497.
- , . Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–346.
- , , , . The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
- , , , et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 suppl):S146–S153.
- , , , . The use of life‐sustaining treatments in hospitalized persons aged 80 and older. J Am Geriatr Soc. 2002;50(5):930–934.
- , , . Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770.
- , , , et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34.
- , , . The MacCAT‐T: a clinical tool to assess patients' capacities to make treatment decisions. Psychiatr Serv. 1997;48(11):1415–1419.
- , , , . Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076–2080.
- , , , . Dying trajectory in the last year of life: does cancer trajectory fit other diseases?J Palliat Med. 2001;4(4):457–464.
- , , , . Trends and Variation in End‐of‐Life Care for Medicare Beneficiaries with Severe Chronic Illness.The Dartmouth Institute for Health Policy 32(3):638–643.
- , , , , . The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment. J Pain Symptom Manage. 1999;17(4):248–255.
- , , , . Family reports of pain in dying hospitalized patients: a structured telephone survey. West J Med. 2000;172(6):374–377.
- , , , et al. Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;126(2):97–106.
- , , , , . Death in the hospital. Arch Intern Med. 1998;158(14):1570–1572.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291(1):88–93.
- , , , , . Association between advance directives and quality of end‐of‐life care: a national study. J Am Geriatr Soc. 2007;55(2):189–194.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Health care costs in the last week of life: associations with end‐of‐life conversations. Arch Intern Med. 2009;169(5):480–488.
- , , , et al. End‐of‐life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012;156(3):204–210.
- , , , et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: cross‐sectional study. Lancet. 2004;364(9443):1421–1427.
- , . Assessing patients' capacities to consent to treatment. N Engl J Med 1998;319:1635–1638.
- . Clinical practice. Assessment of patients' competence to consent to treatment. N Engl J Med. 2007;357(18):1834–1840.
- , , , . The physician‐surrogate relationship. Arch Intern Med. 2007;167(11):1117–1121.
- , , , , . Physicians' experience with surrogate decision making for hospitalized adults. J Gen Intern Med. 2009;24(9):1023–1028.
- , , , . The surrogate's experience in authorizing a do not resuscitate order. Palliat Support Care. 2008;6(1):13–19.
- , , , et al. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975–2004. Bethesda, MD:National Cancer Institute. Based on November 2006 SEER data submission, posted to the SEER Web site,2007. Available at: http://seer.cancer.gov/csr/1975_2004/. Accessed on November 1, 2007.
- , , , , , . End‐of‐life decision‐making in the hospital: current practice and future prospects. J Pain Symptom Manage. 1999;17(1):6–15.
- , , . The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166(5):493–497.
- , . Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–346.
- , , , . The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
- , , , et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 suppl):S146–S153.
- , , , . The use of life‐sustaining treatments in hospitalized persons aged 80 and older. J Am Geriatr Soc. 2002;50(5):930–934.
- , , . Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770.
- , , , et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34.
- , , . The MacCAT‐T: a clinical tool to assess patients' capacities to make treatment decisions. Psychiatr Serv. 1997;48(11):1415–1419.
Copyright © 2012 Society of Hospital Medicine