User login
Synthetic snake venom to the rescue? Potential uses in skin health and rejuvenation
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
Cellular senescence, skin aging, and cosmeceuticals
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.
Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
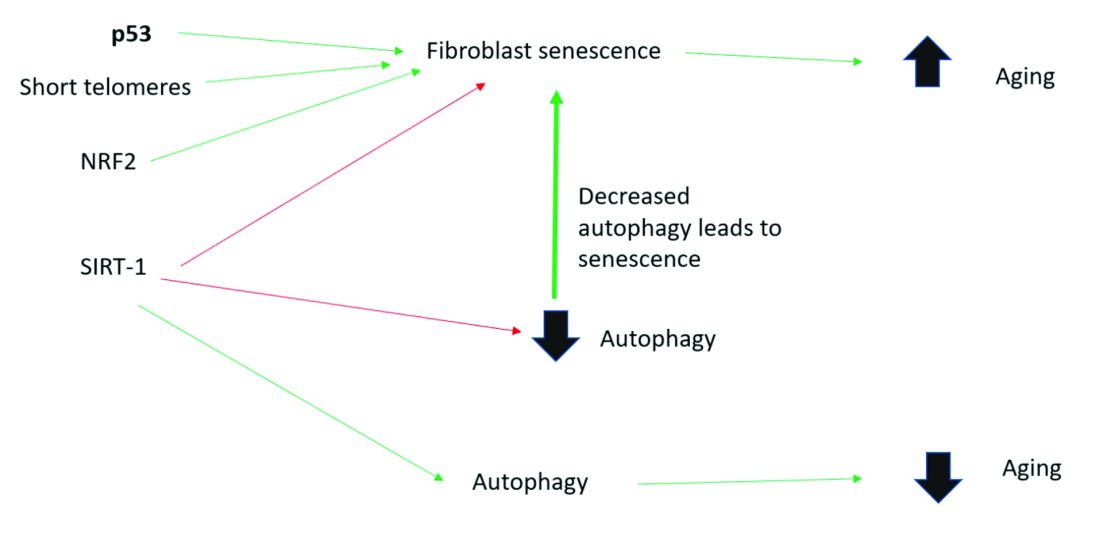
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
Seaweed and other marine-derived products in skin care, Part II: Cosmetic formulations, fucoidan, and salmon eggs
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
Seaweed and other marine-derived products in skin care, part 1: Current indications
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
The cutaneous benefits of bee venom, Part II: Acupuncture, wound healing, and various potential indications
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
The cutaneous benefits of bee venom, Part I: Atopic dermatitis and acne
Honeybees, Apis mellifera, play an important role in the web of life. We rely on bees for pollinating approximately one-third of our crops, including multiple fruits, vegetables, nuts, and seeds.1,2 Bees are also instrumental in the propagation of other plants, flower nectar, and flower pollen. A. mellifera, the European honeybee, is the main pollinator in Europe and North America, but other species, including A. cerana, A. dorsata, A. floria, A. andreniformis, A. koschevnikov, and A. laboriosa, yield honey.3 Honey, propolis, and royal jelly, along with beeswax and bee pollen, are among some of the celebrated bee products that have been found to confer health benefits to human beings.4,5 Bee venom, a toxin bees use for protection, is a convoluted combination of peptides and toxic proteins such as phospholipase A2 (PLA2) and melittin that has garnered significant scientific attention of late and is used to treat various inflammatory conditions.6-8 This column will focus on the investigation of the use of bee venom to treat atopic dermatitis (AD) and acne.
Atopic dermatitis
In 2013, Kim et al. assessed the impact of bee venom on AD-related symptoms in mice, finding that it attenuated the effects of AD-simulating compounds in 48 of 80 patients injected subcutaneously. They concluded that bee venom acted by suppressing mast cell degranulation and proinflammatory cytokine expression.9 Three years later, You et al. conducted a double-blind, randomized, base-controlled multicenter study of 136 patients with AD to ascertain the effects of a bee venom emollient. For 4 weeks, patients applied an emollient with bee venom and silk protein or a vehicle lacking bee venom twice daily. Eczema area and severity index (EASI) scores were significantly lower in the bee venom group, as were the visual analogue scale (VAS) scores. The investigators concluded that bee venom is an effective and safe therapeutic choice for treating patients with AD.10 Further, in 2018, Shin et al. demonstrated that PLA2 derived from bee venom mitigates atopic skin inflammation via the CD206 mannose receptor. They had previously shown in a mouse model that PLA2 from bee venom exerts such activity against AD-like lesions induced by 2,4-dinitrochlorobenzene (DNCB) and house dust mite (Dermatophagoides farinae) extract.11 Gu et al. observed later that year that intraperitoneal administration of bee venom eased the symptoms of ovalbumin-induced AD-like skin lesions in an experimental mouse model. Bee venom also lowered serum immunoglobulin E levels and suppressed infiltration of eosinophils and mast cells. They concluded that bee venom is a viable alternative for attenuating the allergic skin inflammation characteristic of AD.12 At the end of 2018, An et al. reported on the use of an in vivo female Balb/c mouse AD model in which 1-chloro-DNCB acted as inducer in cultures of human keratinocytes, stimulated by TNF-alpha/IFN-gamma. The investigators found that bee venom and melittin displayed robust antiatopic effects as evidenced by reduced lesions. The bee products were also found to have hindered elevated expression of various chemokines and proinflammatory cytokines. The authors suggested that bee venom and melittin appear to warrant consideration as a topical treatment for AD.13 In 2019, Kim et al. demonstrated in mice that bee venom eases the symptoms of AD by inactivating the complement system, particularly through CD55 induction, which might account for its effectiveness in AD treatment in humans, they suggested.6 Early in 2020, Lee et al. demonstrated in a Balb/c mouse model that bee venom appears to be a possible therapeutic macromolecule for treating phthalic anhydride-induced AD.7
Acne
In 2013, in vitro experiments by Han et al. showed that purified bee venom exhibited antimicrobial activity, in a concentration-dependent manner, against Cutibacterium acnes (or Propionibacterium acnes). They followed up with a small randomized, double-blind, controlled trial with 12 subjects who were treated with cosmetics with pure bee venom or cosmetics without it for two weeks. The group receiving bee venom experienced significantly fewer inflammatory and noninflammatory lesions, and a significant decline in adenosine triphosphate levels (a 57.5% reduction) was noted in subjects in the bee venom group, with a nonsignificant decrease of 4.7% observed in the control group. The investigators concluded the purified bee venom may be suitable as an antiacne agent.14 Using a mouse model, An et al. studied the therapeutic effects of bee venom against C. acnes–induced skin inflammation. They found that bee venom significantly diminished the volume of infiltrated inflammatory cells in the treated mice, compared with untreated mice. Bee venom also decreased expression levels of tumor necrosis factor (TNF)-α, and interleukin (IL)-1beta and suppressed Toll-like receptor (TLR)2 and CD14 expression in C. acnes–injected tissue. The investigators concluded that bee venom imparts notable anti-inflammatory activity and has potential for use in treating acne and as an anti-inflammatory agent in skin care.15
In 2015, Kim et al. studied the influence of bee venom against C. acnes–induced inflammation in human keratinocytes (HaCaT) and monocytes (THP-1). They found that bee venom successfully suppressed the secretion of interferon-gamma, IL-1beta, IL-8, and TNF-alpha. It also galvanized the expression of IL-8 and TLR2 in HaCaT cells but hampered their expression in heat-killed C. acnes. The researchers concluded that bee venom displays considerable anti-inflammatory activity against C. acnes and warrants consideration as an alternative to antibiotic acne treatment.16 It is worth noting that early that year, in a comprehensive database review to evaluate the effects and safety of a wide range of complementary treatments for acne, Cao et al. found, among 35 studies including parallel-group randomized controlled trials, that one trial indicated bee venom was superior to control in lowering the number of acne lesions.17
Conclusion
More research, in the form of randomized, controlled trials, is required before bee venom can be incorporated into the dermatologic armamentarium as a first-line therapy for common and vexing cutaneous conditions. Nevertheless, .
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Walsh B. The plight of the honeybee: Mass deaths in bee colonies may mean disaster for farmers – and your favorite Foods. Time Magazine, 2013 Aug 19.
2. Klein AM et al. Proc Biol Sci. 2007 Feb 7;274(1608):303-13. doi: 10.1098/rspb.2006.3721.
3. Ediriweera ER and Premarathna NY. AYU. 2012 Apr;33(2):178-82. doi: 10.4103/0974-8520.105233.
4. Baumann, L. Honey/Propolis/Royal Jelly. In Cosmeceuticals and Cosmetic Ingredients. New York:McGraw-Hill; 2014:203-212.
5. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412. doi: 10.3389/fphar.2017.00412.
6. Kim Y et al. Toxins (Basel). 2019 Apr 26;11(5):239. doi: 10.3390/toxins11050239.
7. Lee YJ et al. Inflammopharmacology. 2020 Feb;28(1):253-63. doi: 10.1007/s10787-019-00646-w.
8. Lee G and Bae H. Molecules. 2016 May 11;21(5):616. doi: 10.3390/molecules21050616.
9. Kim KH et al. Int J Clin Exp Pathol. 2013 Nov 15;6(12):2896-903.
10. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. doi: 10.5021/ad.2016.28.5.593.
11. Shin D et al. Toxins (Basel). 2018 Apr 2;10(4):146. doi: 10.3390/toxins10040146.
12. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. doi: 10.3892/mmr.2018.9398.
13. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24. doi: 10.1111/bph.14487.
14. Han SM et al. J Integr Med. 2013 Sep;11(5):320-6. doi: 10.3736/jintegrmed2013043.
15. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. doi: 10.3892/ijmm.2014.1933.
16. Kim JY et al. Int J Mol Med. 2015 Jun;35(6):1651-6. doi: 10.3892/ijmm.2015.2180.
17. Cao H et al. Cochrane Database Syst Rev. 2015 Jan 19;1:CD009436. doi: 10.1002/14651858.CD009436.pub2.
Honeybees, Apis mellifera, play an important role in the web of life. We rely on bees for pollinating approximately one-third of our crops, including multiple fruits, vegetables, nuts, and seeds.1,2 Bees are also instrumental in the propagation of other plants, flower nectar, and flower pollen. A. mellifera, the European honeybee, is the main pollinator in Europe and North America, but other species, including A. cerana, A. dorsata, A. floria, A. andreniformis, A. koschevnikov, and A. laboriosa, yield honey.3 Honey, propolis, and royal jelly, along with beeswax and bee pollen, are among some of the celebrated bee products that have been found to confer health benefits to human beings.4,5 Bee venom, a toxin bees use for protection, is a convoluted combination of peptides and toxic proteins such as phospholipase A2 (PLA2) and melittin that has garnered significant scientific attention of late and is used to treat various inflammatory conditions.6-8 This column will focus on the investigation of the use of bee venom to treat atopic dermatitis (AD) and acne.
Atopic dermatitis
In 2013, Kim et al. assessed the impact of bee venom on AD-related symptoms in mice, finding that it attenuated the effects of AD-simulating compounds in 48 of 80 patients injected subcutaneously. They concluded that bee venom acted by suppressing mast cell degranulation and proinflammatory cytokine expression.9 Three years later, You et al. conducted a double-blind, randomized, base-controlled multicenter study of 136 patients with AD to ascertain the effects of a bee venom emollient. For 4 weeks, patients applied an emollient with bee venom and silk protein or a vehicle lacking bee venom twice daily. Eczema area and severity index (EASI) scores were significantly lower in the bee venom group, as were the visual analogue scale (VAS) scores. The investigators concluded that bee venom is an effective and safe therapeutic choice for treating patients with AD.10 Further, in 2018, Shin et al. demonstrated that PLA2 derived from bee venom mitigates atopic skin inflammation via the CD206 mannose receptor. They had previously shown in a mouse model that PLA2 from bee venom exerts such activity against AD-like lesions induced by 2,4-dinitrochlorobenzene (DNCB) and house dust mite (Dermatophagoides farinae) extract.11 Gu et al. observed later that year that intraperitoneal administration of bee venom eased the symptoms of ovalbumin-induced AD-like skin lesions in an experimental mouse model. Bee venom also lowered serum immunoglobulin E levels and suppressed infiltration of eosinophils and mast cells. They concluded that bee venom is a viable alternative for attenuating the allergic skin inflammation characteristic of AD.12 At the end of 2018, An et al. reported on the use of an in vivo female Balb/c mouse AD model in which 1-chloro-DNCB acted as inducer in cultures of human keratinocytes, stimulated by TNF-alpha/IFN-gamma. The investigators found that bee venom and melittin displayed robust antiatopic effects as evidenced by reduced lesions. The bee products were also found to have hindered elevated expression of various chemokines and proinflammatory cytokines. The authors suggested that bee venom and melittin appear to warrant consideration as a topical treatment for AD.13 In 2019, Kim et al. demonstrated in mice that bee venom eases the symptoms of AD by inactivating the complement system, particularly through CD55 induction, which might account for its effectiveness in AD treatment in humans, they suggested.6 Early in 2020, Lee et al. demonstrated in a Balb/c mouse model that bee venom appears to be a possible therapeutic macromolecule for treating phthalic anhydride-induced AD.7
Acne
In 2013, in vitro experiments by Han et al. showed that purified bee venom exhibited antimicrobial activity, in a concentration-dependent manner, against Cutibacterium acnes (or Propionibacterium acnes). They followed up with a small randomized, double-blind, controlled trial with 12 subjects who were treated with cosmetics with pure bee venom or cosmetics without it for two weeks. The group receiving bee venom experienced significantly fewer inflammatory and noninflammatory lesions, and a significant decline in adenosine triphosphate levels (a 57.5% reduction) was noted in subjects in the bee venom group, with a nonsignificant decrease of 4.7% observed in the control group. The investigators concluded the purified bee venom may be suitable as an antiacne agent.14 Using a mouse model, An et al. studied the therapeutic effects of bee venom against C. acnes–induced skin inflammation. They found that bee venom significantly diminished the volume of infiltrated inflammatory cells in the treated mice, compared with untreated mice. Bee venom also decreased expression levels of tumor necrosis factor (TNF)-α, and interleukin (IL)-1beta and suppressed Toll-like receptor (TLR)2 and CD14 expression in C. acnes–injected tissue. The investigators concluded that bee venom imparts notable anti-inflammatory activity and has potential for use in treating acne and as an anti-inflammatory agent in skin care.15
In 2015, Kim et al. studied the influence of bee venom against C. acnes–induced inflammation in human keratinocytes (HaCaT) and monocytes (THP-1). They found that bee venom successfully suppressed the secretion of interferon-gamma, IL-1beta, IL-8, and TNF-alpha. It also galvanized the expression of IL-8 and TLR2 in HaCaT cells but hampered their expression in heat-killed C. acnes. The researchers concluded that bee venom displays considerable anti-inflammatory activity against C. acnes and warrants consideration as an alternative to antibiotic acne treatment.16 It is worth noting that early that year, in a comprehensive database review to evaluate the effects and safety of a wide range of complementary treatments for acne, Cao et al. found, among 35 studies including parallel-group randomized controlled trials, that one trial indicated bee venom was superior to control in lowering the number of acne lesions.17
Conclusion
More research, in the form of randomized, controlled trials, is required before bee venom can be incorporated into the dermatologic armamentarium as a first-line therapy for common and vexing cutaneous conditions. Nevertheless, .
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Walsh B. The plight of the honeybee: Mass deaths in bee colonies may mean disaster for farmers – and your favorite Foods. Time Magazine, 2013 Aug 19.
2. Klein AM et al. Proc Biol Sci. 2007 Feb 7;274(1608):303-13. doi: 10.1098/rspb.2006.3721.
3. Ediriweera ER and Premarathna NY. AYU. 2012 Apr;33(2):178-82. doi: 10.4103/0974-8520.105233.
4. Baumann, L. Honey/Propolis/Royal Jelly. In Cosmeceuticals and Cosmetic Ingredients. New York:McGraw-Hill; 2014:203-212.
5. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412. doi: 10.3389/fphar.2017.00412.
6. Kim Y et al. Toxins (Basel). 2019 Apr 26;11(5):239. doi: 10.3390/toxins11050239.
7. Lee YJ et al. Inflammopharmacology. 2020 Feb;28(1):253-63. doi: 10.1007/s10787-019-00646-w.
8. Lee G and Bae H. Molecules. 2016 May 11;21(5):616. doi: 10.3390/molecules21050616.
9. Kim KH et al. Int J Clin Exp Pathol. 2013 Nov 15;6(12):2896-903.
10. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. doi: 10.5021/ad.2016.28.5.593.
11. Shin D et al. Toxins (Basel). 2018 Apr 2;10(4):146. doi: 10.3390/toxins10040146.
12. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. doi: 10.3892/mmr.2018.9398.
13. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24. doi: 10.1111/bph.14487.
14. Han SM et al. J Integr Med. 2013 Sep;11(5):320-6. doi: 10.3736/jintegrmed2013043.
15. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. doi: 10.3892/ijmm.2014.1933.
16. Kim JY et al. Int J Mol Med. 2015 Jun;35(6):1651-6. doi: 10.3892/ijmm.2015.2180.
17. Cao H et al. Cochrane Database Syst Rev. 2015 Jan 19;1:CD009436. doi: 10.1002/14651858.CD009436.pub2.
Honeybees, Apis mellifera, play an important role in the web of life. We rely on bees for pollinating approximately one-third of our crops, including multiple fruits, vegetables, nuts, and seeds.1,2 Bees are also instrumental in the propagation of other plants, flower nectar, and flower pollen. A. mellifera, the European honeybee, is the main pollinator in Europe and North America, but other species, including A. cerana, A. dorsata, A. floria, A. andreniformis, A. koschevnikov, and A. laboriosa, yield honey.3 Honey, propolis, and royal jelly, along with beeswax and bee pollen, are among some of the celebrated bee products that have been found to confer health benefits to human beings.4,5 Bee venom, a toxin bees use for protection, is a convoluted combination of peptides and toxic proteins such as phospholipase A2 (PLA2) and melittin that has garnered significant scientific attention of late and is used to treat various inflammatory conditions.6-8 This column will focus on the investigation of the use of bee venom to treat atopic dermatitis (AD) and acne.
Atopic dermatitis
In 2013, Kim et al. assessed the impact of bee venom on AD-related symptoms in mice, finding that it attenuated the effects of AD-simulating compounds in 48 of 80 patients injected subcutaneously. They concluded that bee venom acted by suppressing mast cell degranulation and proinflammatory cytokine expression.9 Three years later, You et al. conducted a double-blind, randomized, base-controlled multicenter study of 136 patients with AD to ascertain the effects of a bee venom emollient. For 4 weeks, patients applied an emollient with bee venom and silk protein or a vehicle lacking bee venom twice daily. Eczema area and severity index (EASI) scores were significantly lower in the bee venom group, as were the visual analogue scale (VAS) scores. The investigators concluded that bee venom is an effective and safe therapeutic choice for treating patients with AD.10 Further, in 2018, Shin et al. demonstrated that PLA2 derived from bee venom mitigates atopic skin inflammation via the CD206 mannose receptor. They had previously shown in a mouse model that PLA2 from bee venom exerts such activity against AD-like lesions induced by 2,4-dinitrochlorobenzene (DNCB) and house dust mite (Dermatophagoides farinae) extract.11 Gu et al. observed later that year that intraperitoneal administration of bee venom eased the symptoms of ovalbumin-induced AD-like skin lesions in an experimental mouse model. Bee venom also lowered serum immunoglobulin E levels and suppressed infiltration of eosinophils and mast cells. They concluded that bee venom is a viable alternative for attenuating the allergic skin inflammation characteristic of AD.12 At the end of 2018, An et al. reported on the use of an in vivo female Balb/c mouse AD model in which 1-chloro-DNCB acted as inducer in cultures of human keratinocytes, stimulated by TNF-alpha/IFN-gamma. The investigators found that bee venom and melittin displayed robust antiatopic effects as evidenced by reduced lesions. The bee products were also found to have hindered elevated expression of various chemokines and proinflammatory cytokines. The authors suggested that bee venom and melittin appear to warrant consideration as a topical treatment for AD.13 In 2019, Kim et al. demonstrated in mice that bee venom eases the symptoms of AD by inactivating the complement system, particularly through CD55 induction, which might account for its effectiveness in AD treatment in humans, they suggested.6 Early in 2020, Lee et al. demonstrated in a Balb/c mouse model that bee venom appears to be a possible therapeutic macromolecule for treating phthalic anhydride-induced AD.7
Acne
In 2013, in vitro experiments by Han et al. showed that purified bee venom exhibited antimicrobial activity, in a concentration-dependent manner, against Cutibacterium acnes (or Propionibacterium acnes). They followed up with a small randomized, double-blind, controlled trial with 12 subjects who were treated with cosmetics with pure bee venom or cosmetics without it for two weeks. The group receiving bee venom experienced significantly fewer inflammatory and noninflammatory lesions, and a significant decline in adenosine triphosphate levels (a 57.5% reduction) was noted in subjects in the bee venom group, with a nonsignificant decrease of 4.7% observed in the control group. The investigators concluded the purified bee venom may be suitable as an antiacne agent.14 Using a mouse model, An et al. studied the therapeutic effects of bee venom against C. acnes–induced skin inflammation. They found that bee venom significantly diminished the volume of infiltrated inflammatory cells in the treated mice, compared with untreated mice. Bee venom also decreased expression levels of tumor necrosis factor (TNF)-α, and interleukin (IL)-1beta and suppressed Toll-like receptor (TLR)2 and CD14 expression in C. acnes–injected tissue. The investigators concluded that bee venom imparts notable anti-inflammatory activity and has potential for use in treating acne and as an anti-inflammatory agent in skin care.15
In 2015, Kim et al. studied the influence of bee venom against C. acnes–induced inflammation in human keratinocytes (HaCaT) and monocytes (THP-1). They found that bee venom successfully suppressed the secretion of interferon-gamma, IL-1beta, IL-8, and TNF-alpha. It also galvanized the expression of IL-8 and TLR2 in HaCaT cells but hampered their expression in heat-killed C. acnes. The researchers concluded that bee venom displays considerable anti-inflammatory activity against C. acnes and warrants consideration as an alternative to antibiotic acne treatment.16 It is worth noting that early that year, in a comprehensive database review to evaluate the effects and safety of a wide range of complementary treatments for acne, Cao et al. found, among 35 studies including parallel-group randomized controlled trials, that one trial indicated bee venom was superior to control in lowering the number of acne lesions.17
Conclusion
More research, in the form of randomized, controlled trials, is required before bee venom can be incorporated into the dermatologic armamentarium as a first-line therapy for common and vexing cutaneous conditions. Nevertheless, .
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Walsh B. The plight of the honeybee: Mass deaths in bee colonies may mean disaster for farmers – and your favorite Foods. Time Magazine, 2013 Aug 19.
2. Klein AM et al. Proc Biol Sci. 2007 Feb 7;274(1608):303-13. doi: 10.1098/rspb.2006.3721.
3. Ediriweera ER and Premarathna NY. AYU. 2012 Apr;33(2):178-82. doi: 10.4103/0974-8520.105233.
4. Baumann, L. Honey/Propolis/Royal Jelly. In Cosmeceuticals and Cosmetic Ingredients. New York:McGraw-Hill; 2014:203-212.
5. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412. doi: 10.3389/fphar.2017.00412.
6. Kim Y et al. Toxins (Basel). 2019 Apr 26;11(5):239. doi: 10.3390/toxins11050239.
7. Lee YJ et al. Inflammopharmacology. 2020 Feb;28(1):253-63. doi: 10.1007/s10787-019-00646-w.
8. Lee G and Bae H. Molecules. 2016 May 11;21(5):616. doi: 10.3390/molecules21050616.
9. Kim KH et al. Int J Clin Exp Pathol. 2013 Nov 15;6(12):2896-903.
10. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. doi: 10.5021/ad.2016.28.5.593.
11. Shin D et al. Toxins (Basel). 2018 Apr 2;10(4):146. doi: 10.3390/toxins10040146.
12. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. doi: 10.3892/mmr.2018.9398.
13. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24. doi: 10.1111/bph.14487.
14. Han SM et al. J Integr Med. 2013 Sep;11(5):320-6. doi: 10.3736/jintegrmed2013043.
15. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. doi: 10.3892/ijmm.2014.1933.
16. Kim JY et al. Int J Mol Med. 2015 Jun;35(6):1651-6. doi: 10.3892/ijmm.2015.2180.
17. Cao H et al. Cochrane Database Syst Rev. 2015 Jan 19;1:CD009436. doi: 10.1002/14651858.CD009436.pub2.
Circadian rhythms, part 2: Can treating cutaneous conditions at different times of the day improve outcomes?
We continue with a focus on when possible, as well as clinical studies that may shed light on how to time skin care treatments.
It is important to remember that several studies in the last 20 years have revealed cutaneous tendencies based on the time of day. For instance, sebum production is known to be highest around noon, and pH also peaks during the day and is at its lowest at night.1-5
Skin aging
In 2019, Dong and associates showed that blue light at 410 nm reduces PER1 transcription in keratinocytes, indicating that epidermal cells have the capacity to directly sense light and regulate their own clock gene expression. With the introduction of blue light at night, circadian rhythm is disrupted as epidermal skin cells act as if it is daytime. The investigators also considered blue light–induced damage to skin cells at various doses and exposure times in comparison with cells that remained unexposed to light. The production of reactive oxygen species increased in the exposed cells, as did DNA impairment and the emergence of inflammatory mediators, all of which have the potential to hasten aging.6
Early this year, Dong and associates demonstrated that melatonin can dose-dependently stimulate PER1 clock gene expression in normal human dermal fibroblasts and normal human epidermal keratinocytes, and verified that the MT-1 melatonin receptor in such fibroblasts manifests a marked decline with age. The researchers concluded that the melatonin pathway contributes significantly in cutaneous aging and impairment, and that its relationship with skin circadian rhythm points to a possible role in slowing the rate of skin aging through the modulation of cutaneous melatonin receptors.7
Wound healing
In 2019, Walker and associates investigated the effects of dim artificial light at night on wound healing in female C57BL/6 mice, and found that those conditions prior to wounding reduced healing. They concluded that such information might warrant consideration in prescribing treatment.8
Atopic dermatitis
Vaughn and associates contended that alterations in circadian rhythm may contribute to the development of atopic dermatitis.9 A good example of the impact of circadian rhythms on cutaneous health is the nocturnal exacerbation of atopic dermatitis, particularly in children.10
Psoriasis
According to Plikus and associates, recent evidence has emerged showing that the circadian clock regulates UVB-induced DNA damage and cutaneous cancers, and it is also associated with the immune-mediated disorder psoriasis.11
Clinical studies
In 2018, Deshayes and associates conducted a clinical study to evaluate the precursors and stem cell attributes of hHF (human hair follicle keratinocytes), hEpi (human interfollicular epidermal keratinocytes), and hHFDP (hair follicle dermal papilla stem cells) in response to clock pathway changes caused by long-term deregulation of circadian rhythms. A total of 20 women participated in the study, 10 in each group (day workers were the control group and compared with shift workers). Two 3-mm fresh punch biopsies were collected from the occipital region of each participant. The investigators reported that chronic circadian rhythm deregulation influenced clock pathway protein expression and correlated with changes in hHF, hEpi, and hHFDP. They concluded that their findings represented the first data in humans suggesting that deregulation of the clock pathway modulates regenerative activity in human cutaneous and hair precursor cells.12
Later that year, Wu and associates reported on the role of the circadian clock in the transcriptional regulation of human epidermis. Investigators sampled 20 human participants through a 24-hour period and a population of 219 people once, finding a potent circadian oscillator in human epidermis at the population level, hundreds of rhythmically expressed genes, as well as a biomarker set for human epidermis that can, with one sample, highlight circadian phase within a 3-hour time frame. The team concluded that rhythms in human epidermis persist at the population level, and that they were able to present an effective single-sample circadian biomarker.13 This is important, as Morris pointed out, because the standard practice for measuring an individual’s internal clock is to use a dim-light melatonin onset assay over the course of a day.14 In 2019, Jia and associates studied the skin surface lipid profiles of young women to evaluate and characterize circadian human facial surface lipid composition. The investigators identified significant markers of circadian rhythm, with glycerolipids most affected. They ascribed changes in skin barrier function, such as variable pH and transepidermal water loss, to alterations in triacylglycerol levels as well as free fatty acid chain lengths and content that were affected by variations in circadian rhythm.15
Sleep and the timing of topicals
Based on their recent review of the literature on circadian rhythm and skin, Lyons and associates argued that an understanding of circadian rhythm helps dermatologists in recommending the optimal times for patients to apply topical medications. They added that urging patients to get sufficient sleep is important because DNA repair of the skin occurs best at that time.16
Conclusions
Doctors have known for half a century that timing drug delivery to a patient’s circadian clock can enhance outcomes. Chronobiological research into how circadian rhythms work at the cellular level, and in cutaneous cells in particular, is a fascinating and expanding area of inquiry that could help dermatologists more accurately recommend timing for skin care regimens. Much more research, especially in clinical trials, is necessary to further elucidate how to best work with the skin’s natural rhythms.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on skin care technologies. Write to her at dermnews@mdedge.com.
References
1. Mehling A et al. Skin Pharmacol Physiol. 2006;19(4):182-9.
2. Latreille J et al. Skin Pharmacol Physiol. 2004 May-Jun;17(3):133-40.
3. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
4. Verschoore M et al. Chronobiol Int. 1993 Oct;10(5):349-59.
5. Yosipovitch G et al. J Invest Dermatol. 1998 Jan;110(1):20-3.
6. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
7. Dong K et al. Int J Mol Sci. 2020 Jan 3;21(1):326.
8. Walker WH II et al. Arch Dermatol Res. 2019 Sep;311(7):573-6.
9. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
10. Fishbein AB et al. J Allergy Clin Immunol. 2015 Nov;136(5):1170-7.
11. Plikus MV et al. J Biol Rhythms. 2015 Jun;30(3):163-82.
12. Deshayes N et al. Eur J Dermatol. 2018 Aug 1;28(4):467-75.
13. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
14. Morris A. Nat Rev Endocrinol. 2018 Dec;15(1):3.
15. Jia Y et al. Exp Dermatol. 2019 Jul;28(7):858-62.
16. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
We continue with a focus on when possible, as well as clinical studies that may shed light on how to time skin care treatments.
It is important to remember that several studies in the last 20 years have revealed cutaneous tendencies based on the time of day. For instance, sebum production is known to be highest around noon, and pH also peaks during the day and is at its lowest at night.1-5
Skin aging
In 2019, Dong and associates showed that blue light at 410 nm reduces PER1 transcription in keratinocytes, indicating that epidermal cells have the capacity to directly sense light and regulate their own clock gene expression. With the introduction of blue light at night, circadian rhythm is disrupted as epidermal skin cells act as if it is daytime. The investigators also considered blue light–induced damage to skin cells at various doses and exposure times in comparison with cells that remained unexposed to light. The production of reactive oxygen species increased in the exposed cells, as did DNA impairment and the emergence of inflammatory mediators, all of which have the potential to hasten aging.6
Early this year, Dong and associates demonstrated that melatonin can dose-dependently stimulate PER1 clock gene expression in normal human dermal fibroblasts and normal human epidermal keratinocytes, and verified that the MT-1 melatonin receptor in such fibroblasts manifests a marked decline with age. The researchers concluded that the melatonin pathway contributes significantly in cutaneous aging and impairment, and that its relationship with skin circadian rhythm points to a possible role in slowing the rate of skin aging through the modulation of cutaneous melatonin receptors.7
Wound healing
In 2019, Walker and associates investigated the effects of dim artificial light at night on wound healing in female C57BL/6 mice, and found that those conditions prior to wounding reduced healing. They concluded that such information might warrant consideration in prescribing treatment.8
Atopic dermatitis
Vaughn and associates contended that alterations in circadian rhythm may contribute to the development of atopic dermatitis.9 A good example of the impact of circadian rhythms on cutaneous health is the nocturnal exacerbation of atopic dermatitis, particularly in children.10
Psoriasis
According to Plikus and associates, recent evidence has emerged showing that the circadian clock regulates UVB-induced DNA damage and cutaneous cancers, and it is also associated with the immune-mediated disorder psoriasis.11
Clinical studies
In 2018, Deshayes and associates conducted a clinical study to evaluate the precursors and stem cell attributes of hHF (human hair follicle keratinocytes), hEpi (human interfollicular epidermal keratinocytes), and hHFDP (hair follicle dermal papilla stem cells) in response to clock pathway changes caused by long-term deregulation of circadian rhythms. A total of 20 women participated in the study, 10 in each group (day workers were the control group and compared with shift workers). Two 3-mm fresh punch biopsies were collected from the occipital region of each participant. The investigators reported that chronic circadian rhythm deregulation influenced clock pathway protein expression and correlated with changes in hHF, hEpi, and hHFDP. They concluded that their findings represented the first data in humans suggesting that deregulation of the clock pathway modulates regenerative activity in human cutaneous and hair precursor cells.12
Later that year, Wu and associates reported on the role of the circadian clock in the transcriptional regulation of human epidermis. Investigators sampled 20 human participants through a 24-hour period and a population of 219 people once, finding a potent circadian oscillator in human epidermis at the population level, hundreds of rhythmically expressed genes, as well as a biomarker set for human epidermis that can, with one sample, highlight circadian phase within a 3-hour time frame. The team concluded that rhythms in human epidermis persist at the population level, and that they were able to present an effective single-sample circadian biomarker.13 This is important, as Morris pointed out, because the standard practice for measuring an individual’s internal clock is to use a dim-light melatonin onset assay over the course of a day.14 In 2019, Jia and associates studied the skin surface lipid profiles of young women to evaluate and characterize circadian human facial surface lipid composition. The investigators identified significant markers of circadian rhythm, with glycerolipids most affected. They ascribed changes in skin barrier function, such as variable pH and transepidermal water loss, to alterations in triacylglycerol levels as well as free fatty acid chain lengths and content that were affected by variations in circadian rhythm.15
Sleep and the timing of topicals
Based on their recent review of the literature on circadian rhythm and skin, Lyons and associates argued that an understanding of circadian rhythm helps dermatologists in recommending the optimal times for patients to apply topical medications. They added that urging patients to get sufficient sleep is important because DNA repair of the skin occurs best at that time.16
Conclusions
Doctors have known for half a century that timing drug delivery to a patient’s circadian clock can enhance outcomes. Chronobiological research into how circadian rhythms work at the cellular level, and in cutaneous cells in particular, is a fascinating and expanding area of inquiry that could help dermatologists more accurately recommend timing for skin care regimens. Much more research, especially in clinical trials, is necessary to further elucidate how to best work with the skin’s natural rhythms.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on skin care technologies. Write to her at dermnews@mdedge.com.
References
1. Mehling A et al. Skin Pharmacol Physiol. 2006;19(4):182-9.
2. Latreille J et al. Skin Pharmacol Physiol. 2004 May-Jun;17(3):133-40.
3. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
4. Verschoore M et al. Chronobiol Int. 1993 Oct;10(5):349-59.
5. Yosipovitch G et al. J Invest Dermatol. 1998 Jan;110(1):20-3.
6. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
7. Dong K et al. Int J Mol Sci. 2020 Jan 3;21(1):326.
8. Walker WH II et al. Arch Dermatol Res. 2019 Sep;311(7):573-6.
9. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
10. Fishbein AB et al. J Allergy Clin Immunol. 2015 Nov;136(5):1170-7.
11. Plikus MV et al. J Biol Rhythms. 2015 Jun;30(3):163-82.
12. Deshayes N et al. Eur J Dermatol. 2018 Aug 1;28(4):467-75.
13. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
14. Morris A. Nat Rev Endocrinol. 2018 Dec;15(1):3.
15. Jia Y et al. Exp Dermatol. 2019 Jul;28(7):858-62.
16. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
We continue with a focus on when possible, as well as clinical studies that may shed light on how to time skin care treatments.
It is important to remember that several studies in the last 20 years have revealed cutaneous tendencies based on the time of day. For instance, sebum production is known to be highest around noon, and pH also peaks during the day and is at its lowest at night.1-5
Skin aging
In 2019, Dong and associates showed that blue light at 410 nm reduces PER1 transcription in keratinocytes, indicating that epidermal cells have the capacity to directly sense light and regulate their own clock gene expression. With the introduction of blue light at night, circadian rhythm is disrupted as epidermal skin cells act as if it is daytime. The investigators also considered blue light–induced damage to skin cells at various doses and exposure times in comparison with cells that remained unexposed to light. The production of reactive oxygen species increased in the exposed cells, as did DNA impairment and the emergence of inflammatory mediators, all of which have the potential to hasten aging.6
Early this year, Dong and associates demonstrated that melatonin can dose-dependently stimulate PER1 clock gene expression in normal human dermal fibroblasts and normal human epidermal keratinocytes, and verified that the MT-1 melatonin receptor in such fibroblasts manifests a marked decline with age. The researchers concluded that the melatonin pathway contributes significantly in cutaneous aging and impairment, and that its relationship with skin circadian rhythm points to a possible role in slowing the rate of skin aging through the modulation of cutaneous melatonin receptors.7
Wound healing
In 2019, Walker and associates investigated the effects of dim artificial light at night on wound healing in female C57BL/6 mice, and found that those conditions prior to wounding reduced healing. They concluded that such information might warrant consideration in prescribing treatment.8
Atopic dermatitis
Vaughn and associates contended that alterations in circadian rhythm may contribute to the development of atopic dermatitis.9 A good example of the impact of circadian rhythms on cutaneous health is the nocturnal exacerbation of atopic dermatitis, particularly in children.10
Psoriasis
According to Plikus and associates, recent evidence has emerged showing that the circadian clock regulates UVB-induced DNA damage and cutaneous cancers, and it is also associated with the immune-mediated disorder psoriasis.11
Clinical studies
In 2018, Deshayes and associates conducted a clinical study to evaluate the precursors and stem cell attributes of hHF (human hair follicle keratinocytes), hEpi (human interfollicular epidermal keratinocytes), and hHFDP (hair follicle dermal papilla stem cells) in response to clock pathway changes caused by long-term deregulation of circadian rhythms. A total of 20 women participated in the study, 10 in each group (day workers were the control group and compared with shift workers). Two 3-mm fresh punch biopsies were collected from the occipital region of each participant. The investigators reported that chronic circadian rhythm deregulation influenced clock pathway protein expression and correlated with changes in hHF, hEpi, and hHFDP. They concluded that their findings represented the first data in humans suggesting that deregulation of the clock pathway modulates regenerative activity in human cutaneous and hair precursor cells.12
Later that year, Wu and associates reported on the role of the circadian clock in the transcriptional regulation of human epidermis. Investigators sampled 20 human participants through a 24-hour period and a population of 219 people once, finding a potent circadian oscillator in human epidermis at the population level, hundreds of rhythmically expressed genes, as well as a biomarker set for human epidermis that can, with one sample, highlight circadian phase within a 3-hour time frame. The team concluded that rhythms in human epidermis persist at the population level, and that they were able to present an effective single-sample circadian biomarker.13 This is important, as Morris pointed out, because the standard practice for measuring an individual’s internal clock is to use a dim-light melatonin onset assay over the course of a day.14 In 2019, Jia and associates studied the skin surface lipid profiles of young women to evaluate and characterize circadian human facial surface lipid composition. The investigators identified significant markers of circadian rhythm, with glycerolipids most affected. They ascribed changes in skin barrier function, such as variable pH and transepidermal water loss, to alterations in triacylglycerol levels as well as free fatty acid chain lengths and content that were affected by variations in circadian rhythm.15
Sleep and the timing of topicals
Based on their recent review of the literature on circadian rhythm and skin, Lyons and associates argued that an understanding of circadian rhythm helps dermatologists in recommending the optimal times for patients to apply topical medications. They added that urging patients to get sufficient sleep is important because DNA repair of the skin occurs best at that time.16
Conclusions
Doctors have known for half a century that timing drug delivery to a patient’s circadian clock can enhance outcomes. Chronobiological research into how circadian rhythms work at the cellular level, and in cutaneous cells in particular, is a fascinating and expanding area of inquiry that could help dermatologists more accurately recommend timing for skin care regimens. Much more research, especially in clinical trials, is necessary to further elucidate how to best work with the skin’s natural rhythms.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on skin care technologies. Write to her at dermnews@mdedge.com.
References
1. Mehling A et al. Skin Pharmacol Physiol. 2006;19(4):182-9.
2. Latreille J et al. Skin Pharmacol Physiol. 2004 May-Jun;17(3):133-40.
3. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
4. Verschoore M et al. Chronobiol Int. 1993 Oct;10(5):349-59.
5. Yosipovitch G et al. J Invest Dermatol. 1998 Jan;110(1):20-3.
6. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
7. Dong K et al. Int J Mol Sci. 2020 Jan 3;21(1):326.
8. Walker WH II et al. Arch Dermatol Res. 2019 Sep;311(7):573-6.
9. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
10. Fishbein AB et al. J Allergy Clin Immunol. 2015 Nov;136(5):1170-7.
11. Plikus MV et al. J Biol Rhythms. 2015 Jun;30(3):163-82.
12. Deshayes N et al. Eur J Dermatol. 2018 Aug 1;28(4):467-75.
13. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
14. Morris A. Nat Rev Endocrinol. 2018 Dec;15(1):3.
15. Jia Y et al. Exp Dermatol. 2019 Jul;28(7):858-62.
16. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
Circadian rhythms: Does the time of day you use a skin care product matter?
The majority of human cells, including skin and hair cells, keep their own time; that is, they manifest autonomous clocks and the genes that regulate their functioning.1 During the day, one primary function of the skin is protection; at night, repairing any damage (particularly DNA impairment) incurred during the day prevails.2-4 These activities are driven through circadian rhythms using clock genes that exist in all cutaneous cells.2 Important cutaneous functions such as blood flow, transepidermal water loss, and capacitance are affected by circadian rhythms.5 Hydration and inflammation are also among the several functions pertaining to epidermal homeostasis affected by circadian rhythms.6 In addition, some collagens and extracellular matrix proteases are diurnally regulated, and approximately 10% of the transcriptome, including the extracellular matrix, is thought to be controlled by circadian rhythms.7
Cutaneous cell migration and proliferation, wound healing, and tissue vulnerability to harm from UV exposure, oxidative stress, and protease activity, for example, are affected by circadian rhythms, Sherratt et al. noted in suggesting that chronotherapy presents promise for enhancing skin therapy.7 Indeed, recent research has led to the understanding that cutaneous aging, cellular repair, optimal timing for drug delivery to the skin, and skin cancer development are all affected by the chronobiological functioning of the skin.8
We have known for several years that certain types of products should be used at different times of the day. For instance, antioxidants should be used in the morning to protect skin from sun exposure and retinols should be used in the evening because of its induction of light sensitivity. The remainder of this column focuses on research in the last 2 decades that reinforces the notion of circadian rhythms working in the skin, and may alter how we view the timing of skin care. Next month’s column, part two on the circadian rhythms of the skin, will address recent clinical trials and the implications for timing treatments for certain cutaneous conditions.
Emerging data on the circadian rhythms of the skin
In 2001, Le Fur et al. studied the cutaneous circadian rhythms in the facial and forearm skin of eight healthy White women during a 48-hour period. They were able to detect such rhythms in facial sebum excretion, transepidermal water loss (TEWL) in the face and forearm, pH in the face, forearm skin temperature, and forearm capacitance using cosinor or analysis of variance methods. The investigators also observed 8- and 12-hour rhythms in TEWL in both areas, and 12 hours for forearm skin temperature. They verified that such rhythms could be measured and that they vary between skin sites. In addition, they were the first to show that ultradian and/or component rhythms can also be found in TEWL, sebum excretion, and skin temperature.9
A year later, Kawara et al. showed that mRNA of the circadian clock genes Per1, Clock, and bmal1/mop3 are expressed in normal human-cultured keratinocytes and that low-dose UVB down-regulates these genes and changes their express in keratinocyte cell cultures. They concluded that UV targeting of keratinocytes could alter circadian rhythms.10
In 2011, Spörl and colleagues characterized an in vitro functional cell autonomous circadian clock in adult human low calcium temperature keratinocytes, demonstrating that the molecular composition of the keratinocyte clock was comparable with peripheral tissue clocks. Notably, they observed that temperature acts as a robust time cue for epidermal traits, such as cholesterol homeostasis and differentiation.11
The next year, Sandu et al. investigated the kinetics of clock gene expression in epidermal and dermal cells collected from the same donor and compared their characteristics. They were able to reveal the presence of functional circadian machinery in primary cultures of fibroblasts, keratinocytes, and melanocytes, with oscillators identified in all skin cell types and thought to be involved in spurring cutaneous rhythmic functions as they exhibited discrete periods and phase relationships between clock genes.12
Three years later, Sandu et al. characterized the circadian clocks in rat skin and dermal fibroblasts. They found that skin has a self-sustaining circadian clock that experiences age-dependent alterations, and that dermal fibroblasts manifest circadian rhythms that can be modulated by endogenous (e.g., melatonin) and exogenous (e.g., temperature) influences.13
In 2019, Park et al. demonstrated that the diurnal expression of the gene TIMP3, which is thought to evince a circadian rhythm in synchronized human keratinocytes, experiences disruptions in such rhythms by UVB exposure. The inflammation that results can be blocked, they argued, by recovering the circadian expression of TIMP3 using synthetic TIMP3 peptides or bioactive natural ingredients, such as green tea extracts.6
Conclusion
Circadian rhythms and the biological clocks by which most cells, including skin and hair cells, regulate themselves represent a ripe and fascinating area of research. Applying evidence in this realm to skin care has been occurring over time and is likely to enhance our practice even more as we continue to elucidate the behavior of cutaneous cells based on the solar day. Based on this information, my recommendations are to use antioxidants and protective products in the morning, and use DNA repair enzymes, retinoids, and other repair products at night.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Dong K et al. Int J Mol Sci. 2020 Jan 3. doi: 10.3390/ijms21010326.
2. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
3. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
4. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
5. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
6. Park S et al. Int J Mol Sci. 2019 Feb 16. doi: 10.3390/ijms20040862.
7. Sherratt MJ et al. Matrix Biol. 2019 Nov;84:97-110.
8. Luber AJ et al. J Drugs Dermatol. 2014 Feb;13(2):130-4.
9. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
10. Kawara S et al. J Invest Dermatol. 2002 Dec;119(6):1220-3.
11. Spörl F et al. J Invest Dermatol. 2011 Feb;131(2):338-48.
12. Sandu C et al. Cell Mol Life Sci. 2012 Oct;69(19):3329-39.
13. Sandu C et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48.
The majority of human cells, including skin and hair cells, keep their own time; that is, they manifest autonomous clocks and the genes that regulate their functioning.1 During the day, one primary function of the skin is protection; at night, repairing any damage (particularly DNA impairment) incurred during the day prevails.2-4 These activities are driven through circadian rhythms using clock genes that exist in all cutaneous cells.2 Important cutaneous functions such as blood flow, transepidermal water loss, and capacitance are affected by circadian rhythms.5 Hydration and inflammation are also among the several functions pertaining to epidermal homeostasis affected by circadian rhythms.6 In addition, some collagens and extracellular matrix proteases are diurnally regulated, and approximately 10% of the transcriptome, including the extracellular matrix, is thought to be controlled by circadian rhythms.7
Cutaneous cell migration and proliferation, wound healing, and tissue vulnerability to harm from UV exposure, oxidative stress, and protease activity, for example, are affected by circadian rhythms, Sherratt et al. noted in suggesting that chronotherapy presents promise for enhancing skin therapy.7 Indeed, recent research has led to the understanding that cutaneous aging, cellular repair, optimal timing for drug delivery to the skin, and skin cancer development are all affected by the chronobiological functioning of the skin.8
We have known for several years that certain types of products should be used at different times of the day. For instance, antioxidants should be used in the morning to protect skin from sun exposure and retinols should be used in the evening because of its induction of light sensitivity. The remainder of this column focuses on research in the last 2 decades that reinforces the notion of circadian rhythms working in the skin, and may alter how we view the timing of skin care. Next month’s column, part two on the circadian rhythms of the skin, will address recent clinical trials and the implications for timing treatments for certain cutaneous conditions.
Emerging data on the circadian rhythms of the skin
In 2001, Le Fur et al. studied the cutaneous circadian rhythms in the facial and forearm skin of eight healthy White women during a 48-hour period. They were able to detect such rhythms in facial sebum excretion, transepidermal water loss (TEWL) in the face and forearm, pH in the face, forearm skin temperature, and forearm capacitance using cosinor or analysis of variance methods. The investigators also observed 8- and 12-hour rhythms in TEWL in both areas, and 12 hours for forearm skin temperature. They verified that such rhythms could be measured and that they vary between skin sites. In addition, they were the first to show that ultradian and/or component rhythms can also be found in TEWL, sebum excretion, and skin temperature.9
A year later, Kawara et al. showed that mRNA of the circadian clock genes Per1, Clock, and bmal1/mop3 are expressed in normal human-cultured keratinocytes and that low-dose UVB down-regulates these genes and changes their express in keratinocyte cell cultures. They concluded that UV targeting of keratinocytes could alter circadian rhythms.10
In 2011, Spörl and colleagues characterized an in vitro functional cell autonomous circadian clock in adult human low calcium temperature keratinocytes, demonstrating that the molecular composition of the keratinocyte clock was comparable with peripheral tissue clocks. Notably, they observed that temperature acts as a robust time cue for epidermal traits, such as cholesterol homeostasis and differentiation.11
The next year, Sandu et al. investigated the kinetics of clock gene expression in epidermal and dermal cells collected from the same donor and compared their characteristics. They were able to reveal the presence of functional circadian machinery in primary cultures of fibroblasts, keratinocytes, and melanocytes, with oscillators identified in all skin cell types and thought to be involved in spurring cutaneous rhythmic functions as they exhibited discrete periods and phase relationships between clock genes.12
Three years later, Sandu et al. characterized the circadian clocks in rat skin and dermal fibroblasts. They found that skin has a self-sustaining circadian clock that experiences age-dependent alterations, and that dermal fibroblasts manifest circadian rhythms that can be modulated by endogenous (e.g., melatonin) and exogenous (e.g., temperature) influences.13
In 2019, Park et al. demonstrated that the diurnal expression of the gene TIMP3, which is thought to evince a circadian rhythm in synchronized human keratinocytes, experiences disruptions in such rhythms by UVB exposure. The inflammation that results can be blocked, they argued, by recovering the circadian expression of TIMP3 using synthetic TIMP3 peptides or bioactive natural ingredients, such as green tea extracts.6
Conclusion
Circadian rhythms and the biological clocks by which most cells, including skin and hair cells, regulate themselves represent a ripe and fascinating area of research. Applying evidence in this realm to skin care has been occurring over time and is likely to enhance our practice even more as we continue to elucidate the behavior of cutaneous cells based on the solar day. Based on this information, my recommendations are to use antioxidants and protective products in the morning, and use DNA repair enzymes, retinoids, and other repair products at night.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Dong K et al. Int J Mol Sci. 2020 Jan 3. doi: 10.3390/ijms21010326.
2. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
3. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
4. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
5. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
6. Park S et al. Int J Mol Sci. 2019 Feb 16. doi: 10.3390/ijms20040862.
7. Sherratt MJ et al. Matrix Biol. 2019 Nov;84:97-110.
8. Luber AJ et al. J Drugs Dermatol. 2014 Feb;13(2):130-4.
9. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
10. Kawara S et al. J Invest Dermatol. 2002 Dec;119(6):1220-3.
11. Spörl F et al. J Invest Dermatol. 2011 Feb;131(2):338-48.
12. Sandu C et al. Cell Mol Life Sci. 2012 Oct;69(19):3329-39.
13. Sandu C et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48.
The majority of human cells, including skin and hair cells, keep their own time; that is, they manifest autonomous clocks and the genes that regulate their functioning.1 During the day, one primary function of the skin is protection; at night, repairing any damage (particularly DNA impairment) incurred during the day prevails.2-4 These activities are driven through circadian rhythms using clock genes that exist in all cutaneous cells.2 Important cutaneous functions such as blood flow, transepidermal water loss, and capacitance are affected by circadian rhythms.5 Hydration and inflammation are also among the several functions pertaining to epidermal homeostasis affected by circadian rhythms.6 In addition, some collagens and extracellular matrix proteases are diurnally regulated, and approximately 10% of the transcriptome, including the extracellular matrix, is thought to be controlled by circadian rhythms.7
Cutaneous cell migration and proliferation, wound healing, and tissue vulnerability to harm from UV exposure, oxidative stress, and protease activity, for example, are affected by circadian rhythms, Sherratt et al. noted in suggesting that chronotherapy presents promise for enhancing skin therapy.7 Indeed, recent research has led to the understanding that cutaneous aging, cellular repair, optimal timing for drug delivery to the skin, and skin cancer development are all affected by the chronobiological functioning of the skin.8
We have known for several years that certain types of products should be used at different times of the day. For instance, antioxidants should be used in the morning to protect skin from sun exposure and retinols should be used in the evening because of its induction of light sensitivity. The remainder of this column focuses on research in the last 2 decades that reinforces the notion of circadian rhythms working in the skin, and may alter how we view the timing of skin care. Next month’s column, part two on the circadian rhythms of the skin, will address recent clinical trials and the implications for timing treatments for certain cutaneous conditions.
Emerging data on the circadian rhythms of the skin
In 2001, Le Fur et al. studied the cutaneous circadian rhythms in the facial and forearm skin of eight healthy White women during a 48-hour period. They were able to detect such rhythms in facial sebum excretion, transepidermal water loss (TEWL) in the face and forearm, pH in the face, forearm skin temperature, and forearm capacitance using cosinor or analysis of variance methods. The investigators also observed 8- and 12-hour rhythms in TEWL in both areas, and 12 hours for forearm skin temperature. They verified that such rhythms could be measured and that they vary between skin sites. In addition, they were the first to show that ultradian and/or component rhythms can also be found in TEWL, sebum excretion, and skin temperature.9
A year later, Kawara et al. showed that mRNA of the circadian clock genes Per1, Clock, and bmal1/mop3 are expressed in normal human-cultured keratinocytes and that low-dose UVB down-regulates these genes and changes their express in keratinocyte cell cultures. They concluded that UV targeting of keratinocytes could alter circadian rhythms.10
In 2011, Spörl and colleagues characterized an in vitro functional cell autonomous circadian clock in adult human low calcium temperature keratinocytes, demonstrating that the molecular composition of the keratinocyte clock was comparable with peripheral tissue clocks. Notably, they observed that temperature acts as a robust time cue for epidermal traits, such as cholesterol homeostasis and differentiation.11
The next year, Sandu et al. investigated the kinetics of clock gene expression in epidermal and dermal cells collected from the same donor and compared their characteristics. They were able to reveal the presence of functional circadian machinery in primary cultures of fibroblasts, keratinocytes, and melanocytes, with oscillators identified in all skin cell types and thought to be involved in spurring cutaneous rhythmic functions as they exhibited discrete periods and phase relationships between clock genes.12
Three years later, Sandu et al. characterized the circadian clocks in rat skin and dermal fibroblasts. They found that skin has a self-sustaining circadian clock that experiences age-dependent alterations, and that dermal fibroblasts manifest circadian rhythms that can be modulated by endogenous (e.g., melatonin) and exogenous (e.g., temperature) influences.13
In 2019, Park et al. demonstrated that the diurnal expression of the gene TIMP3, which is thought to evince a circadian rhythm in synchronized human keratinocytes, experiences disruptions in such rhythms by UVB exposure. The inflammation that results can be blocked, they argued, by recovering the circadian expression of TIMP3 using synthetic TIMP3 peptides or bioactive natural ingredients, such as green tea extracts.6
Conclusion
Circadian rhythms and the biological clocks by which most cells, including skin and hair cells, regulate themselves represent a ripe and fascinating area of research. Applying evidence in this realm to skin care has been occurring over time and is likely to enhance our practice even more as we continue to elucidate the behavior of cutaneous cells based on the solar day. Based on this information, my recommendations are to use antioxidants and protective products in the morning, and use DNA repair enzymes, retinoids, and other repair products at night.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Dong K et al. Int J Mol Sci. 2020 Jan 3. doi: 10.3390/ijms21010326.
2. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
3. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
4. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
5. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
6. Park S et al. Int J Mol Sci. 2019 Feb 16. doi: 10.3390/ijms20040862.
7. Sherratt MJ et al. Matrix Biol. 2019 Nov;84:97-110.
8. Luber AJ et al. J Drugs Dermatol. 2014 Feb;13(2):130-4.
9. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
10. Kawara S et al. J Invest Dermatol. 2002 Dec;119(6):1220-3.
11. Spörl F et al. J Invest Dermatol. 2011 Feb;131(2):338-48.
12. Sandu C et al. Cell Mol Life Sci. 2012 Oct;69(19):3329-39.
13. Sandu C et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48.
Dermatologists and the history of skin care and beauty devices: Part 4
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
Dermatologists’ role in the development of the skin care industry
This is the third in a series of columns discussing the important roles that dermatologists have played in the skin care industry. with the cosmetic industry, rather than developing their own skin care lines.
Norman Orentreich, MD
Dr. Orentreich was a successful New York City dermatologist and the first to perform hair transplants. This new technique brought him fame and notoriety and arguably made him the first “celebrity dermatologist.” (He was also a member of the original advisory board of Dermatology News, at that time Skin & Allergy News, in January 1970.) Dr. Orentreich was a seminal figure in the trend to link the cosmetic industry and dermatology. In August 1967, Vogue magazine1 published an article on him, titled “Can Great Skin be Created?” This popular article caught the attention of Leonard Lauder, of Estée Lauder, who recruited Dr. Orentreich to help create the skin care line Clinique. Clinique was intended to be a brand with a medical look that promoted its products as “allergy tested,” with packaging that has an antiseptic look and beauty counter salespeople wearing white coats.
Dr. Orentreich’s input into the development of a skin type–based skin care line was fundamental to the development of this brand. The four-question questionnaire with an iconic plastic lever that customers slide left or right instantly provided them with an assessment of their skin type at the beauty counter, with one of four skin types: Very Dry to Dry Skin (Skin Type 1), Dry Combination (Skin Type 2), Combination Oily (Skin Type 3), and Oily (Skin Type 4).
Although this skin-typing system was not scientifically accurate (there is no scientific definition of combination skin), it was reminiscent of the system developed by cosmetic company tycoon Helena Rubinstein in the 1940s that classified people into four skin types: oily, dry, combination, and sensitive. Clinique became a blockbuster skin care brand and was one of the first developed by a dermatologist – although Dr. Orentreich did not put his name on it.
In 1972, Dr. Orentreich filed a patent2 for an exfoliating pad for the skin that later became known as the “Buf-Puf.” I heard years ago that he got the idea from the machines used to buff the floors in the hospital. The buffing pad had a hole in the center where the machine attached. Dr. Orentreich purportedly thought “I wonder what they do with the cut-out centers?” He looked into this, and subsequently used the centers to create the Buf-Puf. I cannot find a reference for this, but I love this story and hope it’s true. If any readers have any knowledge of this, please let me know, so I can amend my story if it is incorrect.
Almay
Almay, an amalgamation of the founders’ names, Alfred and Fanny May Woititz, was the first hypoallergenic brand, established in 1931, and the first to provide hypoallergenic cosmetics, long before Clinique. In addition, the company was the first skin care brand to become available by prescription only (as it was initially), fully disclose all individual ingredients in its products (well before this became mandatory in 1976), provide totally fragrance-free products, develop a hypoallergenic fragrance – and provide patch tests and other materials to physicians to identify contact allergens.
Over 90 years, the company was also the first among skin care brands to do the following:
- Provide custom formulations to individuals proven to be allergic to a specific ingredient, through their physicians.
- Perform a full range of premarket safety testing on all products for allergy and irritation, and test all its products for comedogenicity.
- Formulate cosmetics for use around the eye area (eye shadows and eyeliners) specifically for contact lens wearers.
- Formulate hypoallergenic regimens for specific skin types in the mass market.
- Provide a specific cosmetic regimen for acne-prone women, including a silicone-based makeup and active ingredients for treatment in cosmetics and skin care.
I recently interviewed Stanley Levy, MD, who was one of the consultants to Almay, and practices in Chapel Hill, N.C., where he has an academic niche related to skin care formulation and safety. He told me how Almay provided patch test materials to dermatologists to help identify contact dermatitis to cosmetic ingredients, and described Almay’s relationship with the dermatology field as follows: “From the outset, Almay was linked to dermatology. In 1930, a chemist and pharmacist in New York City, Al Woititz, was looking to compound cosmetics for his wife suffering from cosmetic allergies, Fannie May. He enlisted the counsel of the preeminent dermatologic expert in contact dermatitis at the time, Dr. Marion Sulzberger, to suggest ingredients to avoid. [Dr. Sulzberger was also a member of the original Dermatology News editorial advisory board.] Soon, dermatologists around New York City were recommending these formulations. This led to a product line free of the known allergens and a fledgling company trademarked as Almay. For the past 90 years, [the company] has kept a close relationship with dermatologists, well before that was the norm.”
The Almay research overseen by Dr. Levy and others contributed greatly to our understanding of the allergenicity of skin care.
Albert Kligman, MD
The turning point for the interface of dermatology with the cosmetic industry was the shift from a safety-based approach (hypoallergenic and noncomedogenic) to an emphasis on efficacy claims in the 1980s. Part of the impetus for this was the Dr. Kligman’s observation that retinoids could improve photoaging.
Dr. Kligman, a well-known dermatologist at the University of Pennsylvania, Philadelphia, showed that retinoids were an effective treatment for acne. For more about this, listen to my interview on the Dermatology Weekly podcast, with James Leyden, MD, about his work at the University of Pennsylvania with Dr. Kligman on the development of oral and topical retinoids. During Dr. Kligman’s research on acne, he noticed that wrinkles improved after treatment with tretinoin, and in 1986, he and Dr. Leyden (and several other authors) published the first article about tretinoin’s use for photoaged skin.3 This led to a double-blind study4 conducted by John J. Voorhees, MD, University of Michigan, Ann Arbor, and coauthors that showed statistically significant improvement of photoaged skin when treated with topical tretinoin. Dr. Voorhees and his group did many more studies on retinoids5,6 and photoaging7 – so many that, at one time, he was (and maybe still is) the most widely published dermatologist in the United States. These studies showed that, not only did prescription tretinoin improve the appearance of wrinkles, but so did over-the-counter retinol.8 Retinoids remain the most efficacious prescription and cosmeceutical ingredients to treat wrinkled skin.
When studies conducted by Dr. Kligman, Dr. Voorhees, and by Barbara Gilcrest, MD, 9,10 showed that retinoids improved wrinkles, a major change in the focus in the skin care industry occurred.
During the same time period, the studies on alpha hydroxy acids by Chérie Ditre, MD, Eugene Van Scott, MD, and colleages11,12; and studies by Sheldon Pinnell, MD, on Vitamin C (see part 1 of this series) all demonstrated the efficacy of cosmetic ingredients on photoaged skin. This triggered a major change in how skin care products were marketed, with an efficacy approach rather than a safety approach.
With the shift from safety (hypoallergenic and noncomedogenic issues) to efficacy claims in the 1980s, and as nondrug active ingredients like retinol were shown to have biologic effects, the lines between the Food and Drug Administration’s definition of a drug versus a cosmetic became blurred. In 1984, Dr. Kligman suggested a new classification for the ingredients that fell in the middle, proposing the term “cosmeceutical” and thus, the concept of a cosmeceutical was introduced. To this day, cosmeceutical is not an official definition and the FDA has yet to deal with it as a quasi-drug category. FDA regulations as to what constitutes a drug versus a cosmetic date back to the 1938 Food, Drug and Cosmetic Act.
Once marketing focused on efficacy, many companies made outrageous claims. During the second half of the 1980s, the FDA issued some warning letters to some companies in an effort to control these claims.
Now efficacy claims abound and we, as dermatologists, should be the experts who back up these claims with scientific data. As the cosmeceutical market has evolved and grown, consumers are bewildered by the myriad of active ingredients being promoted and the number of products in the marketplace. As dermatologic innovation has led to more efficacious active ingredients, our patients look to us as knowledgeable and credible sources of information and for recommendations about the best skin care routines for their skin issues. This is all reflected in the fact that physician-dispensed skin care is becoming the fastest growing segment in this market. It is incumbent upon dermatologists to be knowledgeable and conversant about skin care products and skin care routines, and is particularly true for those of us who sell skin care products in our offices.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Burt’s Bees, Evolus, Galderma, and Revance. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Vogue Magazine, 1967 Aug 15. “Can Great Skin be Created?”
2. https://patents.google.com/patent/US3910284.
3. Kligman AM et al. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836-59.
4. Weiss JS et al. JAMA. 1988 Jan 22-29;259(4):527-32.
5. Goldfarb MT et al. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):645-50.
6. Ellis CN et al. J Am Acad Dermatol. 1990 Oct;23(4 Pt 1):629-37.
7. Kang S; Voorhees JJ. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S55-61.
8. Kafi R et al. Arch Dermatol. 2007 May;143(5):606-12.
9. Gilchrest BA. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):610-3.
10. Bhawan J et al. Arch Dermatol. 1991 May;127(5):666-72.
11. Griffin TD et al. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):196-203.
12. Ditre CM et al. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):187-95.
This is the third in a series of columns discussing the important roles that dermatologists have played in the skin care industry. with the cosmetic industry, rather than developing their own skin care lines.
Norman Orentreich, MD
Dr. Orentreich was a successful New York City dermatologist and the first to perform hair transplants. This new technique brought him fame and notoriety and arguably made him the first “celebrity dermatologist.” (He was also a member of the original advisory board of Dermatology News, at that time Skin & Allergy News, in January 1970.) Dr. Orentreich was a seminal figure in the trend to link the cosmetic industry and dermatology. In August 1967, Vogue magazine1 published an article on him, titled “Can Great Skin be Created?” This popular article caught the attention of Leonard Lauder, of Estée Lauder, who recruited Dr. Orentreich to help create the skin care line Clinique. Clinique was intended to be a brand with a medical look that promoted its products as “allergy tested,” with packaging that has an antiseptic look and beauty counter salespeople wearing white coats.
Dr. Orentreich’s input into the development of a skin type–based skin care line was fundamental to the development of this brand. The four-question questionnaire with an iconic plastic lever that customers slide left or right instantly provided them with an assessment of their skin type at the beauty counter, with one of four skin types: Very Dry to Dry Skin (Skin Type 1), Dry Combination (Skin Type 2), Combination Oily (Skin Type 3), and Oily (Skin Type 4).
Although this skin-typing system was not scientifically accurate (there is no scientific definition of combination skin), it was reminiscent of the system developed by cosmetic company tycoon Helena Rubinstein in the 1940s that classified people into four skin types: oily, dry, combination, and sensitive. Clinique became a blockbuster skin care brand and was one of the first developed by a dermatologist – although Dr. Orentreich did not put his name on it.
In 1972, Dr. Orentreich filed a patent2 for an exfoliating pad for the skin that later became known as the “Buf-Puf.” I heard years ago that he got the idea from the machines used to buff the floors in the hospital. The buffing pad had a hole in the center where the machine attached. Dr. Orentreich purportedly thought “I wonder what they do with the cut-out centers?” He looked into this, and subsequently used the centers to create the Buf-Puf. I cannot find a reference for this, but I love this story and hope it’s true. If any readers have any knowledge of this, please let me know, so I can amend my story if it is incorrect.
Almay
Almay, an amalgamation of the founders’ names, Alfred and Fanny May Woititz, was the first hypoallergenic brand, established in 1931, and the first to provide hypoallergenic cosmetics, long before Clinique. In addition, the company was the first skin care brand to become available by prescription only (as it was initially), fully disclose all individual ingredients in its products (well before this became mandatory in 1976), provide totally fragrance-free products, develop a hypoallergenic fragrance – and provide patch tests and other materials to physicians to identify contact allergens.
Over 90 years, the company was also the first among skin care brands to do the following:
- Provide custom formulations to individuals proven to be allergic to a specific ingredient, through their physicians.
- Perform a full range of premarket safety testing on all products for allergy and irritation, and test all its products for comedogenicity.
- Formulate cosmetics for use around the eye area (eye shadows and eyeliners) specifically for contact lens wearers.
- Formulate hypoallergenic regimens for specific skin types in the mass market.
- Provide a specific cosmetic regimen for acne-prone women, including a silicone-based makeup and active ingredients for treatment in cosmetics and skin care.
I recently interviewed Stanley Levy, MD, who was one of the consultants to Almay, and practices in Chapel Hill, N.C., where he has an academic niche related to skin care formulation and safety. He told me how Almay provided patch test materials to dermatologists to help identify contact dermatitis to cosmetic ingredients, and described Almay’s relationship with the dermatology field as follows: “From the outset, Almay was linked to dermatology. In 1930, a chemist and pharmacist in New York City, Al Woititz, was looking to compound cosmetics for his wife suffering from cosmetic allergies, Fannie May. He enlisted the counsel of the preeminent dermatologic expert in contact dermatitis at the time, Dr. Marion Sulzberger, to suggest ingredients to avoid. [Dr. Sulzberger was also a member of the original Dermatology News editorial advisory board.] Soon, dermatologists around New York City were recommending these formulations. This led to a product line free of the known allergens and a fledgling company trademarked as Almay. For the past 90 years, [the company] has kept a close relationship with dermatologists, well before that was the norm.”
The Almay research overseen by Dr. Levy and others contributed greatly to our understanding of the allergenicity of skin care.
Albert Kligman, MD
The turning point for the interface of dermatology with the cosmetic industry was the shift from a safety-based approach (hypoallergenic and noncomedogenic) to an emphasis on efficacy claims in the 1980s. Part of the impetus for this was the Dr. Kligman’s observation that retinoids could improve photoaging.
Dr. Kligman, a well-known dermatologist at the University of Pennsylvania, Philadelphia, showed that retinoids were an effective treatment for acne. For more about this, listen to my interview on the Dermatology Weekly podcast, with James Leyden, MD, about his work at the University of Pennsylvania with Dr. Kligman on the development of oral and topical retinoids. During Dr. Kligman’s research on acne, he noticed that wrinkles improved after treatment with tretinoin, and in 1986, he and Dr. Leyden (and several other authors) published the first article about tretinoin’s use for photoaged skin.3 This led to a double-blind study4 conducted by John J. Voorhees, MD, University of Michigan, Ann Arbor, and coauthors that showed statistically significant improvement of photoaged skin when treated with topical tretinoin. Dr. Voorhees and his group did many more studies on retinoids5,6 and photoaging7 – so many that, at one time, he was (and maybe still is) the most widely published dermatologist in the United States. These studies showed that, not only did prescription tretinoin improve the appearance of wrinkles, but so did over-the-counter retinol.8 Retinoids remain the most efficacious prescription and cosmeceutical ingredients to treat wrinkled skin.
When studies conducted by Dr. Kligman, Dr. Voorhees, and by Barbara Gilcrest, MD, 9,10 showed that retinoids improved wrinkles, a major change in the focus in the skin care industry occurred.
During the same time period, the studies on alpha hydroxy acids by Chérie Ditre, MD, Eugene Van Scott, MD, and colleages11,12; and studies by Sheldon Pinnell, MD, on Vitamin C (see part 1 of this series) all demonstrated the efficacy of cosmetic ingredients on photoaged skin. This triggered a major change in how skin care products were marketed, with an efficacy approach rather than a safety approach.
With the shift from safety (hypoallergenic and noncomedogenic issues) to efficacy claims in the 1980s, and as nondrug active ingredients like retinol were shown to have biologic effects, the lines between the Food and Drug Administration’s definition of a drug versus a cosmetic became blurred. In 1984, Dr. Kligman suggested a new classification for the ingredients that fell in the middle, proposing the term “cosmeceutical” and thus, the concept of a cosmeceutical was introduced. To this day, cosmeceutical is not an official definition and the FDA has yet to deal with it as a quasi-drug category. FDA regulations as to what constitutes a drug versus a cosmetic date back to the 1938 Food, Drug and Cosmetic Act.
Once marketing focused on efficacy, many companies made outrageous claims. During the second half of the 1980s, the FDA issued some warning letters to some companies in an effort to control these claims.
Now efficacy claims abound and we, as dermatologists, should be the experts who back up these claims with scientific data. As the cosmeceutical market has evolved and grown, consumers are bewildered by the myriad of active ingredients being promoted and the number of products in the marketplace. As dermatologic innovation has led to more efficacious active ingredients, our patients look to us as knowledgeable and credible sources of information and for recommendations about the best skin care routines for their skin issues. This is all reflected in the fact that physician-dispensed skin care is becoming the fastest growing segment in this market. It is incumbent upon dermatologists to be knowledgeable and conversant about skin care products and skin care routines, and is particularly true for those of us who sell skin care products in our offices.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Burt’s Bees, Evolus, Galderma, and Revance. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Vogue Magazine, 1967 Aug 15. “Can Great Skin be Created?”
2. https://patents.google.com/patent/US3910284.
3. Kligman AM et al. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836-59.
4. Weiss JS et al. JAMA. 1988 Jan 22-29;259(4):527-32.
5. Goldfarb MT et al. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):645-50.
6. Ellis CN et al. J Am Acad Dermatol. 1990 Oct;23(4 Pt 1):629-37.
7. Kang S; Voorhees JJ. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S55-61.
8. Kafi R et al. Arch Dermatol. 2007 May;143(5):606-12.
9. Gilchrest BA. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):610-3.
10. Bhawan J et al. Arch Dermatol. 1991 May;127(5):666-72.
11. Griffin TD et al. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):196-203.
12. Ditre CM et al. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):187-95.
This is the third in a series of columns discussing the important roles that dermatologists have played in the skin care industry. with the cosmetic industry, rather than developing their own skin care lines.
Norman Orentreich, MD
Dr. Orentreich was a successful New York City dermatologist and the first to perform hair transplants. This new technique brought him fame and notoriety and arguably made him the first “celebrity dermatologist.” (He was also a member of the original advisory board of Dermatology News, at that time Skin & Allergy News, in January 1970.) Dr. Orentreich was a seminal figure in the trend to link the cosmetic industry and dermatology. In August 1967, Vogue magazine1 published an article on him, titled “Can Great Skin be Created?” This popular article caught the attention of Leonard Lauder, of Estée Lauder, who recruited Dr. Orentreich to help create the skin care line Clinique. Clinique was intended to be a brand with a medical look that promoted its products as “allergy tested,” with packaging that has an antiseptic look and beauty counter salespeople wearing white coats.
Dr. Orentreich’s input into the development of a skin type–based skin care line was fundamental to the development of this brand. The four-question questionnaire with an iconic plastic lever that customers slide left or right instantly provided them with an assessment of their skin type at the beauty counter, with one of four skin types: Very Dry to Dry Skin (Skin Type 1), Dry Combination (Skin Type 2), Combination Oily (Skin Type 3), and Oily (Skin Type 4).
Although this skin-typing system was not scientifically accurate (there is no scientific definition of combination skin), it was reminiscent of the system developed by cosmetic company tycoon Helena Rubinstein in the 1940s that classified people into four skin types: oily, dry, combination, and sensitive. Clinique became a blockbuster skin care brand and was one of the first developed by a dermatologist – although Dr. Orentreich did not put his name on it.
In 1972, Dr. Orentreich filed a patent2 for an exfoliating pad for the skin that later became known as the “Buf-Puf.” I heard years ago that he got the idea from the machines used to buff the floors in the hospital. The buffing pad had a hole in the center where the machine attached. Dr. Orentreich purportedly thought “I wonder what they do with the cut-out centers?” He looked into this, and subsequently used the centers to create the Buf-Puf. I cannot find a reference for this, but I love this story and hope it’s true. If any readers have any knowledge of this, please let me know, so I can amend my story if it is incorrect.
Almay
Almay, an amalgamation of the founders’ names, Alfred and Fanny May Woititz, was the first hypoallergenic brand, established in 1931, and the first to provide hypoallergenic cosmetics, long before Clinique. In addition, the company was the first skin care brand to become available by prescription only (as it was initially), fully disclose all individual ingredients in its products (well before this became mandatory in 1976), provide totally fragrance-free products, develop a hypoallergenic fragrance – and provide patch tests and other materials to physicians to identify contact allergens.
Over 90 years, the company was also the first among skin care brands to do the following:
- Provide custom formulations to individuals proven to be allergic to a specific ingredient, through their physicians.
- Perform a full range of premarket safety testing on all products for allergy and irritation, and test all its products for comedogenicity.
- Formulate cosmetics for use around the eye area (eye shadows and eyeliners) specifically for contact lens wearers.
- Formulate hypoallergenic regimens for specific skin types in the mass market.
- Provide a specific cosmetic regimen for acne-prone women, including a silicone-based makeup and active ingredients for treatment in cosmetics and skin care.
I recently interviewed Stanley Levy, MD, who was one of the consultants to Almay, and practices in Chapel Hill, N.C., where he has an academic niche related to skin care formulation and safety. He told me how Almay provided patch test materials to dermatologists to help identify contact dermatitis to cosmetic ingredients, and described Almay’s relationship with the dermatology field as follows: “From the outset, Almay was linked to dermatology. In 1930, a chemist and pharmacist in New York City, Al Woititz, was looking to compound cosmetics for his wife suffering from cosmetic allergies, Fannie May. He enlisted the counsel of the preeminent dermatologic expert in contact dermatitis at the time, Dr. Marion Sulzberger, to suggest ingredients to avoid. [Dr. Sulzberger was also a member of the original Dermatology News editorial advisory board.] Soon, dermatologists around New York City were recommending these formulations. This led to a product line free of the known allergens and a fledgling company trademarked as Almay. For the past 90 years, [the company] has kept a close relationship with dermatologists, well before that was the norm.”
The Almay research overseen by Dr. Levy and others contributed greatly to our understanding of the allergenicity of skin care.
Albert Kligman, MD
The turning point for the interface of dermatology with the cosmetic industry was the shift from a safety-based approach (hypoallergenic and noncomedogenic) to an emphasis on efficacy claims in the 1980s. Part of the impetus for this was the Dr. Kligman’s observation that retinoids could improve photoaging.
Dr. Kligman, a well-known dermatologist at the University of Pennsylvania, Philadelphia, showed that retinoids were an effective treatment for acne. For more about this, listen to my interview on the Dermatology Weekly podcast, with James Leyden, MD, about his work at the University of Pennsylvania with Dr. Kligman on the development of oral and topical retinoids. During Dr. Kligman’s research on acne, he noticed that wrinkles improved after treatment with tretinoin, and in 1986, he and Dr. Leyden (and several other authors) published the first article about tretinoin’s use for photoaged skin.3 This led to a double-blind study4 conducted by John J. Voorhees, MD, University of Michigan, Ann Arbor, and coauthors that showed statistically significant improvement of photoaged skin when treated with topical tretinoin. Dr. Voorhees and his group did many more studies on retinoids5,6 and photoaging7 – so many that, at one time, he was (and maybe still is) the most widely published dermatologist in the United States. These studies showed that, not only did prescription tretinoin improve the appearance of wrinkles, but so did over-the-counter retinol.8 Retinoids remain the most efficacious prescription and cosmeceutical ingredients to treat wrinkled skin.
When studies conducted by Dr. Kligman, Dr. Voorhees, and by Barbara Gilcrest, MD, 9,10 showed that retinoids improved wrinkles, a major change in the focus in the skin care industry occurred.
During the same time period, the studies on alpha hydroxy acids by Chérie Ditre, MD, Eugene Van Scott, MD, and colleages11,12; and studies by Sheldon Pinnell, MD, on Vitamin C (see part 1 of this series) all demonstrated the efficacy of cosmetic ingredients on photoaged skin. This triggered a major change in how skin care products were marketed, with an efficacy approach rather than a safety approach.
With the shift from safety (hypoallergenic and noncomedogenic issues) to efficacy claims in the 1980s, and as nondrug active ingredients like retinol were shown to have biologic effects, the lines between the Food and Drug Administration’s definition of a drug versus a cosmetic became blurred. In 1984, Dr. Kligman suggested a new classification for the ingredients that fell in the middle, proposing the term “cosmeceutical” and thus, the concept of a cosmeceutical was introduced. To this day, cosmeceutical is not an official definition and the FDA has yet to deal with it as a quasi-drug category. FDA regulations as to what constitutes a drug versus a cosmetic date back to the 1938 Food, Drug and Cosmetic Act.
Once marketing focused on efficacy, many companies made outrageous claims. During the second half of the 1980s, the FDA issued some warning letters to some companies in an effort to control these claims.
Now efficacy claims abound and we, as dermatologists, should be the experts who back up these claims with scientific data. As the cosmeceutical market has evolved and grown, consumers are bewildered by the myriad of active ingredients being promoted and the number of products in the marketplace. As dermatologic innovation has led to more efficacious active ingredients, our patients look to us as knowledgeable and credible sources of information and for recommendations about the best skin care routines for their skin issues. This is all reflected in the fact that physician-dispensed skin care is becoming the fastest growing segment in this market. It is incumbent upon dermatologists to be knowledgeable and conversant about skin care products and skin care routines, and is particularly true for those of us who sell skin care products in our offices.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Burt’s Bees, Evolus, Galderma, and Revance. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Vogue Magazine, 1967 Aug 15. “Can Great Skin be Created?”
2. https://patents.google.com/patent/US3910284.
3. Kligman AM et al. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836-59.
4. Weiss JS et al. JAMA. 1988 Jan 22-29;259(4):527-32.
5. Goldfarb MT et al. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):645-50.
6. Ellis CN et al. J Am Acad Dermatol. 1990 Oct;23(4 Pt 1):629-37.
7. Kang S; Voorhees JJ. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S55-61.
8. Kafi R et al. Arch Dermatol. 2007 May;143(5):606-12.
9. Gilchrest BA. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):610-3.
10. Bhawan J et al. Arch Dermatol. 1991 May;127(5):666-72.
11. Griffin TD et al. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):196-203.
12. Ditre CM et al. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):187-95.















