User login
How can we effectively treat stress urinary incontinence without drugs or surgery?
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
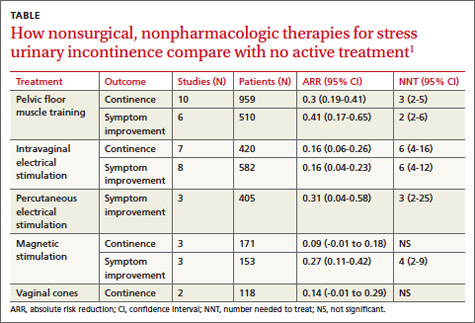
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1
Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Evidence-based answers from the Family Physicians Inquiries Network
Which drugs are most effective for recurrent herpes labialis?
Daily oral acyclovir or valacyclovir may help prevent herpes simplex labialis (HSL) recurrences (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] with heterogeneous results).
No trials compare oral or topical treatments for HSL outbreaks against each other. Oral antivirals modestly reduce healing time and duration of pain, varying according to the agent used: valacyclovir reduces both healing time and duration of pain, famciclovir reduces both in one dosage form but not another, and acyclovir reduces only pain duration (SOR: B, single RCTs).
Several topical medications (acyclovir, penciclovir, docosanol) modestly decrease healing time and pain duration—typically by less than a day—and require multiple doses per day (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A systematic review and meta-analysis of the effectiveness of oral and topical nucleoside antiviral agents to prevent recurrent HSL in immunocompetent people found 11 RCTs with a total of 1250 patients that compared an active drug against placebo.1 The medications were topical 5% acyclovir, topical 1% penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. The primary outcome was recurrence of herpes simplex virus type 1 lesions during the treatment period. The relative risk (RR) of recurrence ranged from 0.22 to 1.22. Pooled results found a benefit favoring antiviral agents (RR of recurrence=0.70; 95% confidence interval [CI], 0.55-0.89).
Seven of the trials looked at acyclovir (5 oral, 2 topical). A subgroup analysis demonstrated that oral acyclovir (800-1000 mg/d) was more effective than placebo (RR=0.51; 95% CI, 0.29-0.88), whereas topical acyclovir wasn’t. Oral valacyclovir (2 studies; 500 mg/d for 4 months) also reduced recurrence (RR=0.65; 95% CI, 0.43-0.91). The authors of the meta-analysis noted that although 9 studies favored the use of an antiviral drug, only 4 showed statistically significant differences when compared with placebo, and none of them had a low risk of bias. They concluded that the review supported using oral acyclovir and valacyclovir to prevent recurrent HSL.1
Oral antivirals produce variable treatment results
Three RCTs evaluated oral antiviral medications against placebo to treat recurrent HSL, with mixed results. The largest RCT found that valacyclovir (2000 mg twice in 24 hours, with or without an additional 1000 mg twice in another 24 hours) modestly but significantly reduced both healing time and duration of pain (by 0.5-0.8 day).2 The second RCT showed that a higher, single dose of famciclovir (1500 mg) reduced healing time (by 1.8 days) and pain duration (by 1.2 days) and that a smaller, repeated dose (750 mg twice in 24 hours) reduced healing time alone (by 2.2 days).3
The third RCT demonstrated that acyclovir (400 mg 5 times a day for 5 days) reduced pain duration (by 0.9 day) but didn’t shorten healing time. If acyclovir was started during the prodrome, it decreased the time to disappearance of the lesion’s hard crust (2.1 days’ less time; P=.03), but the clinical significance of this finding is unclear.4
Topical treatment shows modest success
Two trials demonstrated that topical acyclovir (5% cream) modestly improved healing time and duration of pain (by as much as half a day). Patients in the first trial (paired RCTs reported together) began treatment within an hour of prodromal symptoms or signs, applying the medication 5 times daily for 4 days.5
Patients in the second trial used ME-609 cream (5% acyclovir plus 1% hydrocortisone), 5% acyclovir cream, or placebo, all applied 5 times daily for 5 days.6 Although the cream with acyclovir and hydrocortisone showed a slight benefit compared with placebo (lessening healing time by 0.8 day and pain duration by 1 day), it didn’t improve healing more than acyclovir alone. Other topical agents (penciclovir 1%; docosanol 10%) produced results similar to topical acyclovir.7,8
RECOMMENDATIONS
No national guidelines on this topic exist. An online resource notes that most patients don’t require treatment for mild self-limited HSL.9 For patients with prodromal symptoms, the authors recommend episodic oral antiviral therapy. Patients who have no prodome but multiple painful or disfiguring lesions may choose to use chronic suppressive therapy with an oral antiviral drug.
1. Rahimi H, Mara T, Costella J, et al. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:618-627.
2. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother. 2003;47:1072-1080.
3. Spruance SL, Bodsworth N, Resnick H, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55:47-53.
4. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis. 1990;161:185-190.
5. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238-2243.
6. Hull CM, Harmenberg J, Arlander E, et al; ME-609 Studt Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, doubleblind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64:696.e1-696.e11.
7. Raborn GW, Martel AY, Lassonde M, et al; Worldwide Topical Penciclovir Collaborative Study Group. Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. J Am Dent Assoc. 2002;133:303-309.
8. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
9. Klein RS. Treatment of herpes simplex virus type 1 infection in immunocompetent patients. Waltham, MA: UpToDate; 2012. Available at: www.uptodate.com/contents/treatment-of-herpessimplex-virus-type-1-infection-in-immunocompetentpatients. Accessed January 19, 2012.
Daily oral acyclovir or valacyclovir may help prevent herpes simplex labialis (HSL) recurrences (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] with heterogeneous results).
No trials compare oral or topical treatments for HSL outbreaks against each other. Oral antivirals modestly reduce healing time and duration of pain, varying according to the agent used: valacyclovir reduces both healing time and duration of pain, famciclovir reduces both in one dosage form but not another, and acyclovir reduces only pain duration (SOR: B, single RCTs).
Several topical medications (acyclovir, penciclovir, docosanol) modestly decrease healing time and pain duration—typically by less than a day—and require multiple doses per day (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A systematic review and meta-analysis of the effectiveness of oral and topical nucleoside antiviral agents to prevent recurrent HSL in immunocompetent people found 11 RCTs with a total of 1250 patients that compared an active drug against placebo.1 The medications were topical 5% acyclovir, topical 1% penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. The primary outcome was recurrence of herpes simplex virus type 1 lesions during the treatment period. The relative risk (RR) of recurrence ranged from 0.22 to 1.22. Pooled results found a benefit favoring antiviral agents (RR of recurrence=0.70; 95% confidence interval [CI], 0.55-0.89).
Seven of the trials looked at acyclovir (5 oral, 2 topical). A subgroup analysis demonstrated that oral acyclovir (800-1000 mg/d) was more effective than placebo (RR=0.51; 95% CI, 0.29-0.88), whereas topical acyclovir wasn’t. Oral valacyclovir (2 studies; 500 mg/d for 4 months) also reduced recurrence (RR=0.65; 95% CI, 0.43-0.91). The authors of the meta-analysis noted that although 9 studies favored the use of an antiviral drug, only 4 showed statistically significant differences when compared with placebo, and none of them had a low risk of bias. They concluded that the review supported using oral acyclovir and valacyclovir to prevent recurrent HSL.1
Oral antivirals produce variable treatment results
Three RCTs evaluated oral antiviral medications against placebo to treat recurrent HSL, with mixed results. The largest RCT found that valacyclovir (2000 mg twice in 24 hours, with or without an additional 1000 mg twice in another 24 hours) modestly but significantly reduced both healing time and duration of pain (by 0.5-0.8 day).2 The second RCT showed that a higher, single dose of famciclovir (1500 mg) reduced healing time (by 1.8 days) and pain duration (by 1.2 days) and that a smaller, repeated dose (750 mg twice in 24 hours) reduced healing time alone (by 2.2 days).3
The third RCT demonstrated that acyclovir (400 mg 5 times a day for 5 days) reduced pain duration (by 0.9 day) but didn’t shorten healing time. If acyclovir was started during the prodrome, it decreased the time to disappearance of the lesion’s hard crust (2.1 days’ less time; P=.03), but the clinical significance of this finding is unclear.4
Topical treatment shows modest success
Two trials demonstrated that topical acyclovir (5% cream) modestly improved healing time and duration of pain (by as much as half a day). Patients in the first trial (paired RCTs reported together) began treatment within an hour of prodromal symptoms or signs, applying the medication 5 times daily for 4 days.5
Patients in the second trial used ME-609 cream (5% acyclovir plus 1% hydrocortisone), 5% acyclovir cream, or placebo, all applied 5 times daily for 5 days.6 Although the cream with acyclovir and hydrocortisone showed a slight benefit compared with placebo (lessening healing time by 0.8 day and pain duration by 1 day), it didn’t improve healing more than acyclovir alone. Other topical agents (penciclovir 1%; docosanol 10%) produced results similar to topical acyclovir.7,8
RECOMMENDATIONS
No national guidelines on this topic exist. An online resource notes that most patients don’t require treatment for mild self-limited HSL.9 For patients with prodromal symptoms, the authors recommend episodic oral antiviral therapy. Patients who have no prodome but multiple painful or disfiguring lesions may choose to use chronic suppressive therapy with an oral antiviral drug.
Daily oral acyclovir or valacyclovir may help prevent herpes simplex labialis (HSL) recurrences (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] with heterogeneous results).
No trials compare oral or topical treatments for HSL outbreaks against each other. Oral antivirals modestly reduce healing time and duration of pain, varying according to the agent used: valacyclovir reduces both healing time and duration of pain, famciclovir reduces both in one dosage form but not another, and acyclovir reduces only pain duration (SOR: B, single RCTs).
Several topical medications (acyclovir, penciclovir, docosanol) modestly decrease healing time and pain duration—typically by less than a day—and require multiple doses per day (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A systematic review and meta-analysis of the effectiveness of oral and topical nucleoside antiviral agents to prevent recurrent HSL in immunocompetent people found 11 RCTs with a total of 1250 patients that compared an active drug against placebo.1 The medications were topical 5% acyclovir, topical 1% penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. The primary outcome was recurrence of herpes simplex virus type 1 lesions during the treatment period. The relative risk (RR) of recurrence ranged from 0.22 to 1.22. Pooled results found a benefit favoring antiviral agents (RR of recurrence=0.70; 95% confidence interval [CI], 0.55-0.89).
Seven of the trials looked at acyclovir (5 oral, 2 topical). A subgroup analysis demonstrated that oral acyclovir (800-1000 mg/d) was more effective than placebo (RR=0.51; 95% CI, 0.29-0.88), whereas topical acyclovir wasn’t. Oral valacyclovir (2 studies; 500 mg/d for 4 months) also reduced recurrence (RR=0.65; 95% CI, 0.43-0.91). The authors of the meta-analysis noted that although 9 studies favored the use of an antiviral drug, only 4 showed statistically significant differences when compared with placebo, and none of them had a low risk of bias. They concluded that the review supported using oral acyclovir and valacyclovir to prevent recurrent HSL.1
Oral antivirals produce variable treatment results
Three RCTs evaluated oral antiviral medications against placebo to treat recurrent HSL, with mixed results. The largest RCT found that valacyclovir (2000 mg twice in 24 hours, with or without an additional 1000 mg twice in another 24 hours) modestly but significantly reduced both healing time and duration of pain (by 0.5-0.8 day).2 The second RCT showed that a higher, single dose of famciclovir (1500 mg) reduced healing time (by 1.8 days) and pain duration (by 1.2 days) and that a smaller, repeated dose (750 mg twice in 24 hours) reduced healing time alone (by 2.2 days).3
The third RCT demonstrated that acyclovir (400 mg 5 times a day for 5 days) reduced pain duration (by 0.9 day) but didn’t shorten healing time. If acyclovir was started during the prodrome, it decreased the time to disappearance of the lesion’s hard crust (2.1 days’ less time; P=.03), but the clinical significance of this finding is unclear.4
Topical treatment shows modest success
Two trials demonstrated that topical acyclovir (5% cream) modestly improved healing time and duration of pain (by as much as half a day). Patients in the first trial (paired RCTs reported together) began treatment within an hour of prodromal symptoms or signs, applying the medication 5 times daily for 4 days.5
Patients in the second trial used ME-609 cream (5% acyclovir plus 1% hydrocortisone), 5% acyclovir cream, or placebo, all applied 5 times daily for 5 days.6 Although the cream with acyclovir and hydrocortisone showed a slight benefit compared with placebo (lessening healing time by 0.8 day and pain duration by 1 day), it didn’t improve healing more than acyclovir alone. Other topical agents (penciclovir 1%; docosanol 10%) produced results similar to topical acyclovir.7,8
RECOMMENDATIONS
No national guidelines on this topic exist. An online resource notes that most patients don’t require treatment for mild self-limited HSL.9 For patients with prodromal symptoms, the authors recommend episodic oral antiviral therapy. Patients who have no prodome but multiple painful or disfiguring lesions may choose to use chronic suppressive therapy with an oral antiviral drug.
1. Rahimi H, Mara T, Costella J, et al. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:618-627.
2. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother. 2003;47:1072-1080.
3. Spruance SL, Bodsworth N, Resnick H, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55:47-53.
4. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis. 1990;161:185-190.
5. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238-2243.
6. Hull CM, Harmenberg J, Arlander E, et al; ME-609 Studt Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, doubleblind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64:696.e1-696.e11.
7. Raborn GW, Martel AY, Lassonde M, et al; Worldwide Topical Penciclovir Collaborative Study Group. Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. J Am Dent Assoc. 2002;133:303-309.
8. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
9. Klein RS. Treatment of herpes simplex virus type 1 infection in immunocompetent patients. Waltham, MA: UpToDate; 2012. Available at: www.uptodate.com/contents/treatment-of-herpessimplex-virus-type-1-infection-in-immunocompetentpatients. Accessed January 19, 2012.
1. Rahimi H, Mara T, Costella J, et al. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:618-627.
2. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother. 2003;47:1072-1080.
3. Spruance SL, Bodsworth N, Resnick H, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55:47-53.
4. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis. 1990;161:185-190.
5. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238-2243.
6. Hull CM, Harmenberg J, Arlander E, et al; ME-609 Studt Group. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, doubleblind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64:696.e1-696.e11.
7. Raborn GW, Martel AY, Lassonde M, et al; Worldwide Topical Penciclovir Collaborative Study Group. Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. J Am Dent Assoc. 2002;133:303-309.
8. Sacks SL, Thisted RA, Jones TM, et al; Docosanol 10% Cream Study Group. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45:222-230.
9. Klein RS. Treatment of herpes simplex virus type 1 infection in immunocompetent patients. Waltham, MA: UpToDate; 2012. Available at: www.uptodate.com/contents/treatment-of-herpessimplex-virus-type-1-infection-in-immunocompetentpatients. Accessed January 19, 2012.
Evidence-based answers from the Family Physicians Inquiries Network