User login
Quality Measure Attainment After Add-on Therapy of Both Saxagliptin and Dapagliflozin to Metformin Versus Single Add-On of Saxagliptin or Dapagliflozin
From the Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA (Dr. Blonde), and AstraZeneca, Gaithersburg, MD (Drs. Sheehan, Barrett, and Garcia-Sanchez).
Abstract
- Objective: To evaluate diabetes care quality measure attainment, specifically, blood glucose and blood pressure (BP) control, with saxagliptin, a dipeptidyl peptidase-4 inhibitor, and dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, added singly or as dual add-on therapy in patients with type 2 diabetes inadequately controlled with metformin alone.
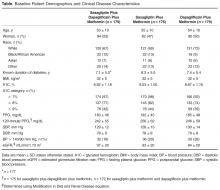
- Methods: Analysis of a phase 3, randomized, double-blind, active-controlled, parallel-group trial was conducted. Patients were randomized 1:1:1 to receive saxagliptin 5 mg/d plus dapagliflozin 10 mg/d, saxagliptin 5 mg/d, or dapagliflozin 10 mg/d as add-on to metformin 1500 to 2000 mg/d. Assessments included attainment of individual and composite glycated hemoglobin (A1C) and BP measures at 24 weeks of treatment.
- Results: Compared with single add-on saxagliptin or dapagliflozin, dual add-on saxagliptin plus dapagliflozin to metformin was associated with significantly more patients attaining the individual quality measures of A1C < 7% and A1C < 8%. Similarly, dual add-on saxagliptin plus dapagliflozin was associated with significantly more patients attaining the composite quality measures A1C < 7% and BP < 140/90 mm Hg and A1C < 8% and BP < 140/90 mmHg (vs saxagliptin plus metformin).
- Conclusion: Dual add-on saxagliptin plus dapagliflozin to metformin was associated with a higher proportion of patients achieving glycemic and BP quality measures compared with single add-on saxagliptin or dapagliflozin.
Assessment of performance is a focus of many health care organizations as a means to evaluate and improve the quality of health care. Standardized performance measures have been developed to improve quality of care as well as to allow for comparative assessment of health plans and to support pay for performance models [1]. A widely used set of performance measures is the Healthcare Effectiveness Data and Information Set or HEDIS [2,3], measures that are maintained by the National Committee for Quality Assurance [4,5] and used by most US health plans [6].
Type 2 diabetes (T2D) is a focus of quality measure assessment and performance improvement because of its high prevalence, substantial personal and economic impact on society, high morbidity and mortality, and because it is a condition that requires coordinated care. Important outcome measures for diabetes include blood glucose control and blood pressure (BP) control. HEDIS measures for T2D include a glycated hemoglobin (A1C) > 9%, indicating poor glucose control, < 8%, indicating good control, and < 7%, a more stringent measure of good glycemic control. The HEDIS measure for BP in T2D is < 140/90 mm Hg, which is considered good BP control. All of these HEDIS measures are currently or were previously (A1C < 7%) endorsed by the National Quality Forum [1,7–10]. Endorsement of a quality measure by the NQF indicates that the measure has been thoroughly evaluated, meets specific criteria, and is based on recognized standards of care grounded in evidence-based medicine [1].
A number of oral agents are utilized in the treatment of diabetes. Saxagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, and dapagliflozin, an oral sodium-glucose cotransporter-2 (SGLT-2) inhibitor, are indicated as adjuncts to diet and exercise in adults with T2D [11,12]. Saxagliptin inhibits DPP-4, and thereby reduces fasting and postprandial glucose concentrations by preventing degradation of the incretin hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide [13]. Dapagliflozin reduces blood glucose concentrations by inhibiting glucose reabsorption in the proximal tubule of the kidney, which results in enhanced urinary glucose excretion [14]. Because their mechanisms of action are glucose-dependent, both saxagliptin and dapagliflozin have a low intrinsic potential to cause hypoglycemia [13,14]. In a randomized, double-blind study of patients with T2D inadequately controlled with metformin, Rosenstock et al assessed the efficacy and safety of dual add-on of saxagliptin plus dapagliflozin versus saxagliptin and dapagliflozin added on alone (ClinicalTrials.gov identifier, NCT01606007) [15]. The dual add-on therapy resulted in a greater adjusted mean reduction from baseline in A1C at week 24 (–1.47%) compared with either saxagliptin (–0.88%) or dapagliflozin (–1.20%) alone added to metformin; the difference for dual add-on saxagliptin plus dapagliflozin to metformin vs. single add-on saxagliptin and single add-on dapagliflozin was –0.59% (P < 0.001) and –0.27% (P = 0.0166), respectively. The incidence of adverse events was similar across the 3 treatment groups, and hypo-glycemia was infrequent (1%), with no reports of severe hypoglycemia events (symptomatic events with glucose ≤ 54 mg/dL requiring assistance).
In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
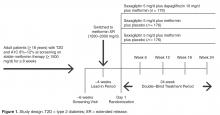
Study Design and Patients
Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.
Results
Patients
Individual Quality Measures
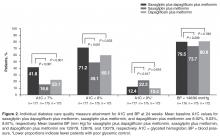
The dual addition of saxagliptin plus dapagliflozin to metformin resulted in a significantly greater proportion of patients achieving A1C < 8.0% compared with saxagliptin plus metformin (71.2% vs 49.1%; P < 0.001; NNT 5 [95% CI 3–8]) or dapagliflozin plus metformin (60.1%; P = 0.033; NNT 9 [95% CI 5–85]; Figure 2). Similar results (proportions of patients: 66.4% vs 40.0% vs 51.9%; P ≤ 0.02; NNTs 4 [95% CI 3–7]) and 7 [95% CI 4–34]) were attained when the analysis excluded patients with baseline A1C < 8.0%.
Significantly fewer patients had A1C > 9% (a measure of poor glycemic control) with saxagliptin plus dapagliflozin plus metformin (12.4%) compared with saxagliptin plus metformin (22.3%; P = 0.017; NNT –10 [95% CI –50 to –6]; Figure 2). The proportion of patients with A1C > 9% was similar for both regimens that included dapagliflozin (12.4% vs 10.4%; P = 0.616).
No significant difference was observed among treatment groups in the proportion of patients with BP < 140/90 mm Hg (Figure 2). However, most patients had BP < 140/90 mm Hg (72%–82%) at baseline, which was generally maintained at week 24.
Composite Quality Measures
Discussion
This post hoc analysis evaluated attainment of glycemic and BP quality measures for diabetes. A significantly greater proportion of patients achieved the individual quality measures of A1C < 7% and A1C < 8% with dual add-on saxagliptin plus dapagliflozin to metformin compared with single add-on saxagliptin or dapagliflozin to metformin after 24 weeks. Similar results were seen when the analysis excluded patients with A1C < 7% and < 8% at baseline. All measures of good glycemic control had clinically relevant NNTs ≤ 10 after 24 weeks with saxagliptin plus dapagliflozin plus metformin compared with saxagliptin or dapagliflozin plus metformin, regardless of baseline status. Very few patients experienced lackof improvement in glycemic control, evidenced by small proportions of patients with A1C > 9%.
There was little difference in BP between dual add-on saxagliptin plus dapagliflozin or single add-on saxagliptin or dapagliflozin to metformin. The proportion of patients who attained the BP quality measure of BP < 140/90 mm Hg was similar across the 3 treatments, as might be expected because most patients already met this target at baseline. However, as might be expected based on the mild diuretic effect and weight loss associated with SGLT-2 inhibitors [16,17], trends in BP favored groups treated with dapagliflozin.
Attainment of multiple treatment targets is desirable in reducing complications of diabetes. A significantly greater proportion of patients achieved both A1C < 7% and BP < 140/90 mm Hg when both saxagliptin and dapagliflozin were added to metformin compared with single-agent addition of either saxagliptin or dapagliflozin plus metformin. Similarly, a significantly greater proportion of patients achieved both A1C < 8% and BP < 140/90 mm Hg with dual addition of saxagliptin and dapagliflozin plus metformin compared with saxagliptin plus metformin. There was also a numerically greater number of patients who achieved both of these goals with triple therapy compared with dapagliflozin plus metformin, but this finding did not reach statistical significance. Clinically relevant NNT values ≤ 10 were observed for both composite outcomes for saxagliptin plus dapagliflozin plus metformin compared with saxagliptin plus metformin or dapagliflozin plus metformin after 24 weeks.
Despite advances in the medical management of T2D, a report published in 2013 showed that between 2007 and 2010, only 53% of patients achieved an A1C < 7.0% and only 19% simultaneously achieved all 3 American Diabetes Association (ADA) goals recommended for most patients at that time: A1C < 7.0%, BP < 130/80 mm Hg, and low-density lipoprotein cholesterol LDL-C < 100 mg/dL [18]. These data highlight a need for new approaches to help patients attain glycemic, BP, and cholesterol goals. Our results demonstrated that a higher proportion of patients attained glycemic and BP quality measures with dual add-on saxagliptin plus dapagliflozin compared with single add-on saxagliptin or dapagliflozin to metformin. As a result of recent updates for cholesterol management from the American College of Cardiology and the American Heart Association [19], attainment of a cholesterol level was retired as a diabetes quality measure and replaced with a recommendation for statin therapy use [20,21]. Although the current analysis did not include assessment of LDL, DPP-4 inhibitors have demonstrated neutral effects on lipids [22,23], and SGLT-2 inhibitors have demonstrated generally modest increases in LDL-C (placebo-adjusted change from baseline: 4.5%–8.0% for canagliflozin 100 and 300 mg/d, 3.9% for dapagliflozin 10 mg, and 2.3%–4.2% for empagliflozin 10 and 25 mg/d) [12,24,25], as well as increases in high-density lipoprotein cholesterol and reductions in triglycerides [26].
Current ADA guidelines recommend an individualized, stepwise approach to treatment with sequential addition of single oral antihyperglycemic agents for patients who do not achieve their glycemic goal in 3 months [27]. Although T2D may progress at different rates in different patients, T2D does generally progress over time [28], and the ADA and American Association of Clinical Endocrinologists treatment guidelines recommend initial dual add-on therapy for individuals with higher A1C, which is suggestive of more advanced disease [27,29]. For individuals requiring initial combination therapy, guidelines note that antihyperglycemic agents that have a low risk of hypoglycemia and low potential for weight gain should be preferentially selected [29]. Attainment of A1C ≤ 7%, the guideline recommendation considered appropriate for many patients, is associated with reductions in microvascular disease and, if attained soon after diagnosis of diabetes, studies have shown reductions in macrovascular disease with long-term follow-up [27,30,31]. However, it may be challenging to achieve A1C < 7% with the addition of single oral antihyperglycemic agents, especially in patients with higher A1C [32]. Less stringent A1C goals (eg, A1C < 8%) may be appropriate in individuals with a long duration of diabetes that is difficult to control, history of severe hypoglycemia, limited life expectancy, numerous comorbidities, and extensive complications or comorbidities, especially cardiovascular disease [27]. Given the shift toward individualized treatment plans with patient-specific treatment goals, it is valuable to understand how different treatment strategies effect attainment of guideline-recommended less stringent and more stringent glycemic targets that may be appropriate for certain patients.
In addition to quality measures that assess glucose lowering with pharmacotherapy, it is important to consider measures that assess other aspects of diabetes care. For example, quality measures related to hypoglycemia and hyperglycemia may help avoid potentially adverse glucose levels, and quality measures related to weight may provide insight on treatment and lifestyle efforts directed at weight loss and management. NQF-endorsed measures of hypoglycemia and hyperglycemia are currently moving through annual review and are paired measures, intended to be interpreted with respect to one another to ensure balanced outcomes [33,34]. This underscores the value of efficacious antihyperglycemic agents with low intrinsic potential for hypoglycemia. Although this analysis did not include quality measures related to hypoglycemia or weight, future studies evaluating these aspects of diabetes care will likely further contribute to a more comprehensive and holistic treatment approach.
In addition to assessing a broad range of quality measures, an important aspect of care to consider is patient affordability. Affordability for an individual patient will depend on access in the patient’s individual plan, the financial resources of the patient, and the potential for medical cost offsets from improved control of the patient’s disease. For example, fixed-dose combination products are associated with increased patient adherence and may increase pharmacy costs but decrease medical costs [35].
Limitations of this study include the post hoc design and that quality measure attainment was assessed over a shorter duration of time (24 weeks) than is commonly assessed in the real-world/community setting (~12 months).
Dual add-on therapy with oral antihyperglycemic agents that have complementary mechanisms of action should lead to enhanced reductions in A1C. The results reported here and from the primary study, in which saxagliptin and dapagliflozin added to metformin significantly reduced mean A1C from baseline to week 24 compared with single add-on saxagliptin or dapagliflozin [15], showed that greater reductions in A1C were attained with the coadministration of saxagliptin and dapagliflozin. The glucuretic effect of SGLT-2 inhibitors has been associated with increased plasma glucagon concentrations and increased endogenous glucose production, which may impair the full glucose-lowering potential of SGLT-2 inhibitors [36,37]. Administering saxagliptin with dapagliflozin as dual therapy was shown to blunt the rise in plasma glucagon caused by dapagliflozin [38], and this may have contributed to the greater glucose control achieved with dual add-on of these 2 antihyperglycemic drugs [15].
By targeting multiple aspects of the underlying pathophysiology in T2D, greater improvements in A1C can be achieved. Dual add-on saxagliptin plus dapagliflozin to metformin resulted in a greater proportion of patients achieving NQF-endorsed HEDIS quality measures, as well as A1C < 7% (no longer an NQF-endorsed measure). As health care shifts to a more value-based payment structure, measuring quality outcomes will assume a greater role in guiding decision making and influence the care that patients receive. Understanding how antihyperglycemic medication regimens affect quality measures can help clinicians make informed decisions.
Corresponding author: Lawrence Blonde, MD, Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA.
Funding/support: This study was supported by AstraZeneca. Medical writing support for the preparation of this manuscript was provided by Lauren D’Angelo, PhD, and Janet Matsuura, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA), with funding from AstraZeneca.
Financial disclosures: Dr. Blonde has received grant and research support from AstraZeneca, Jansen Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis and has received honoraria for participating as a speaker from AstraZeneca, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis as well as honoraria for consultant work from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis. R. Garcia-Sanchez is an employee of AstraZeneca. J. Sheehan and Y. C. Barrett were employees of AstraZeneca at the time of this research.
1. National Quality Forum. ABCs of measurement. Accessed 11 Mar 2016 at www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx.
2. National Committee for Quality Assurance. HEDIS measure development process. Accessed 14 Mar 2016 at www.ncqa.org/tabid/414/Default.aspx.
3. National Committee for Quality Assurance. HEDIS measures. Accessed 11 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
4. National Committee for Quality Assurance. About NCQA: overview. Accessed 14 Mar 2016 at www.ncqa.org/AboutNCQA.aspx.
5. National Committee for Quality Assurance. Health care program evaluations. Accessed 11 Mar 2016 at www.ncqa.org/Programs.aspx.
6. National Committee for Quality Assurance. HEDIS and Performance Measurement. Accessed 14 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement.aspx.
7. National Committee for Quality Assurance. HEDIS 2015 technical specifications for ACO measurement. Washington, DC: National Committee for Quality Assurance; 2014.
8. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (> 9.0%). NQF identifier: 0059. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
9. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) control (< 8.0%). NQF identifier: 0575. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
10. National Quality Forum. Comprehensive diabetes care: blood pressure control (< 140/90 mm Hg). NQF identifier: 0061. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
11. Onglyza(saxagliptin). Full prescribing information. AstraZeneca, Wilmington, DE; 2014.
12. Farxiga (dapagliflozin). Full prescribing information. AstraZeneca, Wilmington, DE; March 2015.
13. Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009;26:488–99.
14. Kasichayanula S, Liu X, Lacreta F, et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014;53:17–27.
15. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2014;38:376–83.
16. Bailey CJ. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. British Journal of Diabetes & Vascular Disease 2010;10:193-9.
17. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53.
18. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9.
19. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
20. National Quality Forum. Comprehensive diabetes care (composite). NQF identifier: #0731. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
21. National Quality Forum. Optimal diabetes care (composite measure). NQF identifier: 0729. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206.
23. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25.
24. Invokana(canagliflzoin). Full prescribing information. Janssen Pharmaceuticals, Titusville, NJ; 2013.
25. Jardiance(empagliflozin). Full prescribing information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly, Ingelheim, Germany and Indianapolis, IN; 2014.
26. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100.
27. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016;39:S1–S119.
28. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32:S151–S6.
29. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 executive summary. Endocr Pract 2016;22:84–113.
30. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
31. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
32. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007;30:890–5.
33. National Quality Forum. Glycemic control: hypoglycemia. NQF identifier: 2363. Accessed 29 Oct 2015 at www.qualityforum.org/QPS/QPSTool.aspx.
34. National Quality Forum. Glycemic control: hyperglycemia. NQF identifier: 2362. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
35. Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009;11:527–33.
36. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508.
37. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.
38. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 2014;20:1187–97.
From the Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA (Dr. Blonde), and AstraZeneca, Gaithersburg, MD (Drs. Sheehan, Barrett, and Garcia-Sanchez).
Abstract
- Objective: To evaluate diabetes care quality measure attainment, specifically, blood glucose and blood pressure (BP) control, with saxagliptin, a dipeptidyl peptidase-4 inhibitor, and dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, added singly or as dual add-on therapy in patients with type 2 diabetes inadequately controlled with metformin alone.
- Methods: Analysis of a phase 3, randomized, double-blind, active-controlled, parallel-group trial was conducted. Patients were randomized 1:1:1 to receive saxagliptin 5 mg/d plus dapagliflozin 10 mg/d, saxagliptin 5 mg/d, or dapagliflozin 10 mg/d as add-on to metformin 1500 to 2000 mg/d. Assessments included attainment of individual and composite glycated hemoglobin (A1C) and BP measures at 24 weeks of treatment.
- Results: Compared with single add-on saxagliptin or dapagliflozin, dual add-on saxagliptin plus dapagliflozin to metformin was associated with significantly more patients attaining the individual quality measures of A1C < 7% and A1C < 8%. Similarly, dual add-on saxagliptin plus dapagliflozin was associated with significantly more patients attaining the composite quality measures A1C < 7% and BP < 140/90 mm Hg and A1C < 8% and BP < 140/90 mmHg (vs saxagliptin plus metformin).
- Conclusion: Dual add-on saxagliptin plus dapagliflozin to metformin was associated with a higher proportion of patients achieving glycemic and BP quality measures compared with single add-on saxagliptin or dapagliflozin.
Assessment of performance is a focus of many health care organizations as a means to evaluate and improve the quality of health care. Standardized performance measures have been developed to improve quality of care as well as to allow for comparative assessment of health plans and to support pay for performance models [1]. A widely used set of performance measures is the Healthcare Effectiveness Data and Information Set or HEDIS [2,3], measures that are maintained by the National Committee for Quality Assurance [4,5] and used by most US health plans [6].
Type 2 diabetes (T2D) is a focus of quality measure assessment and performance improvement because of its high prevalence, substantial personal and economic impact on society, high morbidity and mortality, and because it is a condition that requires coordinated care. Important outcome measures for diabetes include blood glucose control and blood pressure (BP) control. HEDIS measures for T2D include a glycated hemoglobin (A1C) > 9%, indicating poor glucose control, < 8%, indicating good control, and < 7%, a more stringent measure of good glycemic control. The HEDIS measure for BP in T2D is < 140/90 mm Hg, which is considered good BP control. All of these HEDIS measures are currently or were previously (A1C < 7%) endorsed by the National Quality Forum [1,7–10]. Endorsement of a quality measure by the NQF indicates that the measure has been thoroughly evaluated, meets specific criteria, and is based on recognized standards of care grounded in evidence-based medicine [1].
A number of oral agents are utilized in the treatment of diabetes. Saxagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, and dapagliflozin, an oral sodium-glucose cotransporter-2 (SGLT-2) inhibitor, are indicated as adjuncts to diet and exercise in adults with T2D [11,12]. Saxagliptin inhibits DPP-4, and thereby reduces fasting and postprandial glucose concentrations by preventing degradation of the incretin hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide [13]. Dapagliflozin reduces blood glucose concentrations by inhibiting glucose reabsorption in the proximal tubule of the kidney, which results in enhanced urinary glucose excretion [14]. Because their mechanisms of action are glucose-dependent, both saxagliptin and dapagliflozin have a low intrinsic potential to cause hypoglycemia [13,14]. In a randomized, double-blind study of patients with T2D inadequately controlled with metformin, Rosenstock et al assessed the efficacy and safety of dual add-on of saxagliptin plus dapagliflozin versus saxagliptin and dapagliflozin added on alone (ClinicalTrials.gov identifier, NCT01606007) [15]. The dual add-on therapy resulted in a greater adjusted mean reduction from baseline in A1C at week 24 (–1.47%) compared with either saxagliptin (–0.88%) or dapagliflozin (–1.20%) alone added to metformin; the difference for dual add-on saxagliptin plus dapagliflozin to metformin vs. single add-on saxagliptin and single add-on dapagliflozin was –0.59% (P < 0.001) and –0.27% (P = 0.0166), respectively. The incidence of adverse events was similar across the 3 treatment groups, and hypo-glycemia was infrequent (1%), with no reports of severe hypoglycemia events (symptomatic events with glucose ≤ 54 mg/dL requiring assistance).
In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
Study Design and Patients
Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.
Results
Patients
Individual Quality Measures
The dual addition of saxagliptin plus dapagliflozin to metformin resulted in a significantly greater proportion of patients achieving A1C < 8.0% compared with saxagliptin plus metformin (71.2% vs 49.1%; P < 0.001; NNT 5 [95% CI 3–8]) or dapagliflozin plus metformin (60.1%; P = 0.033; NNT 9 [95% CI 5–85]; Figure 2). Similar results (proportions of patients: 66.4% vs 40.0% vs 51.9%; P ≤ 0.02; NNTs 4 [95% CI 3–7]) and 7 [95% CI 4–34]) were attained when the analysis excluded patients with baseline A1C < 8.0%.
Significantly fewer patients had A1C > 9% (a measure of poor glycemic control) with saxagliptin plus dapagliflozin plus metformin (12.4%) compared with saxagliptin plus metformin (22.3%; P = 0.017; NNT –10 [95% CI –50 to –6]; Figure 2). The proportion of patients with A1C > 9% was similar for both regimens that included dapagliflozin (12.4% vs 10.4%; P = 0.616).
No significant difference was observed among treatment groups in the proportion of patients with BP < 140/90 mm Hg (Figure 2). However, most patients had BP < 140/90 mm Hg (72%–82%) at baseline, which was generally maintained at week 24.
Composite Quality Measures
Discussion
This post hoc analysis evaluated attainment of glycemic and BP quality measures for diabetes. A significantly greater proportion of patients achieved the individual quality measures of A1C < 7% and A1C < 8% with dual add-on saxagliptin plus dapagliflozin to metformin compared with single add-on saxagliptin or dapagliflozin to metformin after 24 weeks. Similar results were seen when the analysis excluded patients with A1C < 7% and < 8% at baseline. All measures of good glycemic control had clinically relevant NNTs ≤ 10 after 24 weeks with saxagliptin plus dapagliflozin plus metformin compared with saxagliptin or dapagliflozin plus metformin, regardless of baseline status. Very few patients experienced lackof improvement in glycemic control, evidenced by small proportions of patients with A1C > 9%.
There was little difference in BP between dual add-on saxagliptin plus dapagliflozin or single add-on saxagliptin or dapagliflozin to metformin. The proportion of patients who attained the BP quality measure of BP < 140/90 mm Hg was similar across the 3 treatments, as might be expected because most patients already met this target at baseline. However, as might be expected based on the mild diuretic effect and weight loss associated with SGLT-2 inhibitors [16,17], trends in BP favored groups treated with dapagliflozin.
Attainment of multiple treatment targets is desirable in reducing complications of diabetes. A significantly greater proportion of patients achieved both A1C < 7% and BP < 140/90 mm Hg when both saxagliptin and dapagliflozin were added to metformin compared with single-agent addition of either saxagliptin or dapagliflozin plus metformin. Similarly, a significantly greater proportion of patients achieved both A1C < 8% and BP < 140/90 mm Hg with dual addition of saxagliptin and dapagliflozin plus metformin compared with saxagliptin plus metformin. There was also a numerically greater number of patients who achieved both of these goals with triple therapy compared with dapagliflozin plus metformin, but this finding did not reach statistical significance. Clinically relevant NNT values ≤ 10 were observed for both composite outcomes for saxagliptin plus dapagliflozin plus metformin compared with saxagliptin plus metformin or dapagliflozin plus metformin after 24 weeks.
Despite advances in the medical management of T2D, a report published in 2013 showed that between 2007 and 2010, only 53% of patients achieved an A1C < 7.0% and only 19% simultaneously achieved all 3 American Diabetes Association (ADA) goals recommended for most patients at that time: A1C < 7.0%, BP < 130/80 mm Hg, and low-density lipoprotein cholesterol LDL-C < 100 mg/dL [18]. These data highlight a need for new approaches to help patients attain glycemic, BP, and cholesterol goals. Our results demonstrated that a higher proportion of patients attained glycemic and BP quality measures with dual add-on saxagliptin plus dapagliflozin compared with single add-on saxagliptin or dapagliflozin to metformin. As a result of recent updates for cholesterol management from the American College of Cardiology and the American Heart Association [19], attainment of a cholesterol level was retired as a diabetes quality measure and replaced with a recommendation for statin therapy use [20,21]. Although the current analysis did not include assessment of LDL, DPP-4 inhibitors have demonstrated neutral effects on lipids [22,23], and SGLT-2 inhibitors have demonstrated generally modest increases in LDL-C (placebo-adjusted change from baseline: 4.5%–8.0% for canagliflozin 100 and 300 mg/d, 3.9% for dapagliflozin 10 mg, and 2.3%–4.2% for empagliflozin 10 and 25 mg/d) [12,24,25], as well as increases in high-density lipoprotein cholesterol and reductions in triglycerides [26].
Current ADA guidelines recommend an individualized, stepwise approach to treatment with sequential addition of single oral antihyperglycemic agents for patients who do not achieve their glycemic goal in 3 months [27]. Although T2D may progress at different rates in different patients, T2D does generally progress over time [28], and the ADA and American Association of Clinical Endocrinologists treatment guidelines recommend initial dual add-on therapy for individuals with higher A1C, which is suggestive of more advanced disease [27,29]. For individuals requiring initial combination therapy, guidelines note that antihyperglycemic agents that have a low risk of hypoglycemia and low potential for weight gain should be preferentially selected [29]. Attainment of A1C ≤ 7%, the guideline recommendation considered appropriate for many patients, is associated with reductions in microvascular disease and, if attained soon after diagnosis of diabetes, studies have shown reductions in macrovascular disease with long-term follow-up [27,30,31]. However, it may be challenging to achieve A1C < 7% with the addition of single oral antihyperglycemic agents, especially in patients with higher A1C [32]. Less stringent A1C goals (eg, A1C < 8%) may be appropriate in individuals with a long duration of diabetes that is difficult to control, history of severe hypoglycemia, limited life expectancy, numerous comorbidities, and extensive complications or comorbidities, especially cardiovascular disease [27]. Given the shift toward individualized treatment plans with patient-specific treatment goals, it is valuable to understand how different treatment strategies effect attainment of guideline-recommended less stringent and more stringent glycemic targets that may be appropriate for certain patients.
In addition to quality measures that assess glucose lowering with pharmacotherapy, it is important to consider measures that assess other aspects of diabetes care. For example, quality measures related to hypoglycemia and hyperglycemia may help avoid potentially adverse glucose levels, and quality measures related to weight may provide insight on treatment and lifestyle efforts directed at weight loss and management. NQF-endorsed measures of hypoglycemia and hyperglycemia are currently moving through annual review and are paired measures, intended to be interpreted with respect to one another to ensure balanced outcomes [33,34]. This underscores the value of efficacious antihyperglycemic agents with low intrinsic potential for hypoglycemia. Although this analysis did not include quality measures related to hypoglycemia or weight, future studies evaluating these aspects of diabetes care will likely further contribute to a more comprehensive and holistic treatment approach.
In addition to assessing a broad range of quality measures, an important aspect of care to consider is patient affordability. Affordability for an individual patient will depend on access in the patient’s individual plan, the financial resources of the patient, and the potential for medical cost offsets from improved control of the patient’s disease. For example, fixed-dose combination products are associated with increased patient adherence and may increase pharmacy costs but decrease medical costs [35].
Limitations of this study include the post hoc design and that quality measure attainment was assessed over a shorter duration of time (24 weeks) than is commonly assessed in the real-world/community setting (~12 months).
Dual add-on therapy with oral antihyperglycemic agents that have complementary mechanisms of action should lead to enhanced reductions in A1C. The results reported here and from the primary study, in which saxagliptin and dapagliflozin added to metformin significantly reduced mean A1C from baseline to week 24 compared with single add-on saxagliptin or dapagliflozin [15], showed that greater reductions in A1C were attained with the coadministration of saxagliptin and dapagliflozin. The glucuretic effect of SGLT-2 inhibitors has been associated with increased plasma glucagon concentrations and increased endogenous glucose production, which may impair the full glucose-lowering potential of SGLT-2 inhibitors [36,37]. Administering saxagliptin with dapagliflozin as dual therapy was shown to blunt the rise in plasma glucagon caused by dapagliflozin [38], and this may have contributed to the greater glucose control achieved with dual add-on of these 2 antihyperglycemic drugs [15].
By targeting multiple aspects of the underlying pathophysiology in T2D, greater improvements in A1C can be achieved. Dual add-on saxagliptin plus dapagliflozin to metformin resulted in a greater proportion of patients achieving NQF-endorsed HEDIS quality measures, as well as A1C < 7% (no longer an NQF-endorsed measure). As health care shifts to a more value-based payment structure, measuring quality outcomes will assume a greater role in guiding decision making and influence the care that patients receive. Understanding how antihyperglycemic medication regimens affect quality measures can help clinicians make informed decisions.
Corresponding author: Lawrence Blonde, MD, Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA.
Funding/support: This study was supported by AstraZeneca. Medical writing support for the preparation of this manuscript was provided by Lauren D’Angelo, PhD, and Janet Matsuura, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA), with funding from AstraZeneca.
Financial disclosures: Dr. Blonde has received grant and research support from AstraZeneca, Jansen Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis and has received honoraria for participating as a speaker from AstraZeneca, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis as well as honoraria for consultant work from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis. R. Garcia-Sanchez is an employee of AstraZeneca. J. Sheehan and Y. C. Barrett were employees of AstraZeneca at the time of this research.
From the Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA (Dr. Blonde), and AstraZeneca, Gaithersburg, MD (Drs. Sheehan, Barrett, and Garcia-Sanchez).
Abstract
- Objective: To evaluate diabetes care quality measure attainment, specifically, blood glucose and blood pressure (BP) control, with saxagliptin, a dipeptidyl peptidase-4 inhibitor, and dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, added singly or as dual add-on therapy in patients with type 2 diabetes inadequately controlled with metformin alone.
- Methods: Analysis of a phase 3, randomized, double-blind, active-controlled, parallel-group trial was conducted. Patients were randomized 1:1:1 to receive saxagliptin 5 mg/d plus dapagliflozin 10 mg/d, saxagliptin 5 mg/d, or dapagliflozin 10 mg/d as add-on to metformin 1500 to 2000 mg/d. Assessments included attainment of individual and composite glycated hemoglobin (A1C) and BP measures at 24 weeks of treatment.
- Results: Compared with single add-on saxagliptin or dapagliflozin, dual add-on saxagliptin plus dapagliflozin to metformin was associated with significantly more patients attaining the individual quality measures of A1C < 7% and A1C < 8%. Similarly, dual add-on saxagliptin plus dapagliflozin was associated with significantly more patients attaining the composite quality measures A1C < 7% and BP < 140/90 mm Hg and A1C < 8% and BP < 140/90 mmHg (vs saxagliptin plus metformin).
- Conclusion: Dual add-on saxagliptin plus dapagliflozin to metformin was associated with a higher proportion of patients achieving glycemic and BP quality measures compared with single add-on saxagliptin or dapagliflozin.
Assessment of performance is a focus of many health care organizations as a means to evaluate and improve the quality of health care. Standardized performance measures have been developed to improve quality of care as well as to allow for comparative assessment of health plans and to support pay for performance models [1]. A widely used set of performance measures is the Healthcare Effectiveness Data and Information Set or HEDIS [2,3], measures that are maintained by the National Committee for Quality Assurance [4,5] and used by most US health plans [6].
Type 2 diabetes (T2D) is a focus of quality measure assessment and performance improvement because of its high prevalence, substantial personal and economic impact on society, high morbidity and mortality, and because it is a condition that requires coordinated care. Important outcome measures for diabetes include blood glucose control and blood pressure (BP) control. HEDIS measures for T2D include a glycated hemoglobin (A1C) > 9%, indicating poor glucose control, < 8%, indicating good control, and < 7%, a more stringent measure of good glycemic control. The HEDIS measure for BP in T2D is < 140/90 mm Hg, which is considered good BP control. All of these HEDIS measures are currently or were previously (A1C < 7%) endorsed by the National Quality Forum [1,7–10]. Endorsement of a quality measure by the NQF indicates that the measure has been thoroughly evaluated, meets specific criteria, and is based on recognized standards of care grounded in evidence-based medicine [1].
A number of oral agents are utilized in the treatment of diabetes. Saxagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, and dapagliflozin, an oral sodium-glucose cotransporter-2 (SGLT-2) inhibitor, are indicated as adjuncts to diet and exercise in adults with T2D [11,12]. Saxagliptin inhibits DPP-4, and thereby reduces fasting and postprandial glucose concentrations by preventing degradation of the incretin hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide [13]. Dapagliflozin reduces blood glucose concentrations by inhibiting glucose reabsorption in the proximal tubule of the kidney, which results in enhanced urinary glucose excretion [14]. Because their mechanisms of action are glucose-dependent, both saxagliptin and dapagliflozin have a low intrinsic potential to cause hypoglycemia [13,14]. In a randomized, double-blind study of patients with T2D inadequately controlled with metformin, Rosenstock et al assessed the efficacy and safety of dual add-on of saxagliptin plus dapagliflozin versus saxagliptin and dapagliflozin added on alone (ClinicalTrials.gov identifier, NCT01606007) [15]. The dual add-on therapy resulted in a greater adjusted mean reduction from baseline in A1C at week 24 (–1.47%) compared with either saxagliptin (–0.88%) or dapagliflozin (–1.20%) alone added to metformin; the difference for dual add-on saxagliptin plus dapagliflozin to metformin vs. single add-on saxagliptin and single add-on dapagliflozin was –0.59% (P < 0.001) and –0.27% (P = 0.0166), respectively. The incidence of adverse events was similar across the 3 treatment groups, and hypo-glycemia was infrequent (1%), with no reports of severe hypoglycemia events (symptomatic events with glucose ≤ 54 mg/dL requiring assistance).
In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
Study Design and Patients
Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.
Results
Patients
Individual Quality Measures
The dual addition of saxagliptin plus dapagliflozin to metformin resulted in a significantly greater proportion of patients achieving A1C < 8.0% compared with saxagliptin plus metformin (71.2% vs 49.1%; P < 0.001; NNT 5 [95% CI 3–8]) or dapagliflozin plus metformin (60.1%; P = 0.033; NNT 9 [95% CI 5–85]; Figure 2). Similar results (proportions of patients: 66.4% vs 40.0% vs 51.9%; P ≤ 0.02; NNTs 4 [95% CI 3–7]) and 7 [95% CI 4–34]) were attained when the analysis excluded patients with baseline A1C < 8.0%.
Significantly fewer patients had A1C > 9% (a measure of poor glycemic control) with saxagliptin plus dapagliflozin plus metformin (12.4%) compared with saxagliptin plus metformin (22.3%; P = 0.017; NNT –10 [95% CI –50 to –6]; Figure 2). The proportion of patients with A1C > 9% was similar for both regimens that included dapagliflozin (12.4% vs 10.4%; P = 0.616).
No significant difference was observed among treatment groups in the proportion of patients with BP < 140/90 mm Hg (Figure 2). However, most patients had BP < 140/90 mm Hg (72%–82%) at baseline, which was generally maintained at week 24.
Composite Quality Measures
Discussion
This post hoc analysis evaluated attainment of glycemic and BP quality measures for diabetes. A significantly greater proportion of patients achieved the individual quality measures of A1C < 7% and A1C < 8% with dual add-on saxagliptin plus dapagliflozin to metformin compared with single add-on saxagliptin or dapagliflozin to metformin after 24 weeks. Similar results were seen when the analysis excluded patients with A1C < 7% and < 8% at baseline. All measures of good glycemic control had clinically relevant NNTs ≤ 10 after 24 weeks with saxagliptin plus dapagliflozin plus metformin compared with saxagliptin or dapagliflozin plus metformin, regardless of baseline status. Very few patients experienced lackof improvement in glycemic control, evidenced by small proportions of patients with A1C > 9%.
There was little difference in BP between dual add-on saxagliptin plus dapagliflozin or single add-on saxagliptin or dapagliflozin to metformin. The proportion of patients who attained the BP quality measure of BP < 140/90 mm Hg was similar across the 3 treatments, as might be expected because most patients already met this target at baseline. However, as might be expected based on the mild diuretic effect and weight loss associated with SGLT-2 inhibitors [16,17], trends in BP favored groups treated with dapagliflozin.
Attainment of multiple treatment targets is desirable in reducing complications of diabetes. A significantly greater proportion of patients achieved both A1C < 7% and BP < 140/90 mm Hg when both saxagliptin and dapagliflozin were added to metformin compared with single-agent addition of either saxagliptin or dapagliflozin plus metformin. Similarly, a significantly greater proportion of patients achieved both A1C < 8% and BP < 140/90 mm Hg with dual addition of saxagliptin and dapagliflozin plus metformin compared with saxagliptin plus metformin. There was also a numerically greater number of patients who achieved both of these goals with triple therapy compared with dapagliflozin plus metformin, but this finding did not reach statistical significance. Clinically relevant NNT values ≤ 10 were observed for both composite outcomes for saxagliptin plus dapagliflozin plus metformin compared with saxagliptin plus metformin or dapagliflozin plus metformin after 24 weeks.
Despite advances in the medical management of T2D, a report published in 2013 showed that between 2007 and 2010, only 53% of patients achieved an A1C < 7.0% and only 19% simultaneously achieved all 3 American Diabetes Association (ADA) goals recommended for most patients at that time: A1C < 7.0%, BP < 130/80 mm Hg, and low-density lipoprotein cholesterol LDL-C < 100 mg/dL [18]. These data highlight a need for new approaches to help patients attain glycemic, BP, and cholesterol goals. Our results demonstrated that a higher proportion of patients attained glycemic and BP quality measures with dual add-on saxagliptin plus dapagliflozin compared with single add-on saxagliptin or dapagliflozin to metformin. As a result of recent updates for cholesterol management from the American College of Cardiology and the American Heart Association [19], attainment of a cholesterol level was retired as a diabetes quality measure and replaced with a recommendation for statin therapy use [20,21]. Although the current analysis did not include assessment of LDL, DPP-4 inhibitors have demonstrated neutral effects on lipids [22,23], and SGLT-2 inhibitors have demonstrated generally modest increases in LDL-C (placebo-adjusted change from baseline: 4.5%–8.0% for canagliflozin 100 and 300 mg/d, 3.9% for dapagliflozin 10 mg, and 2.3%–4.2% for empagliflozin 10 and 25 mg/d) [12,24,25], as well as increases in high-density lipoprotein cholesterol and reductions in triglycerides [26].
Current ADA guidelines recommend an individualized, stepwise approach to treatment with sequential addition of single oral antihyperglycemic agents for patients who do not achieve their glycemic goal in 3 months [27]. Although T2D may progress at different rates in different patients, T2D does generally progress over time [28], and the ADA and American Association of Clinical Endocrinologists treatment guidelines recommend initial dual add-on therapy for individuals with higher A1C, which is suggestive of more advanced disease [27,29]. For individuals requiring initial combination therapy, guidelines note that antihyperglycemic agents that have a low risk of hypoglycemia and low potential for weight gain should be preferentially selected [29]. Attainment of A1C ≤ 7%, the guideline recommendation considered appropriate for many patients, is associated with reductions in microvascular disease and, if attained soon after diagnosis of diabetes, studies have shown reductions in macrovascular disease with long-term follow-up [27,30,31]. However, it may be challenging to achieve A1C < 7% with the addition of single oral antihyperglycemic agents, especially in patients with higher A1C [32]. Less stringent A1C goals (eg, A1C < 8%) may be appropriate in individuals with a long duration of diabetes that is difficult to control, history of severe hypoglycemia, limited life expectancy, numerous comorbidities, and extensive complications or comorbidities, especially cardiovascular disease [27]. Given the shift toward individualized treatment plans with patient-specific treatment goals, it is valuable to understand how different treatment strategies effect attainment of guideline-recommended less stringent and more stringent glycemic targets that may be appropriate for certain patients.
In addition to quality measures that assess glucose lowering with pharmacotherapy, it is important to consider measures that assess other aspects of diabetes care. For example, quality measures related to hypoglycemia and hyperglycemia may help avoid potentially adverse glucose levels, and quality measures related to weight may provide insight on treatment and lifestyle efforts directed at weight loss and management. NQF-endorsed measures of hypoglycemia and hyperglycemia are currently moving through annual review and are paired measures, intended to be interpreted with respect to one another to ensure balanced outcomes [33,34]. This underscores the value of efficacious antihyperglycemic agents with low intrinsic potential for hypoglycemia. Although this analysis did not include quality measures related to hypoglycemia or weight, future studies evaluating these aspects of diabetes care will likely further contribute to a more comprehensive and holistic treatment approach.
In addition to assessing a broad range of quality measures, an important aspect of care to consider is patient affordability. Affordability for an individual patient will depend on access in the patient’s individual plan, the financial resources of the patient, and the potential for medical cost offsets from improved control of the patient’s disease. For example, fixed-dose combination products are associated with increased patient adherence and may increase pharmacy costs but decrease medical costs [35].
Limitations of this study include the post hoc design and that quality measure attainment was assessed over a shorter duration of time (24 weeks) than is commonly assessed in the real-world/community setting (~12 months).
Dual add-on therapy with oral antihyperglycemic agents that have complementary mechanisms of action should lead to enhanced reductions in A1C. The results reported here and from the primary study, in which saxagliptin and dapagliflozin added to metformin significantly reduced mean A1C from baseline to week 24 compared with single add-on saxagliptin or dapagliflozin [15], showed that greater reductions in A1C were attained with the coadministration of saxagliptin and dapagliflozin. The glucuretic effect of SGLT-2 inhibitors has been associated with increased plasma glucagon concentrations and increased endogenous glucose production, which may impair the full glucose-lowering potential of SGLT-2 inhibitors [36,37]. Administering saxagliptin with dapagliflozin as dual therapy was shown to blunt the rise in plasma glucagon caused by dapagliflozin [38], and this may have contributed to the greater glucose control achieved with dual add-on of these 2 antihyperglycemic drugs [15].
By targeting multiple aspects of the underlying pathophysiology in T2D, greater improvements in A1C can be achieved. Dual add-on saxagliptin plus dapagliflozin to metformin resulted in a greater proportion of patients achieving NQF-endorsed HEDIS quality measures, as well as A1C < 7% (no longer an NQF-endorsed measure). As health care shifts to a more value-based payment structure, measuring quality outcomes will assume a greater role in guiding decision making and influence the care that patients receive. Understanding how antihyperglycemic medication regimens affect quality measures can help clinicians make informed decisions.
Corresponding author: Lawrence Blonde, MD, Ochsner Diabetes Clinical Research Unit, Frank Riddick Diabetes Institute, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA.
Funding/support: This study was supported by AstraZeneca. Medical writing support for the preparation of this manuscript was provided by Lauren D’Angelo, PhD, and Janet Matsuura, PhD, from Complete Healthcare Communications, LLC (Chadds Ford, PA), with funding from AstraZeneca.
Financial disclosures: Dr. Blonde has received grant and research support from AstraZeneca, Jansen Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis and has received honoraria for participating as a speaker from AstraZeneca, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis as well as honoraria for consultant work from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi-Aventis. R. Garcia-Sanchez is an employee of AstraZeneca. J. Sheehan and Y. C. Barrett were employees of AstraZeneca at the time of this research.
1. National Quality Forum. ABCs of measurement. Accessed 11 Mar 2016 at www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx.
2. National Committee for Quality Assurance. HEDIS measure development process. Accessed 14 Mar 2016 at www.ncqa.org/tabid/414/Default.aspx.
3. National Committee for Quality Assurance. HEDIS measures. Accessed 11 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
4. National Committee for Quality Assurance. About NCQA: overview. Accessed 14 Mar 2016 at www.ncqa.org/AboutNCQA.aspx.
5. National Committee for Quality Assurance. Health care program evaluations. Accessed 11 Mar 2016 at www.ncqa.org/Programs.aspx.
6. National Committee for Quality Assurance. HEDIS and Performance Measurement. Accessed 14 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement.aspx.
7. National Committee for Quality Assurance. HEDIS 2015 technical specifications for ACO measurement. Washington, DC: National Committee for Quality Assurance; 2014.
8. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (> 9.0%). NQF identifier: 0059. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
9. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) control (< 8.0%). NQF identifier: 0575. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
10. National Quality Forum. Comprehensive diabetes care: blood pressure control (< 140/90 mm Hg). NQF identifier: 0061. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
11. Onglyza(saxagliptin). Full prescribing information. AstraZeneca, Wilmington, DE; 2014.
12. Farxiga (dapagliflozin). Full prescribing information. AstraZeneca, Wilmington, DE; March 2015.
13. Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009;26:488–99.
14. Kasichayanula S, Liu X, Lacreta F, et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014;53:17–27.
15. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2014;38:376–83.
16. Bailey CJ. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. British Journal of Diabetes & Vascular Disease 2010;10:193-9.
17. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53.
18. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9.
19. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
20. National Quality Forum. Comprehensive diabetes care (composite). NQF identifier: #0731. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
21. National Quality Forum. Optimal diabetes care (composite measure). NQF identifier: 0729. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206.
23. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25.
24. Invokana(canagliflzoin). Full prescribing information. Janssen Pharmaceuticals, Titusville, NJ; 2013.
25. Jardiance(empagliflozin). Full prescribing information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly, Ingelheim, Germany and Indianapolis, IN; 2014.
26. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100.
27. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016;39:S1–S119.
28. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32:S151–S6.
29. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 executive summary. Endocr Pract 2016;22:84–113.
30. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
31. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
32. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007;30:890–5.
33. National Quality Forum. Glycemic control: hypoglycemia. NQF identifier: 2363. Accessed 29 Oct 2015 at www.qualityforum.org/QPS/QPSTool.aspx.
34. National Quality Forum. Glycemic control: hyperglycemia. NQF identifier: 2362. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
35. Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009;11:527–33.
36. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508.
37. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.
38. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 2014;20:1187–97.
1. National Quality Forum. ABCs of measurement. Accessed 11 Mar 2016 at www.qualityforum.org/Measuring_Performance/ABCs_of_Measurement.aspx.
2. National Committee for Quality Assurance. HEDIS measure development process. Accessed 14 Mar 2016 at www.ncqa.org/tabid/414/Default.aspx.
3. National Committee for Quality Assurance. HEDIS measures. Accessed 11 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
4. National Committee for Quality Assurance. About NCQA: overview. Accessed 14 Mar 2016 at www.ncqa.org/AboutNCQA.aspx.
5. National Committee for Quality Assurance. Health care program evaluations. Accessed 11 Mar 2016 at www.ncqa.org/Programs.aspx.
6. National Committee for Quality Assurance. HEDIS and Performance Measurement. Accessed 14 Mar 2016 at www.ncqa.org/HEDISQualityMeasurement.aspx.
7. National Committee for Quality Assurance. HEDIS 2015 technical specifications for ACO measurement. Washington, DC: National Committee for Quality Assurance; 2014.
8. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) poor control (> 9.0%). NQF identifier: 0059. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
9. National Quality Forum. Comprehensive diabetes care: hemoglobin A1c (HbA1c) control (< 8.0%). NQF identifier: 0575. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
10. National Quality Forum. Comprehensive diabetes care: blood pressure control (< 140/90 mm Hg). NQF identifier: 0061. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
11. Onglyza(saxagliptin). Full prescribing information. AstraZeneca, Wilmington, DE; 2014.
12. Farxiga (dapagliflozin). Full prescribing information. AstraZeneca, Wilmington, DE; March 2015.
13. Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009;26:488–99.
14. Kasichayanula S, Liu X, Lacreta F, et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 2014;53:17–27.
15. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2014;38:376–83.
16. Bailey CJ. SGLT2 inhibitors: glucuretic treatment for type 2 diabetes. British Journal of Diabetes & Vascular Disease 2010;10:193-9.
17. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53.
18. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9.
19. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
20. National Quality Forum. Comprehensive diabetes care (composite). NQF identifier: #0731. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
21. National Quality Forum. Optimal diabetes care (composite measure). NQF identifier: 0729. Accessed 14 Mar 2016 at www.qualityforum.org/ProjectMeasures.aspx?projectID=73652.
22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206.
23. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14–25.
24. Invokana(canagliflzoin). Full prescribing information. Janssen Pharmaceuticals, Titusville, NJ; 2013.
25. Jardiance(empagliflozin). Full prescribing information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly, Ingelheim, Germany and Indianapolis, IN; 2014.
26. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100.
27. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016;39:S1–S119.
28. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32:S151–S6.
29. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 executive summary. Endocr Pract 2016;22:84–113.
30. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
31. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
32. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007;30:890–5.
33. National Quality Forum. Glycemic control: hypoglycemia. NQF identifier: 2363. Accessed 29 Oct 2015 at www.qualityforum.org/QPS/QPSTool.aspx.
34. National Quality Forum. Glycemic control: hyperglycemia. NQF identifier: 2362. Accessed 11 Mar 2016 at www.qualityforum.org/QPS/QPSTool.aspx.
35. Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009;11:527–33.
36. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508.
37. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.
38. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 2014;20:1187–97.
Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus
Data from the Centers for Disease Control and Prevention indicate that almost 24 million Americans, or 7.8% of the population, have diabetes; 90% to 95% of these have type 2 diabetes mellitus (T2DM).1 Diabetes and excessive weight often coexist. An analysis of data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) showed that among individuals with diabetes, 85% were overweight or obese and 55% were obese.2
Gaps remain in the management of T2DM between the goals for clinical parameters of care (eg, control of glucose, blood pressure [BP], and lipids) and actual clinical practice.3 NHANES data reveal that glycemic control improved from a mean glycosylated hemoglobin A1c (HbA1c) of 7.82% in 1999–2000 to 7.18% in 2003–2004.4 Hazard models based on the United Kingdom Prospective Diabetes Study (UKPDS) 10-year outcomes data in 4,320 newly diagnosed T2DM patients suggest that a sustained decrease in HbA1c of 0.511 percentage points could reduce diabetes complications by 10.7%.4,5
Additional analysis of NHANES data showed that in 2003–2004, about 57% of individuals achieved glycemic control, 48% reached BP targets, and 50% achieved target cholesterol goals.Only about 13% of diabetes patients achieved their target goals for all three parameters concurrently.6
This article reviews the association between cardiometabolic risk and the current antihyperglycemic treatments for patients with T2DM, with a focus on the role of incretin-related therapies.
THE IMPORTANCE OF CARDIOMETABOLIC RISK IN T2DM
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among people with diabetes and is the reported cause of mortality in up to 65% of deaths in persons with diabetes in the United States.7 The risk of CVD is two- to fourfold greater among adults with diabetes than among adults who do not have diabetes.8 The risk of CVD in patients with T2DM was evident in the UKPDS 17, where macrovascular complications, including CVD, were about twice as common as microvascular complications (20% vs 9%) after 9 years of follow-up.9 A study that involved more than 44,000 patients showed an almost double rate of mortality from all causes among individuals with T2DM compared with those with no diabetes (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.97).10 Current guidelines recommend aggressive management of CV risk factors, including BP control, correction of atherogenic dyslipidemia, glycemic control, weight reduction for those who are overweight or obese, and smoking cessation for those who smoke.3,11 Lifestyle interventions, including weight reduction and appropriately prescribed physical activity, result in reduced CV risk factors, which can help slow the progression of T2DM.12
GOALS OF T2DM THERAPY
Several studies have demonstrated that glycemic control can delay or prevent the development and progression of microvascular complications.13,14 UKPDS 33 showed that more intensive blood glucose control (median HbA1c 7.0%) in patients with T2DM followed over 10 years significantly (P = .029) reduced the risk for any diabetes-related end point by 12% compared with conventional therapy (median HbA1c 7.9%). Most of the risk reduction was accounted for by a 25% risk reduction in microvascular end points (P = .0099).13 Another report (UKPDS 35) demonstrated that HbA1c was strongly related to microvascular effects, with a 1% reduction in HbA1c associated with a 37% reduction in microvascular complications.14
Does intensive glucose control reduce CV risk?
To resolve the ongoing question of whether intensive glucose control can lead to a reduction in CV risk in patients with T2DM, three large, long-term trials were conducted within the last decade.15–18 Two of these, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, each enrolled more than 10,000 previously treated patients with long-standing T2DM. Patients were randomized to standard or intensive glycemic control for 3.5 years in the ACCORD trial and for 5 years in the ADVANCE trial.15,16
The ACCORD and ADVANCE trials, along with the smaller Veterans Administration Diabetes Trial (VADT) (N = 1,791), failed to show that more intensive glycemic control significantly reduced CVD.15–17 Additionally, the glycemic control component of ACCORD was halted because of increased mortality in the intensive arm compared with the standard arm.15 Further analyses of ACCORD data presented at the 69th Scientific Sessions of the American Diabetes Association (ADA) showed that HbA1c values lower than 7.0% did not explain the increased mortality. The 20% higher risk of death for every 1.0% increase in HbA1c greater than 6.0% suggests that glucose concentrations even lower than the general HbA1c goal of less than 7.0% may be appropriate in some patients.18 The most recent finding from VADT was that CV risk was dependent on disease duration and presence of comorbidities. Intensive therapy seemed to work best in patients with diabetes of less than 15 years’ duration, while risk of a CV event was more than doubled with intensive therapy in patients having diabetes for more than 21 years.
Clarification of treatment goals
A position statement of the ADA and a scientific statement of the American College of Cardiology Foundation and the American Heart Association19 concluded that the “evidence obtained from ACCORD, ADVANCE, and VADT does not suggest the need for major changes in glycemic control targets but, rather, additional clarification of the language that has consistently stressed individualization.” They state that while the general HbA1c goal of less than 7.0% seems reasonable, even lower HbA1c goals may be appropriate for some patients if they can be achieved without significant hypoglycemia or other adverse effects. Such patients might include those with diabetes of short duration, long life expectancy, or no significant CVD or hypoglycemia. Conversely, higher HbA1c goals may be appropriate for patients with limited life expectancy, a history of severe hypoglycemia, established microvascular or macrovascular complications, significant other comorbid conditions, or longstanding diabetes in whom an HbA1c of less than 7.0% has been difficult to attain despite optimal treatment and diabetes self-management education.19
Long-term risk reduction
A 10-year, postinterventional follow-up study (UKPDS 80) of the UKPDS survivor cohort was reported recently.20 Results showed that despite an early loss of glycemic differences between patients treated with diet and those treated with intensive regimens (sulfonylurea or insulin; metformin in overweight patients), the pharmacotherapy group demonstrated a prolonged reduction in microvascular risk as well as a significant reduction in the risk for myocardial infarction (15% [P = .01] in the sulfonylurea-insulin group and 33% [P = .005] in the metformin group) and death from any cause.20 This suggests that early improvement in glycemic control is associated with long-term benefits in the micro- and macrovascular health of patients with T2DM.
Additionally, the recent long-term follow-up of the Steno-2 study21 showed that a multifactorial intervention striving for intensive glucose, BP, and lipid control that included the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents not only reduced the risk of nonfatal CVD among patients with T2DM and microalbuminuria, but also had sustained beneficial effects on vascular complications and on rates of death from any cause and from CV causes. From a health care payer perspective, intensive multifactorial intervention was more likely to be cost-effective than conventional treatment in Denmark, especially if applied in a primary care setting.22
Comprehensive care needed
The lower-than-expected rates of CV outcomes in the ACCORD, ADVANCE, VADT, and Steno-2 studies reinforce the importance of comprehensive diabetes care that treats not only hyperglycemia but also elevated BP and dyslipidemia; these are considered the “ABCs” of diabetes.11,19 The 2009 ADA standards of medical care guidelines recommend that for most T2DM patients, HbA1c should be maintained at less than 7.0%,3 while the American Association of Clinical Endocrinologists (AACE) 2007 guidelines state that HbA1c should be 6.5% or less.11 Both organizations stress the importance of individualized goals, as discussed above, and advocate BP goals of less than 130/80 mm Hg and dyslipidemia goals of low-density lipoprotein cholesterol (LDL-C) less than 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) greater than 40 mg/dL for men and 50 mg/dL for women, and triglycerides less than 150 mg/dL. It is recommended that an optional LDL-C goal of less than 70 mg/dL be considered for individuals with overt CVD.
CURRENT ANTIHYPERGLYCEMIC TREATMENT STRATEGIES
In response to new insights from clinical research and emerging treatment strategies, disease-specific organizations and medical specialty societies regularly revise and update their treatment guidelines and algorithms. These resources recommend that glycemic progress should be regularly monitored and pharmacologic therapy titrated or new drugs added promptly if glycemic goals are not met after 2 to 3 months.
Several algorithms combine scientific evidence with expert clinical opinion to guide physicians in treating their patients with T2DM. The American College of Endocrinology (ACE)/AACE road maps are designed to help develop individualized treatment regimens to achieve an HbA1c of 6.5% or less.23 The algorithm from a writing group assembled by the ADA and the European Association for the Study of Diabetes (EASD) similarly promotes pharmacologic treatment together with lifestyle modifications to maintain a glycemic goal of HbA1c less than 7.0%.24
OVERVIEW OF ANTIHYPERGLYCEMIC TREATMENT APPROACHES
Initial oral therapy
T2DM is usually treated initially with a single oral agent. Consistent with the progressive nature of the disease, patients often eventually require one or more additional oral agents and in many cases insulin.13,27 Choice of specific agents is based on individual patient circumstances, including the need for weight loss and control of fasting versus postprandial glucose, the presence of dyslipidemia and hypertension, and the risk for and potential consequences of hypoglycemia.24 T2DM patients with severely uncontrolled and symptomatic hyperglycemia are best treated, at least initially, with a combination of insulin therapy and lifestyle intervention, often with metformin.
Metformin. The recently revised ADA/EASD writing group algorithm recommends that patients not requiring initial insulin begin treatment with metformin at the time of diagnosis unless there are contraindications.24 Metformin is not associated with hypoglycemia and is considered weight-neutral, although some patients may lose weight.28
Sulfonylureas. Sulfonylureas stimulate insulin secretion from pancreatic beta cells; their use may be associated with hypoglycemia and weight gain. Mechanisms for weight gain with sulfonylureas include reduction of glucosuria and increased caloric intake to prevent or treat hypoglycemia.11,28 Nateglinide and repaglinide are nonsulfonylurea oral insulin secretagogues. They result in rapid and relatively short-lived insulin responses and are usually administered three times a day, before each meal. Their use may be associated with weight gain and hypoglycemia.11
Thiazolidinediones. Thiazolidinediones (TZD) increase insulin sensitivity in muscle, adipose tissue, and the liver. Hypoglycemia is uncommon with TZD monotherapy but weight gain related to increased and redistributed adiposity and fluid retention frequently occurs.
Alpha-glucosidase inhibitors. The alpha-glucosidase inhibitors are administered before meals and primarily reduce postprandial hyperglycemia. They are generally weight-neutral.28
Insulin. Insulin and insulin analogues are the most effective antihyperglycemic agents, but their use can be associated with hypoglycemia and clinically significant weight gain.28
Colesevelam. Colesevelam is a bile acid sequestrant that was recently approved by the US Food and Drug Administration as an antihyperglycemic therapy in people with T2DM. At a dosage of 1.875 g BID or 3.75 g QD in combination with a sulfonylurea, metformin, or insulin therapy, reductions in HbA1c compared with placebo in clinical trials of colesevelam have ranged from –0.5% to –0.7% (P < .02). Frequency of hypoglycemia and weight gain is low with this agent.26
Weight management. Weight reduction is important for overweight or obese patients with T2DM.27,28 Even moderate weight loss (5% of body weight) can be associated with improved insulin action and reduced hyperglycemia.29 Conversely, weight gain has been shown to worsen hyperglycemia and other CV risk factors. Treatment-related weight gain can also lead to decreased regimen adherence, contributing to poor glycemic control.28
THE ROLE OF INCRETIN HORMONES AND INCRETIN-BASED THERAPIES IN T2DM PATIENTS
Over the last few years, the role of incretin hormones and their contribution to diabetes pathophysiology has become more apparent. The incretin effect refers to the observation that orally administered glucose elicits a greater insulin response than does glucose administered intravenously to produce equivalent blood glucose concentrations.30,31 The incretin effect is diminished in patients with T2DM.
Hormone mediation of the incretin effect
The two hormones that mediate the incretin effect are GIP (also known as gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide) and glucagon-like peptide−1 (GLP-1).30,31 GLP-1 has several glucoregulatory actions, including enhancement of endogenous insulin release and suppression of inappropriate glucagon secretion, both in a glucose-dependent manner. Therefore, these effects of GLP-1 occur only when glucose concentrations are elevated, thereby minimizing the risk of hypoglycemia. GLP-1 also regulates gastric emptying; infusions of GLP-1 can slow the accelerated emptying that is often present in T2DM patients. GLP-1 also increases satiety and decreases food intake via a central mechanism.31
Because GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase–4 (DPP-4), therapeutic use of GLP-1 would require continuous infusion, which is impractical.30,31 Two strategies have been used to produce incretin-related therapies. One, inhibition of the DPP-4 enzyme, results in a two- to threefold enhancement of endogenous GLP-1. The other, involving agents that resist breakdown by DPP-4 but bind to and activate the GLP-1 receptor, produces glucoregulatory effects similar to those of GLP-1.30
Following subcutaneous (SC) injection, GLP-1 receptor agonists enhance insulin secretion and suppress inappropriately elevated glucagon, both in a glucose-dependent manner, as well as slow gastric emptying and enhance satiety.30 DPP-4 inhibitors provide glucose-dependent enhanced insulin secretion and glucagon suppression, but they do not have the same effects on gastric emptying or satiety.
Clinically, the GLP-1 receptor agonists improve glycemia and are associated with weight loss.32–35 Adverse gastrointestinal symptoms are relatively common during the first few weeks of treatment. DPP-4 inhibitors improve glycemia but are weight-neutral and are not generally associated with significant gastrointestinal symptoms.32,36–38
Incretin-based therapies
Incretin-based therapies are currently part of the antihyperglycemic armamentarium.25,32 The AACE guidelines11 and the ACE/AACE roadmaps23 include the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin among antihyperglycemic therapies for patients with T2DM. The most recent update of the consensus algorithm statement of a joint ADA/EASD writing group included GLP-1 receptor agonists (but not DPP-4 inhibitors) in tier 2 of preferred agents, especially for patients who have concerns related to weight and hypoglycemia.24 They noted that DPP-4 inhibitors may be appropriate choices in selected patients.
DPP-4 inhibitors: sitagliptin, saxagliptin. Until recently, sitagliptin was the only DPP-4 inhibitor available in the United States. Sitagliptin is approved by the FDA for treatment of T2DM at a recommended oral dosage of 100 mg QD, either as monotherapy or in combination with other oral antihyperglycemic medications. The dosage of sitagliptin should be reduced to 50 mg/day in patients with creatinine clearance (CrCl) levels that are between 30 mL/min and 50 mL/min and to 25 mg/day in those with CrCl less than 30 mL/min.39
In a meta-analysis of incretin-based therapies, DPP-4 inhibitors produced a reduction in HbA1c compared with placebo (weighted mean difference of –0.74%; 95% confidence interval, –0.85% to –0.62%).32 DPP-4 inhibitor antihyperglycemic efficacy has been shown to be similar whether used as a monotherapy or add-on therapy.32,37,38 This same meta-analysis showed DPP-4 inhibitors as having a neutral effect on weight.32 More recently, a single-pill combination of metformin and sitagliptin was approved.40
A study comparing metformin, sitagliptin, and the combination of the two as initial monotherapy in T2DM patients with a baseline HbA1c of 8.8% showed 24-week HbA1c reductions from baseline of –0.66% with sitagliptin 100 mg QD, –0.82% with metformin 500 mg BID, and –1.90% with sitagliptin 50 mg + metformin 1,000 mg BID.41
On July 31, 2009, the FDA approved another DPP-4 inhibitor, saxagliptin, for the treatment of T2DM either as monotherapy or in combination with metformin, a sulfonylurea, or a TZD.42
GLP-1 receptor agonist: exenatide. Exenatide, the only FDA-approved GLP-1 receptor agonist, is the synthetic version of exendin-4, which binds to the human GLP-1 receptor and in vitro possesses many of the glucoregulatory effects of endogenous GLP-1.30,32 Exenatide is indicated as monotherapy or adjunctive therapy for patients with T2DM who have not achieved adequate glycemic control with metformin, a sulfonylurea, a TZD, or metformin in combination with a sulfonylurea or a TZD.43 Exenatide is administered by SC injection BID at a starting dosage of 5 mg BID for 4 weeks, followed by an increase to 10 mg BID.
Exenatide has been shown not only to enhance glucose-dependent insulin secretion but also to restore impaired first-phase insulin response in subjects with T2DM. Exenatide also helps control postprandial glycemic excursions by suppressing inappropriate glucagon secretion, slowing accelerated gastric emptying, and enhancing satiety. The increased satiety results in decreased food intake and weight loss.31,44 In a recent head-to-head crossover study, exenatide was shown to be more effective than sitagliptin in lowering postprandial glucose concentrations, increasing insulin secretion, and reducing postprandial glucagon secretion.45 Exenatide also slowed gastric emptying and reduced caloric intake.
Exenatide, in most studies, resulted in a placebo-subtracted HbA1c reduction of approximately –1.0% and in one study lowered HbA1c from baseline by –1.5%. Completer analyses have shown HbA1c reductions of –1.0% up to 3 years and –0.8% up to 3.5 years. Exenatide has also been associated with a mean weight loss of as much as –3.6 kg at 30 weeks and as much as –5.3 kg at 3.5 years.33–35,46,47 A 1-year study showed that exenatide improved beta-cell secretory function compared with insulin glargine in metformin-treated patients with T2DM.48 Long-term data, including findings from completed and intention-to-treat analyses of 82 weeks49 to at least 3 years47 have demonstrated that exenatide improved CV risk factors, including those related to BP, lipids, and hepatic injury biomarkers.
Therapies in development
Incretin-based therapies in development include a novel once-weekly formulation of exenatide; taspoglutide, another once-weekly GLP-1 receptor agonist; and liraglutide, a GLP-1 receptor agonist that is administered once daily.50 Liraglutide is currently being evaluated in clinical trials as a once-daily SC injection.51–53 Liraglutide has been reported to reduce HbA1c by –1.1% at 26 weeks and up to –1.14% at 52 weeks and result in weight loss (up to –2.8 kg at 26 weeks and up to –2.5 kg at 52 weeks) in patients with T2DM who are treatment-naïve or taking other antidiabetes agents, including metformin, sulfonylurea, and TZD.51–53 Evaluation of the once-weekly formulation of exenatide showed reductions in HbA1c of –1.9% at 30 weeks and –2.0% at 52 weeks with a weight loss of –3.7 kg at 30 weeks and –4.1 kg over 52 weeks of treatment.46,54
CONCLUSION
In the United States, the epidemics of excessive weight and T2DM have contributed to an increased medical risk for many individuals. Comprehensive diabetes treatments targeting not only hyperglycemia but also frequently associated overweight/obesity, hypertension, and dyslipidemia will be required to reduce such risk. Current treatment strategies have evolved based on updated clinical guidelines and trials, as well as practice experience, including those related to newer agents. Incretin-based therapies, such as the GLP-1 receptor agonist, exenatide, and the DPP-4 inhibitors, sitagliptin and saxagliptin, are important additions to the treatment armamentarium, offering a reduction in hyperglycemia and beneficial effects on weight (reduction with exenatide and neutral with sitagliptin), and have been shown to improve several CV risk factors.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, 2008. Available at: http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86.
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006; 49:1761–1769.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004; 140:945–950.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in noninsulin-dependent diabetes mellitus. Ann Intern Med 1996; 124(1 Pt 2):136–145.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Kerr M. ADA 2009: intensive glycemic control not directly linked to excess cardiovascular risk. Medscape Medical News Web site. http://www.medscape.com/viewarticle/704260_print. Published June 11, 2009. Accessed September 16, 2009.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31:1510–1515.
- ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract 2007; 13:260–268.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31:245–259.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131:281–303.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632−2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Januvia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2048–2054.
- Janumet. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2041–2048.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281:E155–E161.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
Data from the Centers for Disease Control and Prevention indicate that almost 24 million Americans, or 7.8% of the population, have diabetes; 90% to 95% of these have type 2 diabetes mellitus (T2DM).1 Diabetes and excessive weight often coexist. An analysis of data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) showed that among individuals with diabetes, 85% were overweight or obese and 55% were obese.2
Gaps remain in the management of T2DM between the goals for clinical parameters of care (eg, control of glucose, blood pressure [BP], and lipids) and actual clinical practice.3 NHANES data reveal that glycemic control improved from a mean glycosylated hemoglobin A1c (HbA1c) of 7.82% in 1999–2000 to 7.18% in 2003–2004.4 Hazard models based on the United Kingdom Prospective Diabetes Study (UKPDS) 10-year outcomes data in 4,320 newly diagnosed T2DM patients suggest that a sustained decrease in HbA1c of 0.511 percentage points could reduce diabetes complications by 10.7%.4,5
Additional analysis of NHANES data showed that in 2003–2004, about 57% of individuals achieved glycemic control, 48% reached BP targets, and 50% achieved target cholesterol goals.Only about 13% of diabetes patients achieved their target goals for all three parameters concurrently.6
This article reviews the association between cardiometabolic risk and the current antihyperglycemic treatments for patients with T2DM, with a focus on the role of incretin-related therapies.
THE IMPORTANCE OF CARDIOMETABOLIC RISK IN T2DM
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among people with diabetes and is the reported cause of mortality in up to 65% of deaths in persons with diabetes in the United States.7 The risk of CVD is two- to fourfold greater among adults with diabetes than among adults who do not have diabetes.8 The risk of CVD in patients with T2DM was evident in the UKPDS 17, where macrovascular complications, including CVD, were about twice as common as microvascular complications (20% vs 9%) after 9 years of follow-up.9 A study that involved more than 44,000 patients showed an almost double rate of mortality from all causes among individuals with T2DM compared with those with no diabetes (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.97).10 Current guidelines recommend aggressive management of CV risk factors, including BP control, correction of atherogenic dyslipidemia, glycemic control, weight reduction for those who are overweight or obese, and smoking cessation for those who smoke.3,11 Lifestyle interventions, including weight reduction and appropriately prescribed physical activity, result in reduced CV risk factors, which can help slow the progression of T2DM.12
GOALS OF T2DM THERAPY
Several studies have demonstrated that glycemic control can delay or prevent the development and progression of microvascular complications.13,14 UKPDS 33 showed that more intensive blood glucose control (median HbA1c 7.0%) in patients with T2DM followed over 10 years significantly (P = .029) reduced the risk for any diabetes-related end point by 12% compared with conventional therapy (median HbA1c 7.9%). Most of the risk reduction was accounted for by a 25% risk reduction in microvascular end points (P = .0099).13 Another report (UKPDS 35) demonstrated that HbA1c was strongly related to microvascular effects, with a 1% reduction in HbA1c associated with a 37% reduction in microvascular complications.14
Does intensive glucose control reduce CV risk?
To resolve the ongoing question of whether intensive glucose control can lead to a reduction in CV risk in patients with T2DM, three large, long-term trials were conducted within the last decade.15–18 Two of these, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, each enrolled more than 10,000 previously treated patients with long-standing T2DM. Patients were randomized to standard or intensive glycemic control for 3.5 years in the ACCORD trial and for 5 years in the ADVANCE trial.15,16
The ACCORD and ADVANCE trials, along with the smaller Veterans Administration Diabetes Trial (VADT) (N = 1,791), failed to show that more intensive glycemic control significantly reduced CVD.15–17 Additionally, the glycemic control component of ACCORD was halted because of increased mortality in the intensive arm compared with the standard arm.15 Further analyses of ACCORD data presented at the 69th Scientific Sessions of the American Diabetes Association (ADA) showed that HbA1c values lower than 7.0% did not explain the increased mortality. The 20% higher risk of death for every 1.0% increase in HbA1c greater than 6.0% suggests that glucose concentrations even lower than the general HbA1c goal of less than 7.0% may be appropriate in some patients.18 The most recent finding from VADT was that CV risk was dependent on disease duration and presence of comorbidities. Intensive therapy seemed to work best in patients with diabetes of less than 15 years’ duration, while risk of a CV event was more than doubled with intensive therapy in patients having diabetes for more than 21 years.
Clarification of treatment goals
A position statement of the ADA and a scientific statement of the American College of Cardiology Foundation and the American Heart Association19 concluded that the “evidence obtained from ACCORD, ADVANCE, and VADT does not suggest the need for major changes in glycemic control targets but, rather, additional clarification of the language that has consistently stressed individualization.” They state that while the general HbA1c goal of less than 7.0% seems reasonable, even lower HbA1c goals may be appropriate for some patients if they can be achieved without significant hypoglycemia or other adverse effects. Such patients might include those with diabetes of short duration, long life expectancy, or no significant CVD or hypoglycemia. Conversely, higher HbA1c goals may be appropriate for patients with limited life expectancy, a history of severe hypoglycemia, established microvascular or macrovascular complications, significant other comorbid conditions, or longstanding diabetes in whom an HbA1c of less than 7.0% has been difficult to attain despite optimal treatment and diabetes self-management education.19
Long-term risk reduction
A 10-year, postinterventional follow-up study (UKPDS 80) of the UKPDS survivor cohort was reported recently.20 Results showed that despite an early loss of glycemic differences between patients treated with diet and those treated with intensive regimens (sulfonylurea or insulin; metformin in overweight patients), the pharmacotherapy group demonstrated a prolonged reduction in microvascular risk as well as a significant reduction in the risk for myocardial infarction (15% [P = .01] in the sulfonylurea-insulin group and 33% [P = .005] in the metformin group) and death from any cause.20 This suggests that early improvement in glycemic control is associated with long-term benefits in the micro- and macrovascular health of patients with T2DM.
Additionally, the recent long-term follow-up of the Steno-2 study21 showed that a multifactorial intervention striving for intensive glucose, BP, and lipid control that included the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents not only reduced the risk of nonfatal CVD among patients with T2DM and microalbuminuria, but also had sustained beneficial effects on vascular complications and on rates of death from any cause and from CV causes. From a health care payer perspective, intensive multifactorial intervention was more likely to be cost-effective than conventional treatment in Denmark, especially if applied in a primary care setting.22
Comprehensive care needed
The lower-than-expected rates of CV outcomes in the ACCORD, ADVANCE, VADT, and Steno-2 studies reinforce the importance of comprehensive diabetes care that treats not only hyperglycemia but also elevated BP and dyslipidemia; these are considered the “ABCs” of diabetes.11,19 The 2009 ADA standards of medical care guidelines recommend that for most T2DM patients, HbA1c should be maintained at less than 7.0%,3 while the American Association of Clinical Endocrinologists (AACE) 2007 guidelines state that HbA1c should be 6.5% or less.11 Both organizations stress the importance of individualized goals, as discussed above, and advocate BP goals of less than 130/80 mm Hg and dyslipidemia goals of low-density lipoprotein cholesterol (LDL-C) less than 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) greater than 40 mg/dL for men and 50 mg/dL for women, and triglycerides less than 150 mg/dL. It is recommended that an optional LDL-C goal of less than 70 mg/dL be considered for individuals with overt CVD.
CURRENT ANTIHYPERGLYCEMIC TREATMENT STRATEGIES
In response to new insights from clinical research and emerging treatment strategies, disease-specific organizations and medical specialty societies regularly revise and update their treatment guidelines and algorithms. These resources recommend that glycemic progress should be regularly monitored and pharmacologic therapy titrated or new drugs added promptly if glycemic goals are not met after 2 to 3 months.
Several algorithms combine scientific evidence with expert clinical opinion to guide physicians in treating their patients with T2DM. The American College of Endocrinology (ACE)/AACE road maps are designed to help develop individualized treatment regimens to achieve an HbA1c of 6.5% or less.23 The algorithm from a writing group assembled by the ADA and the European Association for the Study of Diabetes (EASD) similarly promotes pharmacologic treatment together with lifestyle modifications to maintain a glycemic goal of HbA1c less than 7.0%.24
OVERVIEW OF ANTIHYPERGLYCEMIC TREATMENT APPROACHES
Initial oral therapy
T2DM is usually treated initially with a single oral agent. Consistent with the progressive nature of the disease, patients often eventually require one or more additional oral agents and in many cases insulin.13,27 Choice of specific agents is based on individual patient circumstances, including the need for weight loss and control of fasting versus postprandial glucose, the presence of dyslipidemia and hypertension, and the risk for and potential consequences of hypoglycemia.24 T2DM patients with severely uncontrolled and symptomatic hyperglycemia are best treated, at least initially, with a combination of insulin therapy and lifestyle intervention, often with metformin.
Metformin. The recently revised ADA/EASD writing group algorithm recommends that patients not requiring initial insulin begin treatment with metformin at the time of diagnosis unless there are contraindications.24 Metformin is not associated with hypoglycemia and is considered weight-neutral, although some patients may lose weight.28
Sulfonylureas. Sulfonylureas stimulate insulin secretion from pancreatic beta cells; their use may be associated with hypoglycemia and weight gain. Mechanisms for weight gain with sulfonylureas include reduction of glucosuria and increased caloric intake to prevent or treat hypoglycemia.11,28 Nateglinide and repaglinide are nonsulfonylurea oral insulin secretagogues. They result in rapid and relatively short-lived insulin responses and are usually administered three times a day, before each meal. Their use may be associated with weight gain and hypoglycemia.11
Thiazolidinediones. Thiazolidinediones (TZD) increase insulin sensitivity in muscle, adipose tissue, and the liver. Hypoglycemia is uncommon with TZD monotherapy but weight gain related to increased and redistributed adiposity and fluid retention frequently occurs.
Alpha-glucosidase inhibitors. The alpha-glucosidase inhibitors are administered before meals and primarily reduce postprandial hyperglycemia. They are generally weight-neutral.28
Insulin. Insulin and insulin analogues are the most effective antihyperglycemic agents, but their use can be associated with hypoglycemia and clinically significant weight gain.28
Colesevelam. Colesevelam is a bile acid sequestrant that was recently approved by the US Food and Drug Administration as an antihyperglycemic therapy in people with T2DM. At a dosage of 1.875 g BID or 3.75 g QD in combination with a sulfonylurea, metformin, or insulin therapy, reductions in HbA1c compared with placebo in clinical trials of colesevelam have ranged from –0.5% to –0.7% (P < .02). Frequency of hypoglycemia and weight gain is low with this agent.26
Weight management. Weight reduction is important for overweight or obese patients with T2DM.27,28 Even moderate weight loss (5% of body weight) can be associated with improved insulin action and reduced hyperglycemia.29 Conversely, weight gain has been shown to worsen hyperglycemia and other CV risk factors. Treatment-related weight gain can also lead to decreased regimen adherence, contributing to poor glycemic control.28
THE ROLE OF INCRETIN HORMONES AND INCRETIN-BASED THERAPIES IN T2DM PATIENTS
Over the last few years, the role of incretin hormones and their contribution to diabetes pathophysiology has become more apparent. The incretin effect refers to the observation that orally administered glucose elicits a greater insulin response than does glucose administered intravenously to produce equivalent blood glucose concentrations.30,31 The incretin effect is diminished in patients with T2DM.
Hormone mediation of the incretin effect
The two hormones that mediate the incretin effect are GIP (also known as gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide) and glucagon-like peptide−1 (GLP-1).30,31 GLP-1 has several glucoregulatory actions, including enhancement of endogenous insulin release and suppression of inappropriate glucagon secretion, both in a glucose-dependent manner. Therefore, these effects of GLP-1 occur only when glucose concentrations are elevated, thereby minimizing the risk of hypoglycemia. GLP-1 also regulates gastric emptying; infusions of GLP-1 can slow the accelerated emptying that is often present in T2DM patients. GLP-1 also increases satiety and decreases food intake via a central mechanism.31
Because GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase–4 (DPP-4), therapeutic use of GLP-1 would require continuous infusion, which is impractical.30,31 Two strategies have been used to produce incretin-related therapies. One, inhibition of the DPP-4 enzyme, results in a two- to threefold enhancement of endogenous GLP-1. The other, involving agents that resist breakdown by DPP-4 but bind to and activate the GLP-1 receptor, produces glucoregulatory effects similar to those of GLP-1.30
Following subcutaneous (SC) injection, GLP-1 receptor agonists enhance insulin secretion and suppress inappropriately elevated glucagon, both in a glucose-dependent manner, as well as slow gastric emptying and enhance satiety.30 DPP-4 inhibitors provide glucose-dependent enhanced insulin secretion and glucagon suppression, but they do not have the same effects on gastric emptying or satiety.
Clinically, the GLP-1 receptor agonists improve glycemia and are associated with weight loss.32–35 Adverse gastrointestinal symptoms are relatively common during the first few weeks of treatment. DPP-4 inhibitors improve glycemia but are weight-neutral and are not generally associated with significant gastrointestinal symptoms.32,36–38
Incretin-based therapies
Incretin-based therapies are currently part of the antihyperglycemic armamentarium.25,32 The AACE guidelines11 and the ACE/AACE roadmaps23 include the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin among antihyperglycemic therapies for patients with T2DM. The most recent update of the consensus algorithm statement of a joint ADA/EASD writing group included GLP-1 receptor agonists (but not DPP-4 inhibitors) in tier 2 of preferred agents, especially for patients who have concerns related to weight and hypoglycemia.24 They noted that DPP-4 inhibitors may be appropriate choices in selected patients.
DPP-4 inhibitors: sitagliptin, saxagliptin. Until recently, sitagliptin was the only DPP-4 inhibitor available in the United States. Sitagliptin is approved by the FDA for treatment of T2DM at a recommended oral dosage of 100 mg QD, either as monotherapy or in combination with other oral antihyperglycemic medications. The dosage of sitagliptin should be reduced to 50 mg/day in patients with creatinine clearance (CrCl) levels that are between 30 mL/min and 50 mL/min and to 25 mg/day in those with CrCl less than 30 mL/min.39
In a meta-analysis of incretin-based therapies, DPP-4 inhibitors produced a reduction in HbA1c compared with placebo (weighted mean difference of –0.74%; 95% confidence interval, –0.85% to –0.62%).32 DPP-4 inhibitor antihyperglycemic efficacy has been shown to be similar whether used as a monotherapy or add-on therapy.32,37,38 This same meta-analysis showed DPP-4 inhibitors as having a neutral effect on weight.32 More recently, a single-pill combination of metformin and sitagliptin was approved.40
A study comparing metformin, sitagliptin, and the combination of the two as initial monotherapy in T2DM patients with a baseline HbA1c of 8.8% showed 24-week HbA1c reductions from baseline of –0.66% with sitagliptin 100 mg QD, –0.82% with metformin 500 mg BID, and –1.90% with sitagliptin 50 mg + metformin 1,000 mg BID.41
On July 31, 2009, the FDA approved another DPP-4 inhibitor, saxagliptin, for the treatment of T2DM either as monotherapy or in combination with metformin, a sulfonylurea, or a TZD.42
GLP-1 receptor agonist: exenatide. Exenatide, the only FDA-approved GLP-1 receptor agonist, is the synthetic version of exendin-4, which binds to the human GLP-1 receptor and in vitro possesses many of the glucoregulatory effects of endogenous GLP-1.30,32 Exenatide is indicated as monotherapy or adjunctive therapy for patients with T2DM who have not achieved adequate glycemic control with metformin, a sulfonylurea, a TZD, or metformin in combination with a sulfonylurea or a TZD.43 Exenatide is administered by SC injection BID at a starting dosage of 5 mg BID for 4 weeks, followed by an increase to 10 mg BID.
Exenatide has been shown not only to enhance glucose-dependent insulin secretion but also to restore impaired first-phase insulin response in subjects with T2DM. Exenatide also helps control postprandial glycemic excursions by suppressing inappropriate glucagon secretion, slowing accelerated gastric emptying, and enhancing satiety. The increased satiety results in decreased food intake and weight loss.31,44 In a recent head-to-head crossover study, exenatide was shown to be more effective than sitagliptin in lowering postprandial glucose concentrations, increasing insulin secretion, and reducing postprandial glucagon secretion.45 Exenatide also slowed gastric emptying and reduced caloric intake.
Exenatide, in most studies, resulted in a placebo-subtracted HbA1c reduction of approximately –1.0% and in one study lowered HbA1c from baseline by –1.5%. Completer analyses have shown HbA1c reductions of –1.0% up to 3 years and –0.8% up to 3.5 years. Exenatide has also been associated with a mean weight loss of as much as –3.6 kg at 30 weeks and as much as –5.3 kg at 3.5 years.33–35,46,47 A 1-year study showed that exenatide improved beta-cell secretory function compared with insulin glargine in metformin-treated patients with T2DM.48 Long-term data, including findings from completed and intention-to-treat analyses of 82 weeks49 to at least 3 years47 have demonstrated that exenatide improved CV risk factors, including those related to BP, lipids, and hepatic injury biomarkers.
Therapies in development
Incretin-based therapies in development include a novel once-weekly formulation of exenatide; taspoglutide, another once-weekly GLP-1 receptor agonist; and liraglutide, a GLP-1 receptor agonist that is administered once daily.50 Liraglutide is currently being evaluated in clinical trials as a once-daily SC injection.51–53 Liraglutide has been reported to reduce HbA1c by –1.1% at 26 weeks and up to –1.14% at 52 weeks and result in weight loss (up to –2.8 kg at 26 weeks and up to –2.5 kg at 52 weeks) in patients with T2DM who are treatment-naïve or taking other antidiabetes agents, including metformin, sulfonylurea, and TZD.51–53 Evaluation of the once-weekly formulation of exenatide showed reductions in HbA1c of –1.9% at 30 weeks and –2.0% at 52 weeks with a weight loss of –3.7 kg at 30 weeks and –4.1 kg over 52 weeks of treatment.46,54
CONCLUSION
In the United States, the epidemics of excessive weight and T2DM have contributed to an increased medical risk for many individuals. Comprehensive diabetes treatments targeting not only hyperglycemia but also frequently associated overweight/obesity, hypertension, and dyslipidemia will be required to reduce such risk. Current treatment strategies have evolved based on updated clinical guidelines and trials, as well as practice experience, including those related to newer agents. Incretin-based therapies, such as the GLP-1 receptor agonist, exenatide, and the DPP-4 inhibitors, sitagliptin and saxagliptin, are important additions to the treatment armamentarium, offering a reduction in hyperglycemia and beneficial effects on weight (reduction with exenatide and neutral with sitagliptin), and have been shown to improve several CV risk factors.
Data from the Centers for Disease Control and Prevention indicate that almost 24 million Americans, or 7.8% of the population, have diabetes; 90% to 95% of these have type 2 diabetes mellitus (T2DM).1 Diabetes and excessive weight often coexist. An analysis of data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) showed that among individuals with diabetes, 85% were overweight or obese and 55% were obese.2
Gaps remain in the management of T2DM between the goals for clinical parameters of care (eg, control of glucose, blood pressure [BP], and lipids) and actual clinical practice.3 NHANES data reveal that glycemic control improved from a mean glycosylated hemoglobin A1c (HbA1c) of 7.82% in 1999–2000 to 7.18% in 2003–2004.4 Hazard models based on the United Kingdom Prospective Diabetes Study (UKPDS) 10-year outcomes data in 4,320 newly diagnosed T2DM patients suggest that a sustained decrease in HbA1c of 0.511 percentage points could reduce diabetes complications by 10.7%.4,5
Additional analysis of NHANES data showed that in 2003–2004, about 57% of individuals achieved glycemic control, 48% reached BP targets, and 50% achieved target cholesterol goals.Only about 13% of diabetes patients achieved their target goals for all three parameters concurrently.6
This article reviews the association between cardiometabolic risk and the current antihyperglycemic treatments for patients with T2DM, with a focus on the role of incretin-related therapies.
THE IMPORTANCE OF CARDIOMETABOLIC RISK IN T2DM
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among people with diabetes and is the reported cause of mortality in up to 65% of deaths in persons with diabetes in the United States.7 The risk of CVD is two- to fourfold greater among adults with diabetes than among adults who do not have diabetes.8 The risk of CVD in patients with T2DM was evident in the UKPDS 17, where macrovascular complications, including CVD, were about twice as common as microvascular complications (20% vs 9%) after 9 years of follow-up.9 A study that involved more than 44,000 patients showed an almost double rate of mortality from all causes among individuals with T2DM compared with those with no diabetes (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.97).10 Current guidelines recommend aggressive management of CV risk factors, including BP control, correction of atherogenic dyslipidemia, glycemic control, weight reduction for those who are overweight or obese, and smoking cessation for those who smoke.3,11 Lifestyle interventions, including weight reduction and appropriately prescribed physical activity, result in reduced CV risk factors, which can help slow the progression of T2DM.12
GOALS OF T2DM THERAPY
Several studies have demonstrated that glycemic control can delay or prevent the development and progression of microvascular complications.13,14 UKPDS 33 showed that more intensive blood glucose control (median HbA1c 7.0%) in patients with T2DM followed over 10 years significantly (P = .029) reduced the risk for any diabetes-related end point by 12% compared with conventional therapy (median HbA1c 7.9%). Most of the risk reduction was accounted for by a 25% risk reduction in microvascular end points (P = .0099).13 Another report (UKPDS 35) demonstrated that HbA1c was strongly related to microvascular effects, with a 1% reduction in HbA1c associated with a 37% reduction in microvascular complications.14
Does intensive glucose control reduce CV risk?
To resolve the ongoing question of whether intensive glucose control can lead to a reduction in CV risk in patients with T2DM, three large, long-term trials were conducted within the last decade.15–18 Two of these, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, each enrolled more than 10,000 previously treated patients with long-standing T2DM. Patients were randomized to standard or intensive glycemic control for 3.5 years in the ACCORD trial and for 5 years in the ADVANCE trial.15,16
The ACCORD and ADVANCE trials, along with the smaller Veterans Administration Diabetes Trial (VADT) (N = 1,791), failed to show that more intensive glycemic control significantly reduced CVD.15–17 Additionally, the glycemic control component of ACCORD was halted because of increased mortality in the intensive arm compared with the standard arm.15 Further analyses of ACCORD data presented at the 69th Scientific Sessions of the American Diabetes Association (ADA) showed that HbA1c values lower than 7.0% did not explain the increased mortality. The 20% higher risk of death for every 1.0% increase in HbA1c greater than 6.0% suggests that glucose concentrations even lower than the general HbA1c goal of less than 7.0% may be appropriate in some patients.18 The most recent finding from VADT was that CV risk was dependent on disease duration and presence of comorbidities. Intensive therapy seemed to work best in patients with diabetes of less than 15 years’ duration, while risk of a CV event was more than doubled with intensive therapy in patients having diabetes for more than 21 years.
Clarification of treatment goals
A position statement of the ADA and a scientific statement of the American College of Cardiology Foundation and the American Heart Association19 concluded that the “evidence obtained from ACCORD, ADVANCE, and VADT does not suggest the need for major changes in glycemic control targets but, rather, additional clarification of the language that has consistently stressed individualization.” They state that while the general HbA1c goal of less than 7.0% seems reasonable, even lower HbA1c goals may be appropriate for some patients if they can be achieved without significant hypoglycemia or other adverse effects. Such patients might include those with diabetes of short duration, long life expectancy, or no significant CVD or hypoglycemia. Conversely, higher HbA1c goals may be appropriate for patients with limited life expectancy, a history of severe hypoglycemia, established microvascular or macrovascular complications, significant other comorbid conditions, or longstanding diabetes in whom an HbA1c of less than 7.0% has been difficult to attain despite optimal treatment and diabetes self-management education.19
Long-term risk reduction
A 10-year, postinterventional follow-up study (UKPDS 80) of the UKPDS survivor cohort was reported recently.20 Results showed that despite an early loss of glycemic differences between patients treated with diet and those treated with intensive regimens (sulfonylurea or insulin; metformin in overweight patients), the pharmacotherapy group demonstrated a prolonged reduction in microvascular risk as well as a significant reduction in the risk for myocardial infarction (15% [P = .01] in the sulfonylurea-insulin group and 33% [P = .005] in the metformin group) and death from any cause.20 This suggests that early improvement in glycemic control is associated with long-term benefits in the micro- and macrovascular health of patients with T2DM.
Additionally, the recent long-term follow-up of the Steno-2 study21 showed that a multifactorial intervention striving for intensive glucose, BP, and lipid control that included the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents not only reduced the risk of nonfatal CVD among patients with T2DM and microalbuminuria, but also had sustained beneficial effects on vascular complications and on rates of death from any cause and from CV causes. From a health care payer perspective, intensive multifactorial intervention was more likely to be cost-effective than conventional treatment in Denmark, especially if applied in a primary care setting.22
Comprehensive care needed
The lower-than-expected rates of CV outcomes in the ACCORD, ADVANCE, VADT, and Steno-2 studies reinforce the importance of comprehensive diabetes care that treats not only hyperglycemia but also elevated BP and dyslipidemia; these are considered the “ABCs” of diabetes.11,19 The 2009 ADA standards of medical care guidelines recommend that for most T2DM patients, HbA1c should be maintained at less than 7.0%,3 while the American Association of Clinical Endocrinologists (AACE) 2007 guidelines state that HbA1c should be 6.5% or less.11 Both organizations stress the importance of individualized goals, as discussed above, and advocate BP goals of less than 130/80 mm Hg and dyslipidemia goals of low-density lipoprotein cholesterol (LDL-C) less than 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) greater than 40 mg/dL for men and 50 mg/dL for women, and triglycerides less than 150 mg/dL. It is recommended that an optional LDL-C goal of less than 70 mg/dL be considered for individuals with overt CVD.
CURRENT ANTIHYPERGLYCEMIC TREATMENT STRATEGIES
In response to new insights from clinical research and emerging treatment strategies, disease-specific organizations and medical specialty societies regularly revise and update their treatment guidelines and algorithms. These resources recommend that glycemic progress should be regularly monitored and pharmacologic therapy titrated or new drugs added promptly if glycemic goals are not met after 2 to 3 months.
Several algorithms combine scientific evidence with expert clinical opinion to guide physicians in treating their patients with T2DM. The American College of Endocrinology (ACE)/AACE road maps are designed to help develop individualized treatment regimens to achieve an HbA1c of 6.5% or less.23 The algorithm from a writing group assembled by the ADA and the European Association for the Study of Diabetes (EASD) similarly promotes pharmacologic treatment together with lifestyle modifications to maintain a glycemic goal of HbA1c less than 7.0%.24
OVERVIEW OF ANTIHYPERGLYCEMIC TREATMENT APPROACHES
Initial oral therapy
T2DM is usually treated initially with a single oral agent. Consistent with the progressive nature of the disease, patients often eventually require one or more additional oral agents and in many cases insulin.13,27 Choice of specific agents is based on individual patient circumstances, including the need for weight loss and control of fasting versus postprandial glucose, the presence of dyslipidemia and hypertension, and the risk for and potential consequences of hypoglycemia.24 T2DM patients with severely uncontrolled and symptomatic hyperglycemia are best treated, at least initially, with a combination of insulin therapy and lifestyle intervention, often with metformin.
Metformin. The recently revised ADA/EASD writing group algorithm recommends that patients not requiring initial insulin begin treatment with metformin at the time of diagnosis unless there are contraindications.24 Metformin is not associated with hypoglycemia and is considered weight-neutral, although some patients may lose weight.28
Sulfonylureas. Sulfonylureas stimulate insulin secretion from pancreatic beta cells; their use may be associated with hypoglycemia and weight gain. Mechanisms for weight gain with sulfonylureas include reduction of glucosuria and increased caloric intake to prevent or treat hypoglycemia.11,28 Nateglinide and repaglinide are nonsulfonylurea oral insulin secretagogues. They result in rapid and relatively short-lived insulin responses and are usually administered three times a day, before each meal. Their use may be associated with weight gain and hypoglycemia.11
Thiazolidinediones. Thiazolidinediones (TZD) increase insulin sensitivity in muscle, adipose tissue, and the liver. Hypoglycemia is uncommon with TZD monotherapy but weight gain related to increased and redistributed adiposity and fluid retention frequently occurs.
Alpha-glucosidase inhibitors. The alpha-glucosidase inhibitors are administered before meals and primarily reduce postprandial hyperglycemia. They are generally weight-neutral.28
Insulin. Insulin and insulin analogues are the most effective antihyperglycemic agents, but their use can be associated with hypoglycemia and clinically significant weight gain.28
Colesevelam. Colesevelam is a bile acid sequestrant that was recently approved by the US Food and Drug Administration as an antihyperglycemic therapy in people with T2DM. At a dosage of 1.875 g BID or 3.75 g QD in combination with a sulfonylurea, metformin, or insulin therapy, reductions in HbA1c compared with placebo in clinical trials of colesevelam have ranged from –0.5% to –0.7% (P < .02). Frequency of hypoglycemia and weight gain is low with this agent.26
Weight management. Weight reduction is important for overweight or obese patients with T2DM.27,28 Even moderate weight loss (5% of body weight) can be associated with improved insulin action and reduced hyperglycemia.29 Conversely, weight gain has been shown to worsen hyperglycemia and other CV risk factors. Treatment-related weight gain can also lead to decreased regimen adherence, contributing to poor glycemic control.28
THE ROLE OF INCRETIN HORMONES AND INCRETIN-BASED THERAPIES IN T2DM PATIENTS
Over the last few years, the role of incretin hormones and their contribution to diabetes pathophysiology has become more apparent. The incretin effect refers to the observation that orally administered glucose elicits a greater insulin response than does glucose administered intravenously to produce equivalent blood glucose concentrations.30,31 The incretin effect is diminished in patients with T2DM.
Hormone mediation of the incretin effect
The two hormones that mediate the incretin effect are GIP (also known as gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide) and glucagon-like peptide−1 (GLP-1).30,31 GLP-1 has several glucoregulatory actions, including enhancement of endogenous insulin release and suppression of inappropriate glucagon secretion, both in a glucose-dependent manner. Therefore, these effects of GLP-1 occur only when glucose concentrations are elevated, thereby minimizing the risk of hypoglycemia. GLP-1 also regulates gastric emptying; infusions of GLP-1 can slow the accelerated emptying that is often present in T2DM patients. GLP-1 also increases satiety and decreases food intake via a central mechanism.31
Because GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase–4 (DPP-4), therapeutic use of GLP-1 would require continuous infusion, which is impractical.30,31 Two strategies have been used to produce incretin-related therapies. One, inhibition of the DPP-4 enzyme, results in a two- to threefold enhancement of endogenous GLP-1. The other, involving agents that resist breakdown by DPP-4 but bind to and activate the GLP-1 receptor, produces glucoregulatory effects similar to those of GLP-1.30
Following subcutaneous (SC) injection, GLP-1 receptor agonists enhance insulin secretion and suppress inappropriately elevated glucagon, both in a glucose-dependent manner, as well as slow gastric emptying and enhance satiety.30 DPP-4 inhibitors provide glucose-dependent enhanced insulin secretion and glucagon suppression, but they do not have the same effects on gastric emptying or satiety.
Clinically, the GLP-1 receptor agonists improve glycemia and are associated with weight loss.32–35 Adverse gastrointestinal symptoms are relatively common during the first few weeks of treatment. DPP-4 inhibitors improve glycemia but are weight-neutral and are not generally associated with significant gastrointestinal symptoms.32,36–38
Incretin-based therapies
Incretin-based therapies are currently part of the antihyperglycemic armamentarium.25,32 The AACE guidelines11 and the ACE/AACE roadmaps23 include the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin among antihyperglycemic therapies for patients with T2DM. The most recent update of the consensus algorithm statement of a joint ADA/EASD writing group included GLP-1 receptor agonists (but not DPP-4 inhibitors) in tier 2 of preferred agents, especially for patients who have concerns related to weight and hypoglycemia.24 They noted that DPP-4 inhibitors may be appropriate choices in selected patients.
DPP-4 inhibitors: sitagliptin, saxagliptin. Until recently, sitagliptin was the only DPP-4 inhibitor available in the United States. Sitagliptin is approved by the FDA for treatment of T2DM at a recommended oral dosage of 100 mg QD, either as monotherapy or in combination with other oral antihyperglycemic medications. The dosage of sitagliptin should be reduced to 50 mg/day in patients with creatinine clearance (CrCl) levels that are between 30 mL/min and 50 mL/min and to 25 mg/day in those with CrCl less than 30 mL/min.39
In a meta-analysis of incretin-based therapies, DPP-4 inhibitors produced a reduction in HbA1c compared with placebo (weighted mean difference of –0.74%; 95% confidence interval, –0.85% to –0.62%).32 DPP-4 inhibitor antihyperglycemic efficacy has been shown to be similar whether used as a monotherapy or add-on therapy.32,37,38 This same meta-analysis showed DPP-4 inhibitors as having a neutral effect on weight.32 More recently, a single-pill combination of metformin and sitagliptin was approved.40
A study comparing metformin, sitagliptin, and the combination of the two as initial monotherapy in T2DM patients with a baseline HbA1c of 8.8% showed 24-week HbA1c reductions from baseline of –0.66% with sitagliptin 100 mg QD, –0.82% with metformin 500 mg BID, and –1.90% with sitagliptin 50 mg + metformin 1,000 mg BID.41
On July 31, 2009, the FDA approved another DPP-4 inhibitor, saxagliptin, for the treatment of T2DM either as monotherapy or in combination with metformin, a sulfonylurea, or a TZD.42
GLP-1 receptor agonist: exenatide. Exenatide, the only FDA-approved GLP-1 receptor agonist, is the synthetic version of exendin-4, which binds to the human GLP-1 receptor and in vitro possesses many of the glucoregulatory effects of endogenous GLP-1.30,32 Exenatide is indicated as monotherapy or adjunctive therapy for patients with T2DM who have not achieved adequate glycemic control with metformin, a sulfonylurea, a TZD, or metformin in combination with a sulfonylurea or a TZD.43 Exenatide is administered by SC injection BID at a starting dosage of 5 mg BID for 4 weeks, followed by an increase to 10 mg BID.
Exenatide has been shown not only to enhance glucose-dependent insulin secretion but also to restore impaired first-phase insulin response in subjects with T2DM. Exenatide also helps control postprandial glycemic excursions by suppressing inappropriate glucagon secretion, slowing accelerated gastric emptying, and enhancing satiety. The increased satiety results in decreased food intake and weight loss.31,44 In a recent head-to-head crossover study, exenatide was shown to be more effective than sitagliptin in lowering postprandial glucose concentrations, increasing insulin secretion, and reducing postprandial glucagon secretion.45 Exenatide also slowed gastric emptying and reduced caloric intake.
Exenatide, in most studies, resulted in a placebo-subtracted HbA1c reduction of approximately –1.0% and in one study lowered HbA1c from baseline by –1.5%. Completer analyses have shown HbA1c reductions of –1.0% up to 3 years and –0.8% up to 3.5 years. Exenatide has also been associated with a mean weight loss of as much as –3.6 kg at 30 weeks and as much as –5.3 kg at 3.5 years.33–35,46,47 A 1-year study showed that exenatide improved beta-cell secretory function compared with insulin glargine in metformin-treated patients with T2DM.48 Long-term data, including findings from completed and intention-to-treat analyses of 82 weeks49 to at least 3 years47 have demonstrated that exenatide improved CV risk factors, including those related to BP, lipids, and hepatic injury biomarkers.
Therapies in development
Incretin-based therapies in development include a novel once-weekly formulation of exenatide; taspoglutide, another once-weekly GLP-1 receptor agonist; and liraglutide, a GLP-1 receptor agonist that is administered once daily.50 Liraglutide is currently being evaluated in clinical trials as a once-daily SC injection.51–53 Liraglutide has been reported to reduce HbA1c by –1.1% at 26 weeks and up to –1.14% at 52 weeks and result in weight loss (up to –2.8 kg at 26 weeks and up to –2.5 kg at 52 weeks) in patients with T2DM who are treatment-naïve or taking other antidiabetes agents, including metformin, sulfonylurea, and TZD.51–53 Evaluation of the once-weekly formulation of exenatide showed reductions in HbA1c of –1.9% at 30 weeks and –2.0% at 52 weeks with a weight loss of –3.7 kg at 30 weeks and –4.1 kg over 52 weeks of treatment.46,54
CONCLUSION
In the United States, the epidemics of excessive weight and T2DM have contributed to an increased medical risk for many individuals. Comprehensive diabetes treatments targeting not only hyperglycemia but also frequently associated overweight/obesity, hypertension, and dyslipidemia will be required to reduce such risk. Current treatment strategies have evolved based on updated clinical guidelines and trials, as well as practice experience, including those related to newer agents. Incretin-based therapies, such as the GLP-1 receptor agonist, exenatide, and the DPP-4 inhibitors, sitagliptin and saxagliptin, are important additions to the treatment armamentarium, offering a reduction in hyperglycemia and beneficial effects on weight (reduction with exenatide and neutral with sitagliptin), and have been shown to improve several CV risk factors.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, 2008. Available at: http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86.
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006; 49:1761–1769.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004; 140:945–950.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in noninsulin-dependent diabetes mellitus. Ann Intern Med 1996; 124(1 Pt 2):136–145.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Kerr M. ADA 2009: intensive glycemic control not directly linked to excess cardiovascular risk. Medscape Medical News Web site. http://www.medscape.com/viewarticle/704260_print. Published June 11, 2009. Accessed September 16, 2009.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31:1510–1515.
- ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract 2007; 13:260–268.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31:245–259.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131:281–303.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632−2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Januvia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2048–2054.
- Janumet. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2041–2048.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281:E155–E161.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, 2008. Available at: http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86.
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006; 49:1761–1769.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004; 140:945–950.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in noninsulin-dependent diabetes mellitus. Ann Intern Med 1996; 124(1 Pt 2):136–145.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Kerr M. ADA 2009: intensive glycemic control not directly linked to excess cardiovascular risk. Medscape Medical News Web site. http://www.medscape.com/viewarticle/704260_print. Published June 11, 2009. Accessed September 16, 2009.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31:1510–1515.
- ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract 2007; 13:260–268.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31:245–259.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131:281–303.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632−2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Januvia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2048–2054.
- Janumet. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2041–2048.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281:E155–E161.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
KEY POINTS
- Up to 65% of deaths among people with diabetes are caused by cardiovascular disease.
- Glycemic control can delay or slow the progression of microvascular complications.
- In addition to hyperglycemia, comprehensive diabetes therapy must target cardiovascular disease–related risk factors, including excess weight/obesity, elevated blood pressure, and abnormal lipid concentrations.
- Diminished incretin hormonal activity contributes to the pathophysiology of diabetes.