User login
Caring for Patients on Insulin Pumps
Delivery of insulin via continuous subcutaneous insulin infusion (CSII), or insulin pump, has gained wide acceptance. It is estimated that 400,000 patients with type 1 diabetes mellitus (T1DM) are using insulin pumps.[1] A registry for T1DM in the United States indicated that 50% of the 25,833 participants were using an insulin pump.[2] Use of CSII in type 2 DM (T2DM) is also increasing.[3]
Patients with DM are 3 as likely to be hospitalized than patients without DM.[4] Twenty percent to 30% of adult hospitalized patients have a known diagnosis of DM.[5] It is therefore to be expected that patients on CSII will be seen with increased frequency in the hospital setting. This leads to potential difficultiesinpatient healthcare providers may not be familiar with insulin pumps, and patients may not be aware of complexities associated with pump usage in the hospital.
This article will review CSII usage in the hospital, offering strategies for management in partnership with the patient based on our experiences and processes developed in our institution.
SHOULD CONTINUOUS SUBCUTANEOUS INSULIN INFUSION BE CONTINUED IN THE HOSPITAL?
The American Diabetes Association advocates (1) allowing patients who are physically and mentally able to continue CSII when hospitalized, (2) having a hospital policy for CSII use, and (3) having hospital personnel with expertise on pump management.[6] The American Association of Clinical Endocrinologists echoes much of the same and suggests contacting the specialist responsible for the pump in the ambulatory setting for decisions on adjustments in the hospitalized patient,[7] which at times may not be feasible.
The logic and benefits of basal‐bolus insulin dosing (ie, giving basal insulin to account for fasting requirements, plus bolus insulin to cover nutritional and correctional needs) have been well‐described.[8, 9, 10] In randomized clinical trials on patients admitted to general medical and surgical floors, basal‐bolus insulin (long‐acting basal insulin plus mealtime fast‐acting insulin injections) resulted in better glycemic control and reduced infection rates compared with sliding‐scale therapy (waiting for high blood glucoses before giving insulin, instead of giving it proactively to prevent hyperglycemia).[9, 10] At present, insulin delivery via the insulin pump is the best commercially available method to deliver insulin in a basal‐bolus manner in ambulatory patients. It thus makes sense to continue CSII in the hospital if patients are able to manage their pumps, though there are no randomized trials answering this question as of yet.
Studies on insulin pumps in the hospital are sparse. In one group's latest retrospective study of 136 patients over a 6‐year period, CSII was continued during the entire hospital stay in 65% of the hospitalizations, was used intermittently in 20%, and was discontinued with alternative insulin regimens given in 15%.[11] Mean glucose was 178 47 mg/dL (mean standard deviation), with no significant difference between groups. There were fewer episodes of severe hyperglycemia among those who continued on the pump compared with the other groups, and fewer episodes of hypoglycemia in those who continued on vs those taken off the pump. There was 1 episode of an infusion catheter kinking, resulting in nonfatal hyperglycemia, but no reported pump‐site infections, mechanical pump failure, or diabetic ketoacidosis (DKA) among patients remaining on CSII.
CLINICAL VIGNETTES
The following cases illustrate potential challenges with CSII use that we have encountered in the hospital.
The Patient Needing Transition to Multiple Daily Insulin Injections
A 29‐year‐old male with T1DM, on CSII, was admitted for fever and chills. His latest glycated hemoglobin (HbA1c) level was 6.8%. His glucose levels started rising, and he wished to be taken off the insulin pump. He was started by the primary team on multiple daily insulin injections (MDII) with insulin glargine and insulin lispro. His glucose levels continued to rise, so an intravenous (IV) insulin infusion was started. Endocrinology was then consulted. The patient's condition was concerning for the potential development of DKA, so he was kept on IV insulin. When he was ready for transitioning to subcutaneous insulin, the pump had been taken home by a family member, and the patient could not recall his CSII basal rates but knew his total basal insulin dose, carbohydrate ratio, and sensitivity factor. Endocrinology assisted in transitioning him from the insulin infusion to MDII based on these recalled doses. When the insulin pump was available, the pump settings were interrogated, and he was transitioned back to it.
Key points:
- Having key hospital personnel trained on CSII, including interrogating the pump's settings, facilitates the transitioning of these patients from one hospital unit, or level of care, to another.
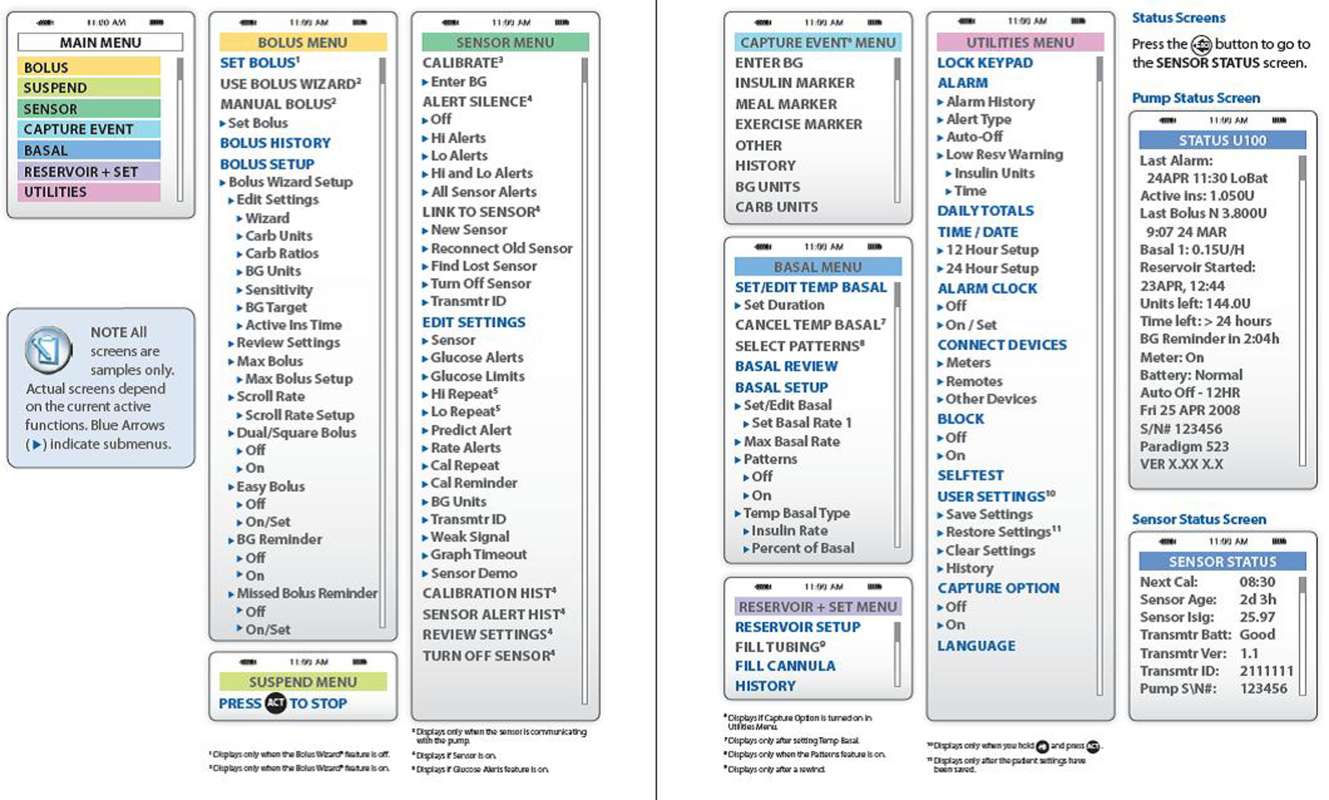
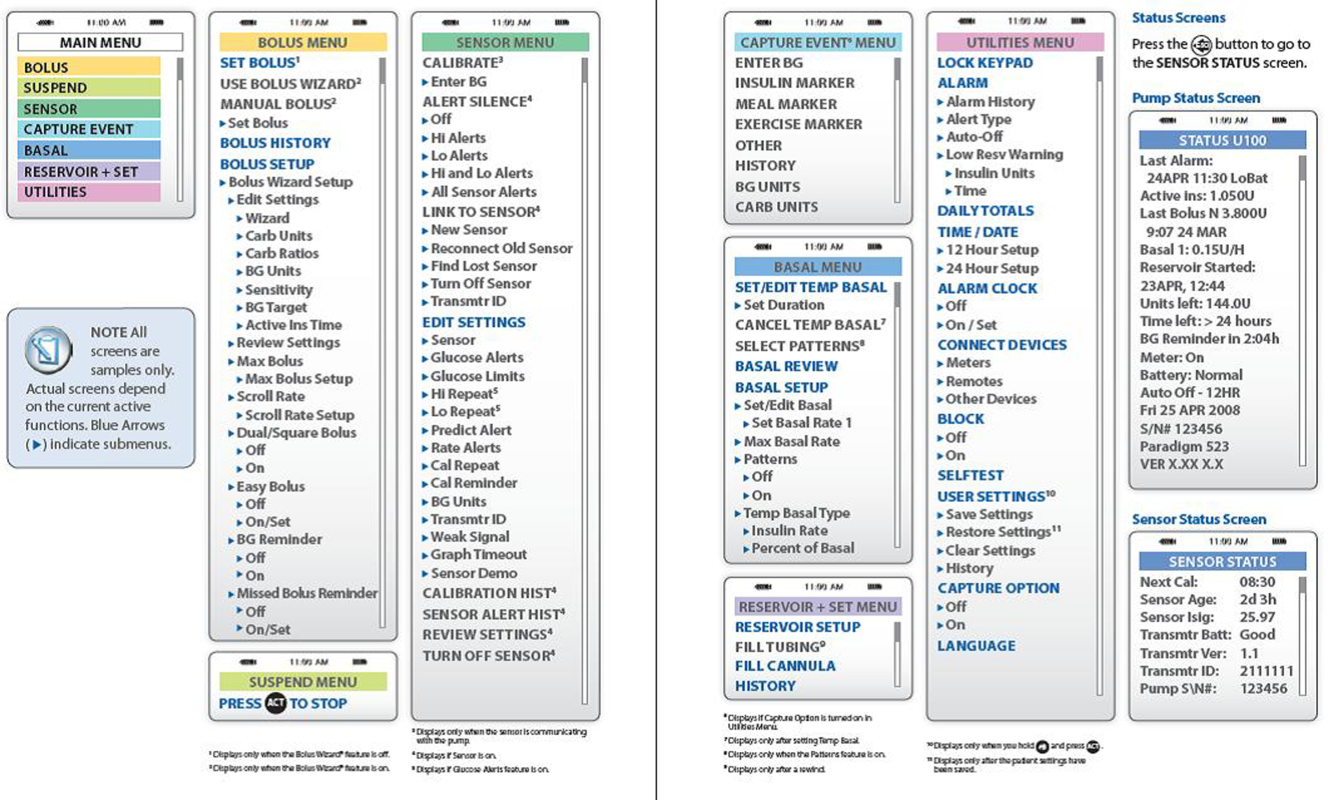
- Accessing the pump's settings involves pushing several buttons on the insulin pump. Because key hospital personnel will encounter patients on different insulin pumps, it may be helpful to keep menu maps handy as a quick reference. A menu map will show at a glance where certain information can be found, such as the basal insulin rate or the sensitivity factor (see examples in Figures 1 and 2).[12, 13]
- Knowing the HbA1c will help determine if pump use has been effective.

The Patient With Technical Problems
An 84‐year‐old gentleman with T2DM was admitted for heart failure and aortic valve replacement. His HbA1c was 6.2%, and he had had several outpatient hypoglycemic events. While on CSII in the hospital, his point of care testing (POCT) glucose readings ranged from 105 to 260 mg/dL. On the afternoon of the third hospital day, POCT readings stayed above 220 mg/dL and rose to 348 mg/dL on the fourth hospital morning, despite multiple blousing and changing the insulin, insertion site, reservoir cartridge, and pump tubing. There was no evidence of infection and no medication change that would have impacted glucose levels. Review of his procedures revealed that he had undergone computed tomography (CT) on the morning of hospital day 3 and wore his pump while being scanned. The pump company was notified.
Key points:
- Patients, and medical and nursing staff, should be reminded to remove insulin pumps for CT scans, magnetic resonance imaging, x‐rays, or other tests with high electromagnetic fields.
- If there is a suspicion of pump malfunction from such a procedure, notify the pump company.
The Patient Who Can Benefit From Inpatient Education
A 70‐year‐old female with T1DM was admitted for heart failure. The patient had been using CSII for 20 years. Her latest HbA1c was 6.9%. She had 1 hypoglycemic event every 1 to 2 weeks. In the hospital, she experienced 2 hypoglycemic events within 3 days, both around bedtime. It was discovered that the patient was giving a bolus of insulin for elevated glucose levels based on the hospital POCT, and when the meal arrived (3060 minutes later), she again delivered a bolus based on her own glucometer reading plus insulin based on the carbohydrates in her meal. The patient was then instructed to request the POCT when her meal tray arrived, and she was taught how to use the pump's built‐in calculator. Glucose excursions improved.
Key points:
- Patients on CSII, though able to exercise autonomy in managing their insulin doses, may also need assistance in dosing insulin properly.
- Although pump education is ideally done on an outpatient basis, hospital‐based providers may encounter patients who need reinforcement of their training while hospitalized. Hospital personnel trained on insulin pumps (such as physicians, nurse practitioners, physician assistants, and certified diabetes educators) can help augment the patient's knowledge while in the hospital. In the absence of such key personnel, patient safety has to be addressed with re‐evaluation of the need to discontinue the pump and switch to multiple doses of subcutaneous insulin.
STEPS IN TAKING CARE OF PATIENTS ON CONTINUOUS SUBCUTANEOUS INSULIN INFUSION
Initial Patient Assessment
On admission, patients are asked whether they use an insulin pump. This is included in the nursing assessment form. If they do, the physician is notified.
The insulin pump might be missed unless specifically asked for because (1) the insulin pump may be thought of more as a device rather than a medication, and (2) the insulin pump may be worn in less obvious areas, not only on the abdomen where providers are more apt to detect it.
Hospital Policy and Insulin Orders
Written hospital policies on how to safely manage patients presenting with an insulin pump will delineate patients who can safely be allowed to continue on the pump, and the responsibilities that come along with this. Our institution has such a policy. Experts from both the legal and biomedical engineering departments were consulted when the policy was crafted. Patients must be fully alert, able, and willing to self‐manage the pump. General contraindications to pump use in the hospital, such as altered mental status or DKA, are listed in Table 1. In addition, patients in the intensive care units are best managed on an IV insulin infusion during their critical illness, in keeping with several society guidelines.[14] Controlling severe hyperglycemia and DKA with multiple boluses through the insulin pump can potentially lead to stacking of insulin with subsequent hypoglycemia.
| Altered state of consciousness |
| Suicidal ideation |
| Prolonged instability of blood glucose levels |
| Diabetic ketoacidosis |
| Patient/family inability or refusal to participate in own care |
| Insulin‐pump malfunction |
| Lack of appropriate supplies for the insulin pump |
| Other circumstances as identified by the physician, resident, or licensed independent practitioner |
In our institution, a computerized insulin pump order set has to be activated. Apart from insulin, POCT, and hypoglycemia‐management orders, this order set contains documents aimed at balancing patient autonomy with delivery of appropriate and safe medical care that the bedside nurse goes over with the patient (Table 2). By policy at our institution, insulin should be dispensed from the hospital's pharmacy (except for that already in the pump), so the order set is linked to the pharmacy and a 3‐mL insulin vial is delivered to the hospital floor and stored in the patient‐specific medication bin. The order set triggers an Endocrinology consult so that the patient can be assessed by key trained personnel.
|
| CSII pump therapy patient agreement |
| Delineates the conditions for continuing on CSII and those for whom it may be discontinued |
| Terms of use and release of liability for patient‐owned equipment |
| Delineates the patient's responsibility for the pump and supplies |
| Patient‐maintained flow sheet for inpatient CSII |
| Includes blood glucose levels (obtained by nurse or patient‐care assistant with the hospital glucose meter) |
| Includes insulin doses (basal, bolus) |
| Includes carbohydrate intake in grams |
Patient Diagnosis
It is important to try to distinguish T1DM vs T2DM, as patients with T1DM are prone to ketoacidosis when the pump is disconnected.
Patient Assessment by the Endocrinology Consult Service
Hospitalized patients on the pump have varying degrees of pump knowledge and skill sets. We have encountered highly trained patients who meticulously count their carbohydrates and double‐check the insulin doses calculated by the built‐in pump calculator, and those who have knowledge gaps because their physicians, and not they themselves, change pump settings at the clinic visits.
Therefore, the Endocrinology consult team members (comprising physicians, nurse practitioners, and certified diabetes educators) go through the following items to be able to order the insulin correctly, assess whether patients are still able and willing to continue on their pump despite their illness, formulate alternative insulin regimens as needed, or help empower patients who may have forgotten some aspects of pump management:
- Insulin pump manufacturer/model.
- Insulin used in the pump.
- Often fast‐acting insulin (lispro, aspart, or glulisine).
- Some patients use regular insulin.
- A few patients use U500 insulin (5 more potent than other insulins).
- Insulin doses/pump settings.
Patients are assessed for:
- Hypoglycemia awareness.
- Previous glucose control.
- Bolus calculation (either using the built‐in calculator, computing this mentally, or using a different calculator).
- Ability to deliver a bolus (including vision and dexterity challenges).
- Ability to change the basal rate, or set a temporary rate, and suspend insulin delivery.
Discussion on Options for Inpatient Management
After assessment, education is provided as needed. If there are concerns on the part of the patient, the primary team, or the Endocrinology team about safe continuation of CSII during the hospitalization, then alternative insulin regimens are discussed. Patients who cannot access their basal rates and cannot adjust the doses are not able to self‐manage; they should be taken off the pump and treated with multiple subcutaneous insulin doses. Conversion to MDII is discussed under Interruption of Continuous Subcutaneous Insulin Infusion for Short and Prolonged Periods.
Provision of Pump Information for Hospital Healthcare Providers
Users of CSII, even if perfectly competent using their pumps in the ambulatory setting, may need assistance in the hospital for various reasons. They may not know what to do for surgical or radiologic procedures (discussed below) and may not be familiar with hospital policies involving CSII. Hospital providers trained on insulin pumps may need a refresher on locating a particular pump setting.
The provider can call the toll‐free number on the back of the pump for assistance (Table 3). Insulin‐pump companies also have menu maps to aid in finding information on pump settings (samples shown in Figures 1 and 2).[12, 13] Documentation of the patient's pump settings will assist in CSII dose changes during the acute illness or assist in switching to MDII if needed. The following information need to be collected:
| Animas Corporation | 877‐937‐7867 |
| Insulet Corporation | 800‐591‐3455 |
| Medtronic | 800‐826‐2099 |
| Roche Diagnostics | 800‐688‐4578 |
Basal Rate
This is the hourly insulin rate delivered for the patient's insulin needs even when not eating. The patient might have one or multiple basal rates in a day, or a different pattern on some days. Because the patient's activity in the hospital will be different from the usual ambulatory activity, we recommend that patients choose only 1 pattern.
Bolus
This is the insulin to cover meals or to correct for hyperglycemia, or both. The patient has to activate buttons for delivery. The patient may or may not be using the built‐in pump calculator.
Carbohydrate Ratio
This is the amount of insulin per quantity of carbohydrate consumed. When patients are initially placed on the insulin pump, they are given a carbohydrate ratio that is derived from a calculation called the rule of 500. In the rule of 500, the number 500 is divided by the patient's total daily insulin dose while on multiple subcutaneous insulin shots. For example, if the patient was on insulin glargine 13 units daily and insulin lispro 4 units 3 daily with meals, 500 divided by 25 gives us a carbohydrate ratio of 20 grams of carbohydrate for 1 unit of insulin (or conversely called insulin‐to‐carbohydrate ratio of 1 unit of insulin for every 20 grams of carbohydrate).
This often comes out to 1 unit for every 1530 grams of carbohydrates in patients with T1DM, and 1 unit for every 515 grams of carbohydrate in patients with T2DM, reflecting the need for a higher insulin dose in the latter.
It is best to ask the patient how many units he or she usually takes for a meal, or to present the patient with an example of a meal and ask how much he or she would take. We have encountered a patient whose carbohydrate ratio was 1, but upon further inquiry, the patient demonstrated that he actually bolused 1 unit for every 1 serving (or 1 unit for every 15 grams) of carbohydrate.
Sensitivity Factor
This is the amount of insulin that would bring the blood glucose to goal. For example, if the patient requires 1 unit of insulin to bring down the blood glucose from 170 mg/dL to 120 mg/dL, then the sensitivity factor of 50 would be seen on the pump screen. Similar to the carbohydrate ratio, a sensitivity factor is calculated when patients are initially placed on the insulin pump. This time, the rule of 1800 is used, where the number 1800 is divided by the patient's total daily insulin dose. In patients with T1DM, this often comes to 30100 mg/dL per 1 unit of insulin; or, conversely, 1 unit for every 30100 mg/dL glucose. For patients with T2DM, this is often 1 unit for every 1025 mg/dL glucose.
This insulin dose is given in addition to the dose resulting from the carbohydrate ratio, or alone if the patient is not eating.
Target
This is the blood glucose goal for the patient, which might be too tight in the presence of acute illness, and therefore would have to be modified. The American Diabetes Association, Endocrine Society, and American Association of Clinical Endocrinologists recommend premeal glucose targets of <140 mg/dL in hospitalized noncritically ill patients on insulin, with re‐evaluation of the insulin dose when premeal glucose levels fall below 100 mg/dL and dose adjustment if they are <70mg/dLunless there is an obvious explanation, such as a missed meal.[14, 15]
Point‐of‐Care Testing for Glucose Monitoring
Our policy specifies that the hospital glucose meter is the meter of record upon which dose adjustments are based. Point‐of‐care testing is performed by our patient care nursing assistants or bedside nurses. The timing is typically before meals, at bedtime, between 2 and 3 AM, and with allowance for other times that patients are used to checking when they were at home, such as after meals. Frequent POCT has to be especially borne in mind for patients with hypoglycemia unawareness. Some patients are used to checking with their own home glucose meters in between these times, and we do work with them with the understanding that dose‐change decisions are based on the hospital glucose meter readings.
Dose Adjustments
Continuous subcutaneous insulin infusion dose adjustments for hypoglycemia and hyperglycemia are usually done in 10% to 20% decrements/emncrements. Our Endocrinology team discusses these with the patients and ensures that the new settings are entered into the pump and into the order set.
INTERRUPTION OF CONTINUOUS SUBCUTANEOUS INSULIN INFUSION FOR SHORT AND PROLONGED PERIODS
Patients with T1DM should not be left without basal insulin. However, pump interruption for 30 minutes to an hour often does not lead to problems. Beyond an hour and certainly closer to 2 to 3 hours off the pump, the patient should be given a subcutaneous insulin injection if the patient is left without easy access to the insulin pump.
The subcutaneous insulin dose for temporary pump suspension can be roughly calculated as hourly basal rate multiplier, where the multiplier is the number of hours that the patient is expected to be disconnected from the pump (for example, hourly basal rate of 0.85 unit/hour 3 hours = 2.55 units, which can be rounded off to 2 or 3 units depending on the patient's general glucose control).
When it has been determined that the patient should come off the pump for substantial periods of time, then subcutaneous insulin injections should be given. This is imperative for the prevention of DKA in patients with T1DM, and highly recommended for maintenance of good glycemic control for patients with T2DM.
Basal Dose
In most cases, these situations stretch for greater than 24 hours, such as surgery and the anticipated recovery from anesthesia. We favor long‐acting insulin for basal needs, given 2 hours before discontinuing the pump. The total basal insulin dose per day can be given as the starting long‐acting insulin dose and then adjusted as needed. The total daily basal insulin dose can be retrieved from the insulin pump. In one study on T1DM patients using insulin lispro on the pump, total daily basal dose was given as insulin glargine without adverse effects.[16] If there is concern for hypoglycemia, then the dose can be reduced by 10% to 20%. Care should be made to ascertain that the basal insulin delivered via the pump is appropriate.
There are several ways to estimate this:
- If the daily total basal and the total prandial insulin requirements approximate a 50:50 ratio, then the basal rate via the pump is appropriate.
- If the daily total basal rate via the pump is similar to a weight‐based estimate of the basal dose (often 0.150.2 units/kg/day in patients with T1DM, 0.20.3 units/kg/day for T2DM, and higher in both cases with longer duration of DM or greater insulin resistance), then the basal rate via the pump is appropriate.
Bolus Dose
Patients can still continue to calculate their sensitivity factor and carbohydrate ratio and request for the equivalent dose of insulin. In the ideal situation, bedside nurses would be taught how to calculate this ratio and dose rapid‐acting insulin accordingly should the patient need to come off the insulin pump. Because of the logistic difficulties of making this uniform in our institution, we have worked instead on providing patients with information on the carbohydrate content of their meal tray. If the pump is discontinued, the patient would continue to calculate their prandial insulin based on their carbohydrates ratio and indicate to the nurse how much he/she would need. Our subcutaneous insulin orders for MDII allow for us to put a range of insulin doses based on the patient's typical insulin needs for mealtime.
Pump Removal for Certain Hospital Procedures
Patients may not remember that the pump has to be removed before entering high‐radiation areas. The pump owner manuals tell patients not to use the pump when going for magnetic resonance imaging, CT scans, or x‐rays, or near equipment with high electromagnetic fields.
Interrogation of the Pump
If there is concern about pump malfunction, patients should be switched to MDII. The pump company can be contacted for pump interrogation and provision of a temporary pump (Table 3).
Pump Disconnection
In our institution, the Radiology department has signage instructing patients to inform the technician if they are wearing an insulin pump. The pump is handed off to a family member or stored until the procedure is over. Another option is to leave it with the bedside nurse or the floor nurse manager for safekeeping. This is less ideal, because the wait for the radiologic procedure might take longer than expected and the patient is left without any insulin on board.
Interruption of Continuous Subcutaneous Insulin Infusion for Surgical Procedures
In our institution, the anesthesiology department has worked with the Endocrinology, Surgery and Medicine departments regarding patients with insulin pumps. Discontinuation of CSII is recommended for surgical procedures longer than 1 hour; patients are asked to continue on the insulin pump until they are taken to the preoperative suite, at which point they are placed on IV insulin infusion. Ideally, there should be an overlap of 1530 minutes. Providing an alternative continuous source of insulin during pump interruption is important, especially for patients with T1DM.[17]
Pump Resumption
Once the patient is ready to resume the pump, any subcutaneous insulin that was delivered and might still be active has to be accounted for and subtracted from the basal pump dose so that hypoglycemia is avoided. An alternative would be to wait until the last basal subcutaneous insulin dose is expected to be cleared before restarting CSII.
SUMMARY
As patients on an insulin pump are increasingly seen in the hospital, inpatient providers have to be able to adapt to these patients' needs. Inpatient providers need to have a working knowledge of the insulin pump. Alternative methods of insulin delivery will have to be discussed with the patient to assure continued safety in the hospital.
Disclosures
Dr. Lansang has served as a Sanofi Advisory Board member.
- http://www.rncos.com/Press_Releases/US‐to‐Dominate‐the‐Global‐Insulin‐Pump‐Market.htm. Accessed on October 25, 2013.
- , , , , , ;T1D Exchange Clinic Network. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389.
- , , , et al. Resource utilization with insulin pump therapy for type 2 diabetes mellitus. Am J Manag Care. 2010;16(12):892–896.
- , , , , . Diabetes‐related hospitalization and hospital‐related utilization. In: Diabetes in America. 2nd ed. Bethesda, MD: National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:553–569. Available at: http://diabetes.niddk.nih.gov/dm/pubs/america/pdf/chapter27.pdf. Accessed February 21, 2013.
- , , , , , . Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–S63.
- , , , et al;AACE Insulin Pump Management Task Force. Statement by the American Association of Clinical Endocrinologists Consensus Panel on insulin pump management. Endocr Pract. 2010;16(5):746–762.
- , , , et al;American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals [published correction appears in Diabetes Care. 2004;27(5):1255]. Diabetes Care. 2004;27(2):553–591.
- , , , et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–2186.
- , , , et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–261.
- , , , , , . Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years' experience with 253 cases. J Diabetes Sci Technol. 2012;6(5):995–1002.
- Medtronic Paradigm Revel insulin pump [menu map]. Northridge, CA: Medtronic. Available at: http://www.medtronicdiabetes.com/sites/default/files/library/download‐library/workbooks/x23_menu_map.pdf. Updated January 22, 2010. Accessed February 2013.
- OneTouch Ping insulin pump [menu map]. West Chester, PA: Animas Corporation.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369.
- , , , et al. Management of hyperglycemia in hospitalized patients in non‐critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
- , , , . Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system. Endocr Pract. 2005;11(3):157–164.
- , , , , . Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des. 2012;18(38):6204–6214.
Delivery of insulin via continuous subcutaneous insulin infusion (CSII), or insulin pump, has gained wide acceptance. It is estimated that 400,000 patients with type 1 diabetes mellitus (T1DM) are using insulin pumps.[1] A registry for T1DM in the United States indicated that 50% of the 25,833 participants were using an insulin pump.[2] Use of CSII in type 2 DM (T2DM) is also increasing.[3]
Patients with DM are 3 as likely to be hospitalized than patients without DM.[4] Twenty percent to 30% of adult hospitalized patients have a known diagnosis of DM.[5] It is therefore to be expected that patients on CSII will be seen with increased frequency in the hospital setting. This leads to potential difficultiesinpatient healthcare providers may not be familiar with insulin pumps, and patients may not be aware of complexities associated with pump usage in the hospital.
This article will review CSII usage in the hospital, offering strategies for management in partnership with the patient based on our experiences and processes developed in our institution.
SHOULD CONTINUOUS SUBCUTANEOUS INSULIN INFUSION BE CONTINUED IN THE HOSPITAL?
The American Diabetes Association advocates (1) allowing patients who are physically and mentally able to continue CSII when hospitalized, (2) having a hospital policy for CSII use, and (3) having hospital personnel with expertise on pump management.[6] The American Association of Clinical Endocrinologists echoes much of the same and suggests contacting the specialist responsible for the pump in the ambulatory setting for decisions on adjustments in the hospitalized patient,[7] which at times may not be feasible.
The logic and benefits of basal‐bolus insulin dosing (ie, giving basal insulin to account for fasting requirements, plus bolus insulin to cover nutritional and correctional needs) have been well‐described.[8, 9, 10] In randomized clinical trials on patients admitted to general medical and surgical floors, basal‐bolus insulin (long‐acting basal insulin plus mealtime fast‐acting insulin injections) resulted in better glycemic control and reduced infection rates compared with sliding‐scale therapy (waiting for high blood glucoses before giving insulin, instead of giving it proactively to prevent hyperglycemia).[9, 10] At present, insulin delivery via the insulin pump is the best commercially available method to deliver insulin in a basal‐bolus manner in ambulatory patients. It thus makes sense to continue CSII in the hospital if patients are able to manage their pumps, though there are no randomized trials answering this question as of yet.
Studies on insulin pumps in the hospital are sparse. In one group's latest retrospective study of 136 patients over a 6‐year period, CSII was continued during the entire hospital stay in 65% of the hospitalizations, was used intermittently in 20%, and was discontinued with alternative insulin regimens given in 15%.[11] Mean glucose was 178 47 mg/dL (mean standard deviation), with no significant difference between groups. There were fewer episodes of severe hyperglycemia among those who continued on the pump compared with the other groups, and fewer episodes of hypoglycemia in those who continued on vs those taken off the pump. There was 1 episode of an infusion catheter kinking, resulting in nonfatal hyperglycemia, but no reported pump‐site infections, mechanical pump failure, or diabetic ketoacidosis (DKA) among patients remaining on CSII.
CLINICAL VIGNETTES
The following cases illustrate potential challenges with CSII use that we have encountered in the hospital.
The Patient Needing Transition to Multiple Daily Insulin Injections
A 29‐year‐old male with T1DM, on CSII, was admitted for fever and chills. His latest glycated hemoglobin (HbA1c) level was 6.8%. His glucose levels started rising, and he wished to be taken off the insulin pump. He was started by the primary team on multiple daily insulin injections (MDII) with insulin glargine and insulin lispro. His glucose levels continued to rise, so an intravenous (IV) insulin infusion was started. Endocrinology was then consulted. The patient's condition was concerning for the potential development of DKA, so he was kept on IV insulin. When he was ready for transitioning to subcutaneous insulin, the pump had been taken home by a family member, and the patient could not recall his CSII basal rates but knew his total basal insulin dose, carbohydrate ratio, and sensitivity factor. Endocrinology assisted in transitioning him from the insulin infusion to MDII based on these recalled doses. When the insulin pump was available, the pump settings were interrogated, and he was transitioned back to it.
Key points:
- Having key hospital personnel trained on CSII, including interrogating the pump's settings, facilitates the transitioning of these patients from one hospital unit, or level of care, to another.
- Accessing the pump's settings involves pushing several buttons on the insulin pump. Because key hospital personnel will encounter patients on different insulin pumps, it may be helpful to keep menu maps handy as a quick reference. A menu map will show at a glance where certain information can be found, such as the basal insulin rate or the sensitivity factor (see examples in Figures 1 and 2).[12, 13]
- Knowing the HbA1c will help determine if pump use has been effective.


The Patient With Technical Problems
An 84‐year‐old gentleman with T2DM was admitted for heart failure and aortic valve replacement. His HbA1c was 6.2%, and he had had several outpatient hypoglycemic events. While on CSII in the hospital, his point of care testing (POCT) glucose readings ranged from 105 to 260 mg/dL. On the afternoon of the third hospital day, POCT readings stayed above 220 mg/dL and rose to 348 mg/dL on the fourth hospital morning, despite multiple blousing and changing the insulin, insertion site, reservoir cartridge, and pump tubing. There was no evidence of infection and no medication change that would have impacted glucose levels. Review of his procedures revealed that he had undergone computed tomography (CT) on the morning of hospital day 3 and wore his pump while being scanned. The pump company was notified.
Key points:
- Patients, and medical and nursing staff, should be reminded to remove insulin pumps for CT scans, magnetic resonance imaging, x‐rays, or other tests with high electromagnetic fields.
- If there is a suspicion of pump malfunction from such a procedure, notify the pump company.
The Patient Who Can Benefit From Inpatient Education
A 70‐year‐old female with T1DM was admitted for heart failure. The patient had been using CSII for 20 years. Her latest HbA1c was 6.9%. She had 1 hypoglycemic event every 1 to 2 weeks. In the hospital, she experienced 2 hypoglycemic events within 3 days, both around bedtime. It was discovered that the patient was giving a bolus of insulin for elevated glucose levels based on the hospital POCT, and when the meal arrived (3060 minutes later), she again delivered a bolus based on her own glucometer reading plus insulin based on the carbohydrates in her meal. The patient was then instructed to request the POCT when her meal tray arrived, and she was taught how to use the pump's built‐in calculator. Glucose excursions improved.
Key points:
- Patients on CSII, though able to exercise autonomy in managing their insulin doses, may also need assistance in dosing insulin properly.
- Although pump education is ideally done on an outpatient basis, hospital‐based providers may encounter patients who need reinforcement of their training while hospitalized. Hospital personnel trained on insulin pumps (such as physicians, nurse practitioners, physician assistants, and certified diabetes educators) can help augment the patient's knowledge while in the hospital. In the absence of such key personnel, patient safety has to be addressed with re‐evaluation of the need to discontinue the pump and switch to multiple doses of subcutaneous insulin.
STEPS IN TAKING CARE OF PATIENTS ON CONTINUOUS SUBCUTANEOUS INSULIN INFUSION
Initial Patient Assessment
On admission, patients are asked whether they use an insulin pump. This is included in the nursing assessment form. If they do, the physician is notified.
The insulin pump might be missed unless specifically asked for because (1) the insulin pump may be thought of more as a device rather than a medication, and (2) the insulin pump may be worn in less obvious areas, not only on the abdomen where providers are more apt to detect it.
Hospital Policy and Insulin Orders
Written hospital policies on how to safely manage patients presenting with an insulin pump will delineate patients who can safely be allowed to continue on the pump, and the responsibilities that come along with this. Our institution has such a policy. Experts from both the legal and biomedical engineering departments were consulted when the policy was crafted. Patients must be fully alert, able, and willing to self‐manage the pump. General contraindications to pump use in the hospital, such as altered mental status or DKA, are listed in Table 1. In addition, patients in the intensive care units are best managed on an IV insulin infusion during their critical illness, in keeping with several society guidelines.[14] Controlling severe hyperglycemia and DKA with multiple boluses through the insulin pump can potentially lead to stacking of insulin with subsequent hypoglycemia.
| Altered state of consciousness |
| Suicidal ideation |
| Prolonged instability of blood glucose levels |
| Diabetic ketoacidosis |
| Patient/family inability or refusal to participate in own care |
| Insulin‐pump malfunction |
| Lack of appropriate supplies for the insulin pump |
| Other circumstances as identified by the physician, resident, or licensed independent practitioner |
In our institution, a computerized insulin pump order set has to be activated. Apart from insulin, POCT, and hypoglycemia‐management orders, this order set contains documents aimed at balancing patient autonomy with delivery of appropriate and safe medical care that the bedside nurse goes over with the patient (Table 2). By policy at our institution, insulin should be dispensed from the hospital's pharmacy (except for that already in the pump), so the order set is linked to the pharmacy and a 3‐mL insulin vial is delivered to the hospital floor and stored in the patient‐specific medication bin. The order set triggers an Endocrinology consult so that the patient can be assessed by key trained personnel.
|
| CSII pump therapy patient agreement |
| Delineates the conditions for continuing on CSII and those for whom it may be discontinued |
| Terms of use and release of liability for patient‐owned equipment |
| Delineates the patient's responsibility for the pump and supplies |
| Patient‐maintained flow sheet for inpatient CSII |
| Includes blood glucose levels (obtained by nurse or patient‐care assistant with the hospital glucose meter) |
| Includes insulin doses (basal, bolus) |
| Includes carbohydrate intake in grams |
Patient Diagnosis
It is important to try to distinguish T1DM vs T2DM, as patients with T1DM are prone to ketoacidosis when the pump is disconnected.
Patient Assessment by the Endocrinology Consult Service
Hospitalized patients on the pump have varying degrees of pump knowledge and skill sets. We have encountered highly trained patients who meticulously count their carbohydrates and double‐check the insulin doses calculated by the built‐in pump calculator, and those who have knowledge gaps because their physicians, and not they themselves, change pump settings at the clinic visits.
Therefore, the Endocrinology consult team members (comprising physicians, nurse practitioners, and certified diabetes educators) go through the following items to be able to order the insulin correctly, assess whether patients are still able and willing to continue on their pump despite their illness, formulate alternative insulin regimens as needed, or help empower patients who may have forgotten some aspects of pump management:
- Insulin pump manufacturer/model.
- Insulin used in the pump.
- Often fast‐acting insulin (lispro, aspart, or glulisine).
- Some patients use regular insulin.
- A few patients use U500 insulin (5 more potent than other insulins).
- Insulin doses/pump settings.
Patients are assessed for:
- Hypoglycemia awareness.
- Previous glucose control.
- Bolus calculation (either using the built‐in calculator, computing this mentally, or using a different calculator).
- Ability to deliver a bolus (including vision and dexterity challenges).
- Ability to change the basal rate, or set a temporary rate, and suspend insulin delivery.
Discussion on Options for Inpatient Management
After assessment, education is provided as needed. If there are concerns on the part of the patient, the primary team, or the Endocrinology team about safe continuation of CSII during the hospitalization, then alternative insulin regimens are discussed. Patients who cannot access their basal rates and cannot adjust the doses are not able to self‐manage; they should be taken off the pump and treated with multiple subcutaneous insulin doses. Conversion to MDII is discussed under Interruption of Continuous Subcutaneous Insulin Infusion for Short and Prolonged Periods.
Provision of Pump Information for Hospital Healthcare Providers
Users of CSII, even if perfectly competent using their pumps in the ambulatory setting, may need assistance in the hospital for various reasons. They may not know what to do for surgical or radiologic procedures (discussed below) and may not be familiar with hospital policies involving CSII. Hospital providers trained on insulin pumps may need a refresher on locating a particular pump setting.
The provider can call the toll‐free number on the back of the pump for assistance (Table 3). Insulin‐pump companies also have menu maps to aid in finding information on pump settings (samples shown in Figures 1 and 2).[12, 13] Documentation of the patient's pump settings will assist in CSII dose changes during the acute illness or assist in switching to MDII if needed. The following information need to be collected:
| Animas Corporation | 877‐937‐7867 |
| Insulet Corporation | 800‐591‐3455 |
| Medtronic | 800‐826‐2099 |
| Roche Diagnostics | 800‐688‐4578 |
Basal Rate
This is the hourly insulin rate delivered for the patient's insulin needs even when not eating. The patient might have one or multiple basal rates in a day, or a different pattern on some days. Because the patient's activity in the hospital will be different from the usual ambulatory activity, we recommend that patients choose only 1 pattern.
Bolus
This is the insulin to cover meals or to correct for hyperglycemia, or both. The patient has to activate buttons for delivery. The patient may or may not be using the built‐in pump calculator.
Carbohydrate Ratio
This is the amount of insulin per quantity of carbohydrate consumed. When patients are initially placed on the insulin pump, they are given a carbohydrate ratio that is derived from a calculation called the rule of 500. In the rule of 500, the number 500 is divided by the patient's total daily insulin dose while on multiple subcutaneous insulin shots. For example, if the patient was on insulin glargine 13 units daily and insulin lispro 4 units 3 daily with meals, 500 divided by 25 gives us a carbohydrate ratio of 20 grams of carbohydrate for 1 unit of insulin (or conversely called insulin‐to‐carbohydrate ratio of 1 unit of insulin for every 20 grams of carbohydrate).
This often comes out to 1 unit for every 1530 grams of carbohydrates in patients with T1DM, and 1 unit for every 515 grams of carbohydrate in patients with T2DM, reflecting the need for a higher insulin dose in the latter.
It is best to ask the patient how many units he or she usually takes for a meal, or to present the patient with an example of a meal and ask how much he or she would take. We have encountered a patient whose carbohydrate ratio was 1, but upon further inquiry, the patient demonstrated that he actually bolused 1 unit for every 1 serving (or 1 unit for every 15 grams) of carbohydrate.
Sensitivity Factor
This is the amount of insulin that would bring the blood glucose to goal. For example, if the patient requires 1 unit of insulin to bring down the blood glucose from 170 mg/dL to 120 mg/dL, then the sensitivity factor of 50 would be seen on the pump screen. Similar to the carbohydrate ratio, a sensitivity factor is calculated when patients are initially placed on the insulin pump. This time, the rule of 1800 is used, where the number 1800 is divided by the patient's total daily insulin dose. In patients with T1DM, this often comes to 30100 mg/dL per 1 unit of insulin; or, conversely, 1 unit for every 30100 mg/dL glucose. For patients with T2DM, this is often 1 unit for every 1025 mg/dL glucose.
This insulin dose is given in addition to the dose resulting from the carbohydrate ratio, or alone if the patient is not eating.
Target
This is the blood glucose goal for the patient, which might be too tight in the presence of acute illness, and therefore would have to be modified. The American Diabetes Association, Endocrine Society, and American Association of Clinical Endocrinologists recommend premeal glucose targets of <140 mg/dL in hospitalized noncritically ill patients on insulin, with re‐evaluation of the insulin dose when premeal glucose levels fall below 100 mg/dL and dose adjustment if they are <70mg/dLunless there is an obvious explanation, such as a missed meal.[14, 15]
Point‐of‐Care Testing for Glucose Monitoring
Our policy specifies that the hospital glucose meter is the meter of record upon which dose adjustments are based. Point‐of‐care testing is performed by our patient care nursing assistants or bedside nurses. The timing is typically before meals, at bedtime, between 2 and 3 AM, and with allowance for other times that patients are used to checking when they were at home, such as after meals. Frequent POCT has to be especially borne in mind for patients with hypoglycemia unawareness. Some patients are used to checking with their own home glucose meters in between these times, and we do work with them with the understanding that dose‐change decisions are based on the hospital glucose meter readings.
Dose Adjustments
Continuous subcutaneous insulin infusion dose adjustments for hypoglycemia and hyperglycemia are usually done in 10% to 20% decrements/emncrements. Our Endocrinology team discusses these with the patients and ensures that the new settings are entered into the pump and into the order set.
INTERRUPTION OF CONTINUOUS SUBCUTANEOUS INSULIN INFUSION FOR SHORT AND PROLONGED PERIODS
Patients with T1DM should not be left without basal insulin. However, pump interruption for 30 minutes to an hour often does not lead to problems. Beyond an hour and certainly closer to 2 to 3 hours off the pump, the patient should be given a subcutaneous insulin injection if the patient is left without easy access to the insulin pump.
The subcutaneous insulin dose for temporary pump suspension can be roughly calculated as hourly basal rate multiplier, where the multiplier is the number of hours that the patient is expected to be disconnected from the pump (for example, hourly basal rate of 0.85 unit/hour 3 hours = 2.55 units, which can be rounded off to 2 or 3 units depending on the patient's general glucose control).
When it has been determined that the patient should come off the pump for substantial periods of time, then subcutaneous insulin injections should be given. This is imperative for the prevention of DKA in patients with T1DM, and highly recommended for maintenance of good glycemic control for patients with T2DM.
Basal Dose
In most cases, these situations stretch for greater than 24 hours, such as surgery and the anticipated recovery from anesthesia. We favor long‐acting insulin for basal needs, given 2 hours before discontinuing the pump. The total basal insulin dose per day can be given as the starting long‐acting insulin dose and then adjusted as needed. The total daily basal insulin dose can be retrieved from the insulin pump. In one study on T1DM patients using insulin lispro on the pump, total daily basal dose was given as insulin glargine without adverse effects.[16] If there is concern for hypoglycemia, then the dose can be reduced by 10% to 20%. Care should be made to ascertain that the basal insulin delivered via the pump is appropriate.
There are several ways to estimate this:
- If the daily total basal and the total prandial insulin requirements approximate a 50:50 ratio, then the basal rate via the pump is appropriate.
- If the daily total basal rate via the pump is similar to a weight‐based estimate of the basal dose (often 0.150.2 units/kg/day in patients with T1DM, 0.20.3 units/kg/day for T2DM, and higher in both cases with longer duration of DM or greater insulin resistance), then the basal rate via the pump is appropriate.
Bolus Dose
Patients can still continue to calculate their sensitivity factor and carbohydrate ratio and request for the equivalent dose of insulin. In the ideal situation, bedside nurses would be taught how to calculate this ratio and dose rapid‐acting insulin accordingly should the patient need to come off the insulin pump. Because of the logistic difficulties of making this uniform in our institution, we have worked instead on providing patients with information on the carbohydrate content of their meal tray. If the pump is discontinued, the patient would continue to calculate their prandial insulin based on their carbohydrates ratio and indicate to the nurse how much he/she would need. Our subcutaneous insulin orders for MDII allow for us to put a range of insulin doses based on the patient's typical insulin needs for mealtime.
Pump Removal for Certain Hospital Procedures
Patients may not remember that the pump has to be removed before entering high‐radiation areas. The pump owner manuals tell patients not to use the pump when going for magnetic resonance imaging, CT scans, or x‐rays, or near equipment with high electromagnetic fields.
Interrogation of the Pump
If there is concern about pump malfunction, patients should be switched to MDII. The pump company can be contacted for pump interrogation and provision of a temporary pump (Table 3).
Pump Disconnection
In our institution, the Radiology department has signage instructing patients to inform the technician if they are wearing an insulin pump. The pump is handed off to a family member or stored until the procedure is over. Another option is to leave it with the bedside nurse or the floor nurse manager for safekeeping. This is less ideal, because the wait for the radiologic procedure might take longer than expected and the patient is left without any insulin on board.
Interruption of Continuous Subcutaneous Insulin Infusion for Surgical Procedures
In our institution, the anesthesiology department has worked with the Endocrinology, Surgery and Medicine departments regarding patients with insulin pumps. Discontinuation of CSII is recommended for surgical procedures longer than 1 hour; patients are asked to continue on the insulin pump until they are taken to the preoperative suite, at which point they are placed on IV insulin infusion. Ideally, there should be an overlap of 1530 minutes. Providing an alternative continuous source of insulin during pump interruption is important, especially for patients with T1DM.[17]
Pump Resumption
Once the patient is ready to resume the pump, any subcutaneous insulin that was delivered and might still be active has to be accounted for and subtracted from the basal pump dose so that hypoglycemia is avoided. An alternative would be to wait until the last basal subcutaneous insulin dose is expected to be cleared before restarting CSII.
SUMMARY
As patients on an insulin pump are increasingly seen in the hospital, inpatient providers have to be able to adapt to these patients' needs. Inpatient providers need to have a working knowledge of the insulin pump. Alternative methods of insulin delivery will have to be discussed with the patient to assure continued safety in the hospital.
Disclosures
Dr. Lansang has served as a Sanofi Advisory Board member.
Delivery of insulin via continuous subcutaneous insulin infusion (CSII), or insulin pump, has gained wide acceptance. It is estimated that 400,000 patients with type 1 diabetes mellitus (T1DM) are using insulin pumps.[1] A registry for T1DM in the United States indicated that 50% of the 25,833 participants were using an insulin pump.[2] Use of CSII in type 2 DM (T2DM) is also increasing.[3]
Patients with DM are 3 as likely to be hospitalized than patients without DM.[4] Twenty percent to 30% of adult hospitalized patients have a known diagnosis of DM.[5] It is therefore to be expected that patients on CSII will be seen with increased frequency in the hospital setting. This leads to potential difficultiesinpatient healthcare providers may not be familiar with insulin pumps, and patients may not be aware of complexities associated with pump usage in the hospital.
This article will review CSII usage in the hospital, offering strategies for management in partnership with the patient based on our experiences and processes developed in our institution.
SHOULD CONTINUOUS SUBCUTANEOUS INSULIN INFUSION BE CONTINUED IN THE HOSPITAL?
The American Diabetes Association advocates (1) allowing patients who are physically and mentally able to continue CSII when hospitalized, (2) having a hospital policy for CSII use, and (3) having hospital personnel with expertise on pump management.[6] The American Association of Clinical Endocrinologists echoes much of the same and suggests contacting the specialist responsible for the pump in the ambulatory setting for decisions on adjustments in the hospitalized patient,[7] which at times may not be feasible.
The logic and benefits of basal‐bolus insulin dosing (ie, giving basal insulin to account for fasting requirements, plus bolus insulin to cover nutritional and correctional needs) have been well‐described.[8, 9, 10] In randomized clinical trials on patients admitted to general medical and surgical floors, basal‐bolus insulin (long‐acting basal insulin plus mealtime fast‐acting insulin injections) resulted in better glycemic control and reduced infection rates compared with sliding‐scale therapy (waiting for high blood glucoses before giving insulin, instead of giving it proactively to prevent hyperglycemia).[9, 10] At present, insulin delivery via the insulin pump is the best commercially available method to deliver insulin in a basal‐bolus manner in ambulatory patients. It thus makes sense to continue CSII in the hospital if patients are able to manage their pumps, though there are no randomized trials answering this question as of yet.
Studies on insulin pumps in the hospital are sparse. In one group's latest retrospective study of 136 patients over a 6‐year period, CSII was continued during the entire hospital stay in 65% of the hospitalizations, was used intermittently in 20%, and was discontinued with alternative insulin regimens given in 15%.[11] Mean glucose was 178 47 mg/dL (mean standard deviation), with no significant difference between groups. There were fewer episodes of severe hyperglycemia among those who continued on the pump compared with the other groups, and fewer episodes of hypoglycemia in those who continued on vs those taken off the pump. There was 1 episode of an infusion catheter kinking, resulting in nonfatal hyperglycemia, but no reported pump‐site infections, mechanical pump failure, or diabetic ketoacidosis (DKA) among patients remaining on CSII.
CLINICAL VIGNETTES
The following cases illustrate potential challenges with CSII use that we have encountered in the hospital.
The Patient Needing Transition to Multiple Daily Insulin Injections
A 29‐year‐old male with T1DM, on CSII, was admitted for fever and chills. His latest glycated hemoglobin (HbA1c) level was 6.8%. His glucose levels started rising, and he wished to be taken off the insulin pump. He was started by the primary team on multiple daily insulin injections (MDII) with insulin glargine and insulin lispro. His glucose levels continued to rise, so an intravenous (IV) insulin infusion was started. Endocrinology was then consulted. The patient's condition was concerning for the potential development of DKA, so he was kept on IV insulin. When he was ready for transitioning to subcutaneous insulin, the pump had been taken home by a family member, and the patient could not recall his CSII basal rates but knew his total basal insulin dose, carbohydrate ratio, and sensitivity factor. Endocrinology assisted in transitioning him from the insulin infusion to MDII based on these recalled doses. When the insulin pump was available, the pump settings were interrogated, and he was transitioned back to it.
Key points:
- Having key hospital personnel trained on CSII, including interrogating the pump's settings, facilitates the transitioning of these patients from one hospital unit, or level of care, to another.
- Accessing the pump's settings involves pushing several buttons on the insulin pump. Because key hospital personnel will encounter patients on different insulin pumps, it may be helpful to keep menu maps handy as a quick reference. A menu map will show at a glance where certain information can be found, such as the basal insulin rate or the sensitivity factor (see examples in Figures 1 and 2).[12, 13]
- Knowing the HbA1c will help determine if pump use has been effective.


The Patient With Technical Problems
An 84‐year‐old gentleman with T2DM was admitted for heart failure and aortic valve replacement. His HbA1c was 6.2%, and he had had several outpatient hypoglycemic events. While on CSII in the hospital, his point of care testing (POCT) glucose readings ranged from 105 to 260 mg/dL. On the afternoon of the third hospital day, POCT readings stayed above 220 mg/dL and rose to 348 mg/dL on the fourth hospital morning, despite multiple blousing and changing the insulin, insertion site, reservoir cartridge, and pump tubing. There was no evidence of infection and no medication change that would have impacted glucose levels. Review of his procedures revealed that he had undergone computed tomography (CT) on the morning of hospital day 3 and wore his pump while being scanned. The pump company was notified.
Key points:
- Patients, and medical and nursing staff, should be reminded to remove insulin pumps for CT scans, magnetic resonance imaging, x‐rays, or other tests with high electromagnetic fields.
- If there is a suspicion of pump malfunction from such a procedure, notify the pump company.
The Patient Who Can Benefit From Inpatient Education
A 70‐year‐old female with T1DM was admitted for heart failure. The patient had been using CSII for 20 years. Her latest HbA1c was 6.9%. She had 1 hypoglycemic event every 1 to 2 weeks. In the hospital, she experienced 2 hypoglycemic events within 3 days, both around bedtime. It was discovered that the patient was giving a bolus of insulin for elevated glucose levels based on the hospital POCT, and when the meal arrived (3060 minutes later), she again delivered a bolus based on her own glucometer reading plus insulin based on the carbohydrates in her meal. The patient was then instructed to request the POCT when her meal tray arrived, and she was taught how to use the pump's built‐in calculator. Glucose excursions improved.
Key points:
- Patients on CSII, though able to exercise autonomy in managing their insulin doses, may also need assistance in dosing insulin properly.
- Although pump education is ideally done on an outpatient basis, hospital‐based providers may encounter patients who need reinforcement of their training while hospitalized. Hospital personnel trained on insulin pumps (such as physicians, nurse practitioners, physician assistants, and certified diabetes educators) can help augment the patient's knowledge while in the hospital. In the absence of such key personnel, patient safety has to be addressed with re‐evaluation of the need to discontinue the pump and switch to multiple doses of subcutaneous insulin.
STEPS IN TAKING CARE OF PATIENTS ON CONTINUOUS SUBCUTANEOUS INSULIN INFUSION
Initial Patient Assessment
On admission, patients are asked whether they use an insulin pump. This is included in the nursing assessment form. If they do, the physician is notified.
The insulin pump might be missed unless specifically asked for because (1) the insulin pump may be thought of more as a device rather than a medication, and (2) the insulin pump may be worn in less obvious areas, not only on the abdomen where providers are more apt to detect it.
Hospital Policy and Insulin Orders
Written hospital policies on how to safely manage patients presenting with an insulin pump will delineate patients who can safely be allowed to continue on the pump, and the responsibilities that come along with this. Our institution has such a policy. Experts from both the legal and biomedical engineering departments were consulted when the policy was crafted. Patients must be fully alert, able, and willing to self‐manage the pump. General contraindications to pump use in the hospital, such as altered mental status or DKA, are listed in Table 1. In addition, patients in the intensive care units are best managed on an IV insulin infusion during their critical illness, in keeping with several society guidelines.[14] Controlling severe hyperglycemia and DKA with multiple boluses through the insulin pump can potentially lead to stacking of insulin with subsequent hypoglycemia.
| Altered state of consciousness |
| Suicidal ideation |
| Prolonged instability of blood glucose levels |
| Diabetic ketoacidosis |
| Patient/family inability or refusal to participate in own care |
| Insulin‐pump malfunction |
| Lack of appropriate supplies for the insulin pump |
| Other circumstances as identified by the physician, resident, or licensed independent practitioner |
In our institution, a computerized insulin pump order set has to be activated. Apart from insulin, POCT, and hypoglycemia‐management orders, this order set contains documents aimed at balancing patient autonomy with delivery of appropriate and safe medical care that the bedside nurse goes over with the patient (Table 2). By policy at our institution, insulin should be dispensed from the hospital's pharmacy (except for that already in the pump), so the order set is linked to the pharmacy and a 3‐mL insulin vial is delivered to the hospital floor and stored in the patient‐specific medication bin. The order set triggers an Endocrinology consult so that the patient can be assessed by key trained personnel.
|
| CSII pump therapy patient agreement |
| Delineates the conditions for continuing on CSII and those for whom it may be discontinued |
| Terms of use and release of liability for patient‐owned equipment |
| Delineates the patient's responsibility for the pump and supplies |
| Patient‐maintained flow sheet for inpatient CSII |
| Includes blood glucose levels (obtained by nurse or patient‐care assistant with the hospital glucose meter) |
| Includes insulin doses (basal, bolus) |
| Includes carbohydrate intake in grams |
Patient Diagnosis
It is important to try to distinguish T1DM vs T2DM, as patients with T1DM are prone to ketoacidosis when the pump is disconnected.
Patient Assessment by the Endocrinology Consult Service
Hospitalized patients on the pump have varying degrees of pump knowledge and skill sets. We have encountered highly trained patients who meticulously count their carbohydrates and double‐check the insulin doses calculated by the built‐in pump calculator, and those who have knowledge gaps because their physicians, and not they themselves, change pump settings at the clinic visits.
Therefore, the Endocrinology consult team members (comprising physicians, nurse practitioners, and certified diabetes educators) go through the following items to be able to order the insulin correctly, assess whether patients are still able and willing to continue on their pump despite their illness, formulate alternative insulin regimens as needed, or help empower patients who may have forgotten some aspects of pump management:
- Insulin pump manufacturer/model.
- Insulin used in the pump.
- Often fast‐acting insulin (lispro, aspart, or glulisine).
- Some patients use regular insulin.
- A few patients use U500 insulin (5 more potent than other insulins).
- Insulin doses/pump settings.
Patients are assessed for:
- Hypoglycemia awareness.
- Previous glucose control.
- Bolus calculation (either using the built‐in calculator, computing this mentally, or using a different calculator).
- Ability to deliver a bolus (including vision and dexterity challenges).
- Ability to change the basal rate, or set a temporary rate, and suspend insulin delivery.
Discussion on Options for Inpatient Management
After assessment, education is provided as needed. If there are concerns on the part of the patient, the primary team, or the Endocrinology team about safe continuation of CSII during the hospitalization, then alternative insulin regimens are discussed. Patients who cannot access their basal rates and cannot adjust the doses are not able to self‐manage; they should be taken off the pump and treated with multiple subcutaneous insulin doses. Conversion to MDII is discussed under Interruption of Continuous Subcutaneous Insulin Infusion for Short and Prolonged Periods.
Provision of Pump Information for Hospital Healthcare Providers
Users of CSII, even if perfectly competent using their pumps in the ambulatory setting, may need assistance in the hospital for various reasons. They may not know what to do for surgical or radiologic procedures (discussed below) and may not be familiar with hospital policies involving CSII. Hospital providers trained on insulin pumps may need a refresher on locating a particular pump setting.
The provider can call the toll‐free number on the back of the pump for assistance (Table 3). Insulin‐pump companies also have menu maps to aid in finding information on pump settings (samples shown in Figures 1 and 2).[12, 13] Documentation of the patient's pump settings will assist in CSII dose changes during the acute illness or assist in switching to MDII if needed. The following information need to be collected:
| Animas Corporation | 877‐937‐7867 |
| Insulet Corporation | 800‐591‐3455 |
| Medtronic | 800‐826‐2099 |
| Roche Diagnostics | 800‐688‐4578 |
Basal Rate
This is the hourly insulin rate delivered for the patient's insulin needs even when not eating. The patient might have one or multiple basal rates in a day, or a different pattern on some days. Because the patient's activity in the hospital will be different from the usual ambulatory activity, we recommend that patients choose only 1 pattern.
Bolus
This is the insulin to cover meals or to correct for hyperglycemia, or both. The patient has to activate buttons for delivery. The patient may or may not be using the built‐in pump calculator.
Carbohydrate Ratio
This is the amount of insulin per quantity of carbohydrate consumed. When patients are initially placed on the insulin pump, they are given a carbohydrate ratio that is derived from a calculation called the rule of 500. In the rule of 500, the number 500 is divided by the patient's total daily insulin dose while on multiple subcutaneous insulin shots. For example, if the patient was on insulin glargine 13 units daily and insulin lispro 4 units 3 daily with meals, 500 divided by 25 gives us a carbohydrate ratio of 20 grams of carbohydrate for 1 unit of insulin (or conversely called insulin‐to‐carbohydrate ratio of 1 unit of insulin for every 20 grams of carbohydrate).
This often comes out to 1 unit for every 1530 grams of carbohydrates in patients with T1DM, and 1 unit for every 515 grams of carbohydrate in patients with T2DM, reflecting the need for a higher insulin dose in the latter.
It is best to ask the patient how many units he or she usually takes for a meal, or to present the patient with an example of a meal and ask how much he or she would take. We have encountered a patient whose carbohydrate ratio was 1, but upon further inquiry, the patient demonstrated that he actually bolused 1 unit for every 1 serving (or 1 unit for every 15 grams) of carbohydrate.
Sensitivity Factor
This is the amount of insulin that would bring the blood glucose to goal. For example, if the patient requires 1 unit of insulin to bring down the blood glucose from 170 mg/dL to 120 mg/dL, then the sensitivity factor of 50 would be seen on the pump screen. Similar to the carbohydrate ratio, a sensitivity factor is calculated when patients are initially placed on the insulin pump. This time, the rule of 1800 is used, where the number 1800 is divided by the patient's total daily insulin dose. In patients with T1DM, this often comes to 30100 mg/dL per 1 unit of insulin; or, conversely, 1 unit for every 30100 mg/dL glucose. For patients with T2DM, this is often 1 unit for every 1025 mg/dL glucose.
This insulin dose is given in addition to the dose resulting from the carbohydrate ratio, or alone if the patient is not eating.
Target
This is the blood glucose goal for the patient, which might be too tight in the presence of acute illness, and therefore would have to be modified. The American Diabetes Association, Endocrine Society, and American Association of Clinical Endocrinologists recommend premeal glucose targets of <140 mg/dL in hospitalized noncritically ill patients on insulin, with re‐evaluation of the insulin dose when premeal glucose levels fall below 100 mg/dL and dose adjustment if they are <70mg/dLunless there is an obvious explanation, such as a missed meal.[14, 15]
Point‐of‐Care Testing for Glucose Monitoring
Our policy specifies that the hospital glucose meter is the meter of record upon which dose adjustments are based. Point‐of‐care testing is performed by our patient care nursing assistants or bedside nurses. The timing is typically before meals, at bedtime, between 2 and 3 AM, and with allowance for other times that patients are used to checking when they were at home, such as after meals. Frequent POCT has to be especially borne in mind for patients with hypoglycemia unawareness. Some patients are used to checking with their own home glucose meters in between these times, and we do work with them with the understanding that dose‐change decisions are based on the hospital glucose meter readings.
Dose Adjustments
Continuous subcutaneous insulin infusion dose adjustments for hypoglycemia and hyperglycemia are usually done in 10% to 20% decrements/emncrements. Our Endocrinology team discusses these with the patients and ensures that the new settings are entered into the pump and into the order set.
INTERRUPTION OF CONTINUOUS SUBCUTANEOUS INSULIN INFUSION FOR SHORT AND PROLONGED PERIODS
Patients with T1DM should not be left without basal insulin. However, pump interruption for 30 minutes to an hour often does not lead to problems. Beyond an hour and certainly closer to 2 to 3 hours off the pump, the patient should be given a subcutaneous insulin injection if the patient is left without easy access to the insulin pump.
The subcutaneous insulin dose for temporary pump suspension can be roughly calculated as hourly basal rate multiplier, where the multiplier is the number of hours that the patient is expected to be disconnected from the pump (for example, hourly basal rate of 0.85 unit/hour 3 hours = 2.55 units, which can be rounded off to 2 or 3 units depending on the patient's general glucose control).
When it has been determined that the patient should come off the pump for substantial periods of time, then subcutaneous insulin injections should be given. This is imperative for the prevention of DKA in patients with T1DM, and highly recommended for maintenance of good glycemic control for patients with T2DM.
Basal Dose
In most cases, these situations stretch for greater than 24 hours, such as surgery and the anticipated recovery from anesthesia. We favor long‐acting insulin for basal needs, given 2 hours before discontinuing the pump. The total basal insulin dose per day can be given as the starting long‐acting insulin dose and then adjusted as needed. The total daily basal insulin dose can be retrieved from the insulin pump. In one study on T1DM patients using insulin lispro on the pump, total daily basal dose was given as insulin glargine without adverse effects.[16] If there is concern for hypoglycemia, then the dose can be reduced by 10% to 20%. Care should be made to ascertain that the basal insulin delivered via the pump is appropriate.
There are several ways to estimate this:
- If the daily total basal and the total prandial insulin requirements approximate a 50:50 ratio, then the basal rate via the pump is appropriate.
- If the daily total basal rate via the pump is similar to a weight‐based estimate of the basal dose (often 0.150.2 units/kg/day in patients with T1DM, 0.20.3 units/kg/day for T2DM, and higher in both cases with longer duration of DM or greater insulin resistance), then the basal rate via the pump is appropriate.
Bolus Dose
Patients can still continue to calculate their sensitivity factor and carbohydrate ratio and request for the equivalent dose of insulin. In the ideal situation, bedside nurses would be taught how to calculate this ratio and dose rapid‐acting insulin accordingly should the patient need to come off the insulin pump. Because of the logistic difficulties of making this uniform in our institution, we have worked instead on providing patients with information on the carbohydrate content of their meal tray. If the pump is discontinued, the patient would continue to calculate their prandial insulin based on their carbohydrates ratio and indicate to the nurse how much he/she would need. Our subcutaneous insulin orders for MDII allow for us to put a range of insulin doses based on the patient's typical insulin needs for mealtime.
Pump Removal for Certain Hospital Procedures
Patients may not remember that the pump has to be removed before entering high‐radiation areas. The pump owner manuals tell patients not to use the pump when going for magnetic resonance imaging, CT scans, or x‐rays, or near equipment with high electromagnetic fields.
Interrogation of the Pump
If there is concern about pump malfunction, patients should be switched to MDII. The pump company can be contacted for pump interrogation and provision of a temporary pump (Table 3).
Pump Disconnection
In our institution, the Radiology department has signage instructing patients to inform the technician if they are wearing an insulin pump. The pump is handed off to a family member or stored until the procedure is over. Another option is to leave it with the bedside nurse or the floor nurse manager for safekeeping. This is less ideal, because the wait for the radiologic procedure might take longer than expected and the patient is left without any insulin on board.
Interruption of Continuous Subcutaneous Insulin Infusion for Surgical Procedures
In our institution, the anesthesiology department has worked with the Endocrinology, Surgery and Medicine departments regarding patients with insulin pumps. Discontinuation of CSII is recommended for surgical procedures longer than 1 hour; patients are asked to continue on the insulin pump until they are taken to the preoperative suite, at which point they are placed on IV insulin infusion. Ideally, there should be an overlap of 1530 minutes. Providing an alternative continuous source of insulin during pump interruption is important, especially for patients with T1DM.[17]
Pump Resumption
Once the patient is ready to resume the pump, any subcutaneous insulin that was delivered and might still be active has to be accounted for and subtracted from the basal pump dose so that hypoglycemia is avoided. An alternative would be to wait until the last basal subcutaneous insulin dose is expected to be cleared before restarting CSII.
SUMMARY
As patients on an insulin pump are increasingly seen in the hospital, inpatient providers have to be able to adapt to these patients' needs. Inpatient providers need to have a working knowledge of the insulin pump. Alternative methods of insulin delivery will have to be discussed with the patient to assure continued safety in the hospital.
Disclosures
Dr. Lansang has served as a Sanofi Advisory Board member.
- http://www.rncos.com/Press_Releases/US‐to‐Dominate‐the‐Global‐Insulin‐Pump‐Market.htm. Accessed on October 25, 2013.
- , , , , , ;T1D Exchange Clinic Network. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389.
- , , , et al. Resource utilization with insulin pump therapy for type 2 diabetes mellitus. Am J Manag Care. 2010;16(12):892–896.
- , , , , . Diabetes‐related hospitalization and hospital‐related utilization. In: Diabetes in America. 2nd ed. Bethesda, MD: National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:553–569. Available at: http://diabetes.niddk.nih.gov/dm/pubs/america/pdf/chapter27.pdf. Accessed February 21, 2013.
- , , , , , . Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–S63.
- , , , et al;AACE Insulin Pump Management Task Force. Statement by the American Association of Clinical Endocrinologists Consensus Panel on insulin pump management. Endocr Pract. 2010;16(5):746–762.
- , , , et al;American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals [published correction appears in Diabetes Care. 2004;27(5):1255]. Diabetes Care. 2004;27(2):553–591.
- , , , et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–2186.
- , , , et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–261.
- , , , , , . Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years' experience with 253 cases. J Diabetes Sci Technol. 2012;6(5):995–1002.
- Medtronic Paradigm Revel insulin pump [menu map]. Northridge, CA: Medtronic. Available at: http://www.medtronicdiabetes.com/sites/default/files/library/download‐library/workbooks/x23_menu_map.pdf. Updated January 22, 2010. Accessed February 2013.
- OneTouch Ping insulin pump [menu map]. West Chester, PA: Animas Corporation.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369.
- , , , et al. Management of hyperglycemia in hospitalized patients in non‐critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
- , , , . Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system. Endocr Pract. 2005;11(3):157–164.
- , , , , . Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des. 2012;18(38):6204–6214.
- http://www.rncos.com/Press_Releases/US‐to‐Dominate‐the‐Global‐Insulin‐Pump‐Market.htm. Accessed on October 25, 2013.
- , , , , , ;T1D Exchange Clinic Network. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389.
- , , , et al. Resource utilization with insulin pump therapy for type 2 diabetes mellitus. Am J Manag Care. 2010;16(12):892–896.
- , , , , . Diabetes‐related hospitalization and hospital‐related utilization. In: Diabetes in America. 2nd ed. Bethesda, MD: National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:553–569. Available at: http://diabetes.niddk.nih.gov/dm/pubs/america/pdf/chapter27.pdf. Accessed February 21, 2013.
- , , , , , . Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–S63.
- , , , et al;AACE Insulin Pump Management Task Force. Statement by the American Association of Clinical Endocrinologists Consensus Panel on insulin pump management. Endocr Pract. 2010;16(5):746–762.
- , , , et al;American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals [published correction appears in Diabetes Care. 2004;27(5):1255]. Diabetes Care. 2004;27(2):553–591.
- , , , et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–2186.
- , , , et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–261.
- , , , , , . Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years' experience with 253 cases. J Diabetes Sci Technol. 2012;6(5):995–1002.
- Medtronic Paradigm Revel insulin pump [menu map]. Northridge, CA: Medtronic. Available at: http://www.medtronicdiabetes.com/sites/default/files/library/download‐library/workbooks/x23_menu_map.pdf. Updated January 22, 2010. Accessed February 2013.
- OneTouch Ping insulin pump [menu map]. West Chester, PA: Animas Corporation.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369.
- , , , et al. Management of hyperglycemia in hospitalized patients in non‐critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
- , , , . Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system. Endocr Pract. 2005;11(3):157–164.
- , , , , . Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des. 2012;18(38):6204–6214.
Incretin-based therapies for type 2 diabetes mellitus: New therapeutic mechanisms
Almost a decade into the 21st century, the global epidemic of diabetes—which accelerated in the 1970s—shows no sign of slowing. At the same time, our insights into both type 1 and type 2 diabetes mellitus (T2DM) have increased at a similarly rapid rate.
At the beginning of the 1970s, it was far from clear whether improved glycemic control made much difference in the long-term well-being of people with diabetes other than to relieve their symptoms of hyperglycemia and decrease the likelihood of diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma. Concerns were expressed about the risk/benefit ratio of antihyperglycemic drugs—so there is nothing new under the sun! The drugs available in the United States were limited to insulin and sulfonylureas. The rest of the world also had access to metformin, but, in truth, its potential was underestimated until much later.
RECOGNIZING THE VALUE OF GLYCEMIC CONTROL
Out of this milieu of scientific uncertainty grew the two clinical trials that effectively ended the debate about the value of glycemic control: the Diabetes Control and Complications Trial (DCCT)1 for type 1 diabetes, and the United Kingdom Prospective Diabetes Study (UKPDS)2,3 for T2DM. The conduct of these trials was facilitated by the timely demonstration of the utility of glycosylated hemoglobin (HbA1c) as an objective measure of glycemic control, and of microalbuminuria as a marker of early nephropathy.
Both the DCCT and the UKPDS, in their initial “end of study” analyses in the 1990s, established the role of glycemic control in reducing the risk of retinopathy, neuropathy, and nephropathy—the microvascular complications of diabetes. Additionally, the UKPDS demonstrated that in T2DM, hypertension management was at least as important as glycemic control in reducing the risk of microvascular complications.
Neither the DCCT nor the UKPDS was powered to determine initially whether glycemic control was a risk factor for cardiovascular disease; however, careful longer-term surveillance of the patient cohorts in the studies has recently borne fruit in this regard. Reports from both studies have shown that efforts to control glycemia early in the course of diabetes are rewarded many years later by a decreased risk of cardiovascular events and death.4,5 This is true even when excellent glycemic control achieved early on is not sustained indefinitely. It has also become widely recognized that the management of diabetes, with prevention of microvascular and cardiovascular disease as major aims, involves much more than a simple preoccupation with glycemic control—important as that is.
NEW TREATMENT OPTIONS
Concurrent with the DCCT and the UKPDS being conducted with, in effect, the therapeutic tools of the 1970s, considerable strides were being made in the development of new classes of antihyperglycemic agents for use in T2DM. These include the thiazolidinediones (TZDs), alpha-glucosidase inhibitors, nonsulfonylurea insulin secretagogues (also known as glinides), and, more recently, the incretin-based drugs that are the focus of this supplement to the Cleveland Clinic Journal of Medicine.
Understandable enthusiasm for tapping into the hitherto unexploited pathways and mechanisms targeted by a new drug class is inevitably tempered by known, or sometimes unforeseen, adverse effects. Some of the adverse effects typically associated with antihyperglycemic drugs used before the incretin-based therapies became available include hypoglycemia, weight gain, and fluid retention; all of these are perceived as possibly increasing the risk of the very thing we are striving to avoid in diabetes—cardiovascular morbidity and mortality. Such is the concern about this risk—epitomized, rightly or wrongly, in the controversial meta-analysis of clinical trials involving rosiglitazone6—that the US Food and Drug Administration now requires new antihyperglycemic drugs not only to meet efficacy standards for improving glycemia but also to show no sign of increased cardiovascular risk. The requirement must be met in preapproval trials, to be followed by postmarketing studies to prove the lack of cardiovascular risk.
As the contributions in this supplement point out, incretin-based therapies generally are either weight neutral or promote weight loss; by their modes of action, they are unlikely to cause hypoglycemia; and, as shown thus far, they are unassociated with fluid retention or increased likelihood of heart failure. Continued vigilance regarding cardiovascular risk will be important for the new incretin-based therapies, however.
BETA-CELL FUNCTION STILL A CHALLENGE
Another aspect of T2DM highlighted by the UKPDS is the degree of pancreatic beta-cell function loss—typically about 50% or more—at the time of clinical diagnosis, and the steady decline in function thereafter.7 This, as much as the understandable fatigue with lifestyle modification that normal humans experience, accounts for the frequent failure of oral antihyperglycemic monotherapy or dual therapy to maintain satisfactory glycemic control over the years. Relieving hyperglycemia at the time of diagnosis by any means usually leads to a temporary improvement in beta-cell function, but the possibility of slowing or even reversing the long-term decline has been an elusive therapeutic goal.
Although direct quantitative assessment of beta-cell function in humans is difficult in routine practice or outside of strict research protocols, a randomized study comparing different monotherapies for T2DM showed that over several years, the rise in HbA1c was more gradual with rosiglitazone than with glyburide or metformin; this suggests that, at least compared with metformin and sulfonylureas, the TZDs may have some longer-term benefit with respect to beta-cell function.8
That incretin-based treatments may help preserve or improve beta-cell function has been suggested by animal data.9 Proving that that is the case in humans will be much more challenging. A recent randomized study in patients with T2DM already taking metformin showed that addition of exenatide for 1 year resulted in improved beta-cell function, assessed by C-peptide responses to glucose and to arginine during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure. The improvement was evident compared with baseline function and with patients randomized to receive insulin glargine in addition to metformin for a year.10 However, 4 weeks after exenatide and glargine were discontinued, the beta-cell function had reverted to the pretreatment level and was not significantly different in the two groups of patients. Moreover, 3 months after treatment discontinuation, the HbA1c levels, which had decreased during the year to a similar extent in both groups, had returned to pretreatment levels. The investigators acknowledged that it was impossible in their study to “discriminate between acute and long-term effects of exenatide on beta-cell function.”10 So, in my opinion, the challenge remains to show that meaningful long-term effects on beta-cell function can be achieved with incretin-based therapy.
That said, there is no doubt that the incretin-based therapies bring a new dimension to our ability to treat diabetes. The articles in this supplement will provide both the specialist and nonspecialist with a better understanding of these relatively new therapies.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44:1249–1258.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355:2427–2443.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
Almost a decade into the 21st century, the global epidemic of diabetes—which accelerated in the 1970s—shows no sign of slowing. At the same time, our insights into both type 1 and type 2 diabetes mellitus (T2DM) have increased at a similarly rapid rate.
At the beginning of the 1970s, it was far from clear whether improved glycemic control made much difference in the long-term well-being of people with diabetes other than to relieve their symptoms of hyperglycemia and decrease the likelihood of diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma. Concerns were expressed about the risk/benefit ratio of antihyperglycemic drugs—so there is nothing new under the sun! The drugs available in the United States were limited to insulin and sulfonylureas. The rest of the world also had access to metformin, but, in truth, its potential was underestimated until much later.
RECOGNIZING THE VALUE OF GLYCEMIC CONTROL
Out of this milieu of scientific uncertainty grew the two clinical trials that effectively ended the debate about the value of glycemic control: the Diabetes Control and Complications Trial (DCCT)1 for type 1 diabetes, and the United Kingdom Prospective Diabetes Study (UKPDS)2,3 for T2DM. The conduct of these trials was facilitated by the timely demonstration of the utility of glycosylated hemoglobin (HbA1c) as an objective measure of glycemic control, and of microalbuminuria as a marker of early nephropathy.
Both the DCCT and the UKPDS, in their initial “end of study” analyses in the 1990s, established the role of glycemic control in reducing the risk of retinopathy, neuropathy, and nephropathy—the microvascular complications of diabetes. Additionally, the UKPDS demonstrated that in T2DM, hypertension management was at least as important as glycemic control in reducing the risk of microvascular complications.
Neither the DCCT nor the UKPDS was powered to determine initially whether glycemic control was a risk factor for cardiovascular disease; however, careful longer-term surveillance of the patient cohorts in the studies has recently borne fruit in this regard. Reports from both studies have shown that efforts to control glycemia early in the course of diabetes are rewarded many years later by a decreased risk of cardiovascular events and death.4,5 This is true even when excellent glycemic control achieved early on is not sustained indefinitely. It has also become widely recognized that the management of diabetes, with prevention of microvascular and cardiovascular disease as major aims, involves much more than a simple preoccupation with glycemic control—important as that is.
NEW TREATMENT OPTIONS
Concurrent with the DCCT and the UKPDS being conducted with, in effect, the therapeutic tools of the 1970s, considerable strides were being made in the development of new classes of antihyperglycemic agents for use in T2DM. These include the thiazolidinediones (TZDs), alpha-glucosidase inhibitors, nonsulfonylurea insulin secretagogues (also known as glinides), and, more recently, the incretin-based drugs that are the focus of this supplement to the Cleveland Clinic Journal of Medicine.
Understandable enthusiasm for tapping into the hitherto unexploited pathways and mechanisms targeted by a new drug class is inevitably tempered by known, or sometimes unforeseen, adverse effects. Some of the adverse effects typically associated with antihyperglycemic drugs used before the incretin-based therapies became available include hypoglycemia, weight gain, and fluid retention; all of these are perceived as possibly increasing the risk of the very thing we are striving to avoid in diabetes—cardiovascular morbidity and mortality. Such is the concern about this risk—epitomized, rightly or wrongly, in the controversial meta-analysis of clinical trials involving rosiglitazone6—that the US Food and Drug Administration now requires new antihyperglycemic drugs not only to meet efficacy standards for improving glycemia but also to show no sign of increased cardiovascular risk. The requirement must be met in preapproval trials, to be followed by postmarketing studies to prove the lack of cardiovascular risk.
As the contributions in this supplement point out, incretin-based therapies generally are either weight neutral or promote weight loss; by their modes of action, they are unlikely to cause hypoglycemia; and, as shown thus far, they are unassociated with fluid retention or increased likelihood of heart failure. Continued vigilance regarding cardiovascular risk will be important for the new incretin-based therapies, however.
BETA-CELL FUNCTION STILL A CHALLENGE
Another aspect of T2DM highlighted by the UKPDS is the degree of pancreatic beta-cell function loss—typically about 50% or more—at the time of clinical diagnosis, and the steady decline in function thereafter.7 This, as much as the understandable fatigue with lifestyle modification that normal humans experience, accounts for the frequent failure of oral antihyperglycemic monotherapy or dual therapy to maintain satisfactory glycemic control over the years. Relieving hyperglycemia at the time of diagnosis by any means usually leads to a temporary improvement in beta-cell function, but the possibility of slowing or even reversing the long-term decline has been an elusive therapeutic goal.
Although direct quantitative assessment of beta-cell function in humans is difficult in routine practice or outside of strict research protocols, a randomized study comparing different monotherapies for T2DM showed that over several years, the rise in HbA1c was more gradual with rosiglitazone than with glyburide or metformin; this suggests that, at least compared with metformin and sulfonylureas, the TZDs may have some longer-term benefit with respect to beta-cell function.8
That incretin-based treatments may help preserve or improve beta-cell function has been suggested by animal data.9 Proving that that is the case in humans will be much more challenging. A recent randomized study in patients with T2DM already taking metformin showed that addition of exenatide for 1 year resulted in improved beta-cell function, assessed by C-peptide responses to glucose and to arginine during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure. The improvement was evident compared with baseline function and with patients randomized to receive insulin glargine in addition to metformin for a year.10 However, 4 weeks after exenatide and glargine were discontinued, the beta-cell function had reverted to the pretreatment level and was not significantly different in the two groups of patients. Moreover, 3 months after treatment discontinuation, the HbA1c levels, which had decreased during the year to a similar extent in both groups, had returned to pretreatment levels. The investigators acknowledged that it was impossible in their study to “discriminate between acute and long-term effects of exenatide on beta-cell function.”10 So, in my opinion, the challenge remains to show that meaningful long-term effects on beta-cell function can be achieved with incretin-based therapy.
That said, there is no doubt that the incretin-based therapies bring a new dimension to our ability to treat diabetes. The articles in this supplement will provide both the specialist and nonspecialist with a better understanding of these relatively new therapies.
Almost a decade into the 21st century, the global epidemic of diabetes—which accelerated in the 1970s—shows no sign of slowing. At the same time, our insights into both type 1 and type 2 diabetes mellitus (T2DM) have increased at a similarly rapid rate.
At the beginning of the 1970s, it was far from clear whether improved glycemic control made much difference in the long-term well-being of people with diabetes other than to relieve their symptoms of hyperglycemia and decrease the likelihood of diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma. Concerns were expressed about the risk/benefit ratio of antihyperglycemic drugs—so there is nothing new under the sun! The drugs available in the United States were limited to insulin and sulfonylureas. The rest of the world also had access to metformin, but, in truth, its potential was underestimated until much later.
RECOGNIZING THE VALUE OF GLYCEMIC CONTROL
Out of this milieu of scientific uncertainty grew the two clinical trials that effectively ended the debate about the value of glycemic control: the Diabetes Control and Complications Trial (DCCT)1 for type 1 diabetes, and the United Kingdom Prospective Diabetes Study (UKPDS)2,3 for T2DM. The conduct of these trials was facilitated by the timely demonstration of the utility of glycosylated hemoglobin (HbA1c) as an objective measure of glycemic control, and of microalbuminuria as a marker of early nephropathy.
Both the DCCT and the UKPDS, in their initial “end of study” analyses in the 1990s, established the role of glycemic control in reducing the risk of retinopathy, neuropathy, and nephropathy—the microvascular complications of diabetes. Additionally, the UKPDS demonstrated that in T2DM, hypertension management was at least as important as glycemic control in reducing the risk of microvascular complications.
Neither the DCCT nor the UKPDS was powered to determine initially whether glycemic control was a risk factor for cardiovascular disease; however, careful longer-term surveillance of the patient cohorts in the studies has recently borne fruit in this regard. Reports from both studies have shown that efforts to control glycemia early in the course of diabetes are rewarded many years later by a decreased risk of cardiovascular events and death.4,5 This is true even when excellent glycemic control achieved early on is not sustained indefinitely. It has also become widely recognized that the management of diabetes, with prevention of microvascular and cardiovascular disease as major aims, involves much more than a simple preoccupation with glycemic control—important as that is.
NEW TREATMENT OPTIONS
Concurrent with the DCCT and the UKPDS being conducted with, in effect, the therapeutic tools of the 1970s, considerable strides were being made in the development of new classes of antihyperglycemic agents for use in T2DM. These include the thiazolidinediones (TZDs), alpha-glucosidase inhibitors, nonsulfonylurea insulin secretagogues (also known as glinides), and, more recently, the incretin-based drugs that are the focus of this supplement to the Cleveland Clinic Journal of Medicine.
Understandable enthusiasm for tapping into the hitherto unexploited pathways and mechanisms targeted by a new drug class is inevitably tempered by known, or sometimes unforeseen, adverse effects. Some of the adverse effects typically associated with antihyperglycemic drugs used before the incretin-based therapies became available include hypoglycemia, weight gain, and fluid retention; all of these are perceived as possibly increasing the risk of the very thing we are striving to avoid in diabetes—cardiovascular morbidity and mortality. Such is the concern about this risk—epitomized, rightly or wrongly, in the controversial meta-analysis of clinical trials involving rosiglitazone6—that the US Food and Drug Administration now requires new antihyperglycemic drugs not only to meet efficacy standards for improving glycemia but also to show no sign of increased cardiovascular risk. The requirement must be met in preapproval trials, to be followed by postmarketing studies to prove the lack of cardiovascular risk.
As the contributions in this supplement point out, incretin-based therapies generally are either weight neutral or promote weight loss; by their modes of action, they are unlikely to cause hypoglycemia; and, as shown thus far, they are unassociated with fluid retention or increased likelihood of heart failure. Continued vigilance regarding cardiovascular risk will be important for the new incretin-based therapies, however.
BETA-CELL FUNCTION STILL A CHALLENGE
Another aspect of T2DM highlighted by the UKPDS is the degree of pancreatic beta-cell function loss—typically about 50% or more—at the time of clinical diagnosis, and the steady decline in function thereafter.7 This, as much as the understandable fatigue with lifestyle modification that normal humans experience, accounts for the frequent failure of oral antihyperglycemic monotherapy or dual therapy to maintain satisfactory glycemic control over the years. Relieving hyperglycemia at the time of diagnosis by any means usually leads to a temporary improvement in beta-cell function, but the possibility of slowing or even reversing the long-term decline has been an elusive therapeutic goal.
Although direct quantitative assessment of beta-cell function in humans is difficult in routine practice or outside of strict research protocols, a randomized study comparing different monotherapies for T2DM showed that over several years, the rise in HbA1c was more gradual with rosiglitazone than with glyburide or metformin; this suggests that, at least compared with metformin and sulfonylureas, the TZDs may have some longer-term benefit with respect to beta-cell function.8
That incretin-based treatments may help preserve or improve beta-cell function has been suggested by animal data.9 Proving that that is the case in humans will be much more challenging. A recent randomized study in patients with T2DM already taking metformin showed that addition of exenatide for 1 year resulted in improved beta-cell function, assessed by C-peptide responses to glucose and to arginine during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure. The improvement was evident compared with baseline function and with patients randomized to receive insulin glargine in addition to metformin for a year.10 However, 4 weeks after exenatide and glargine were discontinued, the beta-cell function had reverted to the pretreatment level and was not significantly different in the two groups of patients. Moreover, 3 months after treatment discontinuation, the HbA1c levels, which had decreased during the year to a similar extent in both groups, had returned to pretreatment levels. The investigators acknowledged that it was impossible in their study to “discriminate between acute and long-term effects of exenatide on beta-cell function.”10 So, in my opinion, the challenge remains to show that meaningful long-term effects on beta-cell function can be achieved with incretin-based therapy.
That said, there is no doubt that the incretin-based therapies bring a new dimension to our ability to treat diabetes. The articles in this supplement will provide both the specialist and nonspecialist with a better understanding of these relatively new therapies.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44:1249–1258.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355:2427–2443.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44:1249–1258.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355:2427–2443.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.