User login
Safety Assessment of a Noninvasive Respiratory Protocol for Adults With COVID-19
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
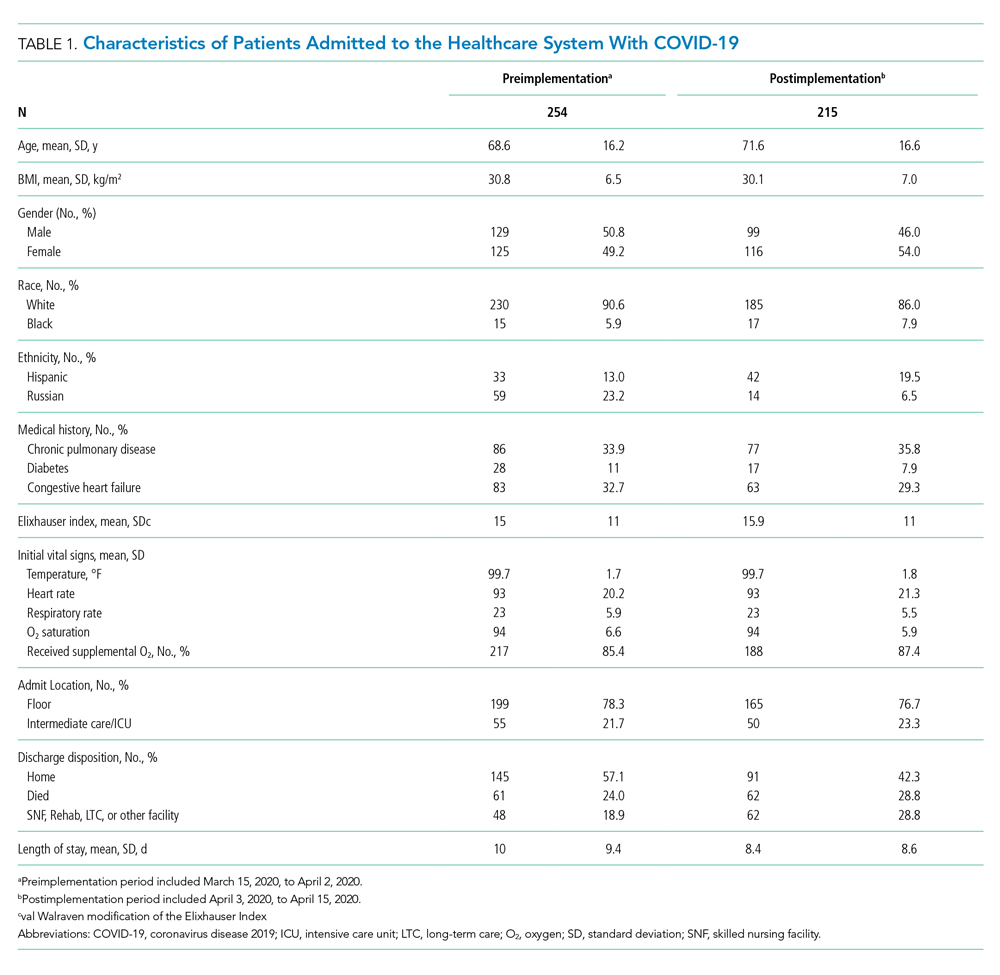
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).
Postimplementation Mortality
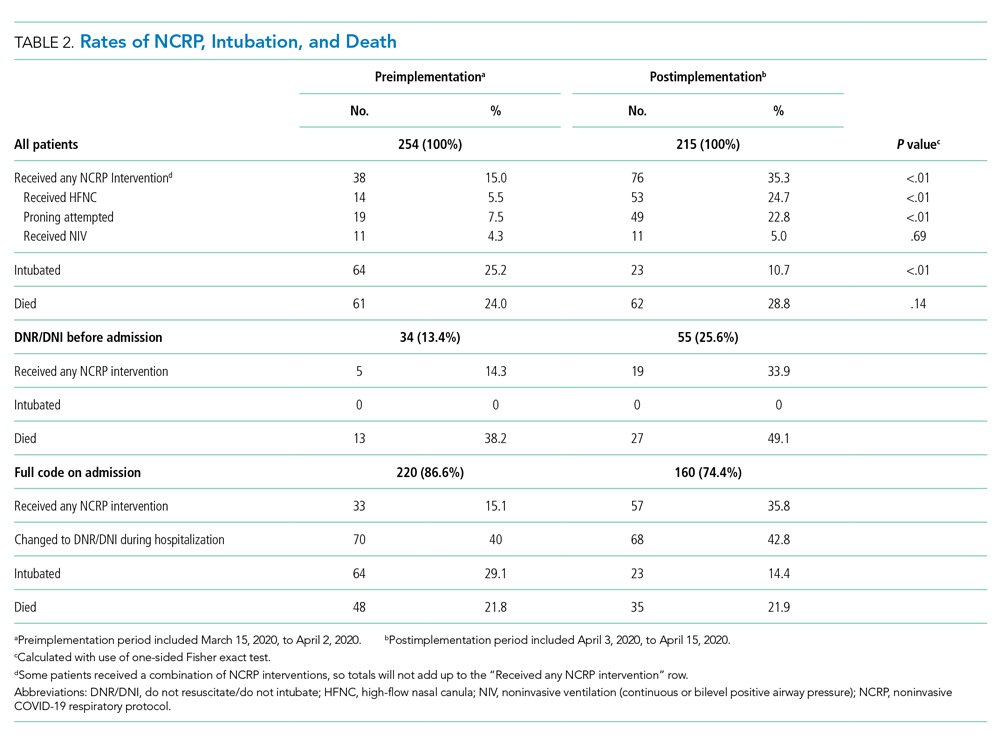
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).
Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
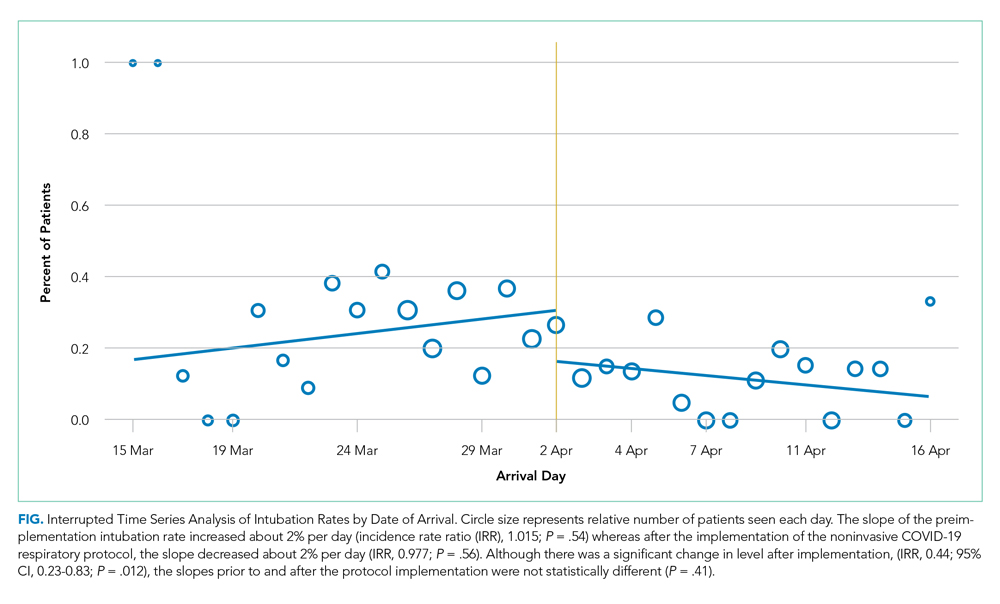
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).
Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).

Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).

Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).

Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).

Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).

Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).

Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
© 2020 Society of Hospital Medicine
A Transdisciplinary COVID-19 Early Respiratory Intervention Protocol: An Implementation Story
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.
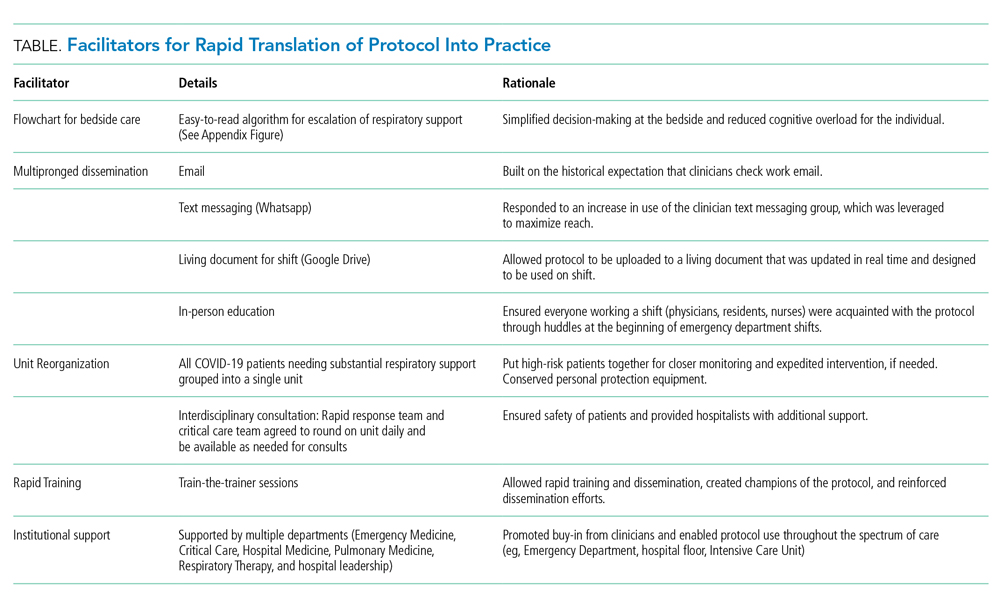
For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
© 2020 Society of Hospital Medicine