User login
Lithium-induced diabetes insipidus: Pathophysiology and treatment
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
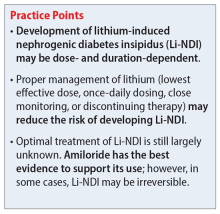
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
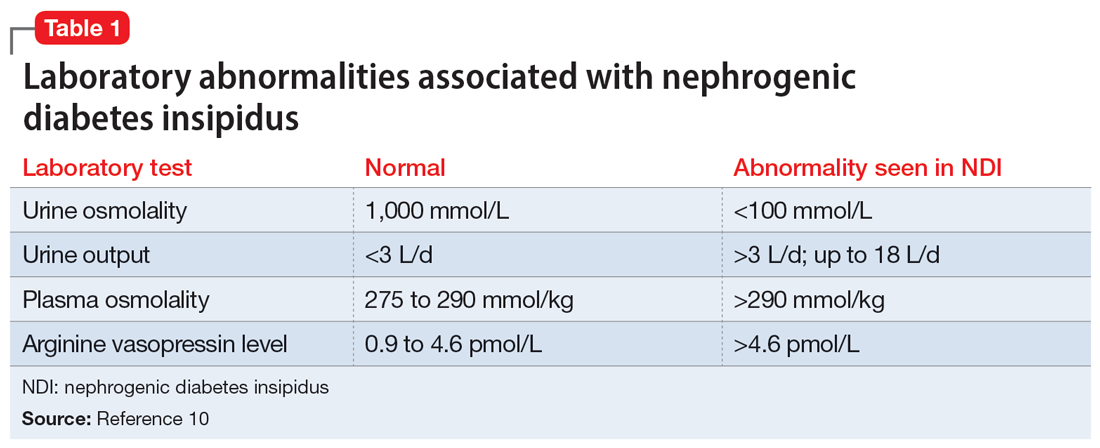
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10
Several treatment options
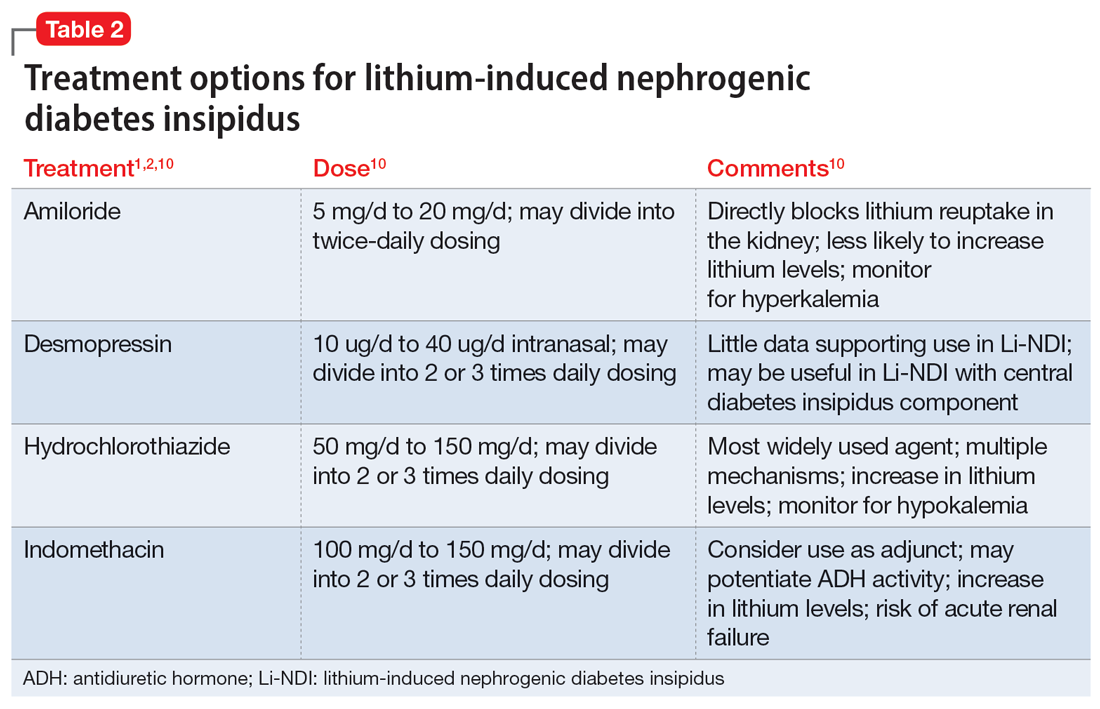
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.
Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Transitioning patients with opioid use disorder from methadone to buprenorphine
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
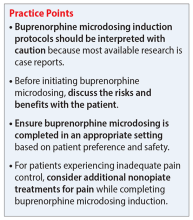
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.