User login
Zollinger-Ellison Syndrome: Not Your Average Peptic Ulcer Disease
IN THIS ARTICLE
- Diagnostic criteria
- Pharmacologic management
- Patient education
A more severe variant of peptic ulcer disease, Zollinger-Ellison syndrome (ZES) is a rare, chronic, and potentially life-threatening ulcerative disorder. Because the syndrome can be easily misdiagnosed based on clinical presentation alone, primary care clinicians need to be aware of its diagnostic features and know when referral to a gastroenterologist is necessary. Clinicians should suspect ZES in patients with peptic ulcer disease that is refractory to traditional medications.
Caused by a gastrin-secreting neuroendocrine tumor of the pancreas or duodenum called a gastrinoma, ZES can be benign or malignant. It typically manifests in white men ages 30 to 50.1 Due to the significant number of patients treated for a benign cause of peptic ulcer disease (eg, Helicobacter pylori or NSAID-induced ulcers) who are never tested for ZES, the exact incidence is difficult to determine.2 However, it is estimated that approximately 0.1 to 3 people per million develop the disease annually.3
PATHOPHYSIOLOGY
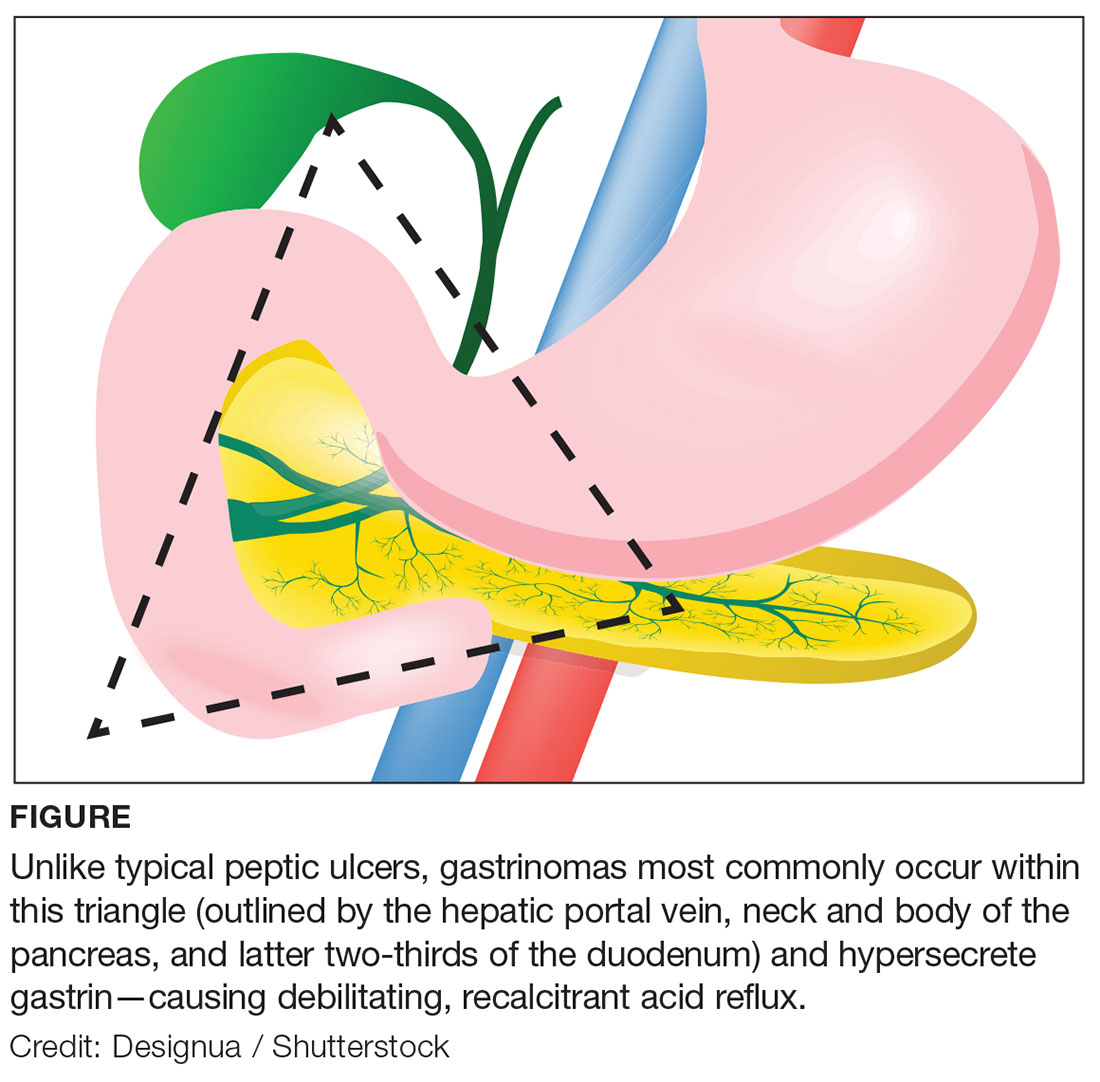
Approximately 80% of gastrinomas occur in the “gastrinoma triangle,” outlined by the hepatic portal vein, neck and body of the pancreas, and latter two-thirds of the duodenum (see Figure).1,4,5 Most gastrinomas involved in ZES occur sporadically, but there is a hereditary component associated with multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder.4
The overproduction and secretion of gastrin by the gastrinoma stimulates hypersecretion of hydrochloric acid.4 This is distinguished from high gastrin levels in the setting of fasting hypochlorhydria or achlorhydria, which may be caused by chronic atrophic gastritis, proton pump inhibitor (PPI) use, or pernicious anemia.5 The chronic hypersecretion of acid causes ulcerations to form. Most commonly, a single ulcer forms in the first portion of the duodenum.3
CLINICAL PRESENTATION
Patients with ZES often report vague abdominal pain that may mimic peptic ulcer disease on initial presentation. The widespread use of PPIs can mask symptoms, and one-fourth of patients present with no abdominal pain at all.6 Patients may also present with
The physical exam may be within normal limits, and no physical finding is considered pathognomonic for ZES. Findings may include epigastric tenderness; pallor, due to an ulcer-related anemia or GI bleed; jaundice, if there is liver involvement; and esophageal or dental erosions, due to excessive acid.8
DIAGNOSIS
Patients with symptoms refractory to medical management should be referred to a specialist for further testing. Once a patient is referred, a gastroenterologist will perform lab tests and imaging studies. In order to be diagnosed with ZES, the patient must exhibit an acidic environment with a pH less than 2 and an inappropriate release of gastrin with a basal acid output greater than 15 mEq/h (or > 5 mEq/h in a patient with prior acid reduction surgery).5,6
Fasting serum gastrin (FSG) is the initial study of choice, followed by a secretin-stimulating test when necessary.9 Diagnosis is established by an FSG level greater than 100 pg/mL; if more than 10-fold the normal level, no further testing is needed. However, results often range from 100 to 1,000 pg/mL.6,10 At these values, further testing with secretin stimulation is warranted.9 The test is performed with an IV injection of secretin, and blood samples are obtained to measure serum gastrin levels.10 An increase greater than 100 pg/mL is considered positive; one greater than 200 pg/mL is diagnostic.3
Once lab tests have been performed, a series of imaging studies are indicated. Endoscopy is used to identify active ulcers and erosions due to long-term acid secretion.3 CT, MRI, and somatostatin receptor scintigraphy (a specialized form of imaging that is the study of choice for localizing gastrinomas) are performed to localize primary tumors and identify any metastatic disease that may be present.10 Finally, after lab tests and imaging studies have been completed, genetic screening for MEN1 is used to determine if the patient has a sporadic or hereditary gastrinoma.3
MANAGEMENT
Once ZES has been diagnosed, the specialist will refer the patient for surgical opinion. The main objectives of surgery are to determine whether the tumor is malignant via biopsy, and to resect the tumor to suppress the acid hypersecretion, if indicated in the absence of liver metastasis and large pancreatic tumor size. Medical management should begin immediately to prevent any further damage from prolonged gastric hypersecretion.1
Pharmacologic options include PPIs, H2-receptor antagonists, and somatostatin analogues; PPIs are considered firstline therapy. Many patients with ZES require a higher dosage than is needed with typical GERD (60-100 mg/d vs 20-40 mg/d). Somatostatin analogues can be used in conjunction with PPIs and have been shown to inhibit tumor growth in patients with malignant ZES.1
Once a ZES diagnosis has been made, the tumor(s) resected (if appropriate), and vagotomy considered or performed, patients will need routine follow-up with their gastroenterologist and their primary care provider, who can manage medications and recommend any lifestyle changes.5
PROGNOSIS
The most important prognostic factor of patients with ZES is whether the gastrinoma is benign or malignant. There are two patterns: aggressive disease (25%) and nonaggressive disease (75%).5 At diagnosis, 40% to 70% of patients with sporadic ZES present with lymph node metastases, and 20% to 40% present with liver metastases. Patients with liver metastases have a 10-year survival rate of 30%, compared to a 15-year survival rate of 83% in patients without liver metastases.11,12
Along with the tumor itself, another prognostic factor to consider is the FSG level at diagnosis. Patients with higher FSG levels have decreased five- and 10-year survival rates compared to patients with lower FSG values. The 10-year survival rate for patients with a lower FSG value (0-499 pg/mL) is 86%, while the 10-year survival rate for those with a greater FSG value (> 1,000 pg/mL) is 73%.11,12 Overall, the prognosis is good for patients with ZES. The 10-year survival rate is high, and management is possible with medications and surgical resection of the gastrinoma.
PATIENT EDUCATION
Once patients are diagnosed, treatment with PPIs is typically lifelong unless they are considered cured by surgical resection. Patients need to understand that compliance is necessary to properly manage symptoms; certain foods, alcohol, and tobacco can affect the condition, and lifestyle modifications should be made, as they would with typical GERD or peptic ulcer disease.
CONCLUSION
ZES is frequently overlooked, and patients often continue to experience unresolved symptoms related to hypergastrinemia. Due to its complexity and ability to mimic other disorders—as well as the implications of duodenal versus pancreatic location, and other disorders of the kidney or endocrine system suggestive of MEN1—ZES should be ruled out in any patient with unexplained persistent GERD, peptic ulcer disease, elevated FSG, chronic diarrhea, and/or abdominal pain.5
The gastrinoma itself is a well-differentiated and slow-growing tumor in the majority of cases, making the prognosis for ZES favorable for long-term survival. Proper pharmacologic management is instrumental for controlling symptoms and decreasing acid production. Surgical resection offers patients the best chance for a complete cure. Clinicians and patients should be well educated about ZES in order to successfully manage the disorder.
1. Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005; 11(35):5423-5432.
2. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2016;10(2):126-130.
3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014; 19(1):44-50.
4. Papadakis M, McPhee S, Rabow M. Current Medical Diagnosis and Treatment 2014. New York, NY: McGraw-Hill Education; 2014:600-601.
5. Feldman M, Friedman LS, Lawrence BJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders/Elsevier; 2016:511-515.
6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012; 18(39):5495-5503.
7. Blonski WC, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17(4):441-444.
8. Roy PK. Zollinger-Ellison syndrome clinical presentation. http://emedicine.medscape.com/article/183555-clinical#b4. Accessed June 14, 2017.
9. Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295-330.
10. Moore AR, Varro A, Pritchard M. Zollinger-Ellison syndrome. Gastrointestinal Nursing. 2012;10(5):44-49.
11. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108(6):1637-1649.
12. Berger AC, Gibril F, Venzon DJ, et al. Prognostic value of initial fasting serum gastrin levels in patients with Zollinger-Ellison syndrome. J Clin Oncol. 2001;19(12):3051-3057.
IN THIS ARTICLE
- Diagnostic criteria
- Pharmacologic management
- Patient education
A more severe variant of peptic ulcer disease, Zollinger-Ellison syndrome (ZES) is a rare, chronic, and potentially life-threatening ulcerative disorder. Because the syndrome can be easily misdiagnosed based on clinical presentation alone, primary care clinicians need to be aware of its diagnostic features and know when referral to a gastroenterologist is necessary. Clinicians should suspect ZES in patients with peptic ulcer disease that is refractory to traditional medications.
Caused by a gastrin-secreting neuroendocrine tumor of the pancreas or duodenum called a gastrinoma, ZES can be benign or malignant. It typically manifests in white men ages 30 to 50.1 Due to the significant number of patients treated for a benign cause of peptic ulcer disease (eg, Helicobacter pylori or NSAID-induced ulcers) who are never tested for ZES, the exact incidence is difficult to determine.2 However, it is estimated that approximately 0.1 to 3 people per million develop the disease annually.3
PATHOPHYSIOLOGY
Approximately 80% of gastrinomas occur in the “gastrinoma triangle,” outlined by the hepatic portal vein, neck and body of the pancreas, and latter two-thirds of the duodenum (see Figure).1,4,5 Most gastrinomas involved in ZES occur sporadically, but there is a hereditary component associated with multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder.4
The overproduction and secretion of gastrin by the gastrinoma stimulates hypersecretion of hydrochloric acid.4 This is distinguished from high gastrin levels in the setting of fasting hypochlorhydria or achlorhydria, which may be caused by chronic atrophic gastritis, proton pump inhibitor (PPI) use, or pernicious anemia.5 The chronic hypersecretion of acid causes ulcerations to form. Most commonly, a single ulcer forms in the first portion of the duodenum.3
CLINICAL PRESENTATION
Patients with ZES often report vague abdominal pain that may mimic peptic ulcer disease on initial presentation. The widespread use of PPIs can mask symptoms, and one-fourth of patients present with no abdominal pain at all.6 Patients may also present with
The physical exam may be within normal limits, and no physical finding is considered pathognomonic for ZES. Findings may include epigastric tenderness; pallor, due to an ulcer-related anemia or GI bleed; jaundice, if there is liver involvement; and esophageal or dental erosions, due to excessive acid.8

DIAGNOSIS
Patients with symptoms refractory to medical management should be referred to a specialist for further testing. Once a patient is referred, a gastroenterologist will perform lab tests and imaging studies. In order to be diagnosed with ZES, the patient must exhibit an acidic environment with a pH less than 2 and an inappropriate release of gastrin with a basal acid output greater than 15 mEq/h (or > 5 mEq/h in a patient with prior acid reduction surgery).5,6
Fasting serum gastrin (FSG) is the initial study of choice, followed by a secretin-stimulating test when necessary.9 Diagnosis is established by an FSG level greater than 100 pg/mL; if more than 10-fold the normal level, no further testing is needed. However, results often range from 100 to 1,000 pg/mL.6,10 At these values, further testing with secretin stimulation is warranted.9 The test is performed with an IV injection of secretin, and blood samples are obtained to measure serum gastrin levels.10 An increase greater than 100 pg/mL is considered positive; one greater than 200 pg/mL is diagnostic.3
Once lab tests have been performed, a series of imaging studies are indicated. Endoscopy is used to identify active ulcers and erosions due to long-term acid secretion.3 CT, MRI, and somatostatin receptor scintigraphy (a specialized form of imaging that is the study of choice for localizing gastrinomas) are performed to localize primary tumors and identify any metastatic disease that may be present.10 Finally, after lab tests and imaging studies have been completed, genetic screening for MEN1 is used to determine if the patient has a sporadic or hereditary gastrinoma.3
MANAGEMENT
Once ZES has been diagnosed, the specialist will refer the patient for surgical opinion. The main objectives of surgery are to determine whether the tumor is malignant via biopsy, and to resect the tumor to suppress the acid hypersecretion, if indicated in the absence of liver metastasis and large pancreatic tumor size. Medical management should begin immediately to prevent any further damage from prolonged gastric hypersecretion.1
Pharmacologic options include PPIs, H2-receptor antagonists, and somatostatin analogues; PPIs are considered firstline therapy. Many patients with ZES require a higher dosage than is needed with typical GERD (60-100 mg/d vs 20-40 mg/d). Somatostatin analogues can be used in conjunction with PPIs and have been shown to inhibit tumor growth in patients with malignant ZES.1
Once a ZES diagnosis has been made, the tumor(s) resected (if appropriate), and vagotomy considered or performed, patients will need routine follow-up with their gastroenterologist and their primary care provider, who can manage medications and recommend any lifestyle changes.5
PROGNOSIS
The most important prognostic factor of patients with ZES is whether the gastrinoma is benign or malignant. There are two patterns: aggressive disease (25%) and nonaggressive disease (75%).5 At diagnosis, 40% to 70% of patients with sporadic ZES present with lymph node metastases, and 20% to 40% present with liver metastases. Patients with liver metastases have a 10-year survival rate of 30%, compared to a 15-year survival rate of 83% in patients without liver metastases.11,12
Along with the tumor itself, another prognostic factor to consider is the FSG level at diagnosis. Patients with higher FSG levels have decreased five- and 10-year survival rates compared to patients with lower FSG values. The 10-year survival rate for patients with a lower FSG value (0-499 pg/mL) is 86%, while the 10-year survival rate for those with a greater FSG value (> 1,000 pg/mL) is 73%.11,12 Overall, the prognosis is good for patients with ZES. The 10-year survival rate is high, and management is possible with medications and surgical resection of the gastrinoma.
PATIENT EDUCATION
Once patients are diagnosed, treatment with PPIs is typically lifelong unless they are considered cured by surgical resection. Patients need to understand that compliance is necessary to properly manage symptoms; certain foods, alcohol, and tobacco can affect the condition, and lifestyle modifications should be made, as they would with typical GERD or peptic ulcer disease.
CONCLUSION
ZES is frequently overlooked, and patients often continue to experience unresolved symptoms related to hypergastrinemia. Due to its complexity and ability to mimic other disorders—as well as the implications of duodenal versus pancreatic location, and other disorders of the kidney or endocrine system suggestive of MEN1—ZES should be ruled out in any patient with unexplained persistent GERD, peptic ulcer disease, elevated FSG, chronic diarrhea, and/or abdominal pain.5
The gastrinoma itself is a well-differentiated and slow-growing tumor in the majority of cases, making the prognosis for ZES favorable for long-term survival. Proper pharmacologic management is instrumental for controlling symptoms and decreasing acid production. Surgical resection offers patients the best chance for a complete cure. Clinicians and patients should be well educated about ZES in order to successfully manage the disorder.
IN THIS ARTICLE
- Diagnostic criteria
- Pharmacologic management
- Patient education
A more severe variant of peptic ulcer disease, Zollinger-Ellison syndrome (ZES) is a rare, chronic, and potentially life-threatening ulcerative disorder. Because the syndrome can be easily misdiagnosed based on clinical presentation alone, primary care clinicians need to be aware of its diagnostic features and know when referral to a gastroenterologist is necessary. Clinicians should suspect ZES in patients with peptic ulcer disease that is refractory to traditional medications.
Caused by a gastrin-secreting neuroendocrine tumor of the pancreas or duodenum called a gastrinoma, ZES can be benign or malignant. It typically manifests in white men ages 30 to 50.1 Due to the significant number of patients treated for a benign cause of peptic ulcer disease (eg, Helicobacter pylori or NSAID-induced ulcers) who are never tested for ZES, the exact incidence is difficult to determine.2 However, it is estimated that approximately 0.1 to 3 people per million develop the disease annually.3
PATHOPHYSIOLOGY
Approximately 80% of gastrinomas occur in the “gastrinoma triangle,” outlined by the hepatic portal vein, neck and body of the pancreas, and latter two-thirds of the duodenum (see Figure).1,4,5 Most gastrinomas involved in ZES occur sporadically, but there is a hereditary component associated with multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder.4
The overproduction and secretion of gastrin by the gastrinoma stimulates hypersecretion of hydrochloric acid.4 This is distinguished from high gastrin levels in the setting of fasting hypochlorhydria or achlorhydria, which may be caused by chronic atrophic gastritis, proton pump inhibitor (PPI) use, or pernicious anemia.5 The chronic hypersecretion of acid causes ulcerations to form. Most commonly, a single ulcer forms in the first portion of the duodenum.3
CLINICAL PRESENTATION
Patients with ZES often report vague abdominal pain that may mimic peptic ulcer disease on initial presentation. The widespread use of PPIs can mask symptoms, and one-fourth of patients present with no abdominal pain at all.6 Patients may also present with
The physical exam may be within normal limits, and no physical finding is considered pathognomonic for ZES. Findings may include epigastric tenderness; pallor, due to an ulcer-related anemia or GI bleed; jaundice, if there is liver involvement; and esophageal or dental erosions, due to excessive acid.8

DIAGNOSIS
Patients with symptoms refractory to medical management should be referred to a specialist for further testing. Once a patient is referred, a gastroenterologist will perform lab tests and imaging studies. In order to be diagnosed with ZES, the patient must exhibit an acidic environment with a pH less than 2 and an inappropriate release of gastrin with a basal acid output greater than 15 mEq/h (or > 5 mEq/h in a patient with prior acid reduction surgery).5,6
Fasting serum gastrin (FSG) is the initial study of choice, followed by a secretin-stimulating test when necessary.9 Diagnosis is established by an FSG level greater than 100 pg/mL; if more than 10-fold the normal level, no further testing is needed. However, results often range from 100 to 1,000 pg/mL.6,10 At these values, further testing with secretin stimulation is warranted.9 The test is performed with an IV injection of secretin, and blood samples are obtained to measure serum gastrin levels.10 An increase greater than 100 pg/mL is considered positive; one greater than 200 pg/mL is diagnostic.3
Once lab tests have been performed, a series of imaging studies are indicated. Endoscopy is used to identify active ulcers and erosions due to long-term acid secretion.3 CT, MRI, and somatostatin receptor scintigraphy (a specialized form of imaging that is the study of choice for localizing gastrinomas) are performed to localize primary tumors and identify any metastatic disease that may be present.10 Finally, after lab tests and imaging studies have been completed, genetic screening for MEN1 is used to determine if the patient has a sporadic or hereditary gastrinoma.3
MANAGEMENT
Once ZES has been diagnosed, the specialist will refer the patient for surgical opinion. The main objectives of surgery are to determine whether the tumor is malignant via biopsy, and to resect the tumor to suppress the acid hypersecretion, if indicated in the absence of liver metastasis and large pancreatic tumor size. Medical management should begin immediately to prevent any further damage from prolonged gastric hypersecretion.1
Pharmacologic options include PPIs, H2-receptor antagonists, and somatostatin analogues; PPIs are considered firstline therapy. Many patients with ZES require a higher dosage than is needed with typical GERD (60-100 mg/d vs 20-40 mg/d). Somatostatin analogues can be used in conjunction with PPIs and have been shown to inhibit tumor growth in patients with malignant ZES.1
Once a ZES diagnosis has been made, the tumor(s) resected (if appropriate), and vagotomy considered or performed, patients will need routine follow-up with their gastroenterologist and their primary care provider, who can manage medications and recommend any lifestyle changes.5
PROGNOSIS
The most important prognostic factor of patients with ZES is whether the gastrinoma is benign or malignant. There are two patterns: aggressive disease (25%) and nonaggressive disease (75%).5 At diagnosis, 40% to 70% of patients with sporadic ZES present with lymph node metastases, and 20% to 40% present with liver metastases. Patients with liver metastases have a 10-year survival rate of 30%, compared to a 15-year survival rate of 83% in patients without liver metastases.11,12
Along with the tumor itself, another prognostic factor to consider is the FSG level at diagnosis. Patients with higher FSG levels have decreased five- and 10-year survival rates compared to patients with lower FSG values. The 10-year survival rate for patients with a lower FSG value (0-499 pg/mL) is 86%, while the 10-year survival rate for those with a greater FSG value (> 1,000 pg/mL) is 73%.11,12 Overall, the prognosis is good for patients with ZES. The 10-year survival rate is high, and management is possible with medications and surgical resection of the gastrinoma.
PATIENT EDUCATION
Once patients are diagnosed, treatment with PPIs is typically lifelong unless they are considered cured by surgical resection. Patients need to understand that compliance is necessary to properly manage symptoms; certain foods, alcohol, and tobacco can affect the condition, and lifestyle modifications should be made, as they would with typical GERD or peptic ulcer disease.
CONCLUSION
ZES is frequently overlooked, and patients often continue to experience unresolved symptoms related to hypergastrinemia. Due to its complexity and ability to mimic other disorders—as well as the implications of duodenal versus pancreatic location, and other disorders of the kidney or endocrine system suggestive of MEN1—ZES should be ruled out in any patient with unexplained persistent GERD, peptic ulcer disease, elevated FSG, chronic diarrhea, and/or abdominal pain.5
The gastrinoma itself is a well-differentiated and slow-growing tumor in the majority of cases, making the prognosis for ZES favorable for long-term survival. Proper pharmacologic management is instrumental for controlling symptoms and decreasing acid production. Surgical resection offers patients the best chance for a complete cure. Clinicians and patients should be well educated about ZES in order to successfully manage the disorder.
1. Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005; 11(35):5423-5432.
2. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2016;10(2):126-130.
3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014; 19(1):44-50.
4. Papadakis M, McPhee S, Rabow M. Current Medical Diagnosis and Treatment 2014. New York, NY: McGraw-Hill Education; 2014:600-601.
5. Feldman M, Friedman LS, Lawrence BJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders/Elsevier; 2016:511-515.
6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012; 18(39):5495-5503.
7. Blonski WC, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17(4):441-444.
8. Roy PK. Zollinger-Ellison syndrome clinical presentation. http://emedicine.medscape.com/article/183555-clinical#b4. Accessed June 14, 2017.
9. Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295-330.
10. Moore AR, Varro A, Pritchard M. Zollinger-Ellison syndrome. Gastrointestinal Nursing. 2012;10(5):44-49.
11. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108(6):1637-1649.
12. Berger AC, Gibril F, Venzon DJ, et al. Prognostic value of initial fasting serum gastrin levels in patients with Zollinger-Ellison syndrome. J Clin Oncol. 2001;19(12):3051-3057.
1. Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005; 11(35):5423-5432.
2. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2016;10(2):126-130.
3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014; 19(1):44-50.
4. Papadakis M, McPhee S, Rabow M. Current Medical Diagnosis and Treatment 2014. New York, NY: McGraw-Hill Education; 2014:600-601.
5. Feldman M, Friedman LS, Lawrence BJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders/Elsevier; 2016:511-515.
6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012; 18(39):5495-5503.
7. Blonski WC, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17(4):441-444.
8. Roy PK. Zollinger-Ellison syndrome clinical presentation. http://emedicine.medscape.com/article/183555-clinical#b4. Accessed June 14, 2017.
9. Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295-330.
10. Moore AR, Varro A, Pritchard M. Zollinger-Ellison syndrome. Gastrointestinal Nursing. 2012;10(5):44-49.
11. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108(6):1637-1649.
12. Berger AC, Gibril F, Venzon DJ, et al. Prognostic value of initial fasting serum gastrin levels in patients with Zollinger-Ellison syndrome. J Clin Oncol. 2001;19(12):3051-3057.