User login
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
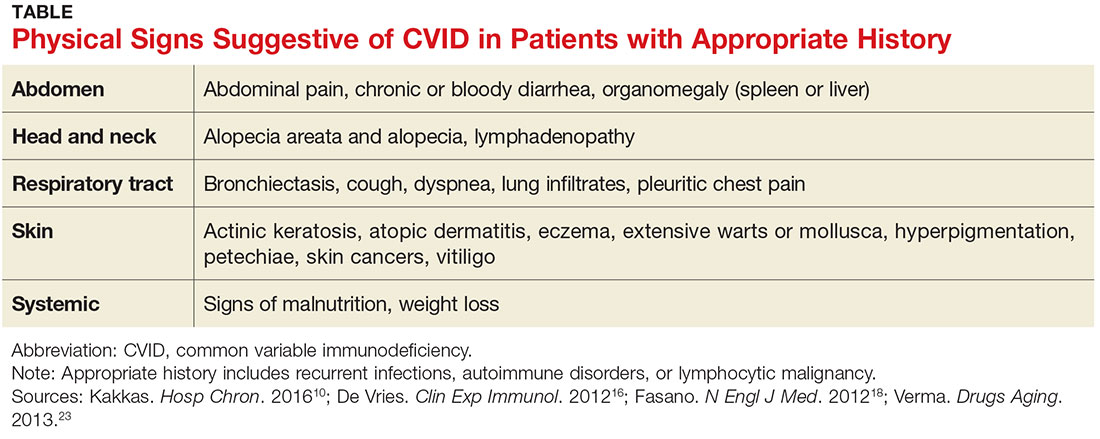
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23
The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23
The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23
The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.