User login
Aortic valve replacement: Options, improvements, and costs
How aortic valve disease is managed continues to evolve, with novel approaches for both aortic valve stenosis and regurgitation.1–8 Indeed, because of the spectrum of procedures, a multispecialty committee was formed to provide a detailed guideline to help physicians work through the various options.4
The paper by Aksoy and colleagues in this issue of the Journal gives further insight into the complexities of decision-making.
As a rule, the indications for a procedure to treat aortic valvular disease continue to be based on whether the patient develops certain symptoms (fatigue, exertional dyspnea, shortness of breath, syncope, chest pain), myocardial deterioration, reduced ejection fraction, or ventricular dilatation.4 Furthermore, the options depend on whether the patient has comorbid disease and is a candidate for surgical aortic valve replacement.
OPEN SURGERY: THE MAINSTAY OF TREATMENT
Open surgery—including in recent years minimally invasive J-incision “keyhole” repair or replacement—has been the mainstay of treatment. The results of surgical aortic valve repair have been excellent, so that 10 years after surgery 95% of patients who have undergone a modified David reimplantation operation have not needed a repeat operation.3 The results are comparable for repair of bicuspid aortic valves.2,3
Furthermore, surgical aortic valve replacement has become very safe. At Cleveland Clinic in 2011, only 3 (0.6%) of 479 patients died during isolated aortic valve replacement, and in 2012 the mortality rate was even better, with only 1 death (0.2%) among 495 patients as of November 2012.
GOOD RESULTS WITH TRANSCATHETER AORTIC VALVE REPLACEMENT
For a new valve procedure to be accepted into practice, it must be easy to do, safe, and consistently good in performance measures such as producing low gradients, eliminating aortic regurgitation, and leading to high rates of long-term freedom from reoperation and of survival. To see if percutaneous aortic valve replacement meets these criteria, it was evaluated by both us at Cleveland Clinic and our colleagues at other institutions in the laboratory and also in feasibility trials in the United States.
The subsequent Placement of Transcatheter Aortic Valves (PARTNER) trial established the benefit of this procedure in terms of superior survival for patients who could not undergo surgery.8 Hence, the transcatheter device was approved for patients who cannot undergo surgery who meet certain criteria (valve area < 0.8 cm2; mean gradient > 40 mm Hg or peak gradient > 64 mm Hg). Of note, the cost per procedure was $78,000, or approximately $50,000 per year of life saved.
The PARTNER A trial showed that the risk of death after transcatheter aortic valve replacement was as low as after open surgery, although the risk of stroke or transient ischemic attack risk was higher—indeed, with the transfemoral approach it was 3 times higher (4.6% vs 1.4%, P < .05).9,10 Furthermore, half the patients had perivalvular leakage after the new procedure, and even mild leakage reduced the survival rate at 2 years.11
Nevertheless, we have now done nearly 400 transcatheter aortic valve replacement procedures in patients who could not undergo open surgery or who would have been at extreme risk during surgery. With the transfemoral approach, in 267 patients, 1 patient died (0.4%), and 2 had strokes (0.7%). (In the rest of the patients, we used alternatives to the transfemoral approach, such as the transaortic, transapical, and transaxillary approaches, also with good results.)
Thus, transcatheter aortic valve replacement in properly selected patients can meet the above criteria.
COSTS AND THE FUTURE
Based on the PARTNER trial results, the Centers for Medicare and Medicaid Services (CMS) agreed to pay for this procedure at the same rate as for surgical aortic valve replacement for patients who cannot or should not undergo surgery, with the approval of two surgeons and within the context of a national registry.10
The reimbursement is adjusted for geographic area. In the United States, for example, hospitals on the East Coast or West Coast receive $88,000 to $94,000 per case, while most other areas receive $32,000 to $62,000.
The surgeon and cardiologist share the professional fee of approximately $2,500, although typically we have a team of eight to 10 physicians (representing the fields of anesthesia, echocardiography, surgery, and cardiology) in the operating room for every procedure, in addition to nursing and technical staff. The challenge for institutions and providers, however, is that the device costs $32,500, and CMS reimbursement does not cover the cost of both the valve and the procedure in many localities. This may affect how widely the valve is eventually used.
While many more options are available now for management of aortic valve disease (minimally invasive repair or replacement, and newer devices), the future usage of transcatheter aortic valve replacement may become dependent on costs, newer devices, cheaper iterations, competition, and CMS reimbursement.
There are now two additional trials, SURTAVI and PARTNER A2, evaluating transcatheter vs open aortic valve replacement in lower-risk patients. The issues that will have to be addressed with new iterations are the risk of stroke and transient ischemic attack, perivalvular leakage, and the costs of the devices.
Newer reports would suggest that the results with transcatheter aortic valve replacement in inoperable and high-risk patients continue to improve as experience evolves.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011; 142:622–629.e1–e3.
- Svensson LG, Batizy LH, Blackstone EH, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011; 142:1491–1498.e7.
- Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg 2013; 10.1016/j.athoracsur.2012.12.027, Epub ahead of print
- Svensson LG, D’Agostino RS. “J” incision minimal-access valve operations”. Ann Thorac Surg 1998; 66:1110–1112.
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012; 144:852–858.e3.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2013; Epub ahead of print.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013; 145(suppl):S11–S16.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
How aortic valve disease is managed continues to evolve, with novel approaches for both aortic valve stenosis and regurgitation.1–8 Indeed, because of the spectrum of procedures, a multispecialty committee was formed to provide a detailed guideline to help physicians work through the various options.4
The paper by Aksoy and colleagues in this issue of the Journal gives further insight into the complexities of decision-making.
As a rule, the indications for a procedure to treat aortic valvular disease continue to be based on whether the patient develops certain symptoms (fatigue, exertional dyspnea, shortness of breath, syncope, chest pain), myocardial deterioration, reduced ejection fraction, or ventricular dilatation.4 Furthermore, the options depend on whether the patient has comorbid disease and is a candidate for surgical aortic valve replacement.
OPEN SURGERY: THE MAINSTAY OF TREATMENT
Open surgery—including in recent years minimally invasive J-incision “keyhole” repair or replacement—has been the mainstay of treatment. The results of surgical aortic valve repair have been excellent, so that 10 years after surgery 95% of patients who have undergone a modified David reimplantation operation have not needed a repeat operation.3 The results are comparable for repair of bicuspid aortic valves.2,3
Furthermore, surgical aortic valve replacement has become very safe. At Cleveland Clinic in 2011, only 3 (0.6%) of 479 patients died during isolated aortic valve replacement, and in 2012 the mortality rate was even better, with only 1 death (0.2%) among 495 patients as of November 2012.
GOOD RESULTS WITH TRANSCATHETER AORTIC VALVE REPLACEMENT
For a new valve procedure to be accepted into practice, it must be easy to do, safe, and consistently good in performance measures such as producing low gradients, eliminating aortic regurgitation, and leading to high rates of long-term freedom from reoperation and of survival. To see if percutaneous aortic valve replacement meets these criteria, it was evaluated by both us at Cleveland Clinic and our colleagues at other institutions in the laboratory and also in feasibility trials in the United States.
The subsequent Placement of Transcatheter Aortic Valves (PARTNER) trial established the benefit of this procedure in terms of superior survival for patients who could not undergo surgery.8 Hence, the transcatheter device was approved for patients who cannot undergo surgery who meet certain criteria (valve area < 0.8 cm2; mean gradient > 40 mm Hg or peak gradient > 64 mm Hg). Of note, the cost per procedure was $78,000, or approximately $50,000 per year of life saved.
The PARTNER A trial showed that the risk of death after transcatheter aortic valve replacement was as low as after open surgery, although the risk of stroke or transient ischemic attack risk was higher—indeed, with the transfemoral approach it was 3 times higher (4.6% vs 1.4%, P < .05).9,10 Furthermore, half the patients had perivalvular leakage after the new procedure, and even mild leakage reduced the survival rate at 2 years.11
Nevertheless, we have now done nearly 400 transcatheter aortic valve replacement procedures in patients who could not undergo open surgery or who would have been at extreme risk during surgery. With the transfemoral approach, in 267 patients, 1 patient died (0.4%), and 2 had strokes (0.7%). (In the rest of the patients, we used alternatives to the transfemoral approach, such as the transaortic, transapical, and transaxillary approaches, also with good results.)
Thus, transcatheter aortic valve replacement in properly selected patients can meet the above criteria.
COSTS AND THE FUTURE
Based on the PARTNER trial results, the Centers for Medicare and Medicaid Services (CMS) agreed to pay for this procedure at the same rate as for surgical aortic valve replacement for patients who cannot or should not undergo surgery, with the approval of two surgeons and within the context of a national registry.10
The reimbursement is adjusted for geographic area. In the United States, for example, hospitals on the East Coast or West Coast receive $88,000 to $94,000 per case, while most other areas receive $32,000 to $62,000.
The surgeon and cardiologist share the professional fee of approximately $2,500, although typically we have a team of eight to 10 physicians (representing the fields of anesthesia, echocardiography, surgery, and cardiology) in the operating room for every procedure, in addition to nursing and technical staff. The challenge for institutions and providers, however, is that the device costs $32,500, and CMS reimbursement does not cover the cost of both the valve and the procedure in many localities. This may affect how widely the valve is eventually used.
While many more options are available now for management of aortic valve disease (minimally invasive repair or replacement, and newer devices), the future usage of transcatheter aortic valve replacement may become dependent on costs, newer devices, cheaper iterations, competition, and CMS reimbursement.
There are now two additional trials, SURTAVI and PARTNER A2, evaluating transcatheter vs open aortic valve replacement in lower-risk patients. The issues that will have to be addressed with new iterations are the risk of stroke and transient ischemic attack, perivalvular leakage, and the costs of the devices.
Newer reports would suggest that the results with transcatheter aortic valve replacement in inoperable and high-risk patients continue to improve as experience evolves.
How aortic valve disease is managed continues to evolve, with novel approaches for both aortic valve stenosis and regurgitation.1–8 Indeed, because of the spectrum of procedures, a multispecialty committee was formed to provide a detailed guideline to help physicians work through the various options.4
The paper by Aksoy and colleagues in this issue of the Journal gives further insight into the complexities of decision-making.
As a rule, the indications for a procedure to treat aortic valvular disease continue to be based on whether the patient develops certain symptoms (fatigue, exertional dyspnea, shortness of breath, syncope, chest pain), myocardial deterioration, reduced ejection fraction, or ventricular dilatation.4 Furthermore, the options depend on whether the patient has comorbid disease and is a candidate for surgical aortic valve replacement.
OPEN SURGERY: THE MAINSTAY OF TREATMENT
Open surgery—including in recent years minimally invasive J-incision “keyhole” repair or replacement—has been the mainstay of treatment. The results of surgical aortic valve repair have been excellent, so that 10 years after surgery 95% of patients who have undergone a modified David reimplantation operation have not needed a repeat operation.3 The results are comparable for repair of bicuspid aortic valves.2,3
Furthermore, surgical aortic valve replacement has become very safe. At Cleveland Clinic in 2011, only 3 (0.6%) of 479 patients died during isolated aortic valve replacement, and in 2012 the mortality rate was even better, with only 1 death (0.2%) among 495 patients as of November 2012.
GOOD RESULTS WITH TRANSCATHETER AORTIC VALVE REPLACEMENT
For a new valve procedure to be accepted into practice, it must be easy to do, safe, and consistently good in performance measures such as producing low gradients, eliminating aortic regurgitation, and leading to high rates of long-term freedom from reoperation and of survival. To see if percutaneous aortic valve replacement meets these criteria, it was evaluated by both us at Cleveland Clinic and our colleagues at other institutions in the laboratory and also in feasibility trials in the United States.
The subsequent Placement of Transcatheter Aortic Valves (PARTNER) trial established the benefit of this procedure in terms of superior survival for patients who could not undergo surgery.8 Hence, the transcatheter device was approved for patients who cannot undergo surgery who meet certain criteria (valve area < 0.8 cm2; mean gradient > 40 mm Hg or peak gradient > 64 mm Hg). Of note, the cost per procedure was $78,000, or approximately $50,000 per year of life saved.
The PARTNER A trial showed that the risk of death after transcatheter aortic valve replacement was as low as after open surgery, although the risk of stroke or transient ischemic attack risk was higher—indeed, with the transfemoral approach it was 3 times higher (4.6% vs 1.4%, P < .05).9,10 Furthermore, half the patients had perivalvular leakage after the new procedure, and even mild leakage reduced the survival rate at 2 years.11
Nevertheless, we have now done nearly 400 transcatheter aortic valve replacement procedures in patients who could not undergo open surgery or who would have been at extreme risk during surgery. With the transfemoral approach, in 267 patients, 1 patient died (0.4%), and 2 had strokes (0.7%). (In the rest of the patients, we used alternatives to the transfemoral approach, such as the transaortic, transapical, and transaxillary approaches, also with good results.)
Thus, transcatheter aortic valve replacement in properly selected patients can meet the above criteria.
COSTS AND THE FUTURE
Based on the PARTNER trial results, the Centers for Medicare and Medicaid Services (CMS) agreed to pay for this procedure at the same rate as for surgical aortic valve replacement for patients who cannot or should not undergo surgery, with the approval of two surgeons and within the context of a national registry.10
The reimbursement is adjusted for geographic area. In the United States, for example, hospitals on the East Coast or West Coast receive $88,000 to $94,000 per case, while most other areas receive $32,000 to $62,000.
The surgeon and cardiologist share the professional fee of approximately $2,500, although typically we have a team of eight to 10 physicians (representing the fields of anesthesia, echocardiography, surgery, and cardiology) in the operating room for every procedure, in addition to nursing and technical staff. The challenge for institutions and providers, however, is that the device costs $32,500, and CMS reimbursement does not cover the cost of both the valve and the procedure in many localities. This may affect how widely the valve is eventually used.
While many more options are available now for management of aortic valve disease (minimally invasive repair or replacement, and newer devices), the future usage of transcatheter aortic valve replacement may become dependent on costs, newer devices, cheaper iterations, competition, and CMS reimbursement.
There are now two additional trials, SURTAVI and PARTNER A2, evaluating transcatheter vs open aortic valve replacement in lower-risk patients. The issues that will have to be addressed with new iterations are the risk of stroke and transient ischemic attack, perivalvular leakage, and the costs of the devices.
Newer reports would suggest that the results with transcatheter aortic valve replacement in inoperable and high-risk patients continue to improve as experience evolves.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011; 142:622–629.e1–e3.
- Svensson LG, Batizy LH, Blackstone EH, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011; 142:1491–1498.e7.
- Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg 2013; 10.1016/j.athoracsur.2012.12.027, Epub ahead of print
- Svensson LG, D’Agostino RS. “J” incision minimal-access valve operations”. Ann Thorac Surg 1998; 66:1110–1112.
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012; 144:852–858.e3.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2013; Epub ahead of print.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013; 145(suppl):S11–S16.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011; 142:622–629.e1–e3.
- Svensson LG, Batizy LH, Blackstone EH, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011; 142:1491–1498.e7.
- Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg 2013; 10.1016/j.athoracsur.2012.12.027, Epub ahead of print
- Svensson LG, D’Agostino RS. “J” incision minimal-access valve operations”. Ann Thorac Surg 1998; 66:1110–1112.
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012; 144:852–858.e3.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2013; Epub ahead of print.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013; 145(suppl):S11–S16.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
Evolution and results of aortic valve surgery, and a ‘disruptive’ technology
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
Acute aortic syndromes: Time to talk of many things
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
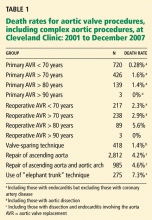
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.