User login
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
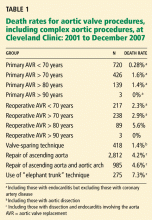
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.