User login
Sudden unexpected death in epilepsy: Finding the missing cardiac links
Sudden death in epilepsy, surgery, and seizure outcomes: The interface between heart and brain
Sudden unexpected death in epilepsy (SUDEP) is most often defined as the sudden, unexpected, nontraumatic, and nondrowning death of patients with epilepsy. The death may be either witnessed or unwitnessed, and may occur with or without evidence of a seizure, excluding documented status epilepticus. In cases of definite SUDEP, postmortem examination does not reveal a structural or toxicologic cause of death.1
The reported incidence of SUDEP ranges from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of epilepsy patients2,3 and from 3.5 to 9.3 cases per 1,000 patient-years in antiepileptic drug registries, medical device registries, and epilepsy surgery programs.4–6 SUDEP is currently accepted as the most important epilepsy-related mode of death5,7 and is associated with standardized mortality ratios in patients with ongoing seizures as high as 24 to 40 times those of the general population.8 The exact mechanism or mechanisms leading to SUDEP remain unknown,1,5,9,10 and despite the identification of several potential risk factors, elimination of seizures still represents the main intervention correlating with risk reduction.5,11
This review will first discuss evidence for a cardiac mechanism of SUDEP, then review data traditionally used to support freedom from seizures following epilepsy surgery as a method for reducing SUDEP risk, and finally examine recent evidence suggesting that both SUDEP and seizure outcomes following epilepsy surgery are governed by a common pathway with certain cardiac manifestations.
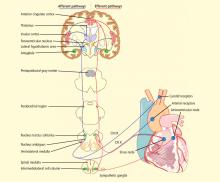
CENTRAL AUTONOMIC NETWORK
EVIDENCE FOR A CARDIAC MECHANISM OF SUDEP
The most significant and broadly discussed cardiac mechanism of SUDEP is cardiac arrhythmia brought about by seizure discharges acting through the autonomic nervous system.5,7,15,16
Clinical evidence
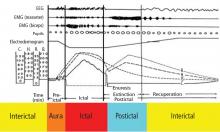
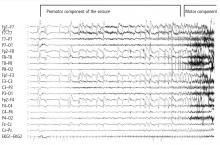
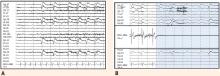
A wide spectrum of cardiac arrhythmias—from ictal asystole to atrial fibrillation to repolarization abnormalities to bundle branch blocks—has been reported during seizures. 14–19 In one study, ictal cardiac arrhythmias occurred in 42% of hospitalized epilepsy patients, the most common being an irregular series of abrupt rate changes near the end of the electroencephalographic (EEG) seizure discharge.17 In another study, R-R interval analysis during the first 10-second period of EEG discharge showed a significant early heart rate increase in 49% of seizures and an early heart rate reduction in 25.5%.18
Certain clinical seizure characteristics have been correlated with the occurrence of ictal electrocardiographic (ECG) abnormalities. One study found that mean seizure duration was longer in patients with ECG abnormalities than in those without such changes.16 Others observed that ictal ECG abnormalities occurred more often and were more severe in generalized tonic-clonic seizures than in complex partial seizures.15,16,19 These same clinical seizure characteristics were correlated with a higher risk of SUDEP,20 which suggests an interrelation among seizure semiology, ECG abnormalities, and SUDEP.
Additionally, direct evidence of seizure-related cardiac changes occurring specifically in SUDEP victims has come from a study that compared EEG and ECG data between 21 SUDEP patients and 43 clinically similar historical controls with refractory partial epilepsy.15 The patients who eventually succumbed to SUDEP had a higher ictal maximal heart rate, a greater increase in heart rate with seizures arising from sleep, and a higher incidence of ictal cardiac repolarization and rhythm abnormalities.15
Experimental evidence
Electrical brain stimulation of the limbic system and insular cortex has repeatedly been shown to provoke heart rate changes, including bradycardia, tachycardia, and asystole.14 Some studies have even suggested a lateralized influence of the insulae on cardiovascular autonomic control, with intraoperative stimulation of the left posterior insula eliciting a cardioinhibitory response and hypotension, and with stimulation of the right anterior insula eliciting tachycardia and hypertension.7 Other studies have suggested that the limbic system has a localization-related influence on cardiovascular responses, with stimulation of the amygdala alone being insufficient to produce the ictal tachycardia so commonly seen in epileptic seizures, which suggests that cortical involvement is essential for the increase in heart rate.21 Such cortical stimulation–induced heart rate changes may explain how massive seizure-related discharges can affect cardiac rhythm during the seizure itself.
There is also, however, evidence of a baseline epilepsy-related autonomic dysfunction. Abnormalities in cardiac uptake of meta-iodobenzylguanidine (MIBG) have been demonstrated interictally in patients with chronic temporal lobe epilepsy relative to controls.22 A recent study specifically showed a pronounced reduction in cardiac MIBG uptake in patients who had ictal asystole compared with other epileptic patients and nonepileptic controls, a finding that suggests a postganglionic cardiac catecholamine disturbance in patients with epilepsy.23 The authors proposed that epilepsy-related impairment of sympathetic cardiac innervation limits adjustment and heart rate modulation, and may thus increase the risk of asystole and, ultimately, SUDEP.23
EPILEPSY SURGERY AND SUDEP RISK
The above findings make it reasonable to postulate that recurrent uncontrolled seizures may lead to significant cardiac changes—namely, arrhythmias—which may then lead to a higher risk of SUDEP. Successful epilepsy surgery, the treatment of choice for uncontrolled partial epilepsy, would eliminate seizures and would thus be expected to reduce SUDEP risk.
Several studies support this hypothesis. Reductions in all-cause mortality and in SUDEP following successful epilepsy surgery have been observed when patients who became seizure-free after surgery were compared either with patients who had ongoing postoperative seizures or with patients with intractable epilepsy who did not undergo epilepsy surgery.11,24 In one study with a mean follow-up of 3.82 years, no deaths occurred among 199 patients who were seizure-free following epilepsy surgery, whereas there were 11 deaths—including 6 cases of SUDEP—among 194 patients who had persistent seizures following epilepsy surgery.11 A separate study compared 202 patients who underwent epilepsy surgery with 46 patients with medically treated intractable epilepsy over a mean follow-up period of greater than 5.7 years.24 In this study, death occurred in only 7% of the surgical patients compared with 20% of the medically treated patients, which suggests that epilepsy surgery (or lack thereof) may be an independent predictor of mortality. Favorable outcome was linked closely to seizure control, as 81% of the patients who died had 2 or more seizures per year at last follow-up, compared with only 47% of survivors in the overall cohort.24
Complete seizure resolution—not just reduction—seems necessary for SUDEP risk reduction
In our experience at the Cleveland Clinic Epilepsy Center, we have found that a reduction in seizure frequency following epilepsy surgery is not enough to eliminate SUDEP risk—complete freedom from seizures is required. Of 37 SUDEP cases identified so far in a cohort of 3,481 patients evaluated in our epilepsy monitoring unit between 1990 and 2005 (Jehi et al, in preparation), 7 patients had undergone epilepsy surgery. None of these 7 patients was seizure-free at the time of death; four had a greater than 50% reduction in seizure frequency. No cases of SUDEP occurred in patients who were seizure-free after surgery.
Our findings mirror earlier results reported by Sperling et al,11 who also found that epilepsy surgery improves mortality only when seizures resolve completely, not when they merely “improve.” It seems plausible, therefore, that elimination of seizures postoperatively eliminates the risk for the several seizure-related cardiac arrhythmogenic changes discussed above, thereby eliminating the risk for the series of events that eventually leads to SUDEP.
A role for stabilized baseline autonomic control?
Alternatively, some data suggest that the “autonomic” impacts of epilepsy surgery may extend beyond the immediate seizure-related manifestations to the baseline interictal period in epilepsy patients. One study found that surgery for temporal lobe epilepsy is followed by a reduction of sympathetic cardiovascular modulation and baroreflex sensitivity.25 The authors proposed that this finding may be attributable to decreased influences of interictal epileptogenic discharges on brain areas involved in cardiovascular autonomic control. These researchers continue to postulate that surgery for temporal lobe epilepsy seems to stabilize cardiovascular control in epilepsy patients by reducing the risk of sympathetically mediated tachyarrhythmias and excessive bradycardiac counterregulation, potentially lowering SUDEP risk.25
CARDIAC FINDINGS, SUDEP, AND SEIZURE OUTCOMES
Whether it is the elimination of seizure-related cardiac arrhythmias achieved by rendering patients seizure-free after surgery, or whether it is the stabilization of baseline autonomic cardiac control by reducing interictal epileptiform discharges, this line of thought assumes that the autonomic dysfunction contributing to SUDEP is caused by epilepsy and that freedom from seizures following epilepsy surgery should therefore be responsible for reducing the risk of death. An alternative hypothesis for the infrequent occurrence of SUDEP in seizure-free patients may be that seizure outcomes following epilepsy surgery and SUDEP risk are actually both governed by the same underlying biologic process. This would suggest that the same patient cohort is at a higher baseline mortality risk and in a prognostically poorer seizure outcome group following epilepsy surgery.
This idea is supported by a recent prospective study among 21 consecutive candidates for temporal lobe epilepsy surgery in which spectral analysis of heart rate variability performed preoperatively was correlated with seizure control 1 year after surgery.26 The study found that patients with poor seizure outcomes (Engel class II to IV, signifying ongoing postoperative seizures) had significantly lower power in all domains of heart rate variability than did patients with favorable seizure outcomes.26
In another study, heart rate was recorded in 16 patients before and after temporal lobe epilepsy surgery, and sympathetic and parasympathetic cardiac modulation was determined as powers of low-frequency (LF, 0.04–0.15 Hz) and high-frequency (HF, 0.15–0.5 Hz) heart rate oscillations.27 The LF/HF ratio was calculated as an index of sympathovagal balance. Cardiac MIBG uptake was measured with MIBG single-photon emission computed tomography and compared with control data. Baseline sympathetic LF power and LF/HF ratio were higher in patients who eventually had persistent seizures than in those who became seizure-free. Following surgery, both measures decreased in seizure-free patients but increased in patients with persistent seizures. MIBG uptake was lower in patients than in controls and even lower yet in patients who had persistent seizures. In this subgroup, MIBG uptake declined further after surgery.
Essentially, both of the above studies26,27 demonstrate findings of autonomic cardiac abnormalities that predated epilepsy surgery and reliably predicted the eventual surgical outcome in terms of seizure continuance. This suggests that “poor candidates” for epilepsy surgery—ie, those with lower chances of achieving seizure freedom with surgery—may a priori have a higher SUDEP risk. A possible explanation for these findings may be epileptogenic zones, including the insula and other components of the central autonomic network, or molecular/genetic diffuse abnormalities that extend beyond a limited surgically removable seizure focus and involve the heart, increasing the risk for cardiac conduction abnormalities.
CONCLUSIONS
SUDEP is the most common cause of death in epilepsy patients. A significant body of literature suggests that a cardiac mechanism contributes to its occurrence. Although the exact relationship between seizure outcomes following epilepsy surgery and SUDEP risk is still being investigated, it is accepted that seizure control correlates with reduced mortality. Cardiac changes and autonomic dysregulation seem to be at the “heart” of the problem.
- Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia 2007; 48:859–871.
- Nilsson L, Tomson T, Farahmand BY, et al Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia 1997; 38:1062–1068.
- Tennis P, Cole TB, Annegers JF, et al Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 1995; 36:29–36.
- Leestma JE, Annegers JF, Brodie MJ, et al Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997; 38:47–55.
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008; 7:1021–1031.
- Nashef L, Fish DR, Sander JW, Shorvon SD. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry 1995; 58:462–464.
- Jehi L, Najm IM. Sudden unexpected death in epilepsy: impact, mechanisms, and prevention. Cleve Clin J Med 2008; 75( suppl 2):S66–S70.
- Lhatoo SD, Johnson AL, Goodridge DM, et al Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol 2001; 49:336–344.
- Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002; 359:1790–1791.
- Schraeder PL, Delin K, McClelland RL, So EL. Coroner and medical examiner documentation of sudden unexplained deaths in epilepsy. Epilepsy Res 2006; 68:137–143.
- Sperling MR, Feldman H, Kinman J, et al Seizure control and mortality in epilepsy. Ann Neurol 1999; 46:45–50.
- Schuele SU, Widdess-Walsh P, Bermeo A, Lüders HO. Sudden unexplained death in epilepsy: the role of the heart. Cleve Clin J Med 2007; 74( suppl 1):S121–S127.
- Britton JW, Benarroch E. Seizures and syncope: anatomic basis and diagnostic considerations. Clin Auton Res 2006; 16:18–28.
- Leung H, Kwan P, Elger CE. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav 2006; 9:19–30.
- Nei M, Ho RT, Abou-Khalil BW, et al EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004; 45:338–345.
- Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000; 41:542–548.
- Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986; 1:1051–1056.
- Galimberti CA, Marchioni E, Barzizza F, et al Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996; 37:742–747.
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res 2002; 52:117–127.
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64:1131–1133.
- Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987; 37:1624–1626.
- Druschky A, Hilz MJ, Hopp P, et al Interictal cardiac autonomic dysfunction in temporal lobe epilepsy demonstrated by [123I] metaiodobenzylguanidine-SPECT. Brain 2001; 124:2372–2382.
- Kerling F, Dütsch M, Linke R, Kuwert T, Stefan H, Hilz MJ. Relation between ictal asystole and cardiac sympathetic dysfunction shown by MIBG-SPECT. Acta Neurol Scand 2009; 120:123–129.
- Vickrey BG, Hays RD, Rausch R, et al Outcomes in 248 patients who had diagnostic evaluations for epilepsy surgery. Lancet 1995; 346:1445–1449.
- Hilz MJ, Devinsky O, Doyle W, et al Decrease of sympathetic cardiovascular modulation after temporal lobe epilepsy surgery. Brain 2002; 125:985–995.
- Persson H, Kumlien E, Ericson M, Tomson T. Preoperative heart rate variability in relation to surgery outcome in refractory epilepsy. Neurology 2005; 65:1021–1025.
- Hilz MJ, Platsch G, Druschky K, et al Outcome of epilepsy surgery correlates with sympathetic modulation and neuroimaging of the heart. J Neurol Sci 2003; 216:153–162.
Sudden unexpected death in epilepsy (SUDEP) is most often defined as the sudden, unexpected, nontraumatic, and nondrowning death of patients with epilepsy. The death may be either witnessed or unwitnessed, and may occur with or without evidence of a seizure, excluding documented status epilepticus. In cases of definite SUDEP, postmortem examination does not reveal a structural or toxicologic cause of death.1
The reported incidence of SUDEP ranges from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of epilepsy patients2,3 and from 3.5 to 9.3 cases per 1,000 patient-years in antiepileptic drug registries, medical device registries, and epilepsy surgery programs.4–6 SUDEP is currently accepted as the most important epilepsy-related mode of death5,7 and is associated with standardized mortality ratios in patients with ongoing seizures as high as 24 to 40 times those of the general population.8 The exact mechanism or mechanisms leading to SUDEP remain unknown,1,5,9,10 and despite the identification of several potential risk factors, elimination of seizures still represents the main intervention correlating with risk reduction.5,11
This review will first discuss evidence for a cardiac mechanism of SUDEP, then review data traditionally used to support freedom from seizures following epilepsy surgery as a method for reducing SUDEP risk, and finally examine recent evidence suggesting that both SUDEP and seizure outcomes following epilepsy surgery are governed by a common pathway with certain cardiac manifestations.
CENTRAL AUTONOMIC NETWORK
EVIDENCE FOR A CARDIAC MECHANISM OF SUDEP
The most significant and broadly discussed cardiac mechanism of SUDEP is cardiac arrhythmia brought about by seizure discharges acting through the autonomic nervous system.5,7,15,16
Clinical evidence
A wide spectrum of cardiac arrhythmias—from ictal asystole to atrial fibrillation to repolarization abnormalities to bundle branch blocks—has been reported during seizures. 14–19 In one study, ictal cardiac arrhythmias occurred in 42% of hospitalized epilepsy patients, the most common being an irregular series of abrupt rate changes near the end of the electroencephalographic (EEG) seizure discharge.17 In another study, R-R interval analysis during the first 10-second period of EEG discharge showed a significant early heart rate increase in 49% of seizures and an early heart rate reduction in 25.5%.18
Certain clinical seizure characteristics have been correlated with the occurrence of ictal electrocardiographic (ECG) abnormalities. One study found that mean seizure duration was longer in patients with ECG abnormalities than in those without such changes.16 Others observed that ictal ECG abnormalities occurred more often and were more severe in generalized tonic-clonic seizures than in complex partial seizures.15,16,19 These same clinical seizure characteristics were correlated with a higher risk of SUDEP,20 which suggests an interrelation among seizure semiology, ECG abnormalities, and SUDEP.
Additionally, direct evidence of seizure-related cardiac changes occurring specifically in SUDEP victims has come from a study that compared EEG and ECG data between 21 SUDEP patients and 43 clinically similar historical controls with refractory partial epilepsy.15 The patients who eventually succumbed to SUDEP had a higher ictal maximal heart rate, a greater increase in heart rate with seizures arising from sleep, and a higher incidence of ictal cardiac repolarization and rhythm abnormalities.15
Experimental evidence
Electrical brain stimulation of the limbic system and insular cortex has repeatedly been shown to provoke heart rate changes, including bradycardia, tachycardia, and asystole.14 Some studies have even suggested a lateralized influence of the insulae on cardiovascular autonomic control, with intraoperative stimulation of the left posterior insula eliciting a cardioinhibitory response and hypotension, and with stimulation of the right anterior insula eliciting tachycardia and hypertension.7 Other studies have suggested that the limbic system has a localization-related influence on cardiovascular responses, with stimulation of the amygdala alone being insufficient to produce the ictal tachycardia so commonly seen in epileptic seizures, which suggests that cortical involvement is essential for the increase in heart rate.21 Such cortical stimulation–induced heart rate changes may explain how massive seizure-related discharges can affect cardiac rhythm during the seizure itself.
There is also, however, evidence of a baseline epilepsy-related autonomic dysfunction. Abnormalities in cardiac uptake of meta-iodobenzylguanidine (MIBG) have been demonstrated interictally in patients with chronic temporal lobe epilepsy relative to controls.22 A recent study specifically showed a pronounced reduction in cardiac MIBG uptake in patients who had ictal asystole compared with other epileptic patients and nonepileptic controls, a finding that suggests a postganglionic cardiac catecholamine disturbance in patients with epilepsy.23 The authors proposed that epilepsy-related impairment of sympathetic cardiac innervation limits adjustment and heart rate modulation, and may thus increase the risk of asystole and, ultimately, SUDEP.23
EPILEPSY SURGERY AND SUDEP RISK
The above findings make it reasonable to postulate that recurrent uncontrolled seizures may lead to significant cardiac changes—namely, arrhythmias—which may then lead to a higher risk of SUDEP. Successful epilepsy surgery, the treatment of choice for uncontrolled partial epilepsy, would eliminate seizures and would thus be expected to reduce SUDEP risk.
Several studies support this hypothesis. Reductions in all-cause mortality and in SUDEP following successful epilepsy surgery have been observed when patients who became seizure-free after surgery were compared either with patients who had ongoing postoperative seizures or with patients with intractable epilepsy who did not undergo epilepsy surgery.11,24 In one study with a mean follow-up of 3.82 years, no deaths occurred among 199 patients who were seizure-free following epilepsy surgery, whereas there were 11 deaths—including 6 cases of SUDEP—among 194 patients who had persistent seizures following epilepsy surgery.11 A separate study compared 202 patients who underwent epilepsy surgery with 46 patients with medically treated intractable epilepsy over a mean follow-up period of greater than 5.7 years.24 In this study, death occurred in only 7% of the surgical patients compared with 20% of the medically treated patients, which suggests that epilepsy surgery (or lack thereof) may be an independent predictor of mortality. Favorable outcome was linked closely to seizure control, as 81% of the patients who died had 2 or more seizures per year at last follow-up, compared with only 47% of survivors in the overall cohort.24
Complete seizure resolution—not just reduction—seems necessary for SUDEP risk reduction
In our experience at the Cleveland Clinic Epilepsy Center, we have found that a reduction in seizure frequency following epilepsy surgery is not enough to eliminate SUDEP risk—complete freedom from seizures is required. Of 37 SUDEP cases identified so far in a cohort of 3,481 patients evaluated in our epilepsy monitoring unit between 1990 and 2005 (Jehi et al, in preparation), 7 patients had undergone epilepsy surgery. None of these 7 patients was seizure-free at the time of death; four had a greater than 50% reduction in seizure frequency. No cases of SUDEP occurred in patients who were seizure-free after surgery.
Our findings mirror earlier results reported by Sperling et al,11 who also found that epilepsy surgery improves mortality only when seizures resolve completely, not when they merely “improve.” It seems plausible, therefore, that elimination of seizures postoperatively eliminates the risk for the several seizure-related cardiac arrhythmogenic changes discussed above, thereby eliminating the risk for the series of events that eventually leads to SUDEP.
A role for stabilized baseline autonomic control?
Alternatively, some data suggest that the “autonomic” impacts of epilepsy surgery may extend beyond the immediate seizure-related manifestations to the baseline interictal period in epilepsy patients. One study found that surgery for temporal lobe epilepsy is followed by a reduction of sympathetic cardiovascular modulation and baroreflex sensitivity.25 The authors proposed that this finding may be attributable to decreased influences of interictal epileptogenic discharges on brain areas involved in cardiovascular autonomic control. These researchers continue to postulate that surgery for temporal lobe epilepsy seems to stabilize cardiovascular control in epilepsy patients by reducing the risk of sympathetically mediated tachyarrhythmias and excessive bradycardiac counterregulation, potentially lowering SUDEP risk.25
CARDIAC FINDINGS, SUDEP, AND SEIZURE OUTCOMES
Whether it is the elimination of seizure-related cardiac arrhythmias achieved by rendering patients seizure-free after surgery, or whether it is the stabilization of baseline autonomic cardiac control by reducing interictal epileptiform discharges, this line of thought assumes that the autonomic dysfunction contributing to SUDEP is caused by epilepsy and that freedom from seizures following epilepsy surgery should therefore be responsible for reducing the risk of death. An alternative hypothesis for the infrequent occurrence of SUDEP in seizure-free patients may be that seizure outcomes following epilepsy surgery and SUDEP risk are actually both governed by the same underlying biologic process. This would suggest that the same patient cohort is at a higher baseline mortality risk and in a prognostically poorer seizure outcome group following epilepsy surgery.
This idea is supported by a recent prospective study among 21 consecutive candidates for temporal lobe epilepsy surgery in which spectral analysis of heart rate variability performed preoperatively was correlated with seizure control 1 year after surgery.26 The study found that patients with poor seizure outcomes (Engel class II to IV, signifying ongoing postoperative seizures) had significantly lower power in all domains of heart rate variability than did patients with favorable seizure outcomes.26
In another study, heart rate was recorded in 16 patients before and after temporal lobe epilepsy surgery, and sympathetic and parasympathetic cardiac modulation was determined as powers of low-frequency (LF, 0.04–0.15 Hz) and high-frequency (HF, 0.15–0.5 Hz) heart rate oscillations.27 The LF/HF ratio was calculated as an index of sympathovagal balance. Cardiac MIBG uptake was measured with MIBG single-photon emission computed tomography and compared with control data. Baseline sympathetic LF power and LF/HF ratio were higher in patients who eventually had persistent seizures than in those who became seizure-free. Following surgery, both measures decreased in seizure-free patients but increased in patients with persistent seizures. MIBG uptake was lower in patients than in controls and even lower yet in patients who had persistent seizures. In this subgroup, MIBG uptake declined further after surgery.
Essentially, both of the above studies26,27 demonstrate findings of autonomic cardiac abnormalities that predated epilepsy surgery and reliably predicted the eventual surgical outcome in terms of seizure continuance. This suggests that “poor candidates” for epilepsy surgery—ie, those with lower chances of achieving seizure freedom with surgery—may a priori have a higher SUDEP risk. A possible explanation for these findings may be epileptogenic zones, including the insula and other components of the central autonomic network, or molecular/genetic diffuse abnormalities that extend beyond a limited surgically removable seizure focus and involve the heart, increasing the risk for cardiac conduction abnormalities.
CONCLUSIONS
SUDEP is the most common cause of death in epilepsy patients. A significant body of literature suggests that a cardiac mechanism contributes to its occurrence. Although the exact relationship between seizure outcomes following epilepsy surgery and SUDEP risk is still being investigated, it is accepted that seizure control correlates with reduced mortality. Cardiac changes and autonomic dysregulation seem to be at the “heart” of the problem.
Sudden unexpected death in epilepsy (SUDEP) is most often defined as the sudden, unexpected, nontraumatic, and nondrowning death of patients with epilepsy. The death may be either witnessed or unwitnessed, and may occur with or without evidence of a seizure, excluding documented status epilepticus. In cases of definite SUDEP, postmortem examination does not reveal a structural or toxicologic cause of death.1
The reported incidence of SUDEP ranges from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of epilepsy patients2,3 and from 3.5 to 9.3 cases per 1,000 patient-years in antiepileptic drug registries, medical device registries, and epilepsy surgery programs.4–6 SUDEP is currently accepted as the most important epilepsy-related mode of death5,7 and is associated with standardized mortality ratios in patients with ongoing seizures as high as 24 to 40 times those of the general population.8 The exact mechanism or mechanisms leading to SUDEP remain unknown,1,5,9,10 and despite the identification of several potential risk factors, elimination of seizures still represents the main intervention correlating with risk reduction.5,11
This review will first discuss evidence for a cardiac mechanism of SUDEP, then review data traditionally used to support freedom from seizures following epilepsy surgery as a method for reducing SUDEP risk, and finally examine recent evidence suggesting that both SUDEP and seizure outcomes following epilepsy surgery are governed by a common pathway with certain cardiac manifestations.
CENTRAL AUTONOMIC NETWORK
EVIDENCE FOR A CARDIAC MECHANISM OF SUDEP
The most significant and broadly discussed cardiac mechanism of SUDEP is cardiac arrhythmia brought about by seizure discharges acting through the autonomic nervous system.5,7,15,16
Clinical evidence
A wide spectrum of cardiac arrhythmias—from ictal asystole to atrial fibrillation to repolarization abnormalities to bundle branch blocks—has been reported during seizures. 14–19 In one study, ictal cardiac arrhythmias occurred in 42% of hospitalized epilepsy patients, the most common being an irregular series of abrupt rate changes near the end of the electroencephalographic (EEG) seizure discharge.17 In another study, R-R interval analysis during the first 10-second period of EEG discharge showed a significant early heart rate increase in 49% of seizures and an early heart rate reduction in 25.5%.18
Certain clinical seizure characteristics have been correlated with the occurrence of ictal electrocardiographic (ECG) abnormalities. One study found that mean seizure duration was longer in patients with ECG abnormalities than in those without such changes.16 Others observed that ictal ECG abnormalities occurred more often and were more severe in generalized tonic-clonic seizures than in complex partial seizures.15,16,19 These same clinical seizure characteristics were correlated with a higher risk of SUDEP,20 which suggests an interrelation among seizure semiology, ECG abnormalities, and SUDEP.
Additionally, direct evidence of seizure-related cardiac changes occurring specifically in SUDEP victims has come from a study that compared EEG and ECG data between 21 SUDEP patients and 43 clinically similar historical controls with refractory partial epilepsy.15 The patients who eventually succumbed to SUDEP had a higher ictal maximal heart rate, a greater increase in heart rate with seizures arising from sleep, and a higher incidence of ictal cardiac repolarization and rhythm abnormalities.15
Experimental evidence
Electrical brain stimulation of the limbic system and insular cortex has repeatedly been shown to provoke heart rate changes, including bradycardia, tachycardia, and asystole.14 Some studies have even suggested a lateralized influence of the insulae on cardiovascular autonomic control, with intraoperative stimulation of the left posterior insula eliciting a cardioinhibitory response and hypotension, and with stimulation of the right anterior insula eliciting tachycardia and hypertension.7 Other studies have suggested that the limbic system has a localization-related influence on cardiovascular responses, with stimulation of the amygdala alone being insufficient to produce the ictal tachycardia so commonly seen in epileptic seizures, which suggests that cortical involvement is essential for the increase in heart rate.21 Such cortical stimulation–induced heart rate changes may explain how massive seizure-related discharges can affect cardiac rhythm during the seizure itself.
There is also, however, evidence of a baseline epilepsy-related autonomic dysfunction. Abnormalities in cardiac uptake of meta-iodobenzylguanidine (MIBG) have been demonstrated interictally in patients with chronic temporal lobe epilepsy relative to controls.22 A recent study specifically showed a pronounced reduction in cardiac MIBG uptake in patients who had ictal asystole compared with other epileptic patients and nonepileptic controls, a finding that suggests a postganglionic cardiac catecholamine disturbance in patients with epilepsy.23 The authors proposed that epilepsy-related impairment of sympathetic cardiac innervation limits adjustment and heart rate modulation, and may thus increase the risk of asystole and, ultimately, SUDEP.23
EPILEPSY SURGERY AND SUDEP RISK
The above findings make it reasonable to postulate that recurrent uncontrolled seizures may lead to significant cardiac changes—namely, arrhythmias—which may then lead to a higher risk of SUDEP. Successful epilepsy surgery, the treatment of choice for uncontrolled partial epilepsy, would eliminate seizures and would thus be expected to reduce SUDEP risk.
Several studies support this hypothesis. Reductions in all-cause mortality and in SUDEP following successful epilepsy surgery have been observed when patients who became seizure-free after surgery were compared either with patients who had ongoing postoperative seizures or with patients with intractable epilepsy who did not undergo epilepsy surgery.11,24 In one study with a mean follow-up of 3.82 years, no deaths occurred among 199 patients who were seizure-free following epilepsy surgery, whereas there were 11 deaths—including 6 cases of SUDEP—among 194 patients who had persistent seizures following epilepsy surgery.11 A separate study compared 202 patients who underwent epilepsy surgery with 46 patients with medically treated intractable epilepsy over a mean follow-up period of greater than 5.7 years.24 In this study, death occurred in only 7% of the surgical patients compared with 20% of the medically treated patients, which suggests that epilepsy surgery (or lack thereof) may be an independent predictor of mortality. Favorable outcome was linked closely to seizure control, as 81% of the patients who died had 2 or more seizures per year at last follow-up, compared with only 47% of survivors in the overall cohort.24
Complete seizure resolution—not just reduction—seems necessary for SUDEP risk reduction
In our experience at the Cleveland Clinic Epilepsy Center, we have found that a reduction in seizure frequency following epilepsy surgery is not enough to eliminate SUDEP risk—complete freedom from seizures is required. Of 37 SUDEP cases identified so far in a cohort of 3,481 patients evaluated in our epilepsy monitoring unit between 1990 and 2005 (Jehi et al, in preparation), 7 patients had undergone epilepsy surgery. None of these 7 patients was seizure-free at the time of death; four had a greater than 50% reduction in seizure frequency. No cases of SUDEP occurred in patients who were seizure-free after surgery.
Our findings mirror earlier results reported by Sperling et al,11 who also found that epilepsy surgery improves mortality only when seizures resolve completely, not when they merely “improve.” It seems plausible, therefore, that elimination of seizures postoperatively eliminates the risk for the several seizure-related cardiac arrhythmogenic changes discussed above, thereby eliminating the risk for the series of events that eventually leads to SUDEP.
A role for stabilized baseline autonomic control?
Alternatively, some data suggest that the “autonomic” impacts of epilepsy surgery may extend beyond the immediate seizure-related manifestations to the baseline interictal period in epilepsy patients. One study found that surgery for temporal lobe epilepsy is followed by a reduction of sympathetic cardiovascular modulation and baroreflex sensitivity.25 The authors proposed that this finding may be attributable to decreased influences of interictal epileptogenic discharges on brain areas involved in cardiovascular autonomic control. These researchers continue to postulate that surgery for temporal lobe epilepsy seems to stabilize cardiovascular control in epilepsy patients by reducing the risk of sympathetically mediated tachyarrhythmias and excessive bradycardiac counterregulation, potentially lowering SUDEP risk.25
CARDIAC FINDINGS, SUDEP, AND SEIZURE OUTCOMES
Whether it is the elimination of seizure-related cardiac arrhythmias achieved by rendering patients seizure-free after surgery, or whether it is the stabilization of baseline autonomic cardiac control by reducing interictal epileptiform discharges, this line of thought assumes that the autonomic dysfunction contributing to SUDEP is caused by epilepsy and that freedom from seizures following epilepsy surgery should therefore be responsible for reducing the risk of death. An alternative hypothesis for the infrequent occurrence of SUDEP in seizure-free patients may be that seizure outcomes following epilepsy surgery and SUDEP risk are actually both governed by the same underlying biologic process. This would suggest that the same patient cohort is at a higher baseline mortality risk and in a prognostically poorer seizure outcome group following epilepsy surgery.
This idea is supported by a recent prospective study among 21 consecutive candidates for temporal lobe epilepsy surgery in which spectral analysis of heart rate variability performed preoperatively was correlated with seizure control 1 year after surgery.26 The study found that patients with poor seizure outcomes (Engel class II to IV, signifying ongoing postoperative seizures) had significantly lower power in all domains of heart rate variability than did patients with favorable seizure outcomes.26
In another study, heart rate was recorded in 16 patients before and after temporal lobe epilepsy surgery, and sympathetic and parasympathetic cardiac modulation was determined as powers of low-frequency (LF, 0.04–0.15 Hz) and high-frequency (HF, 0.15–0.5 Hz) heart rate oscillations.27 The LF/HF ratio was calculated as an index of sympathovagal balance. Cardiac MIBG uptake was measured with MIBG single-photon emission computed tomography and compared with control data. Baseline sympathetic LF power and LF/HF ratio were higher in patients who eventually had persistent seizures than in those who became seizure-free. Following surgery, both measures decreased in seizure-free patients but increased in patients with persistent seizures. MIBG uptake was lower in patients than in controls and even lower yet in patients who had persistent seizures. In this subgroup, MIBG uptake declined further after surgery.
Essentially, both of the above studies26,27 demonstrate findings of autonomic cardiac abnormalities that predated epilepsy surgery and reliably predicted the eventual surgical outcome in terms of seizure continuance. This suggests that “poor candidates” for epilepsy surgery—ie, those with lower chances of achieving seizure freedom with surgery—may a priori have a higher SUDEP risk. A possible explanation for these findings may be epileptogenic zones, including the insula and other components of the central autonomic network, or molecular/genetic diffuse abnormalities that extend beyond a limited surgically removable seizure focus and involve the heart, increasing the risk for cardiac conduction abnormalities.
CONCLUSIONS
SUDEP is the most common cause of death in epilepsy patients. A significant body of literature suggests that a cardiac mechanism contributes to its occurrence. Although the exact relationship between seizure outcomes following epilepsy surgery and SUDEP risk is still being investigated, it is accepted that seizure control correlates with reduced mortality. Cardiac changes and autonomic dysregulation seem to be at the “heart” of the problem.
- Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia 2007; 48:859–871.
- Nilsson L, Tomson T, Farahmand BY, et al Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia 1997; 38:1062–1068.
- Tennis P, Cole TB, Annegers JF, et al Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 1995; 36:29–36.
- Leestma JE, Annegers JF, Brodie MJ, et al Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997; 38:47–55.
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008; 7:1021–1031.
- Nashef L, Fish DR, Sander JW, Shorvon SD. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry 1995; 58:462–464.
- Jehi L, Najm IM. Sudden unexpected death in epilepsy: impact, mechanisms, and prevention. Cleve Clin J Med 2008; 75( suppl 2):S66–S70.
- Lhatoo SD, Johnson AL, Goodridge DM, et al Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol 2001; 49:336–344.
- Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002; 359:1790–1791.
- Schraeder PL, Delin K, McClelland RL, So EL. Coroner and medical examiner documentation of sudden unexplained deaths in epilepsy. Epilepsy Res 2006; 68:137–143.
- Sperling MR, Feldman H, Kinman J, et al Seizure control and mortality in epilepsy. Ann Neurol 1999; 46:45–50.
- Schuele SU, Widdess-Walsh P, Bermeo A, Lüders HO. Sudden unexplained death in epilepsy: the role of the heart. Cleve Clin J Med 2007; 74( suppl 1):S121–S127.
- Britton JW, Benarroch E. Seizures and syncope: anatomic basis and diagnostic considerations. Clin Auton Res 2006; 16:18–28.
- Leung H, Kwan P, Elger CE. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav 2006; 9:19–30.
- Nei M, Ho RT, Abou-Khalil BW, et al EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004; 45:338–345.
- Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000; 41:542–548.
- Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986; 1:1051–1056.
- Galimberti CA, Marchioni E, Barzizza F, et al Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996; 37:742–747.
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res 2002; 52:117–127.
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64:1131–1133.
- Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987; 37:1624–1626.
- Druschky A, Hilz MJ, Hopp P, et al Interictal cardiac autonomic dysfunction in temporal lobe epilepsy demonstrated by [123I] metaiodobenzylguanidine-SPECT. Brain 2001; 124:2372–2382.
- Kerling F, Dütsch M, Linke R, Kuwert T, Stefan H, Hilz MJ. Relation between ictal asystole and cardiac sympathetic dysfunction shown by MIBG-SPECT. Acta Neurol Scand 2009; 120:123–129.
- Vickrey BG, Hays RD, Rausch R, et al Outcomes in 248 patients who had diagnostic evaluations for epilepsy surgery. Lancet 1995; 346:1445–1449.
- Hilz MJ, Devinsky O, Doyle W, et al Decrease of sympathetic cardiovascular modulation after temporal lobe epilepsy surgery. Brain 2002; 125:985–995.
- Persson H, Kumlien E, Ericson M, Tomson T. Preoperative heart rate variability in relation to surgery outcome in refractory epilepsy. Neurology 2005; 65:1021–1025.
- Hilz MJ, Platsch G, Druschky K, et al Outcome of epilepsy surgery correlates with sympathetic modulation and neuroimaging of the heart. J Neurol Sci 2003; 216:153–162.
- Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia 2007; 48:859–871.
- Nilsson L, Tomson T, Farahmand BY, et al Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia 1997; 38:1062–1068.
- Tennis P, Cole TB, Annegers JF, et al Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 1995; 36:29–36.
- Leestma JE, Annegers JF, Brodie MJ, et al Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997; 38:47–55.
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008; 7:1021–1031.
- Nashef L, Fish DR, Sander JW, Shorvon SD. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry 1995; 58:462–464.
- Jehi L, Najm IM. Sudden unexpected death in epilepsy: impact, mechanisms, and prevention. Cleve Clin J Med 2008; 75( suppl 2):S66–S70.
- Lhatoo SD, Johnson AL, Goodridge DM, et al Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol 2001; 49:336–344.
- Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002; 359:1790–1791.
- Schraeder PL, Delin K, McClelland RL, So EL. Coroner and medical examiner documentation of sudden unexplained deaths in epilepsy. Epilepsy Res 2006; 68:137–143.
- Sperling MR, Feldman H, Kinman J, et al Seizure control and mortality in epilepsy. Ann Neurol 1999; 46:45–50.
- Schuele SU, Widdess-Walsh P, Bermeo A, Lüders HO. Sudden unexplained death in epilepsy: the role of the heart. Cleve Clin J Med 2007; 74( suppl 1):S121–S127.
- Britton JW, Benarroch E. Seizures and syncope: anatomic basis and diagnostic considerations. Clin Auton Res 2006; 16:18–28.
- Leung H, Kwan P, Elger CE. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav 2006; 9:19–30.
- Nei M, Ho RT, Abou-Khalil BW, et al EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004; 45:338–345.
- Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000; 41:542–548.
- Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986; 1:1051–1056.
- Galimberti CA, Marchioni E, Barzizza F, et al Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996; 37:742–747.
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res 2002; 52:117–127.
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64:1131–1133.
- Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987; 37:1624–1626.
- Druschky A, Hilz MJ, Hopp P, et al Interictal cardiac autonomic dysfunction in temporal lobe epilepsy demonstrated by [123I] metaiodobenzylguanidine-SPECT. Brain 2001; 124:2372–2382.
- Kerling F, Dütsch M, Linke R, Kuwert T, Stefan H, Hilz MJ. Relation between ictal asystole and cardiac sympathetic dysfunction shown by MIBG-SPECT. Acta Neurol Scand 2009; 120:123–129.
- Vickrey BG, Hays RD, Rausch R, et al Outcomes in 248 patients who had diagnostic evaluations for epilepsy surgery. Lancet 1995; 346:1445–1449.
- Hilz MJ, Devinsky O, Doyle W, et al Decrease of sympathetic cardiovascular modulation after temporal lobe epilepsy surgery. Brain 2002; 125:985–995.
- Persson H, Kumlien E, Ericson M, Tomson T. Preoperative heart rate variability in relation to surgery outcome in refractory epilepsy. Neurology 2005; 65:1021–1025.
- Hilz MJ, Platsch G, Druschky K, et al Outcome of epilepsy surgery correlates with sympathetic modulation and neuroimaging of the heart. J Neurol Sci 2003; 216:153–162.
Sudden unexpected death in epilepsy: Finding the missing cardiac links
Sudden unexpected death in epilepsy: Impact, mechanisms, and prevention
The intimate interplay between heart and brain is well illustrated in epilepsy and may underlie the mechanism of one of its most devastating consequences: sudden unexpected death in epilepsy (SUDEP). This article will briefly describe the potential mechanisms of SUDEP, elaborate on the evidence for a likely cardiac pathophysiology, and review considerations in SUDEP prevention. We begin with a couple of brief case presentations and an epidemiologic overview to illustrate the concept and significance of SUDEP.
CASE PRESENTATIONS
A patient with near-SUDEP
The following is an actual message received by one of the authors:
Dr. Najm: A quick note regarding a 27-year-old male patient of yours with cerebral palsy and seizure disorder. Yesterday, while being transported from floor to floor, he had a cardiac arrest and was successfully resuscitated. Immediately after the code he developed seizures, which were treated with phenytoin and lorazepam. He is now in the neurointensive care unit. Thank you.
This case represents a scenario of near-SUDEP in which death was prevented by the fortuitous presence of immediate medical assistance at the time of cardiac arrest. Had this patient been home at the time of this incident, he almost certainly would have simply been found dead in his bed, like many SUDEP victims.
A typical case with multiple risk factors
A 32-year-old man underwent left temporal lobectomy at the Cleveland Clinic for treatment of medically refractory focal epilepsy. His seizure frequency improved after surgery, but he continued to have rare convulsions. Nevertheless, he discontinued all his anticonvulsant medications on his own. One year later, he was found dead on his bathroom floor. No obvious cause of death was identified.
This case illustrates several characteristics of the patient typically at risk for SUDEP: young, male, with intractable poorly controlled epilepsy, and not taking antiepileptic medications.
EPIDEMIOLOGY AND RISK FACTORS
Epilepsy affects 1% of the US population. Among those affected by epilepsy, SUDEP is a common cause of mortality. Estimates of SUDEP incidence range from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of patients with epilepsy1,2 and from 3.5 to 9.3 cases per 1,000 patient-years in anticonvulsant drug registries, medical device registries, and epilepsy surgery programs.3–5 SUDEP accounts for up to 17% of all deaths in patients with epilepsy6,7 and exceeds the expected rate of sudden death in the general population by nearly 24 times.6,8
Several potential risk factors for SUDEP have been investigated, but results from different studies are conflicting. Consistently identified risk factors include young age, early onset of seizures, refractoriness of epilepsy, the presence of generalized tonic-clonic seizures, male sex, and being in bed at the time of death. Weaker risk factors include being in the prone position at the time of death, having one or more subtherapeutic blood levels of anticonvulsant medication, having a structural brain lesion, and being asleep.9 The current consensus is that SUDEP is primarily a “seizure-related” occurrence, but the exact mechanisms underlying SUDEP are unknown.
PROPOSED MECHANISMS
Pulmonary pathophysiology
Central apnea and acute neurogenic pulmonary edema are the two major proposed pathways linking seizures to SUDEP. Evidence exists for each pathway.
Central apnea. In a prospective study of patients in an epilepsy monitoring unit, central apnea lasting at least 10 seconds was observed postictally in 40% of the recorded seizures.10 Otherwise healthy young epilepsy patients have been reported to develop central apnea immediately following complex partial seizures.11 Neurotransmitters mediating the brain’s own seizure-terminating mechanism could also be inhibiting the brainstem and causing postictal apnea.
Acute neurogenic pulmonary edema has been well described in relation to severe head injury and subarachnoid hemorrhage. Pulmonary edema is frequently found in SUDEP patients at autopsy.12 Intense generalized vasoconstriction induced by massive seizure-related sympathetic outburst can lead to increased pulmonary vascular resistance, and thereby may mediate acute pulmonary edema.
These two mechanisms—central apnea and acute neurogenic pulmonary edema—are not mutually exclusive. In the only animal model of SUDEP, one third of animals died from hypoventilation and had associated pulmonary edema at autopsy.13 Limited opportunities for realistic and practical interventions to reverse SUDEP risks related to pulmonary causes have hindered further development of these concepts.
Cardiac pathophysiology
The most significant and widely discussed cardiac mechanism of SUDEP is cardiac arrhythmia precipitated by seizure discharges acting via the autonomic nervous system.14–19
Experimental evidence. Heart rate changes, including bradycardia, tachycardia, and even asystole, have been repeatedly provoked by electrical brain stimulation of the limbic system and insular cortex.19 Some studies have suggested a lateralized influence of the insulae on cardiovascular autonomic control. In one study, intraoperative stimulation of the left posterior insula elicited a cardioinhibitory response and hypotension, whereas stimulation of the right anterior insula elicited tachycardia and hypertension.20 Such results have not always been reproducible.21–23 Other studies have suggested a localization-related influence of the limbic system on cardiovascular responses. Stimulation of the amygdala has not led to the ictal tachycardia that is commonly seen in epileptic seizures, suggesting that cortical involvement is needed for the development of tachycardia.24
SUDEP PREVENTION
Epilepsy control is first line of defense
A careful consideration of the incidence of SUDEP in various patient populations suggests that controlling patients’ epilepsy might just be the best method of preventing SUDEP. While estimated SUDEP incidence ranges from 0.7 to 1.3 cases per 1,000 patient-years in population-based studies of patients with epilepsy,1,2 this rate escalates by nearly tenfold (3.5 to 9.3 cases per 1,000 patient-years) in cohorts with severe epilepsy, such as those derived from anticonvulsant drug registries, medical device registries, and referral centers.3–5 Therefore, medical control of seizures might reduce the incidence of SUDEP.
Epilepsy surgery cuts SUDEP risk for many patients
Studies involving epilepsy surgery programs also suggest that successful epilepsy surgery reduces the impending risks of SUDEP. In cohorts in which the estimated risk of SUDEP is almost 1% per year without surgery, SUDEP incidence was significantly lower following epilepsy surgery. In a study of 305 patients who underwent temporal lobe epilepsy surgery in the United Kingdom, the incidence of SUDEP following surgery was 2.2 cases per 1,000 person-years, and only one-third of SUDEP cases were among seizure-free patients.31 A similar incidence of 2.4 cases per 1,000 person-years was seen following epilepsy surgery in 596 Swedish patients; none of the 6 SUDEP patients in that study was seizure free.32 In a US study, no SUDEP cases occurred among 256 seizure-free patients with a follow-up of about 5 years after epilepsy surgery.33
In our own experience at the Cleveland Clinic, we have reported on outcomes among 70 patients who underwent frontal lobectomy34 and among 371 patients who underwent temporal lobectomy.35 In the frontal lobectomy study,34 2 of the 39 patients who had persistent seizures following surgery died of SUDEP during follow-up, whereas none of the 31 patients who remained seizure free were dead up to 10 years after surgery. In the temporal lobectomy report,35 2 of the 141 patients with ongoing postoperative seizures died of SUDEP, as compared with none of the 230 patients who were seizure free after a mean follow-up of 5.5 years.
Additional means of prophylaxis needed
Unfortunately, as many as 30% to 40% of patients with epilepsy continue to suffer intractable epilepsy despite all the available treatment modalities, including epilepsy surgery. For these patients, controlling seizures to reduce the risk of SUDEP is neither a possible nor a realistic means of avoiding this devastating condition, and alternative methods of prophylaxis must be sought.
CONCLUSIONS AND FUTURE RESEARCH
Patients with refractory epilepsy currently face a lifelong risk of sudden death as high as 1% per year.3 Elucidating the mechanisms of SUDEP might lead to preventive measures, which could have significant implications in reducing mortality in this patient population. Abundant evidence exists that autonomic dysfunction and cardiac arrhythmias are associated with seizures. The missing links in establishing a cardiac mechanism for SUDEP now include the following: (1) evidence of cardiac arrhythmias generally observed in seizures as a risk factor for SUDEP, (2) determination of clear electrophysiologic characteristics—from EEG and ECG standpoints—of patients at risk for SUDEP, and (3) clarification of the role of cardiac mechanisms in SUDEP and the role that cerebral influences on autonomic function might play. Early identification of patients at risk of SUDEP would offer a unique opportunity for early intervention to prevent this devastating condition.
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet 1999; 353:888–893.
- Tennis P, Cole TB, Annegers JF, Leestma JE, McNutt M, Rajput A. Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 1995; 36:29–36.
- Dasheiff RM. Sudden unexpected death in epilepsy: a series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol 1991; 8:216–222.
- Leestma JE, Annegers JF, Brodie MJ, et al. Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997; 38:47–55.
- Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol 1999; 46:45–50.
- Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia 2000; 41(Suppl 2):S7–S12.
- Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002; 359:1790–1791.
- Ficker DM, So EL, Shen WK, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998; 51:1270–1274.
- Monté CP, Arends JB, Tan IY, Aldenkamp AP, Limburg M, de Krom MC. Sudden unexpected death in epilepsy patients: risk factors. A systematic review. Seizure 2007; 16:1–7.
- Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996; 60:297–300.
- So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000; 41:1494–1497.
- Terrence CF, Rao GR, Perper JA. Neurogenic pulmonary edema in unexpected, unexplained death of epileptic patients. Ann Neurol 1981; 9:458–464.
- Johnston SC, Horn JK, Valente J, Simon RP. The role of hypoventilation in a sheep model of epileptic sudden death. Ann Neurol 1995; 37:531–537.
- Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986; 1:1051–1056.
- Nei M, Ho RT, Abou-Khalil BW, et al. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004; 45:338–345.
- Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000; 41:542–548.
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res 2002; 52:117–127.
- Tigaran S, Mølgaard H, McClelland R, Dam M, Jaffe AS. Evidence of cardiac ischemia during seizures in drug refractory epilepsy patients. Neurology 2003; 60:492–495.
- Leung H, Kwan P, Elger CE. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav 2006; 9:19–30.
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 1991; 303:355–374.
- Jokeit H, Noerpel I, Herbord E, Ebner A. Heart rate does not decrease after right hemispheric amobarbital injection. Neurology 2000; 54:2347–2348.
- Zamrini EY, Meador KJ, Loring DW, et al. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology 1990; 40:1408–1411.
- Yoon BW, Morillo CA, Cechetto DF, Hachinski V. Cerebral hemispheric lateralization in cardiac autonomic control. Arch Neurol 1997; 54:741–744.
- Keilson MJ, Hauser WA, Magrill JP. Electrocardiographic changes during electrographic seizures. Arch Neurol 1989; 46:1169–1170.
- Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987; 37:1624–1626.
- Galimberti CA, Marchioni E, Barzizza F, Manni R, Sartori I, Tartara A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996; 37:742–747.
- Liedholm LJ, Gudjonsson O. Cardiac arrest due to partial epileptic seizures. Neurology 1992; 42:824–829.
- Leung H, Schindler K, Kwan P, Elger C. Asystole induced by electrical stimulation of the left cingulate gyrus. Epileptic Disord 2007; 9:77–81.
- Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia 2000; 41:536–541.
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64:1131–1133.
- Hennessy MJ, Langan Y, Elwes RD, Binnie CD, Polkey CE, Nashef L. A study of mortality after temporal lobe epilepsy surgery. Neurology 1999; 53:1276–1283.
- Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia 2003; 44:575–581.
- Sperling MR, Harris A, Nei M, Liporace JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia 2005; 46(Suppl 11):49–53.
- Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007; 130:574–584.
- Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 2006; 66:1938–1940.
The intimate interplay between heart and brain is well illustrated in epilepsy and may underlie the mechanism of one of its most devastating consequences: sudden unexpected death in epilepsy (SUDEP). This article will briefly describe the potential mechanisms of SUDEP, elaborate on the evidence for a likely cardiac pathophysiology, and review considerations in SUDEP prevention. We begin with a couple of brief case presentations and an epidemiologic overview to illustrate the concept and significance of SUDEP.
CASE PRESENTATIONS
A patient with near-SUDEP
The following is an actual message received by one of the authors:
Dr. Najm: A quick note regarding a 27-year-old male patient of yours with cerebral palsy and seizure disorder. Yesterday, while being transported from floor to floor, he had a cardiac arrest and was successfully resuscitated. Immediately after the code he developed seizures, which were treated with phenytoin and lorazepam. He is now in the neurointensive care unit. Thank you.
This case represents a scenario of near-SUDEP in which death was prevented by the fortuitous presence of immediate medical assistance at the time of cardiac arrest. Had this patient been home at the time of this incident, he almost certainly would have simply been found dead in his bed, like many SUDEP victims.
A typical case with multiple risk factors
A 32-year-old man underwent left temporal lobectomy at the Cleveland Clinic for treatment of medically refractory focal epilepsy. His seizure frequency improved after surgery, but he continued to have rare convulsions. Nevertheless, he discontinued all his anticonvulsant medications on his own. One year later, he was found dead on his bathroom floor. No obvious cause of death was identified.
This case illustrates several characteristics of the patient typically at risk for SUDEP: young, male, with intractable poorly controlled epilepsy, and not taking antiepileptic medications.
EPIDEMIOLOGY AND RISK FACTORS
Epilepsy affects 1% of the US population. Among those affected by epilepsy, SUDEP is a common cause of mortality. Estimates of SUDEP incidence range from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of patients with epilepsy1,2 and from 3.5 to 9.3 cases per 1,000 patient-years in anticonvulsant drug registries, medical device registries, and epilepsy surgery programs.3–5 SUDEP accounts for up to 17% of all deaths in patients with epilepsy6,7 and exceeds the expected rate of sudden death in the general population by nearly 24 times.6,8
Several potential risk factors for SUDEP have been investigated, but results from different studies are conflicting. Consistently identified risk factors include young age, early onset of seizures, refractoriness of epilepsy, the presence of generalized tonic-clonic seizures, male sex, and being in bed at the time of death. Weaker risk factors include being in the prone position at the time of death, having one or more subtherapeutic blood levels of anticonvulsant medication, having a structural brain lesion, and being asleep.9 The current consensus is that SUDEP is primarily a “seizure-related” occurrence, but the exact mechanisms underlying SUDEP are unknown.
PROPOSED MECHANISMS
Pulmonary pathophysiology
Central apnea and acute neurogenic pulmonary edema are the two major proposed pathways linking seizures to SUDEP. Evidence exists for each pathway.
Central apnea. In a prospective study of patients in an epilepsy monitoring unit, central apnea lasting at least 10 seconds was observed postictally in 40% of the recorded seizures.10 Otherwise healthy young epilepsy patients have been reported to develop central apnea immediately following complex partial seizures.11 Neurotransmitters mediating the brain’s own seizure-terminating mechanism could also be inhibiting the brainstem and causing postictal apnea.
Acute neurogenic pulmonary edema has been well described in relation to severe head injury and subarachnoid hemorrhage. Pulmonary edema is frequently found in SUDEP patients at autopsy.12 Intense generalized vasoconstriction induced by massive seizure-related sympathetic outburst can lead to increased pulmonary vascular resistance, and thereby may mediate acute pulmonary edema.
These two mechanisms—central apnea and acute neurogenic pulmonary edema—are not mutually exclusive. In the only animal model of SUDEP, one third of animals died from hypoventilation and had associated pulmonary edema at autopsy.13 Limited opportunities for realistic and practical interventions to reverse SUDEP risks related to pulmonary causes have hindered further development of these concepts.
Cardiac pathophysiology
The most significant and widely discussed cardiac mechanism of SUDEP is cardiac arrhythmia precipitated by seizure discharges acting via the autonomic nervous system.14–19
Experimental evidence. Heart rate changes, including bradycardia, tachycardia, and even asystole, have been repeatedly provoked by electrical brain stimulation of the limbic system and insular cortex.19 Some studies have suggested a lateralized influence of the insulae on cardiovascular autonomic control. In one study, intraoperative stimulation of the left posterior insula elicited a cardioinhibitory response and hypotension, whereas stimulation of the right anterior insula elicited tachycardia and hypertension.20 Such results have not always been reproducible.21–23 Other studies have suggested a localization-related influence of the limbic system on cardiovascular responses. Stimulation of the amygdala has not led to the ictal tachycardia that is commonly seen in epileptic seizures, suggesting that cortical involvement is needed for the development of tachycardia.24
SUDEP PREVENTION
Epilepsy control is first line of defense
A careful consideration of the incidence of SUDEP in various patient populations suggests that controlling patients’ epilepsy might just be the best method of preventing SUDEP. While estimated SUDEP incidence ranges from 0.7 to 1.3 cases per 1,000 patient-years in population-based studies of patients with epilepsy,1,2 this rate escalates by nearly tenfold (3.5 to 9.3 cases per 1,000 patient-years) in cohorts with severe epilepsy, such as those derived from anticonvulsant drug registries, medical device registries, and referral centers.3–5 Therefore, medical control of seizures might reduce the incidence of SUDEP.
Epilepsy surgery cuts SUDEP risk for many patients
Studies involving epilepsy surgery programs also suggest that successful epilepsy surgery reduces the impending risks of SUDEP. In cohorts in which the estimated risk of SUDEP is almost 1% per year without surgery, SUDEP incidence was significantly lower following epilepsy surgery. In a study of 305 patients who underwent temporal lobe epilepsy surgery in the United Kingdom, the incidence of SUDEP following surgery was 2.2 cases per 1,000 person-years, and only one-third of SUDEP cases were among seizure-free patients.31 A similar incidence of 2.4 cases per 1,000 person-years was seen following epilepsy surgery in 596 Swedish patients; none of the 6 SUDEP patients in that study was seizure free.32 In a US study, no SUDEP cases occurred among 256 seizure-free patients with a follow-up of about 5 years after epilepsy surgery.33
In our own experience at the Cleveland Clinic, we have reported on outcomes among 70 patients who underwent frontal lobectomy34 and among 371 patients who underwent temporal lobectomy.35 In the frontal lobectomy study,34 2 of the 39 patients who had persistent seizures following surgery died of SUDEP during follow-up, whereas none of the 31 patients who remained seizure free were dead up to 10 years after surgery. In the temporal lobectomy report,35 2 of the 141 patients with ongoing postoperative seizures died of SUDEP, as compared with none of the 230 patients who were seizure free after a mean follow-up of 5.5 years.
Additional means of prophylaxis needed
Unfortunately, as many as 30% to 40% of patients with epilepsy continue to suffer intractable epilepsy despite all the available treatment modalities, including epilepsy surgery. For these patients, controlling seizures to reduce the risk of SUDEP is neither a possible nor a realistic means of avoiding this devastating condition, and alternative methods of prophylaxis must be sought.
CONCLUSIONS AND FUTURE RESEARCH
Patients with refractory epilepsy currently face a lifelong risk of sudden death as high as 1% per year.3 Elucidating the mechanisms of SUDEP might lead to preventive measures, which could have significant implications in reducing mortality in this patient population. Abundant evidence exists that autonomic dysfunction and cardiac arrhythmias are associated with seizures. The missing links in establishing a cardiac mechanism for SUDEP now include the following: (1) evidence of cardiac arrhythmias generally observed in seizures as a risk factor for SUDEP, (2) determination of clear electrophysiologic characteristics—from EEG and ECG standpoints—of patients at risk for SUDEP, and (3) clarification of the role of cardiac mechanisms in SUDEP and the role that cerebral influences on autonomic function might play. Early identification of patients at risk of SUDEP would offer a unique opportunity for early intervention to prevent this devastating condition.
The intimate interplay between heart and brain is well illustrated in epilepsy and may underlie the mechanism of one of its most devastating consequences: sudden unexpected death in epilepsy (SUDEP). This article will briefly describe the potential mechanisms of SUDEP, elaborate on the evidence for a likely cardiac pathophysiology, and review considerations in SUDEP prevention. We begin with a couple of brief case presentations and an epidemiologic overview to illustrate the concept and significance of SUDEP.
CASE PRESENTATIONS
A patient with near-SUDEP
The following is an actual message received by one of the authors:
Dr. Najm: A quick note regarding a 27-year-old male patient of yours with cerebral palsy and seizure disorder. Yesterday, while being transported from floor to floor, he had a cardiac arrest and was successfully resuscitated. Immediately after the code he developed seizures, which were treated with phenytoin and lorazepam. He is now in the neurointensive care unit. Thank you.
This case represents a scenario of near-SUDEP in which death was prevented by the fortuitous presence of immediate medical assistance at the time of cardiac arrest. Had this patient been home at the time of this incident, he almost certainly would have simply been found dead in his bed, like many SUDEP victims.
A typical case with multiple risk factors
A 32-year-old man underwent left temporal lobectomy at the Cleveland Clinic for treatment of medically refractory focal epilepsy. His seizure frequency improved after surgery, but he continued to have rare convulsions. Nevertheless, he discontinued all his anticonvulsant medications on his own. One year later, he was found dead on his bathroom floor. No obvious cause of death was identified.
This case illustrates several characteristics of the patient typically at risk for SUDEP: young, male, with intractable poorly controlled epilepsy, and not taking antiepileptic medications.
EPIDEMIOLOGY AND RISK FACTORS
Epilepsy affects 1% of the US population. Among those affected by epilepsy, SUDEP is a common cause of mortality. Estimates of SUDEP incidence range from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of patients with epilepsy1,2 and from 3.5 to 9.3 cases per 1,000 patient-years in anticonvulsant drug registries, medical device registries, and epilepsy surgery programs.3–5 SUDEP accounts for up to 17% of all deaths in patients with epilepsy6,7 and exceeds the expected rate of sudden death in the general population by nearly 24 times.6,8
Several potential risk factors for SUDEP have been investigated, but results from different studies are conflicting. Consistently identified risk factors include young age, early onset of seizures, refractoriness of epilepsy, the presence of generalized tonic-clonic seizures, male sex, and being in bed at the time of death. Weaker risk factors include being in the prone position at the time of death, having one or more subtherapeutic blood levels of anticonvulsant medication, having a structural brain lesion, and being asleep.9 The current consensus is that SUDEP is primarily a “seizure-related” occurrence, but the exact mechanisms underlying SUDEP are unknown.
PROPOSED MECHANISMS
Pulmonary pathophysiology
Central apnea and acute neurogenic pulmonary edema are the two major proposed pathways linking seizures to SUDEP. Evidence exists for each pathway.
Central apnea. In a prospective study of patients in an epilepsy monitoring unit, central apnea lasting at least 10 seconds was observed postictally in 40% of the recorded seizures.10 Otherwise healthy young epilepsy patients have been reported to develop central apnea immediately following complex partial seizures.11 Neurotransmitters mediating the brain’s own seizure-terminating mechanism could also be inhibiting the brainstem and causing postictal apnea.
Acute neurogenic pulmonary edema has been well described in relation to severe head injury and subarachnoid hemorrhage. Pulmonary edema is frequently found in SUDEP patients at autopsy.12 Intense generalized vasoconstriction induced by massive seizure-related sympathetic outburst can lead to increased pulmonary vascular resistance, and thereby may mediate acute pulmonary edema.
These two mechanisms—central apnea and acute neurogenic pulmonary edema—are not mutually exclusive. In the only animal model of SUDEP, one third of animals died from hypoventilation and had associated pulmonary edema at autopsy.13 Limited opportunities for realistic and practical interventions to reverse SUDEP risks related to pulmonary causes have hindered further development of these concepts.
Cardiac pathophysiology
The most significant and widely discussed cardiac mechanism of SUDEP is cardiac arrhythmia precipitated by seizure discharges acting via the autonomic nervous system.14–19
Experimental evidence. Heart rate changes, including bradycardia, tachycardia, and even asystole, have been repeatedly provoked by electrical brain stimulation of the limbic system and insular cortex.19 Some studies have suggested a lateralized influence of the insulae on cardiovascular autonomic control. In one study, intraoperative stimulation of the left posterior insula elicited a cardioinhibitory response and hypotension, whereas stimulation of the right anterior insula elicited tachycardia and hypertension.20 Such results have not always been reproducible.21–23 Other studies have suggested a localization-related influence of the limbic system on cardiovascular responses. Stimulation of the amygdala has not led to the ictal tachycardia that is commonly seen in epileptic seizures, suggesting that cortical involvement is needed for the development of tachycardia.24
SUDEP PREVENTION
Epilepsy control is first line of defense
A careful consideration of the incidence of SUDEP in various patient populations suggests that controlling patients’ epilepsy might just be the best method of preventing SUDEP. While estimated SUDEP incidence ranges from 0.7 to 1.3 cases per 1,000 patient-years in population-based studies of patients with epilepsy,1,2 this rate escalates by nearly tenfold (3.5 to 9.3 cases per 1,000 patient-years) in cohorts with severe epilepsy, such as those derived from anticonvulsant drug registries, medical device registries, and referral centers.3–5 Therefore, medical control of seizures might reduce the incidence of SUDEP.
Epilepsy surgery cuts SUDEP risk for many patients
Studies involving epilepsy surgery programs also suggest that successful epilepsy surgery reduces the impending risks of SUDEP. In cohorts in which the estimated risk of SUDEP is almost 1% per year without surgery, SUDEP incidence was significantly lower following epilepsy surgery. In a study of 305 patients who underwent temporal lobe epilepsy surgery in the United Kingdom, the incidence of SUDEP following surgery was 2.2 cases per 1,000 person-years, and only one-third of SUDEP cases were among seizure-free patients.31 A similar incidence of 2.4 cases per 1,000 person-years was seen following epilepsy surgery in 596 Swedish patients; none of the 6 SUDEP patients in that study was seizure free.32 In a US study, no SUDEP cases occurred among 256 seizure-free patients with a follow-up of about 5 years after epilepsy surgery.33
In our own experience at the Cleveland Clinic, we have reported on outcomes among 70 patients who underwent frontal lobectomy34 and among 371 patients who underwent temporal lobectomy.35 In the frontal lobectomy study,34 2 of the 39 patients who had persistent seizures following surgery died of SUDEP during follow-up, whereas none of the 31 patients who remained seizure free were dead up to 10 years after surgery. In the temporal lobectomy report,35 2 of the 141 patients with ongoing postoperative seizures died of SUDEP, as compared with none of the 230 patients who were seizure free after a mean follow-up of 5.5 years.
Additional means of prophylaxis needed
Unfortunately, as many as 30% to 40% of patients with epilepsy continue to suffer intractable epilepsy despite all the available treatment modalities, including epilepsy surgery. For these patients, controlling seizures to reduce the risk of SUDEP is neither a possible nor a realistic means of avoiding this devastating condition, and alternative methods of prophylaxis must be sought.
CONCLUSIONS AND FUTURE RESEARCH
Patients with refractory epilepsy currently face a lifelong risk of sudden death as high as 1% per year.3 Elucidating the mechanisms of SUDEP might lead to preventive measures, which could have significant implications in reducing mortality in this patient population. Abundant evidence exists that autonomic dysfunction and cardiac arrhythmias are associated with seizures. The missing links in establishing a cardiac mechanism for SUDEP now include the following: (1) evidence of cardiac arrhythmias generally observed in seizures as a risk factor for SUDEP, (2) determination of clear electrophysiologic characteristics—from EEG and ECG standpoints—of patients at risk for SUDEP, and (3) clarification of the role of cardiac mechanisms in SUDEP and the role that cerebral influences on autonomic function might play. Early identification of patients at risk of SUDEP would offer a unique opportunity for early intervention to prevent this devastating condition.
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet 1999; 353:888–893.
- Tennis P, Cole TB, Annegers JF, Leestma JE, McNutt M, Rajput A. Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 1995; 36:29–36.
- Dasheiff RM. Sudden unexpected death in epilepsy: a series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol 1991; 8:216–222.
- Leestma JE, Annegers JF, Brodie MJ, et al. Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997; 38:47–55.
- Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol 1999; 46:45–50.
- Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia 2000; 41(Suppl 2):S7–S12.
- Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002; 359:1790–1791.
- Ficker DM, So EL, Shen WK, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998; 51:1270–1274.
- Monté CP, Arends JB, Tan IY, Aldenkamp AP, Limburg M, de Krom MC. Sudden unexpected death in epilepsy patients: risk factors. A systematic review. Seizure 2007; 16:1–7.
- Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996; 60:297–300.
- So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000; 41:1494–1497.
- Terrence CF, Rao GR, Perper JA. Neurogenic pulmonary edema in unexpected, unexplained death of epileptic patients. Ann Neurol 1981; 9:458–464.
- Johnston SC, Horn JK, Valente J, Simon RP. The role of hypoventilation in a sheep model of epileptic sudden death. Ann Neurol 1995; 37:531–537.
- Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986; 1:1051–1056.
- Nei M, Ho RT, Abou-Khalil BW, et al. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004; 45:338–345.
- Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000; 41:542–548.
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res 2002; 52:117–127.
- Tigaran S, Mølgaard H, McClelland R, Dam M, Jaffe AS. Evidence of cardiac ischemia during seizures in drug refractory epilepsy patients. Neurology 2003; 60:492–495.
- Leung H, Kwan P, Elger CE. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav 2006; 9:19–30.
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 1991; 303:355–374.
- Jokeit H, Noerpel I, Herbord E, Ebner A. Heart rate does not decrease after right hemispheric amobarbital injection. Neurology 2000; 54:2347–2348.
- Zamrini EY, Meador KJ, Loring DW, et al. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology 1990; 40:1408–1411.
- Yoon BW, Morillo CA, Cechetto DF, Hachinski V. Cerebral hemispheric lateralization in cardiac autonomic control. Arch Neurol 1997; 54:741–744.
- Keilson MJ, Hauser WA, Magrill JP. Electrocardiographic changes during electrographic seizures. Arch Neurol 1989; 46:1169–1170.
- Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987; 37:1624–1626.
- Galimberti CA, Marchioni E, Barzizza F, Manni R, Sartori I, Tartara A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996; 37:742–747.
- Liedholm LJ, Gudjonsson O. Cardiac arrest due to partial epileptic seizures. Neurology 1992; 42:824–829.
- Leung H, Schindler K, Kwan P, Elger C. Asystole induced by electrical stimulation of the left cingulate gyrus. Epileptic Disord 2007; 9:77–81.
- Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia 2000; 41:536–541.
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64:1131–1133.
- Hennessy MJ, Langan Y, Elwes RD, Binnie CD, Polkey CE, Nashef L. A study of mortality after temporal lobe epilepsy surgery. Neurology 1999; 53:1276–1283.
- Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia 2003; 44:575–581.
- Sperling MR, Harris A, Nei M, Liporace JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia 2005; 46(Suppl 11):49–53.
- Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007; 130:574–584.
- Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 2006; 66:1938–1940.
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet 1999; 353:888–893.
- Tennis P, Cole TB, Annegers JF, Leestma JE, McNutt M, Rajput A. Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 1995; 36:29–36.
- Dasheiff RM. Sudden unexpected death in epilepsy: a series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol 1991; 8:216–222.
- Leestma JE, Annegers JF, Brodie MJ, et al. Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997; 38:47–55.
- Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol 1999; 46:45–50.
- Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia 2000; 41(Suppl 2):S7–S12.
- Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002; 359:1790–1791.
- Ficker DM, So EL, Shen WK, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998; 51:1270–1274.
- Monté CP, Arends JB, Tan IY, Aldenkamp AP, Limburg M, de Krom MC. Sudden unexpected death in epilepsy patients: risk factors. A systematic review. Seizure 2007; 16:1–7.
- Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996; 60:297–300.
- So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000; 41:1494–1497.
- Terrence CF, Rao GR, Perper JA. Neurogenic pulmonary edema in unexpected, unexplained death of epileptic patients. Ann Neurol 1981; 9:458–464.
- Johnston SC, Horn JK, Valente J, Simon RP. The role of hypoventilation in a sheep model of epileptic sudden death. Ann Neurol 1995; 37:531–537.
- Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986; 1:1051–1056.
- Nei M, Ho RT, Abou-Khalil BW, et al. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004; 45:338–345.
- Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000; 41:542–548.
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res 2002; 52:117–127.
- Tigaran S, Mølgaard H, McClelland R, Dam M, Jaffe AS. Evidence of cardiac ischemia during seizures in drug refractory epilepsy patients. Neurology 2003; 60:492–495.
- Leung H, Kwan P, Elger CE. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav 2006; 9:19–30.
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 1991; 303:355–374.
- Jokeit H, Noerpel I, Herbord E, Ebner A. Heart rate does not decrease after right hemispheric amobarbital injection. Neurology 2000; 54:2347–2348.
- Zamrini EY, Meador KJ, Loring DW, et al. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology 1990; 40:1408–1411.
- Yoon BW, Morillo CA, Cechetto DF, Hachinski V. Cerebral hemispheric lateralization in cardiac autonomic control. Arch Neurol 1997; 54:741–744.
- Keilson MJ, Hauser WA, Magrill JP. Electrocardiographic changes during electrographic seizures. Arch Neurol 1989; 46:1169–1170.
- Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987; 37:1624–1626.
- Galimberti CA, Marchioni E, Barzizza F, Manni R, Sartori I, Tartara A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996; 37:742–747.
- Liedholm LJ, Gudjonsson O. Cardiac arrest due to partial epileptic seizures. Neurology 1992; 42:824–829.
- Leung H, Schindler K, Kwan P, Elger C. Asystole induced by electrical stimulation of the left cingulate gyrus. Epileptic Disord 2007; 9:77–81.
- Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia 2000; 41:536–541.
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64:1131–1133.
- Hennessy MJ, Langan Y, Elwes RD, Binnie CD, Polkey CE, Nashef L. A study of mortality after temporal lobe epilepsy surgery. Neurology 1999; 53:1276–1283.
- Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia 2003; 44:575–581.
- Sperling MR, Harris A, Nei M, Liporace JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia 2005; 46(Suppl 11):49–53.
- Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007; 130:574–584.
- Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 2006; 66:1938–1940.