User login
Reverse T3 or perverse T3? Still puzzling after 40 years
Four decades after reverse T3 (3,3´5´-triiodothyronine) was discovered, its physiologic and clinical relevance remains unclear and is still being studied. But scientific uncertainty has not stopped writers in the consumer press and on the Internet from making unsubstantiated claims about this hormone. Many patients believe their hypothyroid symptoms are due to high levels of reverse T3 and want to be tested for it, and some even bring in test results from independent laboratories.
HOW THYROID HORMONES WERE DISCOVERED
In 1970, Braverman et al9 showed that T4 is converted to T3 in athyreotic humans, and Sterling et al10 demonstrated the same in healthy humans. During that decade, techniques for measuring T4 were refined,11 and a specific radioimmunoassay for reverse T3 allowed a glimpse of its physiologic role.12 In 1975, Chopra et al13 noted reciprocal changes in the levels of T3 and reverse T3 in systemic illnesses—ie, when people are sick, their T3 levels go down and their reverse T3 levels go up.
The end of the 70s was marked by a surge of interest in T4 metabolites, including the development of a radioimmunoassay for 3,3´-diiodothyronine (3-3´ T2).18
The observed reciprocal changes in serum levels of T3 and reverse T3 suggested that T4 degradation is regulated into activating (T3) or inactivating (reverse T3) pathways, and that these changes are a presumed homeostatic process of energy conservation.19
HOW THYROID HORMONES ARE METABOLIZED
In the thyroid gland, for thyroid hormones to be synthesized, iodide must be oxidized and incorporated into the precursors 3-monoiodotyrosine (MIT) and 3,5-diiodotyrosine (DIT). This process is mediated by the enzyme thyroid peroxidase in the presence of hydrogen peroxide.20
The thyroid can make T4 and some T3
T4 is the main iodothyronine produced by the thyroid gland, at a rate of 80 to 100 µg per day.21 It is synthesized from the fusion of 2 DIT molecules.
The thyroid can also make T3 by fusing 1 DIT and 1 MIT molecule, but this process accounts for no more than 20% of the circulating T3 in humans. The rest of T3, and 95% to 98% of all reverse T3, is derived from peripheral conversion of T4 through deiodination.
T4 is converted to T3 or reverse T3
The metabolic transformation of thyroid hormones in peripheral tissues determines their biologic potency and regulates their biologic effects.
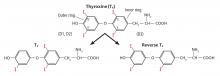
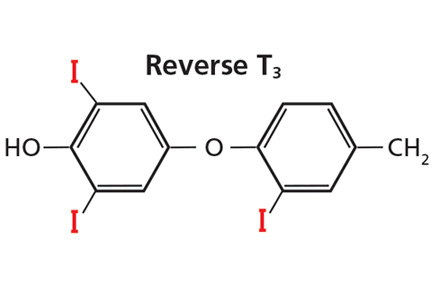
The number 4 in T4 means it has 4 iodine atoms. It can lose 1 of them, yielding either T3 or reverse T3, depending on which iodine atom it loses (Figure 3). Loss of iodine from the five-prime (5´) position on its outer ring yields T3, the most potent thyroid hormone, produced at a rate of 30 to 40 µg per day.21 On the other hand, when T4 loses an iodine atom from the five (5) position on its inner ring it yields reverse T3, produced at a rate slightly less than that of T3, 28 to 40 µg per day.21 Reverse T3 is inactive.
Both T3 and reverse T3 can shed more iodine atoms, forming in turn various isomers of T2, T1, and ultimately T0. Other pathways for thyroid hormone metabolism include glucuronidation, sulfation, oxidative deamination, and ether bond cleavage.20–22
D1 and D2 catalyze T3, D3 catalyzes reverse T3
Three types of enzymes that mediate deiodination have been identified and designated D1, D2, and D3. In humans they are expressed in variable amounts throughout the body:
- D1 mainly in the liver, kidneys, thyroid, and pituitary, but notably absent in the central nervous system
- D2 in the central nervous system, pituitary, brown adipose tissue, thyroid, placenta, skeletal muscle, and heart
- D3 in the central nervous system, skin, hemangiomas, fetal liver, placenta, and fetal tissues.23
D1 and D2 are responsible for converting T4 to T3, and D3 is responsible for converting T4 to reverse T3.
Plasma concentrations of free T4 and free T3 are relatively constant; however, tissue concentrations of free T3 vary in different tissues according to the amount of hormone transported and the activity of local deiodinases.23 Most thyroid hormone actions are initiated after T3 binds to its nuclear receptor. In this setting, deiodinases play a critical role in maintaining tissue and cellular thyroid hormone levels, so that thyroid hormone signaling can change irrespective of serum hormonal concentrations.22–24 For example, in the central nervous system, production of T3 by local D2 is significantly relevant for T3 homeostasis.22,23
Deiodinases also modulate the tissue-specific concentrations of T3 in response to iodine deficiency and to changes in thyroid state.23 During iodine deficiency and hypothyroidism, tissues that express D2, especially brain tissues, increase the activity of this enzyme in order to increase local conversion of T4 to T3. In hyperthyroidism, D1 overexpression contributes to the relative excess of T3 production, while D3 up-regulation in the brain protects the central nervous system from excessive amounts of thyroid hormone.23
REVERSE T3 AND SYSTEMIC ILLNESS
D3 is the main physiologic inactivator of thyroid hormones. This enzyme plays a central role in protecting tissues from an excess of thyroid hormone.23,24 This mechanism is crucial for fetal development and explains the high expression of D3 in the human placenta and fetal tissues.
In adult tissues, the importance of D3 in the regulation of thyroid hormone homeostasis becomes apparent under certain pathophysiologic conditions, such as nonthyroidal illness and malnutrition.
Whenever a reduction in metabolism is homeostatically desirable, such as in critically ill patients or during starvation, conversion to T3 is reduced and, alternatively, conversion to reverse T3 is increased. This pathway represents a metabolic adaptation that may protect the tissues from the catabolic effects of thyroid hormone that could otherwise worsen the patient’s basic clinical condition.
Euthyroid sick syndrome or hypothyroid?
In a variety of systemic illnesses, some patients with low T3, low or normal T4, and normal thyroid-stimulating hormone (TSH) levels could in fact be “sick euthyroid” rather than hypothyroid. The first reports of the euthyroid sick syndrome or low T3 syndrome date back to about 1976, and even though assays for reverse T3 were not widely available, some authors linked the syndrome to high levels of reverse T3.15,16 The syndrome is also known as nonthyroidal illness syndrome.
Advances in techniques for measuring T3, reverse T3, and other iodothyronines filled a gap in the understanding of the alterations that occur in thyroid hormone economy during severe nonthyroidal diseases. In 1982, Wartofsky and Burman25 reviewed the alterations in thyroid function in patients with systemic illness and discussed other factors that may alter thyroid economy, such as age, stress, and diverse drugs.
More recently, the low-T3 syndrome was revisited with a generalized concept regarding the role of D3 in the syndrome.26 D3, normally undetectable in mature tissues, is reactivated in diverse cell types in response to injury and is responsible for a fall in serum T3 levels. Hypoxia induces D3 activity and mRNA in vitro and in vivo.27 Recent studies have focused on the role of cytokines in the low T3 syndrome. For instance, interleukin 6 reduces D1 and D2 activity and increases D3 activity in vitro.28
In the outpatient setting, diverse conditions may affect thyroid hormone homeostasis, compatible with mild or atypical forms of low-T3 syndrome, including caloric deprivation, heart failure, and human immunodeficiency virus infection.29
POSSIBLE CLINICAL UTILITY OF MEASURING REVERSE T3
In inpatients
Unfortunately, measuring serum reverse T3 levels has not, in general, proven clinically useful for the diagnosis of hypothyroidism in systemically ill patients. Burmeister30 demonstrated, in a retrospective study, that when illness complicates the interpretation of thyroid function tests, serum reverse T3 measurements do not reliably distinguish the hypothyroid sick patient from the euthyroid sick patient. The best way to make the diagnosis, Burmeister suggested, is by clinical assessment, combined use of free T4 and TSH measurements, and patient follow-up.
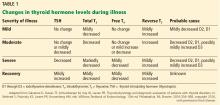
In the outpatient setting, the utility of reverse T3 measurements is controversial. In intensive care units, the differential diagnosis between hypothyroidism and nonthyroidal illness syndrome can sometimes be difficult. Reverse T3 levels can be low, normal, or high regardless of the thyroidal state of the patient.30 Moreover, endogenous changes in the hypothalamic-pituitary-thyroid axis may be further complicated by medications commonly used in intensive care units, such as dopamine and glucocorticoids. Changes in thyroid function should be evaluated in the context of the patient’s clinical condition (Table 1).20 But regardless of the T3 level, treatment with T3 or T4 should not be started without taking into consideration the patient’s general clinical context; controlled trials have not shown such therapy to be beneficial.20
In outpatients
In noncritical conditions that may be associated with mild forms of low T3 syndrome, patients generally present with low T3 concentrations concurrently with low or normal TSH. Not infrequently, however, patients present with a serum reverse T3 measurement and impute their symptoms of hypothyroidism to “abnormal” reverse T3 levels, in spite of normal TSH levels.
There is no rationale for measuring reverse T3 to initiate or to adjust levothyroxine therapy—the single test relevant for these purposes is the TSH measurement. The risks of basing treatment decisions on reverse T3 levels include the use of excessive doses of levothyroxine that may lead to a state of subclinical or even clinical hyperthyroidism.
TAKE-HOME MESSAGE
The existence of an inactivating pathway of thyroid hormones represents a homeostatic mechanism, and in selected circumstances measuring serum reverse T3 may be useful, such as in euthyroid sick patients. The discovery of the molecular mechanisms that lead to the reactivation of D3 in illness is an important field of research.
- Kendall EC. Landmark article, June 19, 1915. The isolation in crystalline form of the compound containing iodin, which occurs in the thyroid. Its chemical nature and physiologic activity. By E.C. Kendall. JAMA 1983; 250(15):2045–2046. doi:10.1001/jama.1983.03340150087037
- Harington CR. Chemistry of thyroxine: isolation of thyroxine from the thyroid gland. Biochem J 1926; 20(2):293–299. pmid: 16743658
- Harington CR, Barger G. Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem J 1927; 21(1):169–183. pmid:16743801
- Gross J, Pitt-Rivers R. The identification of 3,5,3’L-triiodothyronine in human plasma. Lancet 1952; 1(6705):439–441. doi:10.1016/S0140-6736(52)91952-1
- Gross J, Pitt-Rivers R. 3:5:3’-triiodothyronine. 1. Isolation from thyroid gland and synthesis. Biochem J 1953; 53(4):645–650. pmid:13032123
- Pitt-Rivers R, Stanbury JB, Rapp B. Conversion of thyroxine to 3-5-3´-triiodothyronine in vivo. J Clin Endocrinol Metab 1955; 15(5):616–620. doi:10.1210/jcem-15-5-616
- Maclagan NF, Bowden CH, Wilkinson JH. The metabolism of thyroid hormones. 2. Detection of thyroxine and tri-iodothyronine in human plasma. Biochem J. 1957; 67(1):5–11. pmid:13471502
- Galton VA, Pitt-Rivers R. The identification of the acetic acid analogues of thyroxine and tri-iodothyronine in mammalian tissues. Biochem J 1959; 72(2):319–321. pmid: 13662303
- Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest 1970; 49(5):855–864. doi:10.1172/JCI106304
- Sterling K, Brenner MA, Newman ES. Conversion of thyroxine to triiodothyronine in normal human subjects. Science 1970; 169(3950):1099–1100. doi:10.1126/science.169.3950.1099
- Chopra IJ. A radioimmunoassay for measurement of thyroxine in unextracted serum. J Clin Endocrinol Metab 1972; 34:938–947. doi:10.1210/jcem-34-6-938
- Chopra IJ. A radioimmunoassay for measurement of 3,3´,5´-triiodothyronine (reverse T3). J Clin Invest 1974; 54(3):583–592. doi:10.1172/JCI107795
- Chopra IJ, Chopra U, Smith SR, Reza M, Solomon DH. Reciprocal changes in serum concentrations of 3,3´,5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab 1975; 41(6):1043–1049. doi:10.1210/jcem-41-6-1043
- Burman KD, Read J, Dimond RC, Strum D, et al. Measurement of 3,3’,5’-triiodothyroinine (reverse T3), 3,3’-L-diiodothyronine, T3 and T4 in human amniotic fluid and in cord and maternal serum. J Clin Endocrinol Metab 1976; 43(6):1351–1359. doi:10.1210/jcem-43-6-1351
- Rubenfeld S. Euthyroid sick syndrome. N Engl J Med 1978; 299(25):1414. doi:10.1056/NEJM197812212992514
- Burger A, Nicod P, Suter P, Vallotton MB, Vagenakis P, Braverman L. Reduced active thyroid hormone levels in acute illness. Lancet 1976; 1(7961):653–655. doi:10.1016/S0140-6736(76)92774-4
- Burman KD, Dimond RC, Wright FD, Earll JM, Bruton J, Wartofsky L. A radioimmunoassay for 3,3´,5´-L-triiodothyronine (reverse T3): assessment of thyroid gland content and serum measurements in conditions of normal and altered thyroidal economy and following administration of thyrotropin releasing hormone (TRH) and thyrotropin (TSH). J Clin Endocrinol Metab 1977; 44(4):660–672. doi:10.1210/jcem-44-4-660
- Burman KD, Strum D, Dimond RC, et al. A radioimmunoassay for 3,3´-L-diiodothyronine (3,3´T2). J Clin Endocrinol Metab 1977; 45(2):339–352. doi:10.1210/jcem-45-2-339
- Burman KD. Recent developments in thyroid hormone metabolism: interpretation and significance of measurements of reverse T3, 3,3´T2, and thyroglobulin. Metabolism 1978; 27(5):615–630. doi:10.1016/0026-0495(78)90028-8.
- Salvatore D, Davies TF, Schlumberger M, Hay ID, Larsen PR. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 13th ed. Philadelphia, PA; Elsevier; 2016:334–368.
- Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev 1984; 5(2):151–184. doi:10.1210/edrv-5-2-151
- Peeters RP, Visser TJ, Peeters RP. Metabolism of thyroid hormone. Thyroid Disease Manager. www.thyroidmanager.org/chapter/metabolism-of-thyroid-hormone. Accessed March 14, 2018.
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002; 23(1):38–89. doi:10.1210/edrv.23.1.0455
- Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol 2011; 209(3):273–282. doi:10.1530/JOE-11-0002
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab 2008; 4(3):148–155. doi:10.1038/ncpendmet0727
- Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 2008; 118(3):975–983. doi:10.1172/JCI32824
- Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest 2011; 121(5):1834–1845. doi:10.1172/JCI44678
- Moura Neto A, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol 2016; 2016:2157583. doi:10.1155/2016/2157583
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000; 343(3):185–189. doi:10.1056/NEJM200007203430305
Four decades after reverse T3 (3,3´5´-triiodothyronine) was discovered, its physiologic and clinical relevance remains unclear and is still being studied. But scientific uncertainty has not stopped writers in the consumer press and on the Internet from making unsubstantiated claims about this hormone. Many patients believe their hypothyroid symptoms are due to high levels of reverse T3 and want to be tested for it, and some even bring in test results from independent laboratories.
HOW THYROID HORMONES WERE DISCOVERED
In 1970, Braverman et al9 showed that T4 is converted to T3 in athyreotic humans, and Sterling et al10 demonstrated the same in healthy humans. During that decade, techniques for measuring T4 were refined,11 and a specific radioimmunoassay for reverse T3 allowed a glimpse of its physiologic role.12 In 1975, Chopra et al13 noted reciprocal changes in the levels of T3 and reverse T3 in systemic illnesses—ie, when people are sick, their T3 levels go down and their reverse T3 levels go up.
The end of the 70s was marked by a surge of interest in T4 metabolites, including the development of a radioimmunoassay for 3,3´-diiodothyronine (3-3´ T2).18
The observed reciprocal changes in serum levels of T3 and reverse T3 suggested that T4 degradation is regulated into activating (T3) or inactivating (reverse T3) pathways, and that these changes are a presumed homeostatic process of energy conservation.19
HOW THYROID HORMONES ARE METABOLIZED
In the thyroid gland, for thyroid hormones to be synthesized, iodide must be oxidized and incorporated into the precursors 3-monoiodotyrosine (MIT) and 3,5-diiodotyrosine (DIT). This process is mediated by the enzyme thyroid peroxidase in the presence of hydrogen peroxide.20
The thyroid can make T4 and some T3
T4 is the main iodothyronine produced by the thyroid gland, at a rate of 80 to 100 µg per day.21 It is synthesized from the fusion of 2 DIT molecules.
The thyroid can also make T3 by fusing 1 DIT and 1 MIT molecule, but this process accounts for no more than 20% of the circulating T3 in humans. The rest of T3, and 95% to 98% of all reverse T3, is derived from peripheral conversion of T4 through deiodination.
T4 is converted to T3 or reverse T3
The metabolic transformation of thyroid hormones in peripheral tissues determines their biologic potency and regulates their biologic effects.
The number 4 in T4 means it has 4 iodine atoms. It can lose 1 of them, yielding either T3 or reverse T3, depending on which iodine atom it loses (Figure 3). Loss of iodine from the five-prime (5´) position on its outer ring yields T3, the most potent thyroid hormone, produced at a rate of 30 to 40 µg per day.21 On the other hand, when T4 loses an iodine atom from the five (5) position on its inner ring it yields reverse T3, produced at a rate slightly less than that of T3, 28 to 40 µg per day.21 Reverse T3 is inactive.
Both T3 and reverse T3 can shed more iodine atoms, forming in turn various isomers of T2, T1, and ultimately T0. Other pathways for thyroid hormone metabolism include glucuronidation, sulfation, oxidative deamination, and ether bond cleavage.20–22
D1 and D2 catalyze T3, D3 catalyzes reverse T3
Three types of enzymes that mediate deiodination have been identified and designated D1, D2, and D3. In humans they are expressed in variable amounts throughout the body:
- D1 mainly in the liver, kidneys, thyroid, and pituitary, but notably absent in the central nervous system
- D2 in the central nervous system, pituitary, brown adipose tissue, thyroid, placenta, skeletal muscle, and heart
- D3 in the central nervous system, skin, hemangiomas, fetal liver, placenta, and fetal tissues.23
D1 and D2 are responsible for converting T4 to T3, and D3 is responsible for converting T4 to reverse T3.
Plasma concentrations of free T4 and free T3 are relatively constant; however, tissue concentrations of free T3 vary in different tissues according to the amount of hormone transported and the activity of local deiodinases.23 Most thyroid hormone actions are initiated after T3 binds to its nuclear receptor. In this setting, deiodinases play a critical role in maintaining tissue and cellular thyroid hormone levels, so that thyroid hormone signaling can change irrespective of serum hormonal concentrations.22–24 For example, in the central nervous system, production of T3 by local D2 is significantly relevant for T3 homeostasis.22,23
Deiodinases also modulate the tissue-specific concentrations of T3 in response to iodine deficiency and to changes in thyroid state.23 During iodine deficiency and hypothyroidism, tissues that express D2, especially brain tissues, increase the activity of this enzyme in order to increase local conversion of T4 to T3. In hyperthyroidism, D1 overexpression contributes to the relative excess of T3 production, while D3 up-regulation in the brain protects the central nervous system from excessive amounts of thyroid hormone.23
REVERSE T3 AND SYSTEMIC ILLNESS
D3 is the main physiologic inactivator of thyroid hormones. This enzyme plays a central role in protecting tissues from an excess of thyroid hormone.23,24 This mechanism is crucial for fetal development and explains the high expression of D3 in the human placenta and fetal tissues.
In adult tissues, the importance of D3 in the regulation of thyroid hormone homeostasis becomes apparent under certain pathophysiologic conditions, such as nonthyroidal illness and malnutrition.
Whenever a reduction in metabolism is homeostatically desirable, such as in critically ill patients or during starvation, conversion to T3 is reduced and, alternatively, conversion to reverse T3 is increased. This pathway represents a metabolic adaptation that may protect the tissues from the catabolic effects of thyroid hormone that could otherwise worsen the patient’s basic clinical condition.
Euthyroid sick syndrome or hypothyroid?
In a variety of systemic illnesses, some patients with low T3, low or normal T4, and normal thyroid-stimulating hormone (TSH) levels could in fact be “sick euthyroid” rather than hypothyroid. The first reports of the euthyroid sick syndrome or low T3 syndrome date back to about 1976, and even though assays for reverse T3 were not widely available, some authors linked the syndrome to high levels of reverse T3.15,16 The syndrome is also known as nonthyroidal illness syndrome.
Advances in techniques for measuring T3, reverse T3, and other iodothyronines filled a gap in the understanding of the alterations that occur in thyroid hormone economy during severe nonthyroidal diseases. In 1982, Wartofsky and Burman25 reviewed the alterations in thyroid function in patients with systemic illness and discussed other factors that may alter thyroid economy, such as age, stress, and diverse drugs.
More recently, the low-T3 syndrome was revisited with a generalized concept regarding the role of D3 in the syndrome.26 D3, normally undetectable in mature tissues, is reactivated in diverse cell types in response to injury and is responsible for a fall in serum T3 levels. Hypoxia induces D3 activity and mRNA in vitro and in vivo.27 Recent studies have focused on the role of cytokines in the low T3 syndrome. For instance, interleukin 6 reduces D1 and D2 activity and increases D3 activity in vitro.28
In the outpatient setting, diverse conditions may affect thyroid hormone homeostasis, compatible with mild or atypical forms of low-T3 syndrome, including caloric deprivation, heart failure, and human immunodeficiency virus infection.29
POSSIBLE CLINICAL UTILITY OF MEASURING REVERSE T3
In inpatients
Unfortunately, measuring serum reverse T3 levels has not, in general, proven clinically useful for the diagnosis of hypothyroidism in systemically ill patients. Burmeister30 demonstrated, in a retrospective study, that when illness complicates the interpretation of thyroid function tests, serum reverse T3 measurements do not reliably distinguish the hypothyroid sick patient from the euthyroid sick patient. The best way to make the diagnosis, Burmeister suggested, is by clinical assessment, combined use of free T4 and TSH measurements, and patient follow-up.
In the outpatient setting, the utility of reverse T3 measurements is controversial. In intensive care units, the differential diagnosis between hypothyroidism and nonthyroidal illness syndrome can sometimes be difficult. Reverse T3 levels can be low, normal, or high regardless of the thyroidal state of the patient.30 Moreover, endogenous changes in the hypothalamic-pituitary-thyroid axis may be further complicated by medications commonly used in intensive care units, such as dopamine and glucocorticoids. Changes in thyroid function should be evaluated in the context of the patient’s clinical condition (Table 1).20 But regardless of the T3 level, treatment with T3 or T4 should not be started without taking into consideration the patient’s general clinical context; controlled trials have not shown such therapy to be beneficial.20
In outpatients
In noncritical conditions that may be associated with mild forms of low T3 syndrome, patients generally present with low T3 concentrations concurrently with low or normal TSH. Not infrequently, however, patients present with a serum reverse T3 measurement and impute their symptoms of hypothyroidism to “abnormal” reverse T3 levels, in spite of normal TSH levels.
There is no rationale for measuring reverse T3 to initiate or to adjust levothyroxine therapy—the single test relevant for these purposes is the TSH measurement. The risks of basing treatment decisions on reverse T3 levels include the use of excessive doses of levothyroxine that may lead to a state of subclinical or even clinical hyperthyroidism.
TAKE-HOME MESSAGE
The existence of an inactivating pathway of thyroid hormones represents a homeostatic mechanism, and in selected circumstances measuring serum reverse T3 may be useful, such as in euthyroid sick patients. The discovery of the molecular mechanisms that lead to the reactivation of D3 in illness is an important field of research.
Four decades after reverse T3 (3,3´5´-triiodothyronine) was discovered, its physiologic and clinical relevance remains unclear and is still being studied. But scientific uncertainty has not stopped writers in the consumer press and on the Internet from making unsubstantiated claims about this hormone. Many patients believe their hypothyroid symptoms are due to high levels of reverse T3 and want to be tested for it, and some even bring in test results from independent laboratories.
HOW THYROID HORMONES WERE DISCOVERED
In 1970, Braverman et al9 showed that T4 is converted to T3 in athyreotic humans, and Sterling et al10 demonstrated the same in healthy humans. During that decade, techniques for measuring T4 were refined,11 and a specific radioimmunoassay for reverse T3 allowed a glimpse of its physiologic role.12 In 1975, Chopra et al13 noted reciprocal changes in the levels of T3 and reverse T3 in systemic illnesses—ie, when people are sick, their T3 levels go down and their reverse T3 levels go up.
The end of the 70s was marked by a surge of interest in T4 metabolites, including the development of a radioimmunoassay for 3,3´-diiodothyronine (3-3´ T2).18
The observed reciprocal changes in serum levels of T3 and reverse T3 suggested that T4 degradation is regulated into activating (T3) or inactivating (reverse T3) pathways, and that these changes are a presumed homeostatic process of energy conservation.19
HOW THYROID HORMONES ARE METABOLIZED
In the thyroid gland, for thyroid hormones to be synthesized, iodide must be oxidized and incorporated into the precursors 3-monoiodotyrosine (MIT) and 3,5-diiodotyrosine (DIT). This process is mediated by the enzyme thyroid peroxidase in the presence of hydrogen peroxide.20
The thyroid can make T4 and some T3
T4 is the main iodothyronine produced by the thyroid gland, at a rate of 80 to 100 µg per day.21 It is synthesized from the fusion of 2 DIT molecules.
The thyroid can also make T3 by fusing 1 DIT and 1 MIT molecule, but this process accounts for no more than 20% of the circulating T3 in humans. The rest of T3, and 95% to 98% of all reverse T3, is derived from peripheral conversion of T4 through deiodination.
T4 is converted to T3 or reverse T3
The metabolic transformation of thyroid hormones in peripheral tissues determines their biologic potency and regulates their biologic effects.
The number 4 in T4 means it has 4 iodine atoms. It can lose 1 of them, yielding either T3 or reverse T3, depending on which iodine atom it loses (Figure 3). Loss of iodine from the five-prime (5´) position on its outer ring yields T3, the most potent thyroid hormone, produced at a rate of 30 to 40 µg per day.21 On the other hand, when T4 loses an iodine atom from the five (5) position on its inner ring it yields reverse T3, produced at a rate slightly less than that of T3, 28 to 40 µg per day.21 Reverse T3 is inactive.
Both T3 and reverse T3 can shed more iodine atoms, forming in turn various isomers of T2, T1, and ultimately T0. Other pathways for thyroid hormone metabolism include glucuronidation, sulfation, oxidative deamination, and ether bond cleavage.20–22
D1 and D2 catalyze T3, D3 catalyzes reverse T3
Three types of enzymes that mediate deiodination have been identified and designated D1, D2, and D3. In humans they are expressed in variable amounts throughout the body:
- D1 mainly in the liver, kidneys, thyroid, and pituitary, but notably absent in the central nervous system
- D2 in the central nervous system, pituitary, brown adipose tissue, thyroid, placenta, skeletal muscle, and heart
- D3 in the central nervous system, skin, hemangiomas, fetal liver, placenta, and fetal tissues.23
D1 and D2 are responsible for converting T4 to T3, and D3 is responsible for converting T4 to reverse T3.
Plasma concentrations of free T4 and free T3 are relatively constant; however, tissue concentrations of free T3 vary in different tissues according to the amount of hormone transported and the activity of local deiodinases.23 Most thyroid hormone actions are initiated after T3 binds to its nuclear receptor. In this setting, deiodinases play a critical role in maintaining tissue and cellular thyroid hormone levels, so that thyroid hormone signaling can change irrespective of serum hormonal concentrations.22–24 For example, in the central nervous system, production of T3 by local D2 is significantly relevant for T3 homeostasis.22,23
Deiodinases also modulate the tissue-specific concentrations of T3 in response to iodine deficiency and to changes in thyroid state.23 During iodine deficiency and hypothyroidism, tissues that express D2, especially brain tissues, increase the activity of this enzyme in order to increase local conversion of T4 to T3. In hyperthyroidism, D1 overexpression contributes to the relative excess of T3 production, while D3 up-regulation in the brain protects the central nervous system from excessive amounts of thyroid hormone.23
REVERSE T3 AND SYSTEMIC ILLNESS
D3 is the main physiologic inactivator of thyroid hormones. This enzyme plays a central role in protecting tissues from an excess of thyroid hormone.23,24 This mechanism is crucial for fetal development and explains the high expression of D3 in the human placenta and fetal tissues.
In adult tissues, the importance of D3 in the regulation of thyroid hormone homeostasis becomes apparent under certain pathophysiologic conditions, such as nonthyroidal illness and malnutrition.
Whenever a reduction in metabolism is homeostatically desirable, such as in critically ill patients or during starvation, conversion to T3 is reduced and, alternatively, conversion to reverse T3 is increased. This pathway represents a metabolic adaptation that may protect the tissues from the catabolic effects of thyroid hormone that could otherwise worsen the patient’s basic clinical condition.
Euthyroid sick syndrome or hypothyroid?
In a variety of systemic illnesses, some patients with low T3, low or normal T4, and normal thyroid-stimulating hormone (TSH) levels could in fact be “sick euthyroid” rather than hypothyroid. The first reports of the euthyroid sick syndrome or low T3 syndrome date back to about 1976, and even though assays for reverse T3 were not widely available, some authors linked the syndrome to high levels of reverse T3.15,16 The syndrome is also known as nonthyroidal illness syndrome.
Advances in techniques for measuring T3, reverse T3, and other iodothyronines filled a gap in the understanding of the alterations that occur in thyroid hormone economy during severe nonthyroidal diseases. In 1982, Wartofsky and Burman25 reviewed the alterations in thyroid function in patients with systemic illness and discussed other factors that may alter thyroid economy, such as age, stress, and diverse drugs.
More recently, the low-T3 syndrome was revisited with a generalized concept regarding the role of D3 in the syndrome.26 D3, normally undetectable in mature tissues, is reactivated in diverse cell types in response to injury and is responsible for a fall in serum T3 levels. Hypoxia induces D3 activity and mRNA in vitro and in vivo.27 Recent studies have focused on the role of cytokines in the low T3 syndrome. For instance, interleukin 6 reduces D1 and D2 activity and increases D3 activity in vitro.28
In the outpatient setting, diverse conditions may affect thyroid hormone homeostasis, compatible with mild or atypical forms of low-T3 syndrome, including caloric deprivation, heart failure, and human immunodeficiency virus infection.29
POSSIBLE CLINICAL UTILITY OF MEASURING REVERSE T3
In inpatients
Unfortunately, measuring serum reverse T3 levels has not, in general, proven clinically useful for the diagnosis of hypothyroidism in systemically ill patients. Burmeister30 demonstrated, in a retrospective study, that when illness complicates the interpretation of thyroid function tests, serum reverse T3 measurements do not reliably distinguish the hypothyroid sick patient from the euthyroid sick patient. The best way to make the diagnosis, Burmeister suggested, is by clinical assessment, combined use of free T4 and TSH measurements, and patient follow-up.
In the outpatient setting, the utility of reverse T3 measurements is controversial. In intensive care units, the differential diagnosis between hypothyroidism and nonthyroidal illness syndrome can sometimes be difficult. Reverse T3 levels can be low, normal, or high regardless of the thyroidal state of the patient.30 Moreover, endogenous changes in the hypothalamic-pituitary-thyroid axis may be further complicated by medications commonly used in intensive care units, such as dopamine and glucocorticoids. Changes in thyroid function should be evaluated in the context of the patient’s clinical condition (Table 1).20 But regardless of the T3 level, treatment with T3 or T4 should not be started without taking into consideration the patient’s general clinical context; controlled trials have not shown such therapy to be beneficial.20
In outpatients
In noncritical conditions that may be associated with mild forms of low T3 syndrome, patients generally present with low T3 concentrations concurrently with low or normal TSH. Not infrequently, however, patients present with a serum reverse T3 measurement and impute their symptoms of hypothyroidism to “abnormal” reverse T3 levels, in spite of normal TSH levels.
There is no rationale for measuring reverse T3 to initiate or to adjust levothyroxine therapy—the single test relevant for these purposes is the TSH measurement. The risks of basing treatment decisions on reverse T3 levels include the use of excessive doses of levothyroxine that may lead to a state of subclinical or even clinical hyperthyroidism.
TAKE-HOME MESSAGE
The existence of an inactivating pathway of thyroid hormones represents a homeostatic mechanism, and in selected circumstances measuring serum reverse T3 may be useful, such as in euthyroid sick patients. The discovery of the molecular mechanisms that lead to the reactivation of D3 in illness is an important field of research.
- Kendall EC. Landmark article, June 19, 1915. The isolation in crystalline form of the compound containing iodin, which occurs in the thyroid. Its chemical nature and physiologic activity. By E.C. Kendall. JAMA 1983; 250(15):2045–2046. doi:10.1001/jama.1983.03340150087037
- Harington CR. Chemistry of thyroxine: isolation of thyroxine from the thyroid gland. Biochem J 1926; 20(2):293–299. pmid: 16743658
- Harington CR, Barger G. Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem J 1927; 21(1):169–183. pmid:16743801
- Gross J, Pitt-Rivers R. The identification of 3,5,3’L-triiodothyronine in human plasma. Lancet 1952; 1(6705):439–441. doi:10.1016/S0140-6736(52)91952-1
- Gross J, Pitt-Rivers R. 3:5:3’-triiodothyronine. 1. Isolation from thyroid gland and synthesis. Biochem J 1953; 53(4):645–650. pmid:13032123
- Pitt-Rivers R, Stanbury JB, Rapp B. Conversion of thyroxine to 3-5-3´-triiodothyronine in vivo. J Clin Endocrinol Metab 1955; 15(5):616–620. doi:10.1210/jcem-15-5-616
- Maclagan NF, Bowden CH, Wilkinson JH. The metabolism of thyroid hormones. 2. Detection of thyroxine and tri-iodothyronine in human plasma. Biochem J. 1957; 67(1):5–11. pmid:13471502
- Galton VA, Pitt-Rivers R. The identification of the acetic acid analogues of thyroxine and tri-iodothyronine in mammalian tissues. Biochem J 1959; 72(2):319–321. pmid: 13662303
- Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest 1970; 49(5):855–864. doi:10.1172/JCI106304
- Sterling K, Brenner MA, Newman ES. Conversion of thyroxine to triiodothyronine in normal human subjects. Science 1970; 169(3950):1099–1100. doi:10.1126/science.169.3950.1099
- Chopra IJ. A radioimmunoassay for measurement of thyroxine in unextracted serum. J Clin Endocrinol Metab 1972; 34:938–947. doi:10.1210/jcem-34-6-938
- Chopra IJ. A radioimmunoassay for measurement of 3,3´,5´-triiodothyronine (reverse T3). J Clin Invest 1974; 54(3):583–592. doi:10.1172/JCI107795
- Chopra IJ, Chopra U, Smith SR, Reza M, Solomon DH. Reciprocal changes in serum concentrations of 3,3´,5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab 1975; 41(6):1043–1049. doi:10.1210/jcem-41-6-1043
- Burman KD, Read J, Dimond RC, Strum D, et al. Measurement of 3,3’,5’-triiodothyroinine (reverse T3), 3,3’-L-diiodothyronine, T3 and T4 in human amniotic fluid and in cord and maternal serum. J Clin Endocrinol Metab 1976; 43(6):1351–1359. doi:10.1210/jcem-43-6-1351
- Rubenfeld S. Euthyroid sick syndrome. N Engl J Med 1978; 299(25):1414. doi:10.1056/NEJM197812212992514
- Burger A, Nicod P, Suter P, Vallotton MB, Vagenakis P, Braverman L. Reduced active thyroid hormone levels in acute illness. Lancet 1976; 1(7961):653–655. doi:10.1016/S0140-6736(76)92774-4
- Burman KD, Dimond RC, Wright FD, Earll JM, Bruton J, Wartofsky L. A radioimmunoassay for 3,3´,5´-L-triiodothyronine (reverse T3): assessment of thyroid gland content and serum measurements in conditions of normal and altered thyroidal economy and following administration of thyrotropin releasing hormone (TRH) and thyrotropin (TSH). J Clin Endocrinol Metab 1977; 44(4):660–672. doi:10.1210/jcem-44-4-660
- Burman KD, Strum D, Dimond RC, et al. A radioimmunoassay for 3,3´-L-diiodothyronine (3,3´T2). J Clin Endocrinol Metab 1977; 45(2):339–352. doi:10.1210/jcem-45-2-339
- Burman KD. Recent developments in thyroid hormone metabolism: interpretation and significance of measurements of reverse T3, 3,3´T2, and thyroglobulin. Metabolism 1978; 27(5):615–630. doi:10.1016/0026-0495(78)90028-8.
- Salvatore D, Davies TF, Schlumberger M, Hay ID, Larsen PR. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 13th ed. Philadelphia, PA; Elsevier; 2016:334–368.
- Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev 1984; 5(2):151–184. doi:10.1210/edrv-5-2-151
- Peeters RP, Visser TJ, Peeters RP. Metabolism of thyroid hormone. Thyroid Disease Manager. www.thyroidmanager.org/chapter/metabolism-of-thyroid-hormone. Accessed March 14, 2018.
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002; 23(1):38–89. doi:10.1210/edrv.23.1.0455
- Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol 2011; 209(3):273–282. doi:10.1530/JOE-11-0002
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab 2008; 4(3):148–155. doi:10.1038/ncpendmet0727
- Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 2008; 118(3):975–983. doi:10.1172/JCI32824
- Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest 2011; 121(5):1834–1845. doi:10.1172/JCI44678
- Moura Neto A, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol 2016; 2016:2157583. doi:10.1155/2016/2157583
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000; 343(3):185–189. doi:10.1056/NEJM200007203430305
- Kendall EC. Landmark article, June 19, 1915. The isolation in crystalline form of the compound containing iodin, which occurs in the thyroid. Its chemical nature and physiologic activity. By E.C. Kendall. JAMA 1983; 250(15):2045–2046. doi:10.1001/jama.1983.03340150087037
- Harington CR. Chemistry of thyroxine: isolation of thyroxine from the thyroid gland. Biochem J 1926; 20(2):293–299. pmid: 16743658
- Harington CR, Barger G. Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem J 1927; 21(1):169–183. pmid:16743801
- Gross J, Pitt-Rivers R. The identification of 3,5,3’L-triiodothyronine in human plasma. Lancet 1952; 1(6705):439–441. doi:10.1016/S0140-6736(52)91952-1
- Gross J, Pitt-Rivers R. 3:5:3’-triiodothyronine. 1. Isolation from thyroid gland and synthesis. Biochem J 1953; 53(4):645–650. pmid:13032123
- Pitt-Rivers R, Stanbury JB, Rapp B. Conversion of thyroxine to 3-5-3´-triiodothyronine in vivo. J Clin Endocrinol Metab 1955; 15(5):616–620. doi:10.1210/jcem-15-5-616
- Maclagan NF, Bowden CH, Wilkinson JH. The metabolism of thyroid hormones. 2. Detection of thyroxine and tri-iodothyronine in human plasma. Biochem J. 1957; 67(1):5–11. pmid:13471502
- Galton VA, Pitt-Rivers R. The identification of the acetic acid analogues of thyroxine and tri-iodothyronine in mammalian tissues. Biochem J 1959; 72(2):319–321. pmid: 13662303
- Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest 1970; 49(5):855–864. doi:10.1172/JCI106304
- Sterling K, Brenner MA, Newman ES. Conversion of thyroxine to triiodothyronine in normal human subjects. Science 1970; 169(3950):1099–1100. doi:10.1126/science.169.3950.1099
- Chopra IJ. A radioimmunoassay for measurement of thyroxine in unextracted serum. J Clin Endocrinol Metab 1972; 34:938–947. doi:10.1210/jcem-34-6-938
- Chopra IJ. A radioimmunoassay for measurement of 3,3´,5´-triiodothyronine (reverse T3). J Clin Invest 1974; 54(3):583–592. doi:10.1172/JCI107795
- Chopra IJ, Chopra U, Smith SR, Reza M, Solomon DH. Reciprocal changes in serum concentrations of 3,3´,5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab 1975; 41(6):1043–1049. doi:10.1210/jcem-41-6-1043
- Burman KD, Read J, Dimond RC, Strum D, et al. Measurement of 3,3’,5’-triiodothyroinine (reverse T3), 3,3’-L-diiodothyronine, T3 and T4 in human amniotic fluid and in cord and maternal serum. J Clin Endocrinol Metab 1976; 43(6):1351–1359. doi:10.1210/jcem-43-6-1351
- Rubenfeld S. Euthyroid sick syndrome. N Engl J Med 1978; 299(25):1414. doi:10.1056/NEJM197812212992514
- Burger A, Nicod P, Suter P, Vallotton MB, Vagenakis P, Braverman L. Reduced active thyroid hormone levels in acute illness. Lancet 1976; 1(7961):653–655. doi:10.1016/S0140-6736(76)92774-4
- Burman KD, Dimond RC, Wright FD, Earll JM, Bruton J, Wartofsky L. A radioimmunoassay for 3,3´,5´-L-triiodothyronine (reverse T3): assessment of thyroid gland content and serum measurements in conditions of normal and altered thyroidal economy and following administration of thyrotropin releasing hormone (TRH) and thyrotropin (TSH). J Clin Endocrinol Metab 1977; 44(4):660–672. doi:10.1210/jcem-44-4-660
- Burman KD, Strum D, Dimond RC, et al. A radioimmunoassay for 3,3´-L-diiodothyronine (3,3´T2). J Clin Endocrinol Metab 1977; 45(2):339–352. doi:10.1210/jcem-45-2-339
- Burman KD. Recent developments in thyroid hormone metabolism: interpretation and significance of measurements of reverse T3, 3,3´T2, and thyroglobulin. Metabolism 1978; 27(5):615–630. doi:10.1016/0026-0495(78)90028-8.
- Salvatore D, Davies TF, Schlumberger M, Hay ID, Larsen PR. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 13th ed. Philadelphia, PA; Elsevier; 2016:334–368.
- Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev 1984; 5(2):151–184. doi:10.1210/edrv-5-2-151
- Peeters RP, Visser TJ, Peeters RP. Metabolism of thyroid hormone. Thyroid Disease Manager. www.thyroidmanager.org/chapter/metabolism-of-thyroid-hormone. Accessed March 14, 2018.
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002; 23(1):38–89. doi:10.1210/edrv.23.1.0455
- Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol 2011; 209(3):273–282. doi:10.1530/JOE-11-0002
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab 2008; 4(3):148–155. doi:10.1038/ncpendmet0727
- Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 2008; 118(3):975–983. doi:10.1172/JCI32824
- Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest 2011; 121(5):1834–1845. doi:10.1172/JCI44678
- Moura Neto A, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol 2016; 2016:2157583. doi:10.1155/2016/2157583
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000; 343(3):185–189. doi:10.1056/NEJM200007203430305
Iodine deficiency: Clinical implications
A 65-year-old woman is found to have a goiter. She is clinically euthyroid. She is a strict vegan and only uses noniodized Himalayan salt for cooking. Her thyroid gland is diffusely enlarged with no nodules. The estimated weight of the thyroid gland is 50 g (normal 10–20 g) based on ultrasonography. Her thyroid-stimulating hormone (TSH) level is 2.95 mU/L (reference range 0.5–5 mU/L), and her free thyroxine level is 0.8 ng/dL (0.7–1.8 ng/dL). Testing for TSH receptor antibody is negative. Her 24-hour urine iodine is undetectable (urine iodine concentration < 10 μg/L with urine volume 3,175 mL). What may be the cause of her goiter?
Iodine is an essential element needed for the production of thyroid hormone, which controls metabolism and plays a major role in fetal neurodevelopment. Its ionized form is called iodide. Iodine deficiency results in impairment of thyroid hormone synthesis and may lead to several undesirable consequences. Physicians should be aware of the risks iodine deficiency poses, especially during pregnancy, and should be familiar with approaches to testing and current indications for iodine supplementation.
SOURCES OF IODINE AND SALT IODIZATION
The major environmental source of iodine is the ocean. Elemental iodine in the ocean volatilizes into the atmosphere and returns to the soil by rain. The effects of glaciation, flooding, and leaching into soil have resulted in the variable geographic distribution of iodine. Mountainous areas (eg, the Alps, Andes, Himalayas) and areas with frequent flooding typically have iodine-deficient soil due to slow iodine cycling.1 Seafood is a good source of iodine because marine plants and animals concentrate iodine from seawater. The iodine content of other foods varies widely, depending on the source and any additives.
In the United States, the major sources of dietary iodine are dairy products (due to livestock iodine supplements and use of iodophors for cleaning milk udders) and iodized salt.1,2 Seafood contains a higher amount of iodine by weight than dairy products but is consumed far less than dairy.3,4 Further, the iodine content of milk can range from 88 to 168 μg per 250 mL (about 1 cup), depending on the product manufacturer. Also, iodine content is often omitted from the food label. Even if it is reported, the package labeling may not accurately predict the iodine content.5
Less common sources of iodine are radiographic contrast, bread with iodate dough conditioners, red food coloring (erythrosine), and drugs such as amiodarone.1
Using iodized salt is an effective and stable way to ensure adequate iodine intake. In the United States, only table salt is iodized, and the salt typically used in processed food has only minimal iodine content.6 Nearly 70% of the salt we ingest is from processed food. Table salt provides only 15% of dietary salt intake, and only 70% of consumers choose iodized salt for home cooking.7
IODINE REQUIREMENTS
Daily requirements of iodine suggested by the World Health Organization (WHO) and by the US Institute of Medicine are in the range of 90 to 150 µg/day.8,9 The iodine requirement is higher in pregnancy (220–250 µg/day) because of increased maternal thyroid hormone production required to maintain euthyroidism and increased renal iodine clearance, and it is even higher in lactating women (250–290 µg/day).
IODINE STATUS IN POPULATIONS
Since the establishment of universal salt iodization programs under the influence of the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in 1990, global iodine status has continued to improve. Yet only 70% of households worldwide currently have access to adequately iodized salt, because many countries lack a national program for iodine supplementation. The population of the United States was historically iodine-deficient, but since the introduction of salt iodization in the 1920s, the iodine status in the United States has been considered adequate.1
The WHO defines iodine status for a population by the median spot urinary iodine concentration. Because a urinary iodine concentration of 100 μg/L represents an iodine intake of about 150 μg/day, the WHO uses a median urinary iodine concentration of 100 to 199 μg/L to define adequate iodine intake for a nonpregnant population.9
The National Health and Nutrition Examination Survey (NHANES) found that the median urinary iodine concentration decreased by more than 50% from the 1970s to the 1990s, indicating declining iodine status in the US population.2 Of particular concern, the percentage of women of childbearing age with moderate iodine deficiency increased from 4% to 15% over this period.2 Still, the NHANES survey in 2009–2010 indicated that the overall US population is still iodine-sufficient (median urinary iodine concentration 144 μg/L).10 The decline in the US iodine status may be due to reduction of iodine content in dairy products, increased use of noniodized salt by the food industry, and recommendations to avoid salt for blood pressure control.
Although US iodine status has been considered generally adequate, iodine intake varies greatly across the population. Vegans tend to have iodine-deficient diets, while kelp consumers may have excessive iodine intake.11 Individuals with lactose intolerance are at risk of iodine deficiency, given that dairy products are a major source of iodine in the United States. Physicians should be aware of these risk factors for iodine deficiency.
PREGNANCY AND LACTATION
It is crucial to maintain euthyroidism during pregnancy. In early gestation, maternal thyroid hormone production increases 50% due to an increase in thyroid-binding globulin and stimulation by human chorionic gonadotropin. The glomerular filtration rate increases by 30% to 50% during pregnancy, thus increasing renal iodine clearance. Fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodine readily crosses the placenta.12
Women with sufficient iodine intake before and during pregnancy generally have adequate intrathyroidal iodine storage and can adapt to the increased demand for thyroid hormone throughout gestation. But in the setting of even mild iodine deficiency, total body iodine stores decline gradually from the first to third trimester of pregnancy.13
The fetal thyroid gland does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by TSH until the full development of the pituitary-portal vascular system at 20 weeks of gestation.12 Therefore, the fetus relies on maternal thyroid hormone during this critical stage of neurodevelopment. Thyroid hormone is essential for oligodendrocyte differentiation and myelin distribution14 as well as fetal neuronal proliferation and migration in the first and second trimesters. Iodine deficiency leading to maternal hypothyroidism can result in irreversible fetal brain damage.
Because of the greater requirement during pregnancy, the WHO recommends using a median urinary iodine concentration of 150 to 249 μg/L to define a population that has no iodine deficiency.9 The NHANES data from 2007 to 2010 showed that pregnant US women were mildly iodine-deficient (median urinary iodine concentration 135 μg/L),10 and the National Children’s Study of 501 pregnant US women during the third trimester in 2009 to 2010 showed they had adequate iodine intake (median urinary iodine concentration 167 μg/L). Interestingly, pregnant non-Hispanic blacks were the only ethnic group with a median urinary iodine concentration less than 150 μg/L, suggesting that race or ethnicity is a predictor of iodine status in pregnant women.10
Iodine requirements during lactation
During lactation, thyroid hormone production and renal iodine clearance return to the prepregnancy state. However, a significant amount of iodine is excreted into breast milk at a concentration 20 to 50 times greater than that in plasma.15 It is recommended that lactating women continue high iodine intake to ensure sufficient iodine in breast milk to build reserves in the newborn’s thyroid gland.
The iodine requirement during lactation is 225 to 350 μg/day.16 Breast milk containing 100 to 200 μg/L of iodine appears to provide adequate iodine to meet Institute of Medicine recommendations for infants.17 The amount of iodine excreted into breast milk depends on maternal iodine intake. In the setting of iodine sufficiency, the iodine content of breast milk is 150 to 180 μg/L, but it is much lower (9–32 μg/L) in women from iodine-deficient areas, eg, the “goiter belt,” which included the Great Lakes, the Appalachians, and northwestern states. While iodized salt has virtually eliminated the goiter belt, the risk of iodine deficiency remains for people who avoid iodized salt and dairy.15
To ensure adequate iodine intake, the American Thyroid Association recommends that women receive iodine supplementation daily during pregnancy and lactation.11 However, the iodine content of prenatal multivitamins is currently not mandated in the United States. Only half of marketed prenatal vitamins in the United States contain iodine, in the form of either potassium iodide or kelp. Though most iodine-containing products claim to contain at least 150 μg of iodine per daily dose, when measured, the actual iodine content varied between 33 and 610 μg.18
CONSEQUENCES OF IODINE DEFICIENCY
Goiter
Goiter in iodine-deficient areas is considered to be an adaptation to chronic iodine deficiency. Low iodine intake leads to reduced thyroid hormone production, which in turn stimulates TSH secretion from the pituitary. TSH increases iodine uptake by the thyroid, stimulates thyroid growth, and leads to goiter development.
Initially, goiter is characterized by diffuse thyroid enlargement, but over time it may become nodular from progressive accumulation of new thyroid follicles. Goiter in children from iodine-deficient areas is diffusely enlarged, whereas in older adults it tends to be multinodular.
Iodine deficiency and chronic TSH stimulation may play a role in TSH receptor-activating mutations of thyroid follicles. These “gain-of-function” mutations are more common in the glands of patients with goiter in areas of iodine deficiency but are relatively rare in areas of iodine sufficiency.19 Toxic multinodular goiter may eventually develop, and hyperthyroidism may occur if iodine deficiency is not severe.
Goiter generally does not cause obstructive symptoms, since the thyroid usually grows outward. However, a very large goiter may descend to the thoracic inlet and compress the trachea and esophagus. The obstructive effect of a large goiter can be demonstrated by having a patient raise the arms adjacent to the face (the Pemberton maneuver). Signs suggesting obstruction are engorged neck veins, facial plethora, increased dyspnea, and stridor during the maneuver. Computed tomography of the neck and upper thorax may provide information on the degree of tracheal compression.20
Hypothyroidism
A normal or low triiodothyronine (T3), a low serum thyroxine (T4), and a variably elevated TSH are features of thyroid function tests in iodine deficiency.11,21,22 As long as daily iodine intake exceeds 50 μg/day, the absolute uptake of iodine by the thyroid gland usually remains adequate to maintain euthyroidism. Below 50 μg/day, iodine storage in the thyroid becomes depleted, leading to hypothyroidism.1
The clinical manifestations of hypothyroidism from iodine deficiency are similar to those of hypothyroidism from other causes. Because of thyroid hormone’s role in neural and somatic development, the manifestations of hypothyroidism differ among age groups (Table 1).
Cretinism
Before the development of fetal thyroid tissue in the 10th to 12th week of gestation, the fetus is dependent on maternal thyroid hormone, which crosses the placenta to support general and neural development. Iodine deficiency leading to maternal hypothyroidism (in early gestation) or inadequate fetal thyroid hormone production (in late gestation) may result in various degrees of mental retardation or lower than expected IQ.
Severe iodine deficiency during gestation typically results in cretinism, characterized by severe mental retardation accompanied by other neurologic or physical defects. Cretinism is divided into two subtypes according to clinical manifestations (neurologic and myxomatous cretinism; Table 2), which may reflect the different timing of intrauterine insult to the developing fetal nervous system and whether the iodine deficiency continues into the postnatal period. Both types can be prevented by adequate maternal iodine intake before and during pregnancy.23,24
Although mild gestational iodine deficiency does not result in cretinism, it nevertheless has an adverse impact on fetal neurodevelopment and subsequent functioning. Children of mothers with mild gestational iodine deficiency were found to have reductions in spelling, grammar, and English literacy performance despite growing up in iodine-replete environments.25
Impaired cognitive development
Reduction in IQ has been noted in affected youth from regions of severe and mild iodine deficiency. A meta-analysis of studies relating iodine deficiency to cognitive development suggested that chronic moderate to severe iodine deficiency reduced expected average IQ by about 13.5 points.26
The effects of mild iodine deficiency during childhood are more difficult to quantify. The results of one study suggested that mild iodine deficiency was associated with subtle neurodevelopmental deficits and that iodine supplementation might improve cognitive function in mildly iodine-deficient children.27
In a 2009 randomized, placebo-controlled study in New Zealand, 184 children ages 10 to 13 with mild iodine deficiency (median urinary iodine concentration of 63 μg/L) received iodine supplementation (150 μg/day) or placebo for 28 weeks. Iodine supplementation increased the median urinary iodine concentration to 145 μg/L and significantly improved perceptual reasoning measures and overall cognitive score compared with placebo.28
These findings suggest that correcting mild iodine deficiency in children could improve certain components of cognition. More research is needed to understand the effects of mild iodine deficiency and iodine supplementation on cognitive function.
ASSESSING IODINE STATUS
The diagnosis of iodine deficiency is based on clinical and laboratory assessments. Clinical manifestations compatible with iodine deficiency and careful history-taking focused on the patient’s dietary iodine intake and geographic data are keys to the diagnosis.
Four main methods are used to assess iodine status at a population level: urinary iodine, serum thyroglobulin, serum TSH, and thyroid size. Urinary iodine is a sensitive marker for recent iodine intake (within days); thyroglobulin represents iodine nutrition over a period of months and thyroid size over a period of years.1
Urinary iodine
Most dietary iodine is excreted into the urine within 24 hours of ingestion, and the 24-hour urinary iodine is considered a reference standard for the measurement of individual daily iodine intake. However, the process of collection is cumbersome, and the 24-hour urinary iodine can vary from day to day in the same person, depending on the amount of iodine ingested.
A study in healthy women from an iodine-sufficient area suggested that 10 repeated 24-hour urine collections estimated the person’s iodine status at a precision of 20% because of variable daily iodine intake.29 Therefore, when necessary, several 24-hour urine iodine determinations should be performed.
A single, random, spot urinary iodine is expressed as the urinary iodine concentration and is affected by the amount of iodine and fluids the individual ingests in a day, thus resulting in high variation both within an individual person and between individuals. Expressing the urinary iodine concentration as the ratio of urine iodine to creatinine is useful in correcting for the influence of fluid intake. The ratio of urine iodine to creatinine can be used to estimate 24-hour urine iodine with the following formula: urine iodine (μg/L)/creatinine (g/L)× age- and sex-specific estimated 24-hour creatinine excretion (g/day). Another clinical use of the spot urine iodine is to screen for exposure to a large amount of iodine from a source such as radiographic contrast.30
Although individual urine iodine excretion and urine volume can vary from day to day, this variation tends to even out in a large number of samples. In study populations of at least 500, the median value of the spot urinary iodine concentration is considered a reliable measure of iodine intake in that population.30 The spot urine iodine test is convenient, making it the test of choice to study iodine status in a large cohort. The WHO recommends using the median value of the spot urine iodine to evaluate the iodine status of a population.9
Thyroglobulin
Thyroglobulin is a thyroid-specific protein involved in the synthesis of thyroid hormone. Small amounts can be detected in the blood of healthy people. In the absence of thyroid damage, the amount of serum thyroglobulin depends on thyroid cell mass and TSH stimulation. The serum level is elevated in iodine deficiency as a result of chronic TSH stimulation and thyroid hyperplasia. Thus, thyroglobulin can serve as a marker of iodine deficiency.
Serum thyroglobulin assays have been adapted for use on dried whole-blood spots, which require only a few drops of whole blood collected on filter paper and left to air-dry. The results of the dried whole-blood assay correlate closely with those of the serum assay.31 An established international dried whole-blood thyroglobulin reference range for iodine-sufficient school-age children is 4 to 40 μg/L.32 A median level of less than 13 μg/L in school-age children indicates iodine sufficiency in the population.33
Thyroid-stimulating hormone
Iodine deficiency lowers serum T4, which in turn leads to increased serum TSH. Therefore, iodine-deficient populations generally have higher TSH than iodine-sufficient groups. However, the TSH values in older children and adults with iodine deficiency are not significantly different from values of those with adequate iodine intake. Therefore, TSH is not a practical marker of iodine deficiency in the general population.
In contrast, TSH in newborns is a reasonable indicator of population iodine status. The newborn thyroid has limited iodine stores compared with that of an adult and hence a much higher iodine turnover rate. TSH from the cord blood is markedly elevated in newborns of mothers with moderate to severe iodine deficiency.34 A high prevalence of newborns with elevated TSH should therefore reflect iodine deficiency in the area where the mothers of the newborns live.
TSH is now routinely checked in newborns to screen for congenital hypothyroidism. TSH is typically checked 2 to 5 days after delivery to avoid confusion with transient physiologic TSH elevation, which occurs within a few hours after birth and decreases rapidly in 24 hours. The WHO has proposed that a more than 3% prevalence of newborns with TSH values higher than 5 mU/L from blood samples collected 3 to 4 days after birth indicates iodine deficiency in a population.1 This threshold appears to correlate well with the iodine status of the population defined by the WHO’s median urinary iodine concentration.35,36
But several other factors can influence the measurement of newborn TSH, such as prematurity, time of blood collection, maternal or newborn exposure to iodine-containing antiseptics, and the TSH assay methodology. These potential confounding factors limit the role of neonatal TSH as a reliable monitoring tool for iodine deficiency.35,37
Thyroid size
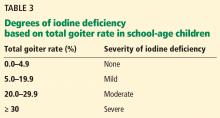
The size of the thyroid gland varies inversely with iodine intake. Thyroid size can be assessed by either palpation or ultrasonography, with the latter being more sensitive. The goiter rate in school-age children can be used to determine the severity of iodine deficiency in the population (Table 3). A goiter rate of 5% or more in school-age children suggests the presence of iodine deficiency in the community.
Although thyroid size is easy to estimate by palpation, it has low sensitivity and specificity to detect iodine deficiency and high interobserver variation. Thyroid ultrasonography provides a more precise measurement of thyroid gland volume. Zimmermann et al38 provided reference data on thyroid volume stratified by age, sex, and body surface area of school-age children in iodine-sufficient areas.38 Results of ultrasonography in a population is then compared with these reference data. The higher the percentage of the population with thyroid volume exceeding the 97th percentile of the reference range, the more severe the iodine deficiency. However, the WHO does not specify how to grade the degree of iodine deficiency based on the thyroid volume obtained with ultrasonography. Follow-up studies showed no significant correlation between urinary iodine concentration and thyroid size.39,40
Thyroid size decreases slowly after iodine repletion. Therefore, the goiter rate may remain high for several years after iodine supplementation begins.1,9
TREATMENT AND PREVENTION
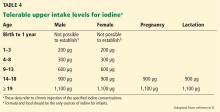
Treatment of iodine deficiency should be instituted at the levels recommended by the Institute of Medicine and the WHO. The tolerable upper intake levels for iodine are outlined in Table 4. In a nonpregnant adult, 150 μg/day is sufficient for normal thyroid function. Iodine intake should be higher for pregnant and lactating women (250 μg/day according to the WHO recommendation).
Iodine supplementation is easily achieved by using iodized salt or an iodine-containing daily multivitamin.
In patients with overt hypothyroidism from iodine deficiency, we recommend initiating levothyroxine treatment along with iodine supplementation to restore euthyroidism, with consideration of possible interruption in 6 to 12 months when the urine iodine has normalized and goiter size has decreased. Thyroid function should be reassessed 4 to 6 weeks after discontinuation of levothyroxine.
At the population level, iodine deficiency can usually be prevented by iodization of food products or the water supply. In developing countries where salt iodization is not practical, iodine deficiency has been eradicated by adding iodine drops to well water or by injecting people with iodized oil.
TAKE-HOME POINTS
Iodine is essential for thyroid hormone synthesis. It can be obtained by eating iodine-containing foods or by using iodized salt. The WHO classifies iodine deficiency based on the median urinary iodine concentration. Iodine nutrition at the community level is best assessed by measurements of urinary iodine, thyroglobulin, serum TSH, and thyroid size.
Adequate iodine intake during pregnancy is important for fetal development. Iodine deficiency is associated with goiter and hypothyroidism. Severe iodine deficiency during pregnancy is associated with cretinism.
There is evidence that mild to moderate iodine deficiency can cause impaired cognitive development and that correcting the iodine deficiency can significantly improve cognitive function.
CASE FOLLOW-UP
This patient had been following a strict vegan diet with very little intake of iodized salt. Her dietary history and the presence of goiter suggested iodine deficiency. She was instructed to take an iodine supplement 150 μg/day to meet her daily requirement. After 2 months of iodine supplementation, her urine iodine concentration had increased to 58 μg/L. She remained biochemically euthyroid.
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30:376–408.
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013; 23:523–528.
- Huang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc 2005; 26:468–469.
- US Census Bureau. The 2012 Statistical abstract. Health and nutrition. www.census.gov/prod/2011pubs/12statab/health.pdf. Accessed December 1, 2016.
- Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004; 89:3421–3424.
- Salt Institute. Production and Industry. www.saltinstitute.org/salt-101/production-industry. Accessed September 20, 2016.
- Dunn JT. Guarding our nation's thyroid health. J Clin Endocrinol Metab 2002; 87:486–488.
- Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- World Health Organization (WHO). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en. Accessed December 1, 2016.
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23:927–937.
- Public Health Committee of the American Thyroid Association; Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006; 16:949–951.
- Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am 2011; 40:765–777.
- Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008; 93:2466–2468.
- Younes-Rapozo V, Berendonk J, Savignon T, Manhaes AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24:445–453.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009; 70:803–809.
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 2007; 10:1571–1583.
- Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev 2001; 59:269–278.
- Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009; 360:939–940.
- Tonacchera M, Agretti P, Chiovato L, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J Clin Endocrinol Metab 2000; 85:2270–2274.
- Medeiros-Neto G, Camargo RY, Tomimori EK. Approach to and treatment of goiters. Med Clin North Am 2012; 96:351–368.
- Heidemann P, Stubbe P. Serum 3,5,3'-triiodothyronine, thyroxine, and thyrotropin in hypothyroid infants with congenital goiter and the response to iodine. J Clin Endocrinol Metab 1978; 47:189–192.
- Patel YC, Pharoah PO, Hornabrook RW, Hetzel BS. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab 1973; 37:783–789.
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid 1993; 3:59–69.
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24:39–50.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98:1954–1962.
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency. 1st ed. New York, NY: Cognizant Communication; 1994:195–200.
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 2010; 24:29–38.
- Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90:1264–1271.
- Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141:2049–2054.
- Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009; 19:1281–1286.
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr 2003; 77:663–667.
- Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91:4881–4887.
- Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013; 98:1271–1280.
- Thilly CH, Delange F, Lagasse R, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab 1978; 47:354–360.
- Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr 1997; 127:55–58.
- Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999; 88:21–24.
- Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24:63–75.
- Zimmermann MB, Moretti D, Chaouki N, Torresani T. Development of a dried whole-blood spot thyroglobulin assay and its evaluation as an indicator of thyroid status in goitrous children receiving iodized salt. Am J Clin Nutr 2003; 77:1453–1458.
- Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India). Eur J Endocrinol 2000; 143:37–46.
- Moradi M, Hashemipour M, Akbari S, Kor Z, Mirbod SA, Kooshanmehr MR. Ultrasonographic evaluation of the thyroid gland volume among 8-15-year-old children in Isfahan, Iran. Adv Biomed Res 2014; 3:9.
A 65-year-old woman is found to have a goiter. She is clinically euthyroid. She is a strict vegan and only uses noniodized Himalayan salt for cooking. Her thyroid gland is diffusely enlarged with no nodules. The estimated weight of the thyroid gland is 50 g (normal 10–20 g) based on ultrasonography. Her thyroid-stimulating hormone (TSH) level is 2.95 mU/L (reference range 0.5–5 mU/L), and her free thyroxine level is 0.8 ng/dL (0.7–1.8 ng/dL). Testing for TSH receptor antibody is negative. Her 24-hour urine iodine is undetectable (urine iodine concentration < 10 μg/L with urine volume 3,175 mL). What may be the cause of her goiter?
Iodine is an essential element needed for the production of thyroid hormone, which controls metabolism and plays a major role in fetal neurodevelopment. Its ionized form is called iodide. Iodine deficiency results in impairment of thyroid hormone synthesis and may lead to several undesirable consequences. Physicians should be aware of the risks iodine deficiency poses, especially during pregnancy, and should be familiar with approaches to testing and current indications for iodine supplementation.
SOURCES OF IODINE AND SALT IODIZATION
The major environmental source of iodine is the ocean. Elemental iodine in the ocean volatilizes into the atmosphere and returns to the soil by rain. The effects of glaciation, flooding, and leaching into soil have resulted in the variable geographic distribution of iodine. Mountainous areas (eg, the Alps, Andes, Himalayas) and areas with frequent flooding typically have iodine-deficient soil due to slow iodine cycling.1 Seafood is a good source of iodine because marine plants and animals concentrate iodine from seawater. The iodine content of other foods varies widely, depending on the source and any additives.
In the United States, the major sources of dietary iodine are dairy products (due to livestock iodine supplements and use of iodophors for cleaning milk udders) and iodized salt.1,2 Seafood contains a higher amount of iodine by weight than dairy products but is consumed far less than dairy.3,4 Further, the iodine content of milk can range from 88 to 168 μg per 250 mL (about 1 cup), depending on the product manufacturer. Also, iodine content is often omitted from the food label. Even if it is reported, the package labeling may not accurately predict the iodine content.5
Less common sources of iodine are radiographic contrast, bread with iodate dough conditioners, red food coloring (erythrosine), and drugs such as amiodarone.1
Using iodized salt is an effective and stable way to ensure adequate iodine intake. In the United States, only table salt is iodized, and the salt typically used in processed food has only minimal iodine content.6 Nearly 70% of the salt we ingest is from processed food. Table salt provides only 15% of dietary salt intake, and only 70% of consumers choose iodized salt for home cooking.7
IODINE REQUIREMENTS
Daily requirements of iodine suggested by the World Health Organization (WHO) and by the US Institute of Medicine are in the range of 90 to 150 µg/day.8,9 The iodine requirement is higher in pregnancy (220–250 µg/day) because of increased maternal thyroid hormone production required to maintain euthyroidism and increased renal iodine clearance, and it is even higher in lactating women (250–290 µg/day).
IODINE STATUS IN POPULATIONS
Since the establishment of universal salt iodization programs under the influence of the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in 1990, global iodine status has continued to improve. Yet only 70% of households worldwide currently have access to adequately iodized salt, because many countries lack a national program for iodine supplementation. The population of the United States was historically iodine-deficient, but since the introduction of salt iodization in the 1920s, the iodine status in the United States has been considered adequate.1
The WHO defines iodine status for a population by the median spot urinary iodine concentration. Because a urinary iodine concentration of 100 μg/L represents an iodine intake of about 150 μg/day, the WHO uses a median urinary iodine concentration of 100 to 199 μg/L to define adequate iodine intake for a nonpregnant population.9
The National Health and Nutrition Examination Survey (NHANES) found that the median urinary iodine concentration decreased by more than 50% from the 1970s to the 1990s, indicating declining iodine status in the US population.2 Of particular concern, the percentage of women of childbearing age with moderate iodine deficiency increased from 4% to 15% over this period.2 Still, the NHANES survey in 2009–2010 indicated that the overall US population is still iodine-sufficient (median urinary iodine concentration 144 μg/L).10 The decline in the US iodine status may be due to reduction of iodine content in dairy products, increased use of noniodized salt by the food industry, and recommendations to avoid salt for blood pressure control.
Although US iodine status has been considered generally adequate, iodine intake varies greatly across the population. Vegans tend to have iodine-deficient diets, while kelp consumers may have excessive iodine intake.11 Individuals with lactose intolerance are at risk of iodine deficiency, given that dairy products are a major source of iodine in the United States. Physicians should be aware of these risk factors for iodine deficiency.
PREGNANCY AND LACTATION
It is crucial to maintain euthyroidism during pregnancy. In early gestation, maternal thyroid hormone production increases 50% due to an increase in thyroid-binding globulin and stimulation by human chorionic gonadotropin. The glomerular filtration rate increases by 30% to 50% during pregnancy, thus increasing renal iodine clearance. Fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodine readily crosses the placenta.12
Women with sufficient iodine intake before and during pregnancy generally have adequate intrathyroidal iodine storage and can adapt to the increased demand for thyroid hormone throughout gestation. But in the setting of even mild iodine deficiency, total body iodine stores decline gradually from the first to third trimester of pregnancy.13
The fetal thyroid gland does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by TSH until the full development of the pituitary-portal vascular system at 20 weeks of gestation.12 Therefore, the fetus relies on maternal thyroid hormone during this critical stage of neurodevelopment. Thyroid hormone is essential for oligodendrocyte differentiation and myelin distribution14 as well as fetal neuronal proliferation and migration in the first and second trimesters. Iodine deficiency leading to maternal hypothyroidism can result in irreversible fetal brain damage.
Because of the greater requirement during pregnancy, the WHO recommends using a median urinary iodine concentration of 150 to 249 μg/L to define a population that has no iodine deficiency.9 The NHANES data from 2007 to 2010 showed that pregnant US women were mildly iodine-deficient (median urinary iodine concentration 135 μg/L),10 and the National Children’s Study of 501 pregnant US women during the third trimester in 2009 to 2010 showed they had adequate iodine intake (median urinary iodine concentration 167 μg/L). Interestingly, pregnant non-Hispanic blacks were the only ethnic group with a median urinary iodine concentration less than 150 μg/L, suggesting that race or ethnicity is a predictor of iodine status in pregnant women.10
Iodine requirements during lactation
During lactation, thyroid hormone production and renal iodine clearance return to the prepregnancy state. However, a significant amount of iodine is excreted into breast milk at a concentration 20 to 50 times greater than that in plasma.15 It is recommended that lactating women continue high iodine intake to ensure sufficient iodine in breast milk to build reserves in the newborn’s thyroid gland.
The iodine requirement during lactation is 225 to 350 μg/day.16 Breast milk containing 100 to 200 μg/L of iodine appears to provide adequate iodine to meet Institute of Medicine recommendations for infants.17 The amount of iodine excreted into breast milk depends on maternal iodine intake. In the setting of iodine sufficiency, the iodine content of breast milk is 150 to 180 μg/L, but it is much lower (9–32 μg/L) in women from iodine-deficient areas, eg, the “goiter belt,” which included the Great Lakes, the Appalachians, and northwestern states. While iodized salt has virtually eliminated the goiter belt, the risk of iodine deficiency remains for people who avoid iodized salt and dairy.15
To ensure adequate iodine intake, the American Thyroid Association recommends that women receive iodine supplementation daily during pregnancy and lactation.11 However, the iodine content of prenatal multivitamins is currently not mandated in the United States. Only half of marketed prenatal vitamins in the United States contain iodine, in the form of either potassium iodide or kelp. Though most iodine-containing products claim to contain at least 150 μg of iodine per daily dose, when measured, the actual iodine content varied between 33 and 610 μg.18
CONSEQUENCES OF IODINE DEFICIENCY
Goiter
Goiter in iodine-deficient areas is considered to be an adaptation to chronic iodine deficiency. Low iodine intake leads to reduced thyroid hormone production, which in turn stimulates TSH secretion from the pituitary. TSH increases iodine uptake by the thyroid, stimulates thyroid growth, and leads to goiter development.
Initially, goiter is characterized by diffuse thyroid enlargement, but over time it may become nodular from progressive accumulation of new thyroid follicles. Goiter in children from iodine-deficient areas is diffusely enlarged, whereas in older adults it tends to be multinodular.
Iodine deficiency and chronic TSH stimulation may play a role in TSH receptor-activating mutations of thyroid follicles. These “gain-of-function” mutations are more common in the glands of patients with goiter in areas of iodine deficiency but are relatively rare in areas of iodine sufficiency.19 Toxic multinodular goiter may eventually develop, and hyperthyroidism may occur if iodine deficiency is not severe.
Goiter generally does not cause obstructive symptoms, since the thyroid usually grows outward. However, a very large goiter may descend to the thoracic inlet and compress the trachea and esophagus. The obstructive effect of a large goiter can be demonstrated by having a patient raise the arms adjacent to the face (the Pemberton maneuver). Signs suggesting obstruction are engorged neck veins, facial plethora, increased dyspnea, and stridor during the maneuver. Computed tomography of the neck and upper thorax may provide information on the degree of tracheal compression.20
Hypothyroidism
A normal or low triiodothyronine (T3), a low serum thyroxine (T4), and a variably elevated TSH are features of thyroid function tests in iodine deficiency.11,21,22 As long as daily iodine intake exceeds 50 μg/day, the absolute uptake of iodine by the thyroid gland usually remains adequate to maintain euthyroidism. Below 50 μg/day, iodine storage in the thyroid becomes depleted, leading to hypothyroidism.1
The clinical manifestations of hypothyroidism from iodine deficiency are similar to those of hypothyroidism from other causes. Because of thyroid hormone’s role in neural and somatic development, the manifestations of hypothyroidism differ among age groups (Table 1).
Cretinism
Before the development of fetal thyroid tissue in the 10th to 12th week of gestation, the fetus is dependent on maternal thyroid hormone, which crosses the placenta to support general and neural development. Iodine deficiency leading to maternal hypothyroidism (in early gestation) or inadequate fetal thyroid hormone production (in late gestation) may result in various degrees of mental retardation or lower than expected IQ.
Severe iodine deficiency during gestation typically results in cretinism, characterized by severe mental retardation accompanied by other neurologic or physical defects. Cretinism is divided into two subtypes according to clinical manifestations (neurologic and myxomatous cretinism; Table 2), which may reflect the different timing of intrauterine insult to the developing fetal nervous system and whether the iodine deficiency continues into the postnatal period. Both types can be prevented by adequate maternal iodine intake before and during pregnancy.23,24
Although mild gestational iodine deficiency does not result in cretinism, it nevertheless has an adverse impact on fetal neurodevelopment and subsequent functioning. Children of mothers with mild gestational iodine deficiency were found to have reductions in spelling, grammar, and English literacy performance despite growing up in iodine-replete environments.25
Impaired cognitive development
Reduction in IQ has been noted in affected youth from regions of severe and mild iodine deficiency. A meta-analysis of studies relating iodine deficiency to cognitive development suggested that chronic moderate to severe iodine deficiency reduced expected average IQ by about 13.5 points.26
The effects of mild iodine deficiency during childhood are more difficult to quantify. The results of one study suggested that mild iodine deficiency was associated with subtle neurodevelopmental deficits and that iodine supplementation might improve cognitive function in mildly iodine-deficient children.27
In a 2009 randomized, placebo-controlled study in New Zealand, 184 children ages 10 to 13 with mild iodine deficiency (median urinary iodine concentration of 63 μg/L) received iodine supplementation (150 μg/day) or placebo for 28 weeks. Iodine supplementation increased the median urinary iodine concentration to 145 μg/L and significantly improved perceptual reasoning measures and overall cognitive score compared with placebo.28
These findings suggest that correcting mild iodine deficiency in children could improve certain components of cognition. More research is needed to understand the effects of mild iodine deficiency and iodine supplementation on cognitive function.
ASSESSING IODINE STATUS
The diagnosis of iodine deficiency is based on clinical and laboratory assessments. Clinical manifestations compatible with iodine deficiency and careful history-taking focused on the patient’s dietary iodine intake and geographic data are keys to the diagnosis.
Four main methods are used to assess iodine status at a population level: urinary iodine, serum thyroglobulin, serum TSH, and thyroid size. Urinary iodine is a sensitive marker for recent iodine intake (within days); thyroglobulin represents iodine nutrition over a period of months and thyroid size over a period of years.1
Urinary iodine
Most dietary iodine is excreted into the urine within 24 hours of ingestion, and the 24-hour urinary iodine is considered a reference standard for the measurement of individual daily iodine intake. However, the process of collection is cumbersome, and the 24-hour urinary iodine can vary from day to day in the same person, depending on the amount of iodine ingested.
A study in healthy women from an iodine-sufficient area suggested that 10 repeated 24-hour urine collections estimated the person’s iodine status at a precision of 20% because of variable daily iodine intake.29 Therefore, when necessary, several 24-hour urine iodine determinations should be performed.
A single, random, spot urinary iodine is expressed as the urinary iodine concentration and is affected by the amount of iodine and fluids the individual ingests in a day, thus resulting in high variation both within an individual person and between individuals. Expressing the urinary iodine concentration as the ratio of urine iodine to creatinine is useful in correcting for the influence of fluid intake. The ratio of urine iodine to creatinine can be used to estimate 24-hour urine iodine with the following formula: urine iodine (μg/L)/creatinine (g/L)× age- and sex-specific estimated 24-hour creatinine excretion (g/day). Another clinical use of the spot urine iodine is to screen for exposure to a large amount of iodine from a source such as radiographic contrast.30
Although individual urine iodine excretion and urine volume can vary from day to day, this variation tends to even out in a large number of samples. In study populations of at least 500, the median value of the spot urinary iodine concentration is considered a reliable measure of iodine intake in that population.30 The spot urine iodine test is convenient, making it the test of choice to study iodine status in a large cohort. The WHO recommends using the median value of the spot urine iodine to evaluate the iodine status of a population.9
Thyroglobulin
Thyroglobulin is a thyroid-specific protein involved in the synthesis of thyroid hormone. Small amounts can be detected in the blood of healthy people. In the absence of thyroid damage, the amount of serum thyroglobulin depends on thyroid cell mass and TSH stimulation. The serum level is elevated in iodine deficiency as a result of chronic TSH stimulation and thyroid hyperplasia. Thus, thyroglobulin can serve as a marker of iodine deficiency.
Serum thyroglobulin assays have been adapted for use on dried whole-blood spots, which require only a few drops of whole blood collected on filter paper and left to air-dry. The results of the dried whole-blood assay correlate closely with those of the serum assay.31 An established international dried whole-blood thyroglobulin reference range for iodine-sufficient school-age children is 4 to 40 μg/L.32 A median level of less than 13 μg/L in school-age children indicates iodine sufficiency in the population.33
Thyroid-stimulating hormone
Iodine deficiency lowers serum T4, which in turn leads to increased serum TSH. Therefore, iodine-deficient populations generally have higher TSH than iodine-sufficient groups. However, the TSH values in older children and adults with iodine deficiency are not significantly different from values of those with adequate iodine intake. Therefore, TSH is not a practical marker of iodine deficiency in the general population.
In contrast, TSH in newborns is a reasonable indicator of population iodine status. The newborn thyroid has limited iodine stores compared with that of an adult and hence a much higher iodine turnover rate. TSH from the cord blood is markedly elevated in newborns of mothers with moderate to severe iodine deficiency.34 A high prevalence of newborns with elevated TSH should therefore reflect iodine deficiency in the area where the mothers of the newborns live.
TSH is now routinely checked in newborns to screen for congenital hypothyroidism. TSH is typically checked 2 to 5 days after delivery to avoid confusion with transient physiologic TSH elevation, which occurs within a few hours after birth and decreases rapidly in 24 hours. The WHO has proposed that a more than 3% prevalence of newborns with TSH values higher than 5 mU/L from blood samples collected 3 to 4 days after birth indicates iodine deficiency in a population.1 This threshold appears to correlate well with the iodine status of the population defined by the WHO’s median urinary iodine concentration.35,36
But several other factors can influence the measurement of newborn TSH, such as prematurity, time of blood collection, maternal or newborn exposure to iodine-containing antiseptics, and the TSH assay methodology. These potential confounding factors limit the role of neonatal TSH as a reliable monitoring tool for iodine deficiency.35,37
Thyroid size
The size of the thyroid gland varies inversely with iodine intake. Thyroid size can be assessed by either palpation or ultrasonography, with the latter being more sensitive. The goiter rate in school-age children can be used to determine the severity of iodine deficiency in the population (Table 3). A goiter rate of 5% or more in school-age children suggests the presence of iodine deficiency in the community.
Although thyroid size is easy to estimate by palpation, it has low sensitivity and specificity to detect iodine deficiency and high interobserver variation. Thyroid ultrasonography provides a more precise measurement of thyroid gland volume. Zimmermann et al38 provided reference data on thyroid volume stratified by age, sex, and body surface area of school-age children in iodine-sufficient areas.38 Results of ultrasonography in a population is then compared with these reference data. The higher the percentage of the population with thyroid volume exceeding the 97th percentile of the reference range, the more severe the iodine deficiency. However, the WHO does not specify how to grade the degree of iodine deficiency based on the thyroid volume obtained with ultrasonography. Follow-up studies showed no significant correlation between urinary iodine concentration and thyroid size.39,40
Thyroid size decreases slowly after iodine repletion. Therefore, the goiter rate may remain high for several years after iodine supplementation begins.1,9
TREATMENT AND PREVENTION
Treatment of iodine deficiency should be instituted at the levels recommended by the Institute of Medicine and the WHO. The tolerable upper intake levels for iodine are outlined in Table 4. In a nonpregnant adult, 150 μg/day is sufficient for normal thyroid function. Iodine intake should be higher for pregnant and lactating women (250 μg/day according to the WHO recommendation).
Iodine supplementation is easily achieved by using iodized salt or an iodine-containing daily multivitamin.
In patients with overt hypothyroidism from iodine deficiency, we recommend initiating levothyroxine treatment along with iodine supplementation to restore euthyroidism, with consideration of possible interruption in 6 to 12 months when the urine iodine has normalized and goiter size has decreased. Thyroid function should be reassessed 4 to 6 weeks after discontinuation of levothyroxine.
At the population level, iodine deficiency can usually be prevented by iodization of food products or the water supply. In developing countries where salt iodization is not practical, iodine deficiency has been eradicated by adding iodine drops to well water or by injecting people with iodized oil.
TAKE-HOME POINTS
Iodine is essential for thyroid hormone synthesis. It can be obtained by eating iodine-containing foods or by using iodized salt. The WHO classifies iodine deficiency based on the median urinary iodine concentration. Iodine nutrition at the community level is best assessed by measurements of urinary iodine, thyroglobulin, serum TSH, and thyroid size.
Adequate iodine intake during pregnancy is important for fetal development. Iodine deficiency is associated with goiter and hypothyroidism. Severe iodine deficiency during pregnancy is associated with cretinism.
There is evidence that mild to moderate iodine deficiency can cause impaired cognitive development and that correcting the iodine deficiency can significantly improve cognitive function.
CASE FOLLOW-UP
This patient had been following a strict vegan diet with very little intake of iodized salt. Her dietary history and the presence of goiter suggested iodine deficiency. She was instructed to take an iodine supplement 150 μg/day to meet her daily requirement. After 2 months of iodine supplementation, her urine iodine concentration had increased to 58 μg/L. She remained biochemically euthyroid.
A 65-year-old woman is found to have a goiter. She is clinically euthyroid. She is a strict vegan and only uses noniodized Himalayan salt for cooking. Her thyroid gland is diffusely enlarged with no nodules. The estimated weight of the thyroid gland is 50 g (normal 10–20 g) based on ultrasonography. Her thyroid-stimulating hormone (TSH) level is 2.95 mU/L (reference range 0.5–5 mU/L), and her free thyroxine level is 0.8 ng/dL (0.7–1.8 ng/dL). Testing for TSH receptor antibody is negative. Her 24-hour urine iodine is undetectable (urine iodine concentration < 10 μg/L with urine volume 3,175 mL). What may be the cause of her goiter?
Iodine is an essential element needed for the production of thyroid hormone, which controls metabolism and plays a major role in fetal neurodevelopment. Its ionized form is called iodide. Iodine deficiency results in impairment of thyroid hormone synthesis and may lead to several undesirable consequences. Physicians should be aware of the risks iodine deficiency poses, especially during pregnancy, and should be familiar with approaches to testing and current indications for iodine supplementation.
SOURCES OF IODINE AND SALT IODIZATION
The major environmental source of iodine is the ocean. Elemental iodine in the ocean volatilizes into the atmosphere and returns to the soil by rain. The effects of glaciation, flooding, and leaching into soil have resulted in the variable geographic distribution of iodine. Mountainous areas (eg, the Alps, Andes, Himalayas) and areas with frequent flooding typically have iodine-deficient soil due to slow iodine cycling.1 Seafood is a good source of iodine because marine plants and animals concentrate iodine from seawater. The iodine content of other foods varies widely, depending on the source and any additives.
In the United States, the major sources of dietary iodine are dairy products (due to livestock iodine supplements and use of iodophors for cleaning milk udders) and iodized salt.1,2 Seafood contains a higher amount of iodine by weight than dairy products but is consumed far less than dairy.3,4 Further, the iodine content of milk can range from 88 to 168 μg per 250 mL (about 1 cup), depending on the product manufacturer. Also, iodine content is often omitted from the food label. Even if it is reported, the package labeling may not accurately predict the iodine content.5
Less common sources of iodine are radiographic contrast, bread with iodate dough conditioners, red food coloring (erythrosine), and drugs such as amiodarone.1
Using iodized salt is an effective and stable way to ensure adequate iodine intake. In the United States, only table salt is iodized, and the salt typically used in processed food has only minimal iodine content.6 Nearly 70% of the salt we ingest is from processed food. Table salt provides only 15% of dietary salt intake, and only 70% of consumers choose iodized salt for home cooking.7
IODINE REQUIREMENTS
Daily requirements of iodine suggested by the World Health Organization (WHO) and by the US Institute of Medicine are in the range of 90 to 150 µg/day.8,9 The iodine requirement is higher in pregnancy (220–250 µg/day) because of increased maternal thyroid hormone production required to maintain euthyroidism and increased renal iodine clearance, and it is even higher in lactating women (250–290 µg/day).
IODINE STATUS IN POPULATIONS
Since the establishment of universal salt iodization programs under the influence of the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in 1990, global iodine status has continued to improve. Yet only 70% of households worldwide currently have access to adequately iodized salt, because many countries lack a national program for iodine supplementation. The population of the United States was historically iodine-deficient, but since the introduction of salt iodization in the 1920s, the iodine status in the United States has been considered adequate.1
The WHO defines iodine status for a population by the median spot urinary iodine concentration. Because a urinary iodine concentration of 100 μg/L represents an iodine intake of about 150 μg/day, the WHO uses a median urinary iodine concentration of 100 to 199 μg/L to define adequate iodine intake for a nonpregnant population.9
The National Health and Nutrition Examination Survey (NHANES) found that the median urinary iodine concentration decreased by more than 50% from the 1970s to the 1990s, indicating declining iodine status in the US population.2 Of particular concern, the percentage of women of childbearing age with moderate iodine deficiency increased from 4% to 15% over this period.2 Still, the NHANES survey in 2009–2010 indicated that the overall US population is still iodine-sufficient (median urinary iodine concentration 144 μg/L).10 The decline in the US iodine status may be due to reduction of iodine content in dairy products, increased use of noniodized salt by the food industry, and recommendations to avoid salt for blood pressure control.
Although US iodine status has been considered generally adequate, iodine intake varies greatly across the population. Vegans tend to have iodine-deficient diets, while kelp consumers may have excessive iodine intake.11 Individuals with lactose intolerance are at risk of iodine deficiency, given that dairy products are a major source of iodine in the United States. Physicians should be aware of these risk factors for iodine deficiency.
PREGNANCY AND LACTATION
It is crucial to maintain euthyroidism during pregnancy. In early gestation, maternal thyroid hormone production increases 50% due to an increase in thyroid-binding globulin and stimulation by human chorionic gonadotropin. The glomerular filtration rate increases by 30% to 50% during pregnancy, thus increasing renal iodine clearance. Fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodine readily crosses the placenta.12
Women with sufficient iodine intake before and during pregnancy generally have adequate intrathyroidal iodine storage and can adapt to the increased demand for thyroid hormone throughout gestation. But in the setting of even mild iodine deficiency, total body iodine stores decline gradually from the first to third trimester of pregnancy.13
The fetal thyroid gland does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by TSH until the full development of the pituitary-portal vascular system at 20 weeks of gestation.12 Therefore, the fetus relies on maternal thyroid hormone during this critical stage of neurodevelopment. Thyroid hormone is essential for oligodendrocyte differentiation and myelin distribution14 as well as fetal neuronal proliferation and migration in the first and second trimesters. Iodine deficiency leading to maternal hypothyroidism can result in irreversible fetal brain damage.
Because of the greater requirement during pregnancy, the WHO recommends using a median urinary iodine concentration of 150 to 249 μg/L to define a population that has no iodine deficiency.9 The NHANES data from 2007 to 2010 showed that pregnant US women were mildly iodine-deficient (median urinary iodine concentration 135 μg/L),10 and the National Children’s Study of 501 pregnant US women during the third trimester in 2009 to 2010 showed they had adequate iodine intake (median urinary iodine concentration 167 μg/L). Interestingly, pregnant non-Hispanic blacks were the only ethnic group with a median urinary iodine concentration less than 150 μg/L, suggesting that race or ethnicity is a predictor of iodine status in pregnant women.10
Iodine requirements during lactation
During lactation, thyroid hormone production and renal iodine clearance return to the prepregnancy state. However, a significant amount of iodine is excreted into breast milk at a concentration 20 to 50 times greater than that in plasma.15 It is recommended that lactating women continue high iodine intake to ensure sufficient iodine in breast milk to build reserves in the newborn’s thyroid gland.
The iodine requirement during lactation is 225 to 350 μg/day.16 Breast milk containing 100 to 200 μg/L of iodine appears to provide adequate iodine to meet Institute of Medicine recommendations for infants.17 The amount of iodine excreted into breast milk depends on maternal iodine intake. In the setting of iodine sufficiency, the iodine content of breast milk is 150 to 180 μg/L, but it is much lower (9–32 μg/L) in women from iodine-deficient areas, eg, the “goiter belt,” which included the Great Lakes, the Appalachians, and northwestern states. While iodized salt has virtually eliminated the goiter belt, the risk of iodine deficiency remains for people who avoid iodized salt and dairy.15
To ensure adequate iodine intake, the American Thyroid Association recommends that women receive iodine supplementation daily during pregnancy and lactation.11 However, the iodine content of prenatal multivitamins is currently not mandated in the United States. Only half of marketed prenatal vitamins in the United States contain iodine, in the form of either potassium iodide or kelp. Though most iodine-containing products claim to contain at least 150 μg of iodine per daily dose, when measured, the actual iodine content varied between 33 and 610 μg.18
CONSEQUENCES OF IODINE DEFICIENCY
Goiter
Goiter in iodine-deficient areas is considered to be an adaptation to chronic iodine deficiency. Low iodine intake leads to reduced thyroid hormone production, which in turn stimulates TSH secretion from the pituitary. TSH increases iodine uptake by the thyroid, stimulates thyroid growth, and leads to goiter development.
Initially, goiter is characterized by diffuse thyroid enlargement, but over time it may become nodular from progressive accumulation of new thyroid follicles. Goiter in children from iodine-deficient areas is diffusely enlarged, whereas in older adults it tends to be multinodular.
Iodine deficiency and chronic TSH stimulation may play a role in TSH receptor-activating mutations of thyroid follicles. These “gain-of-function” mutations are more common in the glands of patients with goiter in areas of iodine deficiency but are relatively rare in areas of iodine sufficiency.19 Toxic multinodular goiter may eventually develop, and hyperthyroidism may occur if iodine deficiency is not severe.
Goiter generally does not cause obstructive symptoms, since the thyroid usually grows outward. However, a very large goiter may descend to the thoracic inlet and compress the trachea and esophagus. The obstructive effect of a large goiter can be demonstrated by having a patient raise the arms adjacent to the face (the Pemberton maneuver). Signs suggesting obstruction are engorged neck veins, facial plethora, increased dyspnea, and stridor during the maneuver. Computed tomography of the neck and upper thorax may provide information on the degree of tracheal compression.20
Hypothyroidism
A normal or low triiodothyronine (T3), a low serum thyroxine (T4), and a variably elevated TSH are features of thyroid function tests in iodine deficiency.11,21,22 As long as daily iodine intake exceeds 50 μg/day, the absolute uptake of iodine by the thyroid gland usually remains adequate to maintain euthyroidism. Below 50 μg/day, iodine storage in the thyroid becomes depleted, leading to hypothyroidism.1
The clinical manifestations of hypothyroidism from iodine deficiency are similar to those of hypothyroidism from other causes. Because of thyroid hormone’s role in neural and somatic development, the manifestations of hypothyroidism differ among age groups (Table 1).
Cretinism
Before the development of fetal thyroid tissue in the 10th to 12th week of gestation, the fetus is dependent on maternal thyroid hormone, which crosses the placenta to support general and neural development. Iodine deficiency leading to maternal hypothyroidism (in early gestation) or inadequate fetal thyroid hormone production (in late gestation) may result in various degrees of mental retardation or lower than expected IQ.
Severe iodine deficiency during gestation typically results in cretinism, characterized by severe mental retardation accompanied by other neurologic or physical defects. Cretinism is divided into two subtypes according to clinical manifestations (neurologic and myxomatous cretinism; Table 2), which may reflect the different timing of intrauterine insult to the developing fetal nervous system and whether the iodine deficiency continues into the postnatal period. Both types can be prevented by adequate maternal iodine intake before and during pregnancy.23,24
Although mild gestational iodine deficiency does not result in cretinism, it nevertheless has an adverse impact on fetal neurodevelopment and subsequent functioning. Children of mothers with mild gestational iodine deficiency were found to have reductions in spelling, grammar, and English literacy performance despite growing up in iodine-replete environments.25
Impaired cognitive development
Reduction in IQ has been noted in affected youth from regions of severe and mild iodine deficiency. A meta-analysis of studies relating iodine deficiency to cognitive development suggested that chronic moderate to severe iodine deficiency reduced expected average IQ by about 13.5 points.26
The effects of mild iodine deficiency during childhood are more difficult to quantify. The results of one study suggested that mild iodine deficiency was associated with subtle neurodevelopmental deficits and that iodine supplementation might improve cognitive function in mildly iodine-deficient children.27
In a 2009 randomized, placebo-controlled study in New Zealand, 184 children ages 10 to 13 with mild iodine deficiency (median urinary iodine concentration of 63 μg/L) received iodine supplementation (150 μg/day) or placebo for 28 weeks. Iodine supplementation increased the median urinary iodine concentration to 145 μg/L and significantly improved perceptual reasoning measures and overall cognitive score compared with placebo.28
These findings suggest that correcting mild iodine deficiency in children could improve certain components of cognition. More research is needed to understand the effects of mild iodine deficiency and iodine supplementation on cognitive function.
ASSESSING IODINE STATUS
The diagnosis of iodine deficiency is based on clinical and laboratory assessments. Clinical manifestations compatible with iodine deficiency and careful history-taking focused on the patient’s dietary iodine intake and geographic data are keys to the diagnosis.
Four main methods are used to assess iodine status at a population level: urinary iodine, serum thyroglobulin, serum TSH, and thyroid size. Urinary iodine is a sensitive marker for recent iodine intake (within days); thyroglobulin represents iodine nutrition over a period of months and thyroid size over a period of years.1
Urinary iodine
Most dietary iodine is excreted into the urine within 24 hours of ingestion, and the 24-hour urinary iodine is considered a reference standard for the measurement of individual daily iodine intake. However, the process of collection is cumbersome, and the 24-hour urinary iodine can vary from day to day in the same person, depending on the amount of iodine ingested.
A study in healthy women from an iodine-sufficient area suggested that 10 repeated 24-hour urine collections estimated the person’s iodine status at a precision of 20% because of variable daily iodine intake.29 Therefore, when necessary, several 24-hour urine iodine determinations should be performed.
A single, random, spot urinary iodine is expressed as the urinary iodine concentration and is affected by the amount of iodine and fluids the individual ingests in a day, thus resulting in high variation both within an individual person and between individuals. Expressing the urinary iodine concentration as the ratio of urine iodine to creatinine is useful in correcting for the influence of fluid intake. The ratio of urine iodine to creatinine can be used to estimate 24-hour urine iodine with the following formula: urine iodine (μg/L)/creatinine (g/L)× age- and sex-specific estimated 24-hour creatinine excretion (g/day). Another clinical use of the spot urine iodine is to screen for exposure to a large amount of iodine from a source such as radiographic contrast.30
Although individual urine iodine excretion and urine volume can vary from day to day, this variation tends to even out in a large number of samples. In study populations of at least 500, the median value of the spot urinary iodine concentration is considered a reliable measure of iodine intake in that population.30 The spot urine iodine test is convenient, making it the test of choice to study iodine status in a large cohort. The WHO recommends using the median value of the spot urine iodine to evaluate the iodine status of a population.9
Thyroglobulin
Thyroglobulin is a thyroid-specific protein involved in the synthesis of thyroid hormone. Small amounts can be detected in the blood of healthy people. In the absence of thyroid damage, the amount of serum thyroglobulin depends on thyroid cell mass and TSH stimulation. The serum level is elevated in iodine deficiency as a result of chronic TSH stimulation and thyroid hyperplasia. Thus, thyroglobulin can serve as a marker of iodine deficiency.
Serum thyroglobulin assays have been adapted for use on dried whole-blood spots, which require only a few drops of whole blood collected on filter paper and left to air-dry. The results of the dried whole-blood assay correlate closely with those of the serum assay.31 An established international dried whole-blood thyroglobulin reference range for iodine-sufficient school-age children is 4 to 40 μg/L.32 A median level of less than 13 μg/L in school-age children indicates iodine sufficiency in the population.33
Thyroid-stimulating hormone
Iodine deficiency lowers serum T4, which in turn leads to increased serum TSH. Therefore, iodine-deficient populations generally have higher TSH than iodine-sufficient groups. However, the TSH values in older children and adults with iodine deficiency are not significantly different from values of those with adequate iodine intake. Therefore, TSH is not a practical marker of iodine deficiency in the general population.
In contrast, TSH in newborns is a reasonable indicator of population iodine status. The newborn thyroid has limited iodine stores compared with that of an adult and hence a much higher iodine turnover rate. TSH from the cord blood is markedly elevated in newborns of mothers with moderate to severe iodine deficiency.34 A high prevalence of newborns with elevated TSH should therefore reflect iodine deficiency in the area where the mothers of the newborns live.
TSH is now routinely checked in newborns to screen for congenital hypothyroidism. TSH is typically checked 2 to 5 days after delivery to avoid confusion with transient physiologic TSH elevation, which occurs within a few hours after birth and decreases rapidly in 24 hours. The WHO has proposed that a more than 3% prevalence of newborns with TSH values higher than 5 mU/L from blood samples collected 3 to 4 days after birth indicates iodine deficiency in a population.1 This threshold appears to correlate well with the iodine status of the population defined by the WHO’s median urinary iodine concentration.35,36
But several other factors can influence the measurement of newborn TSH, such as prematurity, time of blood collection, maternal or newborn exposure to iodine-containing antiseptics, and the TSH assay methodology. These potential confounding factors limit the role of neonatal TSH as a reliable monitoring tool for iodine deficiency.35,37
Thyroid size
The size of the thyroid gland varies inversely with iodine intake. Thyroid size can be assessed by either palpation or ultrasonography, with the latter being more sensitive. The goiter rate in school-age children can be used to determine the severity of iodine deficiency in the population (Table 3). A goiter rate of 5% or more in school-age children suggests the presence of iodine deficiency in the community.
Although thyroid size is easy to estimate by palpation, it has low sensitivity and specificity to detect iodine deficiency and high interobserver variation. Thyroid ultrasonography provides a more precise measurement of thyroid gland volume. Zimmermann et al38 provided reference data on thyroid volume stratified by age, sex, and body surface area of school-age children in iodine-sufficient areas.38 Results of ultrasonography in a population is then compared with these reference data. The higher the percentage of the population with thyroid volume exceeding the 97th percentile of the reference range, the more severe the iodine deficiency. However, the WHO does not specify how to grade the degree of iodine deficiency based on the thyroid volume obtained with ultrasonography. Follow-up studies showed no significant correlation between urinary iodine concentration and thyroid size.39,40
Thyroid size decreases slowly after iodine repletion. Therefore, the goiter rate may remain high for several years after iodine supplementation begins.1,9
TREATMENT AND PREVENTION
Treatment of iodine deficiency should be instituted at the levels recommended by the Institute of Medicine and the WHO. The tolerable upper intake levels for iodine are outlined in Table 4. In a nonpregnant adult, 150 μg/day is sufficient for normal thyroid function. Iodine intake should be higher for pregnant and lactating women (250 μg/day according to the WHO recommendation).
Iodine supplementation is easily achieved by using iodized salt or an iodine-containing daily multivitamin.
In patients with overt hypothyroidism from iodine deficiency, we recommend initiating levothyroxine treatment along with iodine supplementation to restore euthyroidism, with consideration of possible interruption in 6 to 12 months when the urine iodine has normalized and goiter size has decreased. Thyroid function should be reassessed 4 to 6 weeks after discontinuation of levothyroxine.
At the population level, iodine deficiency can usually be prevented by iodization of food products or the water supply. In developing countries where salt iodization is not practical, iodine deficiency has been eradicated by adding iodine drops to well water or by injecting people with iodized oil.
TAKE-HOME POINTS
Iodine is essential for thyroid hormone synthesis. It can be obtained by eating iodine-containing foods or by using iodized salt. The WHO classifies iodine deficiency based on the median urinary iodine concentration. Iodine nutrition at the community level is best assessed by measurements of urinary iodine, thyroglobulin, serum TSH, and thyroid size.
Adequate iodine intake during pregnancy is important for fetal development. Iodine deficiency is associated with goiter and hypothyroidism. Severe iodine deficiency during pregnancy is associated with cretinism.
There is evidence that mild to moderate iodine deficiency can cause impaired cognitive development and that correcting the iodine deficiency can significantly improve cognitive function.
CASE FOLLOW-UP
This patient had been following a strict vegan diet with very little intake of iodized salt. Her dietary history and the presence of goiter suggested iodine deficiency. She was instructed to take an iodine supplement 150 μg/day to meet her daily requirement. After 2 months of iodine supplementation, her urine iodine concentration had increased to 58 μg/L. She remained biochemically euthyroid.
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30:376–408.
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013; 23:523–528.
- Huang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc 2005; 26:468–469.
- US Census Bureau. The 2012 Statistical abstract. Health and nutrition. www.census.gov/prod/2011pubs/12statab/health.pdf. Accessed December 1, 2016.
- Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004; 89:3421–3424.
- Salt Institute. Production and Industry. www.saltinstitute.org/salt-101/production-industry. Accessed September 20, 2016.
- Dunn JT. Guarding our nation's thyroid health. J Clin Endocrinol Metab 2002; 87:486–488.
- Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- World Health Organization (WHO). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en. Accessed December 1, 2016.
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23:927–937.
- Public Health Committee of the American Thyroid Association; Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006; 16:949–951.
- Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am 2011; 40:765–777.
- Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008; 93:2466–2468.
- Younes-Rapozo V, Berendonk J, Savignon T, Manhaes AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24:445–453.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009; 70:803–809.
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 2007; 10:1571–1583.
- Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev 2001; 59:269–278.
- Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009; 360:939–940.
- Tonacchera M, Agretti P, Chiovato L, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J Clin Endocrinol Metab 2000; 85:2270–2274.
- Medeiros-Neto G, Camargo RY, Tomimori EK. Approach to and treatment of goiters. Med Clin North Am 2012; 96:351–368.
- Heidemann P, Stubbe P. Serum 3,5,3'-triiodothyronine, thyroxine, and thyrotropin in hypothyroid infants with congenital goiter and the response to iodine. J Clin Endocrinol Metab 1978; 47:189–192.
- Patel YC, Pharoah PO, Hornabrook RW, Hetzel BS. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab 1973; 37:783–789.
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid 1993; 3:59–69.
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24:39–50.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98:1954–1962.
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency. 1st ed. New York, NY: Cognizant Communication; 1994:195–200.
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 2010; 24:29–38.
- Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90:1264–1271.
- Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141:2049–2054.
- Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009; 19:1281–1286.
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr 2003; 77:663–667.
- Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91:4881–4887.
- Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013; 98:1271–1280.
- Thilly CH, Delange F, Lagasse R, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab 1978; 47:354–360.
- Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr 1997; 127:55–58.
- Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999; 88:21–24.
- Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24:63–75.
- Zimmermann MB, Moretti D, Chaouki N, Torresani T. Development of a dried whole-blood spot thyroglobulin assay and its evaluation as an indicator of thyroid status in goitrous children receiving iodized salt. Am J Clin Nutr 2003; 77:1453–1458.
- Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India). Eur J Endocrinol 2000; 143:37–46.
- Moradi M, Hashemipour M, Akbari S, Kor Z, Mirbod SA, Kooshanmehr MR. Ultrasonographic evaluation of the thyroid gland volume among 8-15-year-old children in Isfahan, Iran. Adv Biomed Res 2014; 3:9.
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30:376–408.
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013; 23:523–528.
- Huang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc 2005; 26:468–469.
- US Census Bureau. The 2012 Statistical abstract. Health and nutrition. www.census.gov/prod/2011pubs/12statab/health.pdf. Accessed December 1, 2016.
- Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004; 89:3421–3424.
- Salt Institute. Production and Industry. www.saltinstitute.org/salt-101/production-industry. Accessed September 20, 2016.
- Dunn JT. Guarding our nation's thyroid health. J Clin Endocrinol Metab 2002; 87:486–488.
- Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- World Health Organization (WHO). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en. Accessed December 1, 2016.
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23:927–937.
- Public Health Committee of the American Thyroid Association; Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006; 16:949–951.
- Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am 2011; 40:765–777.
- Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008; 93:2466–2468.
- Younes-Rapozo V, Berendonk J, Savignon T, Manhaes AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24:445–453.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009; 70:803–809.
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 2007; 10:1571–1583.
- Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev 2001; 59:269–278.
- Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009; 360:939–940.
- Tonacchera M, Agretti P, Chiovato L, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J Clin Endocrinol Metab 2000; 85:2270–2274.
- Medeiros-Neto G, Camargo RY, Tomimori EK. Approach to and treatment of goiters. Med Clin North Am 2012; 96:351–368.
- Heidemann P, Stubbe P. Serum 3,5,3'-triiodothyronine, thyroxine, and thyrotropin in hypothyroid infants with congenital goiter and the response to iodine. J Clin Endocrinol Metab 1978; 47:189–192.
- Patel YC, Pharoah PO, Hornabrook RW, Hetzel BS. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab 1973; 37:783–789.
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid 1993; 3:59–69.
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24:39–50.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98:1954–1962.
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency. 1st ed. New York, NY: Cognizant Communication; 1994:195–200.
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 2010; 24:29–38.
- Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90:1264–1271.
- Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141:2049–2054.
- Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009; 19:1281–1286.
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr 2003; 77:663–667.
- Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91:4881–4887.
- Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013; 98:1271–1280.
- Thilly CH, Delange F, Lagasse R, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab 1978; 47:354–360.
- Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr 1997; 127:55–58.
- Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999; 88:21–24.
- Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24:63–75.
- Zimmermann MB, Moretti D, Chaouki N, Torresani T. Development of a dried whole-blood spot thyroglobulin assay and its evaluation as an indicator of thyroid status in goitrous children receiving iodized salt. Am J Clin Nutr 2003; 77:1453–1458.
- Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India). Eur J Endocrinol 2000; 143:37–46.
- Moradi M, Hashemipour M, Akbari S, Kor Z, Mirbod SA, Kooshanmehr MR. Ultrasonographic evaluation of the thyroid gland volume among 8-15-year-old children in Isfahan, Iran. Adv Biomed Res 2014; 3:9.
KEY POINTS
- Adequate iodine intake during pregnancy is critical for normal fetal development.
- Major sources of dietary iodine in the United States are dairy products and iodized salt.
- The daily iodine requirement for nonpregnant adults is 150 µg, and for pregnant women it is 220 to 250 μg. Pregnant and lactating women should take a daily iodine supplement to ensure adequate iodine intake.
- Assessing the risk of iodine deficiency from clinical signs and from the history is key to diagnosing iodine deficiency. Individual urine iodine concentrations may vary from day to day. Repeated samples can be used to confirm iodine deficiency.