User login
Newer oral and noninsulin therapies to treat type 2 diabetes mellitus
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
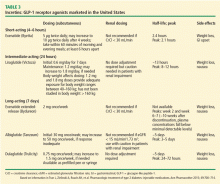
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
In 2013, canagliflozin became the first sodium-glucose cotransporter-2 (SGLT-2) inhibitor to be FDA-approved for treating patients with type 2 DM, followed in 2014 by dapagliflozin and empagliflozin. Several other drugs in this class are available outside the US or are currently undergoing clinical development, including ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin. Currently, no SGLT-2 inhibitors are FDA-approved for type 1 DM, although they have been used off-label and in trials in this patient population.
These drugs work by targeting the SGLT-2 protein in the kidney. In healthy individuals, 99% of filtered glucose is reabsorbed by the kidney with a filtered load of approximately 180 g/day.23 Glucosuria occurs with glucose concentrations above this threshold. In patients with type 2 DM, this threshold and the ability to reabsorb glucose is increased, contributing to hyperglycemia.24 Located in the proximal tubule, the SGLT-2 protein is responsible for 80% to 90% of glucose reabsorption, with SGLT-1 responsible for the other 10% to 20%.25 Inhibition of SGLT-2 reduces the renal threshold for glucose, thus leading to glucosuria and reduction in serum glucose levels.24 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for the SGLT-2 inhibitors.
As a monotherapy, SGLT-2 inhibitors significantly reduce HbA1c levels by 0.4% to 1.1% when compared with placebo.26–28 Reductions may be more significant in patients with HbA1c levels greater than 8.5%, and even more so in patients with HbA1c levels above 10%.29 When compared with other therapeutic options for type 2 DM, SGLT-2 inhibitors have efficacy similar to metformin, sitagliptin, and glipizide; however, some studies have shown superiority to glimepiride and sitagliptin at reducing HbA1c, depending on the dose and duration of treatment.26,27
The SGLT-2 inhibitors do not rely on insulin activity, allowing for their use at any stage of type 2 DM and in combination with other therapies, including insulin. As an add-on medication, SGLT-2 inhibitors reduce HbA1c by 0.5% to 0.7%.27,28 Given that the mechanism of action depends on the filtered load of glucose, they are less effective in patients with a reduced glomerular filtration rate (GFR).
The SGLT-2 inhibitors have benefits beyond that of glycemic control. Studies report weight loss of 1 to 3 kg, which is maintained up to 104 weeks.27–31 Sustained weight loss is secondary to glucosuria, which amounts to a caloric loss of 200 to 300 kcal/day.30 Also, SGLT-2 inhibitors lead to modest reductions in systolic and diastolic blood pressure of approximately 3 to 6 mm Hg and 1 to 2 mm Hg, respectively, due to their diuretic effect.27,31 The risk of hypoglycemia is low—similar to that of metformin and DPP-4 inhibitors—and only slightly higher than placebo when used as monotherapy.26,31 When added to sulfonylureas or insulin, however, the risk of hypoglycemia is increased.26,29
Meta-analyses of SGLT-2 inhibitors showed rates of death and other serious adverse effects were no different than placebo.26,27 A 2015 study on the CV safety of empagliflozin showed lower rates of CV death (38% relative risk [RR] reduction), lower rates of hospitalization due to heart failure (35% RR reduction), and lower rates of all-cause mortality (32% RR reduction) when compared with placebo, with no difference in nonfatal stroke and MI.32 CV safety trials for dapagliflozin and canagliflozin are ongoing, although some trials have shown an increased incidence in CV events in the first 30 days of treatment with canagliflozin.4
Common side effects include genital infections, such as vaginitis and balanitis, as well as urinary tract infections. In a 2013 meta-analysis,27 genital infections carried an odds ratio of 3.5 for SGLT-2 inhibitors compared with placebo, while urinary tract infections carried a 1.34 odds ratio. The increased risk of infection is thought to be secondary to glucosuria combined with immune dysfunction and altered glycosylation uroepithelium cells.30
During clinical trials, more cases of bladder cancer were diagnosed in patients on dapagliflozin than on placebo, leading to a delay in FDA approval. No causal relationship was established, but dapagliflozin is not recommended in patients with active bladder cancer.30
Treatment with SGLT-2 inhibitors can lead to a decrease in GFR, likely secondary to the diuretic effect. In patients with GFR greater than 60 mL/min, this decrease is transient. In patients with GFR below 60 mL/min (moderate renal impairment) who were treated with dapagliflozin, GFR did not quite return to baseline, and they did not show an improvement in HbA1c relative to placebo.33 Canagliflozin at a dose of 300 mg/day caused renal-related adverse events with GFR 45 to 60 mL/min, but a lower dose of 100 mg/day did not.27 A decrease in GFR also occurred in patients with chronic kidney disease treated with empagliflozin, which returned to baseline after discontinuing the drug.31 Despite these findings, renal function stabilizes in patients on SGLT-2 inhibitors over time, whereas it continues to decrease with placebo, suggesting there may be a renal protective effect.30 Their diuretic effect can also lead to volume depletion in patients at risk such as elderly patients or those already taking diuretics.31
Some studies have shown mild increases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels with no change in triglycerides, though long-term effects of this are unknown.31
There are case reports of euglycemic diabetic ketoacidosis occurring in patients with type 1 DM and type 2 DM treated with SGLT-2 inhibitors, which led the FDA in May 2015 to issue a warning that SGLT-2 inhibitors may increase the risk of ketoacidosis.34 There are several possible mechanisms for this increased risk. The SGLT-2 inhibitors may decrease renal clearance of ketones, stimulate glucagon secretion leading to hepatic ketogenesis, or suppress glucose-mediated insulin secretion leading practitioners to decrease insulin doses thus resulting in increased ketone production via lipolysis.34 More studies are needed, but patients and healthcare providers should be aware of potential euglycemic ketoacidosis associated with SGLT-2-inhibitors, as the lack of hyperglycemia can delay the diagnosis.
BILE ACID SEQUESTRANTS
Bile acid sequestrants have been used for years in hyperlipidemia to reduce LDL concentration; however, colesevelam is the only drug in this class approved (2009) for treating type 2 DM, after studies showed colesevelam improves glycemic control.35–37 Though several possibilities have been proposed, the precise mechanism of action for lowering blood glucose levels is unknown.35 Colesevelam is not absorbed systemically and does not affect endogenous insulin levels.4 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for colesevelam.
As monotherapy, studies have shown varying effectiveness in reducing HbA1c relative to placebo ranging from no statistical difference to 0.54% reduction.36,37 As an add-on to other diabetic medications, a Cochrane review of six randomized controlled trials showed a decrease in HbA1c by 0.3% to 0.5% and decrease in fasting glucose of 15 mg/dL.37 Additional benefits of colesevelam include low risk for hypoglycemia, weight neutrality, and reduction in LDL.4 No serious adverse events or deaths have been associated with colesevelam, including CV events; however, more trials on macrovascular outcomes are needed to clarify its side-effect profile.35
Common side effects include constipation, flatulence, and dyspepsia.35 Colesevelam has shown a statistically significant increase in triglycerides, so its use in patients with triglycerides above 500 mg/dL or with hypertriglyceridemia-induced pancreatitis is contraindicated.4 Caution should be used prior to starting treatment in patients with triglyceride levels above 200 mg/dL.35 Colesevelam is contraindicated in patients with a history of small-bowel obstruction, and caution is recommended in patients with decreased gastric motility. This drug may reduce absorption of fat-soluble vitamins and some medications.4
Although further research into the long-term effects of colesevelam is needed, its relatively good safety profile makes it a reasonable choice in diabetic patients with hyperlipidemia not controlled with statins.
DOPAMINE-RECEPTOR AGONIST
Bromocriptine, a dopamine-receptor agonist, was FDA-approved for the treatment of Parkinson disease, hyperprolactinemia, and acromegaly in the 1970s. In 2009, a quick-release formulation of bromocriptine (bromocriptine QR) was approved for treatment of type 2 DM. Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for bromocriptine.
The precise mechanism of action is unclear, but an American Association of Clinical Endocrinologists expert panel recommendation suggests that it may lower glucose levels by improving hypothalamic-mediated, postprandial insulin sensitivity via increasing morning dopaminergic activity (decreased in patients with type 2 DM) and by reducing hypothalamic adrenergic tone.38 It is not currently recommended as monotherapy, although a study of 154 patients showed monotherapy reduced HbA1c by 0.55%.39 When added to other diabetic medications, it reduced HbA1c by 0.4% to 0.7%.4,38
Bromocriptine QR is weight neutral and carries a low risk of hypoglycemia.4 A safety trial with 3,095 patients showed fewer adverse CV events in patients treated with bromocriptine QR compared with placebo, which may be secondary to reduced sympathetic tone or to reduced systemic inflammation.40 Some studies have shown reductions in blood pressure, free fatty acid levels, and triglycerides, with no change in LDL or HDL.38
Common side effects include nausea, headache, dizziness, diarrhea, and fatigue. Administration is recommended with food to reduce GI side effects. It is contraindicated in women who are nursing and those with syncopal migraines. Furthermore, it may be prudent to avoid this medication in patients with a history of psychosis, those currently treated with dopamine agonists or antagonists, or those at risk for hypotension.4
CONCLUSION
The pathophysiology of type 2 DM involves at least seven organs and tissues—the brain, liver, pancreas, intestines, kidneys, fat, and muscle—and no single medication addresses all seven of them. Most patients require more than one medication to adequately treat their diabetes, making availability and development of drugs with unique and complementary mechanisms of action of paramount importance. The medications described here—DPP-4 inhibitors, GLP-1 agonists, SGLT-2 inhibitors, colesevelam, and bromocriptine QR—provide therapeutic options with novel mechanisms of action, all while avoiding weight gain and providing a low risk of hypoglycemia. While not appropriate for every patient, these medications give healthcare providers additional options to individualize treatment and optimize care for patients.
Acknowledgments. The authors gratefully thank Julie Benke-Bennett for assistance with manuscript formatting and transcription. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag 2015; 11:621–632.
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clin Ther 2011; 12:1868–1882.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015; 49:540–556.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet 2012; 380:475–483.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:668–673.
- Green JB, Bethel A, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013; 10:289–301.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162:620–626.
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36:2288–2296.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Eng J Med 2014; 370:794–797.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother 2015; 49:700–714.
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 2015; 6:109–134.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015; 6:19–28.
- Harris KB, McCarty DJ. Efficacy and tolerability of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015; 6:3–18.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gludd LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analysis of randomized controlled trials. BMJ 2012; 344:d7771.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet 2014; 384:1349–1357.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open-label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2:289–297.
- Neumiller JJ. Incretin-based therapies. Med Clin N Am 2015; 99:107–129.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:38–47.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75:1272–1277.
- Defronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36:3169–3176.
- Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303:C348–C354.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:457–466.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70:1149–1158.
- Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7:448–461.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 2014; 5:124–136.
- Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92–102.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Aggarwal W, Loomba RS, Arora RR. Efficacy of colesevelam on lowering glycemia and lipids. J Cardiovasc Pharmacol 2012; 59:198–205.
- Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam Hcl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008; 31:1479–1484.
- Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012; 12:CD009361.
- Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S. The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations. Endocr Pract 2013; 19:100–106.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8:1683–1707.
- Gaziano JM, Concotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
In 2013, canagliflozin became the first sodium-glucose cotransporter-2 (SGLT-2) inhibitor to be FDA-approved for treating patients with type 2 DM, followed in 2014 by dapagliflozin and empagliflozin. Several other drugs in this class are available outside the US or are currently undergoing clinical development, including ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin. Currently, no SGLT-2 inhibitors are FDA-approved for type 1 DM, although they have been used off-label and in trials in this patient population.
These drugs work by targeting the SGLT-2 protein in the kidney. In healthy individuals, 99% of filtered glucose is reabsorbed by the kidney with a filtered load of approximately 180 g/day.23 Glucosuria occurs with glucose concentrations above this threshold. In patients with type 2 DM, this threshold and the ability to reabsorb glucose is increased, contributing to hyperglycemia.24 Located in the proximal tubule, the SGLT-2 protein is responsible for 80% to 90% of glucose reabsorption, with SGLT-1 responsible for the other 10% to 20%.25 Inhibition of SGLT-2 reduces the renal threshold for glucose, thus leading to glucosuria and reduction in serum glucose levels.24 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for the SGLT-2 inhibitors.
As a monotherapy, SGLT-2 inhibitors significantly reduce HbA1c levels by 0.4% to 1.1% when compared with placebo.26–28 Reductions may be more significant in patients with HbA1c levels greater than 8.5%, and even more so in patients with HbA1c levels above 10%.29 When compared with other therapeutic options for type 2 DM, SGLT-2 inhibitors have efficacy similar to metformin, sitagliptin, and glipizide; however, some studies have shown superiority to glimepiride and sitagliptin at reducing HbA1c, depending on the dose and duration of treatment.26,27
The SGLT-2 inhibitors do not rely on insulin activity, allowing for their use at any stage of type 2 DM and in combination with other therapies, including insulin. As an add-on medication, SGLT-2 inhibitors reduce HbA1c by 0.5% to 0.7%.27,28 Given that the mechanism of action depends on the filtered load of glucose, they are less effective in patients with a reduced glomerular filtration rate (GFR).
The SGLT-2 inhibitors have benefits beyond that of glycemic control. Studies report weight loss of 1 to 3 kg, which is maintained up to 104 weeks.27–31 Sustained weight loss is secondary to glucosuria, which amounts to a caloric loss of 200 to 300 kcal/day.30 Also, SGLT-2 inhibitors lead to modest reductions in systolic and diastolic blood pressure of approximately 3 to 6 mm Hg and 1 to 2 mm Hg, respectively, due to their diuretic effect.27,31 The risk of hypoglycemia is low—similar to that of metformin and DPP-4 inhibitors—and only slightly higher than placebo when used as monotherapy.26,31 When added to sulfonylureas or insulin, however, the risk of hypoglycemia is increased.26,29
Meta-analyses of SGLT-2 inhibitors showed rates of death and other serious adverse effects were no different than placebo.26,27 A 2015 study on the CV safety of empagliflozin showed lower rates of CV death (38% relative risk [RR] reduction), lower rates of hospitalization due to heart failure (35% RR reduction), and lower rates of all-cause mortality (32% RR reduction) when compared with placebo, with no difference in nonfatal stroke and MI.32 CV safety trials for dapagliflozin and canagliflozin are ongoing, although some trials have shown an increased incidence in CV events in the first 30 days of treatment with canagliflozin.4
Common side effects include genital infections, such as vaginitis and balanitis, as well as urinary tract infections. In a 2013 meta-analysis,27 genital infections carried an odds ratio of 3.5 for SGLT-2 inhibitors compared with placebo, while urinary tract infections carried a 1.34 odds ratio. The increased risk of infection is thought to be secondary to glucosuria combined with immune dysfunction and altered glycosylation uroepithelium cells.30
During clinical trials, more cases of bladder cancer were diagnosed in patients on dapagliflozin than on placebo, leading to a delay in FDA approval. No causal relationship was established, but dapagliflozin is not recommended in patients with active bladder cancer.30
Treatment with SGLT-2 inhibitors can lead to a decrease in GFR, likely secondary to the diuretic effect. In patients with GFR greater than 60 mL/min, this decrease is transient. In patients with GFR below 60 mL/min (moderate renal impairment) who were treated with dapagliflozin, GFR did not quite return to baseline, and they did not show an improvement in HbA1c relative to placebo.33 Canagliflozin at a dose of 300 mg/day caused renal-related adverse events with GFR 45 to 60 mL/min, but a lower dose of 100 mg/day did not.27 A decrease in GFR also occurred in patients with chronic kidney disease treated with empagliflozin, which returned to baseline after discontinuing the drug.31 Despite these findings, renal function stabilizes in patients on SGLT-2 inhibitors over time, whereas it continues to decrease with placebo, suggesting there may be a renal protective effect.30 Their diuretic effect can also lead to volume depletion in patients at risk such as elderly patients or those already taking diuretics.31
Some studies have shown mild increases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels with no change in triglycerides, though long-term effects of this are unknown.31
There are case reports of euglycemic diabetic ketoacidosis occurring in patients with type 1 DM and type 2 DM treated with SGLT-2 inhibitors, which led the FDA in May 2015 to issue a warning that SGLT-2 inhibitors may increase the risk of ketoacidosis.34 There are several possible mechanisms for this increased risk. The SGLT-2 inhibitors may decrease renal clearance of ketones, stimulate glucagon secretion leading to hepatic ketogenesis, or suppress glucose-mediated insulin secretion leading practitioners to decrease insulin doses thus resulting in increased ketone production via lipolysis.34 More studies are needed, but patients and healthcare providers should be aware of potential euglycemic ketoacidosis associated with SGLT-2-inhibitors, as the lack of hyperglycemia can delay the diagnosis.
BILE ACID SEQUESTRANTS
Bile acid sequestrants have been used for years in hyperlipidemia to reduce LDL concentration; however, colesevelam is the only drug in this class approved (2009) for treating type 2 DM, after studies showed colesevelam improves glycemic control.35–37 Though several possibilities have been proposed, the precise mechanism of action for lowering blood glucose levels is unknown.35 Colesevelam is not absorbed systemically and does not affect endogenous insulin levels.4 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for colesevelam.
As monotherapy, studies have shown varying effectiveness in reducing HbA1c relative to placebo ranging from no statistical difference to 0.54% reduction.36,37 As an add-on to other diabetic medications, a Cochrane review of six randomized controlled trials showed a decrease in HbA1c by 0.3% to 0.5% and decrease in fasting glucose of 15 mg/dL.37 Additional benefits of colesevelam include low risk for hypoglycemia, weight neutrality, and reduction in LDL.4 No serious adverse events or deaths have been associated with colesevelam, including CV events; however, more trials on macrovascular outcomes are needed to clarify its side-effect profile.35
Common side effects include constipation, flatulence, and dyspepsia.35 Colesevelam has shown a statistically significant increase in triglycerides, so its use in patients with triglycerides above 500 mg/dL or with hypertriglyceridemia-induced pancreatitis is contraindicated.4 Caution should be used prior to starting treatment in patients with triglyceride levels above 200 mg/dL.35 Colesevelam is contraindicated in patients with a history of small-bowel obstruction, and caution is recommended in patients with decreased gastric motility. This drug may reduce absorption of fat-soluble vitamins and some medications.4
Although further research into the long-term effects of colesevelam is needed, its relatively good safety profile makes it a reasonable choice in diabetic patients with hyperlipidemia not controlled with statins.
DOPAMINE-RECEPTOR AGONIST
Bromocriptine, a dopamine-receptor agonist, was FDA-approved for the treatment of Parkinson disease, hyperprolactinemia, and acromegaly in the 1970s. In 2009, a quick-release formulation of bromocriptine (bromocriptine QR) was approved for treatment of type 2 DM. Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for bromocriptine.
The precise mechanism of action is unclear, but an American Association of Clinical Endocrinologists expert panel recommendation suggests that it may lower glucose levels by improving hypothalamic-mediated, postprandial insulin sensitivity via increasing morning dopaminergic activity (decreased in patients with type 2 DM) and by reducing hypothalamic adrenergic tone.38 It is not currently recommended as monotherapy, although a study of 154 patients showed monotherapy reduced HbA1c by 0.55%.39 When added to other diabetic medications, it reduced HbA1c by 0.4% to 0.7%.4,38
Bromocriptine QR is weight neutral and carries a low risk of hypoglycemia.4 A safety trial with 3,095 patients showed fewer adverse CV events in patients treated with bromocriptine QR compared with placebo, which may be secondary to reduced sympathetic tone or to reduced systemic inflammation.40 Some studies have shown reductions in blood pressure, free fatty acid levels, and triglycerides, with no change in LDL or HDL.38
Common side effects include nausea, headache, dizziness, diarrhea, and fatigue. Administration is recommended with food to reduce GI side effects. It is contraindicated in women who are nursing and those with syncopal migraines. Furthermore, it may be prudent to avoid this medication in patients with a history of psychosis, those currently treated with dopamine agonists or antagonists, or those at risk for hypotension.4
CONCLUSION
The pathophysiology of type 2 DM involves at least seven organs and tissues—the brain, liver, pancreas, intestines, kidneys, fat, and muscle—and no single medication addresses all seven of them. Most patients require more than one medication to adequately treat their diabetes, making availability and development of drugs with unique and complementary mechanisms of action of paramount importance. The medications described here—DPP-4 inhibitors, GLP-1 agonists, SGLT-2 inhibitors, colesevelam, and bromocriptine QR—provide therapeutic options with novel mechanisms of action, all while avoiding weight gain and providing a low risk of hypoglycemia. While not appropriate for every patient, these medications give healthcare providers additional options to individualize treatment and optimize care for patients.
Acknowledgments. The authors gratefully thank Julie Benke-Bennett for assistance with manuscript formatting and transcription. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
In 2013, canagliflozin became the first sodium-glucose cotransporter-2 (SGLT-2) inhibitor to be FDA-approved for treating patients with type 2 DM, followed in 2014 by dapagliflozin and empagliflozin. Several other drugs in this class are available outside the US or are currently undergoing clinical development, including ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin. Currently, no SGLT-2 inhibitors are FDA-approved for type 1 DM, although they have been used off-label and in trials in this patient population.
These drugs work by targeting the SGLT-2 protein in the kidney. In healthy individuals, 99% of filtered glucose is reabsorbed by the kidney with a filtered load of approximately 180 g/day.23 Glucosuria occurs with glucose concentrations above this threshold. In patients with type 2 DM, this threshold and the ability to reabsorb glucose is increased, contributing to hyperglycemia.24 Located in the proximal tubule, the SGLT-2 protein is responsible for 80% to 90% of glucose reabsorption, with SGLT-1 responsible for the other 10% to 20%.25 Inhibition of SGLT-2 reduces the renal threshold for glucose, thus leading to glucosuria and reduction in serum glucose levels.24 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for the SGLT-2 inhibitors.
As a monotherapy, SGLT-2 inhibitors significantly reduce HbA1c levels by 0.4% to 1.1% when compared with placebo.26–28 Reductions may be more significant in patients with HbA1c levels greater than 8.5%, and even more so in patients with HbA1c levels above 10%.29 When compared with other therapeutic options for type 2 DM, SGLT-2 inhibitors have efficacy similar to metformin, sitagliptin, and glipizide; however, some studies have shown superiority to glimepiride and sitagliptin at reducing HbA1c, depending on the dose and duration of treatment.26,27
The SGLT-2 inhibitors do not rely on insulin activity, allowing for their use at any stage of type 2 DM and in combination with other therapies, including insulin. As an add-on medication, SGLT-2 inhibitors reduce HbA1c by 0.5% to 0.7%.27,28 Given that the mechanism of action depends on the filtered load of glucose, they are less effective in patients with a reduced glomerular filtration rate (GFR).
The SGLT-2 inhibitors have benefits beyond that of glycemic control. Studies report weight loss of 1 to 3 kg, which is maintained up to 104 weeks.27–31 Sustained weight loss is secondary to glucosuria, which amounts to a caloric loss of 200 to 300 kcal/day.30 Also, SGLT-2 inhibitors lead to modest reductions in systolic and diastolic blood pressure of approximately 3 to 6 mm Hg and 1 to 2 mm Hg, respectively, due to their diuretic effect.27,31 The risk of hypoglycemia is low—similar to that of metformin and DPP-4 inhibitors—and only slightly higher than placebo when used as monotherapy.26,31 When added to sulfonylureas or insulin, however, the risk of hypoglycemia is increased.26,29
Meta-analyses of SGLT-2 inhibitors showed rates of death and other serious adverse effects were no different than placebo.26,27 A 2015 study on the CV safety of empagliflozin showed lower rates of CV death (38% relative risk [RR] reduction), lower rates of hospitalization due to heart failure (35% RR reduction), and lower rates of all-cause mortality (32% RR reduction) when compared with placebo, with no difference in nonfatal stroke and MI.32 CV safety trials for dapagliflozin and canagliflozin are ongoing, although some trials have shown an increased incidence in CV events in the first 30 days of treatment with canagliflozin.4
Common side effects include genital infections, such as vaginitis and balanitis, as well as urinary tract infections. In a 2013 meta-analysis,27 genital infections carried an odds ratio of 3.5 for SGLT-2 inhibitors compared with placebo, while urinary tract infections carried a 1.34 odds ratio. The increased risk of infection is thought to be secondary to glucosuria combined with immune dysfunction and altered glycosylation uroepithelium cells.30
During clinical trials, more cases of bladder cancer were diagnosed in patients on dapagliflozin than on placebo, leading to a delay in FDA approval. No causal relationship was established, but dapagliflozin is not recommended in patients with active bladder cancer.30
Treatment with SGLT-2 inhibitors can lead to a decrease in GFR, likely secondary to the diuretic effect. In patients with GFR greater than 60 mL/min, this decrease is transient. In patients with GFR below 60 mL/min (moderate renal impairment) who were treated with dapagliflozin, GFR did not quite return to baseline, and they did not show an improvement in HbA1c relative to placebo.33 Canagliflozin at a dose of 300 mg/day caused renal-related adverse events with GFR 45 to 60 mL/min, but a lower dose of 100 mg/day did not.27 A decrease in GFR also occurred in patients with chronic kidney disease treated with empagliflozin, which returned to baseline after discontinuing the drug.31 Despite these findings, renal function stabilizes in patients on SGLT-2 inhibitors over time, whereas it continues to decrease with placebo, suggesting there may be a renal protective effect.30 Their diuretic effect can also lead to volume depletion in patients at risk such as elderly patients or those already taking diuretics.31
Some studies have shown mild increases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels with no change in triglycerides, though long-term effects of this are unknown.31
There are case reports of euglycemic diabetic ketoacidosis occurring in patients with type 1 DM and type 2 DM treated with SGLT-2 inhibitors, which led the FDA in May 2015 to issue a warning that SGLT-2 inhibitors may increase the risk of ketoacidosis.34 There are several possible mechanisms for this increased risk. The SGLT-2 inhibitors may decrease renal clearance of ketones, stimulate glucagon secretion leading to hepatic ketogenesis, or suppress glucose-mediated insulin secretion leading practitioners to decrease insulin doses thus resulting in increased ketone production via lipolysis.34 More studies are needed, but patients and healthcare providers should be aware of potential euglycemic ketoacidosis associated with SGLT-2-inhibitors, as the lack of hyperglycemia can delay the diagnosis.
BILE ACID SEQUESTRANTS
Bile acid sequestrants have been used for years in hyperlipidemia to reduce LDL concentration; however, colesevelam is the only drug in this class approved (2009) for treating type 2 DM, after studies showed colesevelam improves glycemic control.35–37 Though several possibilities have been proposed, the precise mechanism of action for lowering blood glucose levels is unknown.35 Colesevelam is not absorbed systemically and does not affect endogenous insulin levels.4 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for colesevelam.
As monotherapy, studies have shown varying effectiveness in reducing HbA1c relative to placebo ranging from no statistical difference to 0.54% reduction.36,37 As an add-on to other diabetic medications, a Cochrane review of six randomized controlled trials showed a decrease in HbA1c by 0.3% to 0.5% and decrease in fasting glucose of 15 mg/dL.37 Additional benefits of colesevelam include low risk for hypoglycemia, weight neutrality, and reduction in LDL.4 No serious adverse events or deaths have been associated with colesevelam, including CV events; however, more trials on macrovascular outcomes are needed to clarify its side-effect profile.35
Common side effects include constipation, flatulence, and dyspepsia.35 Colesevelam has shown a statistically significant increase in triglycerides, so its use in patients with triglycerides above 500 mg/dL or with hypertriglyceridemia-induced pancreatitis is contraindicated.4 Caution should be used prior to starting treatment in patients with triglyceride levels above 200 mg/dL.35 Colesevelam is contraindicated in patients with a history of small-bowel obstruction, and caution is recommended in patients with decreased gastric motility. This drug may reduce absorption of fat-soluble vitamins and some medications.4
Although further research into the long-term effects of colesevelam is needed, its relatively good safety profile makes it a reasonable choice in diabetic patients with hyperlipidemia not controlled with statins.
DOPAMINE-RECEPTOR AGONIST
Bromocriptine, a dopamine-receptor agonist, was FDA-approved for the treatment of Parkinson disease, hyperprolactinemia, and acromegaly in the 1970s. In 2009, a quick-release formulation of bromocriptine (bromocriptine QR) was approved for treatment of type 2 DM. Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for bromocriptine.
The precise mechanism of action is unclear, but an American Association of Clinical Endocrinologists expert panel recommendation suggests that it may lower glucose levels by improving hypothalamic-mediated, postprandial insulin sensitivity via increasing morning dopaminergic activity (decreased in patients with type 2 DM) and by reducing hypothalamic adrenergic tone.38 It is not currently recommended as monotherapy, although a study of 154 patients showed monotherapy reduced HbA1c by 0.55%.39 When added to other diabetic medications, it reduced HbA1c by 0.4% to 0.7%.4,38
Bromocriptine QR is weight neutral and carries a low risk of hypoglycemia.4 A safety trial with 3,095 patients showed fewer adverse CV events in patients treated with bromocriptine QR compared with placebo, which may be secondary to reduced sympathetic tone or to reduced systemic inflammation.40 Some studies have shown reductions in blood pressure, free fatty acid levels, and triglycerides, with no change in LDL or HDL.38
Common side effects include nausea, headache, dizziness, diarrhea, and fatigue. Administration is recommended with food to reduce GI side effects. It is contraindicated in women who are nursing and those with syncopal migraines. Furthermore, it may be prudent to avoid this medication in patients with a history of psychosis, those currently treated with dopamine agonists or antagonists, or those at risk for hypotension.4
CONCLUSION
The pathophysiology of type 2 DM involves at least seven organs and tissues—the brain, liver, pancreas, intestines, kidneys, fat, and muscle—and no single medication addresses all seven of them. Most patients require more than one medication to adequately treat their diabetes, making availability and development of drugs with unique and complementary mechanisms of action of paramount importance. The medications described here—DPP-4 inhibitors, GLP-1 agonists, SGLT-2 inhibitors, colesevelam, and bromocriptine QR—provide therapeutic options with novel mechanisms of action, all while avoiding weight gain and providing a low risk of hypoglycemia. While not appropriate for every patient, these medications give healthcare providers additional options to individualize treatment and optimize care for patients.
Acknowledgments. The authors gratefully thank Julie Benke-Bennett for assistance with manuscript formatting and transcription. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag 2015; 11:621–632.
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clin Ther 2011; 12:1868–1882.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015; 49:540–556.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet 2012; 380:475–483.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:668–673.
- Green JB, Bethel A, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013; 10:289–301.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162:620–626.
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36:2288–2296.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Eng J Med 2014; 370:794–797.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother 2015; 49:700–714.
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 2015; 6:109–134.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015; 6:19–28.
- Harris KB, McCarty DJ. Efficacy and tolerability of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015; 6:3–18.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gludd LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analysis of randomized controlled trials. BMJ 2012; 344:d7771.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet 2014; 384:1349–1357.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open-label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2:289–297.
- Neumiller JJ. Incretin-based therapies. Med Clin N Am 2015; 99:107–129.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:38–47.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75:1272–1277.
- Defronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36:3169–3176.
- Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303:C348–C354.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:457–466.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70:1149–1158.
- Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7:448–461.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 2014; 5:124–136.
- Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92–102.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Aggarwal W, Loomba RS, Arora RR. Efficacy of colesevelam on lowering glycemia and lipids. J Cardiovasc Pharmacol 2012; 59:198–205.
- Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam Hcl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008; 31:1479–1484.
- Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012; 12:CD009361.
- Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S. The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations. Endocr Pract 2013; 19:100–106.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8:1683–1707.
- Gaziano JM, Concotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag 2015; 11:621–632.
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clin Ther 2011; 12:1868–1882.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015; 49:540–556.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet 2012; 380:475–483.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:668–673.
- Green JB, Bethel A, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013; 10:289–301.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162:620–626.
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36:2288–2296.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Eng J Med 2014; 370:794–797.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother 2015; 49:700–714.
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 2015; 6:109–134.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015; 6:19–28.
- Harris KB, McCarty DJ. Efficacy and tolerability of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015; 6:3–18.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gludd LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analysis of randomized controlled trials. BMJ 2012; 344:d7771.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet 2014; 384:1349–1357.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open-label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2:289–297.
- Neumiller JJ. Incretin-based therapies. Med Clin N Am 2015; 99:107–129.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:38–47.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75:1272–1277.
- Defronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36:3169–3176.
- Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303:C348–C354.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:457–466.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70:1149–1158.
- Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7:448–461.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 2014; 5:124–136.
- Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92–102.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Aggarwal W, Loomba RS, Arora RR. Efficacy of colesevelam on lowering glycemia and lipids. J Cardiovasc Pharmacol 2012; 59:198–205.
- Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam Hcl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008; 31:1479–1484.
- Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012; 12:CD009361.
- Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S. The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations. Endocr Pract 2013; 19:100–106.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8:1683–1707.
- Gaziano JM, Concotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
KEY POINTS
- The US Food and Drug Administration has approved 14 noninsulin pharmaceuticals in five drug classes in the past decade for type 2 diabetes therapy.
- The noninsulin drug classes of dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, bile acid sequestrants, and dopamine-receptor agonists have different mechanisms of action and therapeutic effects.
- Successful management strategies require a balancing of multiple agents to achieve target glucose while avoiding adverse effects.