User login
The essential value of glycemic control in preventing microvascular and neuropathic complications was established in the Diabetes Control and Complications Trial (DCCT)1 and the United Kingdom Prospective Diabetes Study (UKPDS),2 conducted in patients with type 1 and type 2 diabetes, respectively. However, it took another 10 or more years of observational follow-up of those cohorts to demonstrate statistically significant atherosclerotic cardiovascular (CV) disease benefits resulting from the intensive glycemic control achieved during those trials, as reported in the DCCT-Epidemiology of Diabetes Interventions and Complications (EDIC)3 and the UKPDS follow-up studies.4 Overall, it took more than 20 years of observational follow-up of the original intensive glucose-treatment cohort of DCCT/EDIC to show a significant decline in total deaths compared with the conventional treatment cohort.5
In patients with type 2 diabetes, the relationship between intensive glycemic control and CV benefits is somewhat controversial, particularly in view of negative CV outcomes from several long-term clinical trials in subjects older than the UKPDS subjects and with longer duration of diabetes:
- ACCORD trial (Action to Control Cardiovascular Risk in Diabetes)6,7
- ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation)8,9
- VADT: (Veteran Affairs Diabetes Trial).10,11
The controversy was spurred by an unexplained increase in total deaths in ACCORD,6,7 despite a reduction in ischemic coronary events. In the VADT,10,11 a significant decline was reported for major CV events, but not total deaths, after a median of 9.8 years of observational follow-up.11 In the ADVANCE cohort,8,9 a reduction in total deaths or CV events was not seen after 5.4 years of additional follow-up.9 Some of these differences in outcomes between the UKPDS and these other long-term trials may well reflect the younger aged and the newly diagnosed patients in the UKPDS population, and differences in specific glucose-lowering strategies. Nevertheless, this remains an unsettled issue.
CV OUTCOMES WITH SPECIFIC ANTIHYPERGLYCEMIC AGENTS
Another poorly understood question relates to the impact of specific glucose-lowering agents on CV events, regardless of the glucose control. Table 1 lists the studies of the currently available agents. Studies such as the UKPDS have investigated the question of intensive hyperglycemia control compared with standard, less intensive control.
The question of CV safety with glucose-lowering agents was highlighted in a 2007 meta-analysis of 42 short-term studies with rosiglitazone that reported significantly worse myocardial infarction (MI) risks along with increased mortality from all CV causes that was borderline significant (P = .06).12 This finding, however, was not confirmed in the only randomized controlled trial (RCT) completed with rosiglitazone.13 The controversy led the US Food and Drug Administration (FDA) to issue a 2008 guidance statement recommending that all new diabetes drugs undergo a long-term, noninferiority RCT to prove their CV safety vs an active comparator.14 Before the FDA mandate, few clinical trials had addressed the long-term effects of glucose-lowering drugs on CV outcomes in patients with type 2 diabetes.
In the UKPDS, the only primary prevention trial thus far, investigators used first-generation sulfonylureas (glyburide and chlorpropamide) with or without insulin as the intensive control strategy in 3,867 patients newly diagnosed with type 2 diabetes.2 After a median follow-up of 10 years, the active treatment group had a borderline benefit in fatal and nonfatal MI (16% reduction in relative risk for MI; P = .052) compared with the nondrug treatment.
Additionally, a small subgroup of overweight patients in the UKPDS who were randomized to metformin (N = 342) had 36% (P = .010) lower risk of all-cause mortality and 39% (P = .011) lower risk of MI compared with conventional treatment.15 This benefit occurred despite a more modest hemoglobin A1c (HbA1c) reduction (0.6%) in the metformin group than in the entire UKPDS trial (0.9%).
The mechanism underlying these impressive CV benefits remains unclear in view of the nonglucose effects of metformin, such as lack of weight gain. Metformin also has been reported to reduce generation of advanced glycosylation end products and oxidative damage to apolipoprotein B100 in patients with type 2 diabetes.16
One curious but unexplained finding in the UKPDS was an increase in both diabetes-related deaths (relative risk [RR], 1.96; P = .039) and total deaths (RR, 1.60; P = .04) in a subgroup of 268 patients in whom metformin was added to sulfonylurea therapy; however, the number of total deaths was relatively small, 47 deaths in the metformin added group and 31 deaths in the sulfonylurea group.15 Because of the absence of proven CV benefits with any other diabetes drug thus far, metformin is generally the preferred initial drug in all treatment guidelines.
The relative effects of metformin and sulfonylureas when used as the initial monotherapy regimen have been studied in several large observational studies.17–20 In general, there appears to be a consistent pattern of significantly increased CV events and total mortality—by 20% to 50%—in those treated with sulfonylureas, with or without prior CV disease. However, these analyses were not based on RCTs.
In two RCTs—NAVIGATOR Study Group21 and STOP-NIDDM Trial22—patients with impaired glucose tolerance were recruited with the primary aim of preventing progression to diabetes. In the NAVIGATOR trial, the short-acting insulin secretagogue nateglinide did not reduce CV events or the progression to diabetes.21 In the other study, acarbose, an alpha-glucosidase inhibitor, significantly reduced CV events (hazard ratio [HR], 0.51; 95% confidence interval [CI], 0.28–0.85; P = .03)22 and also prevented progression to diabetes. (P < .002).23 Although the number of CV events in that 3-year study was small, a meta-analyses of seven studies using acarbose therapy in patients with diabetes also found a significant reduction in composite CV events (HR, 0.65; P < .007), including MIs.24 A long-term, much larger RCT with acarbose is in progress.25
Following the demonstration of strikingly protective effects of metformin on CV events in the UKPDS,16 two major trials of thiazolidinediones (TZDs) investigated the effects of insulin sensitization on CV events.13,26 Pioglitazone in patients with long duration type 2 diabetes mellitus and pre-existing CV disease was reported to marginally reduce major CV outcomes (HR, 0.84; 95% CI, 0.72–98; P < .03),26 whereas rosiglitazone in patients with a shorter duration of diabetes was found to be noninferior to the control group.13 In both trials, however, there was a twofold increased risk for hospitalization for heart failure and increased risk for bone fractures in women,13,26 but without an increased risk for mortality.27 Furthermore, in the BARI 2D trial in patients with diabetes and established CV disease, adding rosiglitazone did not significantly reduce propensity-matched CV outcomes, compared with insulin secretagogues or insulin.28 Thus, while TZDs appear to have no major adverse effects on CV outcomes, the other associated adverse effects limit their use.
In a 1-year study of the efficacy and CV safety of the dopaminergic agent quick-release bromocriptine, an FDA-approved drug for diabetes, there was a marked decrease in incidence of composite CV end points (HR, 0.60; 95% CI, 0.37–0.96).29 However, there also was a 47% dropout rate and a small number of total events; thus, the implications remain inconclusive.
Incretin-mimetic agents and CV outcomes
Following the FDA guidance,14 all newer agents, including incretin-mimetic agents (dipeptidyl peptidase-4 [DPP-4] inhibitors and glucagon-like peptide-1 [GLP-1] receptor agonists) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been undergoing well-designed, long-term, noninferiority trials with the comparison group receiving the standard of diabetes and CV care. The goal of these trials, unlike that of most of the studies discussed in this article, was to investigate the safety of individual agents rather than different levels of glycemic control.
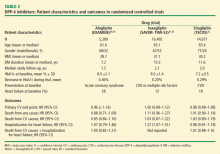
Since 2013, such trials with three of the available DPP-4 inhibitors have been completed (Table 2).30–34 Each trial was conducted in patients with pre-existing CV disease or high risk of it. The mean duration of follow-up in these trials was 1.5 to 3.0 years. There were significant, but only marginal, differences in HbA1c compared with the control groups receiving standard care. In each trial, the primary CV end points showed noninferiority, thus documenting their CV safety. One important difference in secondary end points was a significant increase in hospitalization rates for heart failure with saxagliptin31,33 that was not observed in the trials with alogliptin30,34 or sitagliptin.32 Another secondary end point—hospitalization for heart failure plus CV mortality—also was not increased in the alogliptin34 and sitagliptin32 trials (rates not reported for saxagliptin); however, there was no increase in total deaths from any cause in these trials.
The mechanisms underlying the increased rates of heart failure with saxagliptin are unclear. The baseline characteristics of patients in these three trials were similar (Table 2). Patients with type 2 diabetes have higher rates of heart failure in general, but the effects of concomitant drug therapy on risk of heart failure, other than with TZDs, have not been well studied. In an extensive meta-analysis of 84 RTCs of various durations, the overall risk (OR) of heart failure was higher in patients treated with DPP-4 inhibitors than in those treated with placebo or active comparators (OR, 1.19; 95% CI, 1.03–1.37; P = .015), suggesting that DPP-4 inhibitors as a class could be associated with an increased risk of heart failure.35 A case-control study, however, found no increase in rates of heart failure with DPP-4 inhibitors, although there were very few patients on saxagliptin.36 Yet another large retrospective, propensity-adjusted observational analysis of more than 112,000 patients, which compared those on saxagliptin and sitagliptin, reported no difference in rates of heart failure; however, the median follow-up period was less than 6 months.37
In comparative observational analyses,18,37,38 the risks of heart failure with TZDs and sulfonylureas were increased, compared with DPP-4 inhibitors, particularly with TZDs. On the other hand, a large population-based analysis from Italy found that DPP-4 inhibitors were associated with a propensity-matched 36% lower rate of hospitalization for heart failure compared with sulfonylureas.39 These data point to a need for more well-designed comparative studies to investigate valid differences between drugs in this class.
In the only GLP-1 receptor agonist trial completed thus far, ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome), there were no differences in primary and major secondary CV outcomes in 6,068 very high-risk patients randomized to lixisenatide or placebo after a 25-month follow-up (HR, 1.02; 95%, CI, 0.89–1.17).40 Moreover, the hospitalization rates for heart failure were not increased (HR, 0.96; 95% CI, 0.75–1.23). The earlier meta-analyses of short-term studies with DPP-4 inhibitors reporting significant reductions in CV events41,42 also underscore the need for well-designed long-term RCTs to accurately interpret drug effects.
SGLT-2 inhibitors and CV outcomes
The first CV outcome RCT with the SGLT-2 inhibitor empagliflozin, the EMPA-REG OUTCOME trial,43 was recently reported. Of great importance in this 7,020-patient trial comparing empagliflozin with placebo were the following results:
- 14% reduction in the primary end point (composite of death from CV causes, nonfatal MI, or nonfatal stroke) (P = .04)
- 32% reduction in all-cause deaths (P < .001)
- 35% reduction in hospitalization for heart failure (P = .002).
The mechanism underlying these impressive benefits is not known, although there were modest reductions in HbA1c levels, body weight (~2 kg), waist circumference (~2 cm), and systolic blood pressure (~4 mm Hg) with empagliflozin. The main adverse effects were related to a 3 to 4 times increased incidence of genital infections. Trials with other agents in this class are currently ongoing.
Insulin and CV outcomes
The UKPDS trial is the only primary prevention trial that provided evidence of significant benefits from intensive glucose control (with insulin, with or without sulfonylurea therapy) on CV outcomes and mortality, but only after 10 additional years of follow-up after the end of the trial.4 A few other trials have investigated the long-term effects of insulin compared with conventional therapy in patients with CV disease.
The DIGAMI-1 (Diabetes Insulin-Glucose in Acute Myocardial Infarction) was a RCT conducted between 1990 and 1993 in 620 patients with type 2 diabetes and acute MI randomized to short-term, intensive insulin-based glucose therapy or to conventional glucose-lowering therapy.44 Results showed the intensive treatment group had an 11% decrease in mortality rate at 3.4 years. A 20-year follow-up reassessment showed the overall survival was improved by a mean of 2.3 years at 8 years, particularly in those at lower risk at baseline.45 However, none of these patients were on statin therapy at baseline; thus, the implications of that study with current standards of care are quite uncertain. Subsequent studies—DIGAMI-2 (N = 1,253)46 and the HI-5 (Hyperglycemia: Intensive Insulin Infusion in Infarction) study (N = 240),47 both investigating the effects of intensive insulin therapy in patients with type 2 diabetes and MI—showed no significant effects on mortality in patients at 1 year (DIGAMI-2) and 6 months (HI-5).
The HEART2D trial (Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus), an RCT of 1,115 post-MI patients, investigated the effects of targeting prandial insulin compared with basal insulin. During a mean follow-up of 2.7 years, there were no between-group differences in CV outcomes (HR, 0.98; 95% CI, 0.8–1.21) or glycemic control.48 Also, there was no impact of glycemic variability.49 Finally, the ORIGIN trial (Outcome Reduction With an Initial Glargine Intervention), an RCT of more than 12,000 patients at high risk for CV disease but with relatively recent onset of either type 2 diabetes or prediabetes, randomized patients to basal insulin glargine or noninsulin treatments.50 The baseline HbA1c was relatively low at 6.4%, but it significantly declined by 0.3% by the end of trial, compared with the control group. There was no effect on CV outcomes (HR, 1.02; 95% CI, 0.94–1.11) after a median follow-up of 6.2 years.
However, it remains a perplexing question regarding whether long-term treatment with increasing insulin dosages in a subset of obese patients with poorly controlled type 2 diabetes and increasing insulin resistance could be potentially harmful to the CV system.51
CONCLUSION
The long-term RCTs with antihyperglycemic agents, including DCCT/EDIC in type 1 diabetes and UKPDS, ACCORD, and VADT in type 2 diabetes, with the exception of ADVANCE, have established the value of intensive glycemic control in reducing CV outcomes but only after many years of follow-up. However, the effects of intensive glycemic control on CV disease in type 2 diabetes are inconsistent, with only the primary prevention cohorts of UKPDS showing significant effects on mortality after prolonged follow-up. This is in contrast to the positive effects of statins in relatively short-term trials.
While it is difficult to interpret the CV results of specific drugs from the degree of glycemic control, it is reassuring that the large RCTs with several individual agents, including TZDs (both pioglitazone and rosiglitazone), several DPP-4 inhibitors, and one GLP-1 receptor agonist, have demonstrated no appreciable harm. The increase in the secondary outcome of heart failure but with no increase in mortality observed with saxagliptin requires further mechanistic studies while awaiting the results of other ongoing trials with newer agents including other incretin-based drugs and SGLT-2 inhibitors.
With SGLT-2 inhibitors, the recently published results of the empagliflozin trail (EMPA-REG OUTCOME trial) with type 2 diabetes revealed a significant reduction in CV end points and mortality. Before those data were published, metformin was the only antihyperglycemic drug that had shown a significant effect on CV events and mortality, but it was studied in only a small subgroup of the UKPDS cohort, and there are no RCTs of the relative impact of metformin or other agents as compared to sulfonylureas. The results of ongoing CV trials with SGLT-2 inhibitors are eagerly awaited.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313:45–53.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Gerstein HC, Miller ME, Ismail-Beigi F, et al. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet 2014; 384: 1936–1941.
- The ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Zoungas S, Chalmers J, Neal B, et al; ADAVNCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014; 371:1392–1406.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hayward RA, Reaven PD, Wiitala WL et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372:2197–2206.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009; 373:2125–2135.
- US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. December 2008. Available at: http://www.fda.gov/
downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed March 15, 2016. - UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010; 59:1038–1045.
- Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all-cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009; 339:b4731.
- Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 2012; 157:601–610.
- Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab 2012; 97:4605–4612.
- Li Y, Hu Y, Ley SH, Rajpathak S, Hu FB. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014; 37:3106–3113.
- The NAVIGATOR Study Group, Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362:1463–1476.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003; 290:486–494.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002; 359:2072–2077.
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004; 25:10–16.
- Holman RR, Bethel MA, Chan JC, et al; ACE Study Group. Rationale for the design of the Acarbose Cardiovascular Event (ACE) trial. Am Heart J 2014; 168:23–29. Epub ahead of print April 5, 2014. doi:10.1016/j.ahj.2014.03.021
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitazone clinical trial in macrovascular events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomized clinical trials. Lancet 2007; 370:1129–1136.
- Bach RG, Brooks MM, Lombardero M, et al; BARI 2D Investigators. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 2013; 128:785–794.
- Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- White WB, Cannon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Scirica BM, Braunwald E, Raz I, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014; 130:1579–1588.
- Zannad F, Cannon CP, Cushman WC, EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015; 385:2067–2076.
- Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014; 24:689–697.
- Yu OH, Filion KB, Azoulay L, Patenaude V, Majdan A, Suissa S. Incretin-based drugs and the risk of congestive heart failure. Diabetes Care 2015; 38:277–284.
- Fu AZ, Johnston SS, Ghannam A, et al. Association between hospitalization for heart failure and dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care 2016 [epub ahead of print].
- Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015; 385:2107–2117.
- Fadini GP, Avagaro A, Degli Esposti L, et al; OsMed Health-DB Network. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J 2015; 36:2454–2462.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–2257.
- Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol 2012; 110:826–833.
- Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013; 15:112–120.
- Zinamn B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Malmberg K, Ryden L, Efendic S, et al. Randomised trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57–65.
- Ritsinger V, Malmberg K, Martensson A, Ryden L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol 2014; 2:627–633.
- Malmberg K, Ryden L, Wedel H, et al; DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26:650–661.
- Cheung NW, Wong VW, McLean M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006; 29:765–770.
- Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009; 32:381–386.
- Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care 2011; 34:855–857.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Nolan CJ, Ruderman NB, Kahn SE, et al. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015; 64:673–686.
The essential value of glycemic control in preventing microvascular and neuropathic complications was established in the Diabetes Control and Complications Trial (DCCT)1 and the United Kingdom Prospective Diabetes Study (UKPDS),2 conducted in patients with type 1 and type 2 diabetes, respectively. However, it took another 10 or more years of observational follow-up of those cohorts to demonstrate statistically significant atherosclerotic cardiovascular (CV) disease benefits resulting from the intensive glycemic control achieved during those trials, as reported in the DCCT-Epidemiology of Diabetes Interventions and Complications (EDIC)3 and the UKPDS follow-up studies.4 Overall, it took more than 20 years of observational follow-up of the original intensive glucose-treatment cohort of DCCT/EDIC to show a significant decline in total deaths compared with the conventional treatment cohort.5
In patients with type 2 diabetes, the relationship between intensive glycemic control and CV benefits is somewhat controversial, particularly in view of negative CV outcomes from several long-term clinical trials in subjects older than the UKPDS subjects and with longer duration of diabetes:
- ACCORD trial (Action to Control Cardiovascular Risk in Diabetes)6,7
- ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation)8,9
- VADT: (Veteran Affairs Diabetes Trial).10,11
The controversy was spurred by an unexplained increase in total deaths in ACCORD,6,7 despite a reduction in ischemic coronary events. In the VADT,10,11 a significant decline was reported for major CV events, but not total deaths, after a median of 9.8 years of observational follow-up.11 In the ADVANCE cohort,8,9 a reduction in total deaths or CV events was not seen after 5.4 years of additional follow-up.9 Some of these differences in outcomes between the UKPDS and these other long-term trials may well reflect the younger aged and the newly diagnosed patients in the UKPDS population, and differences in specific glucose-lowering strategies. Nevertheless, this remains an unsettled issue.
CV OUTCOMES WITH SPECIFIC ANTIHYPERGLYCEMIC AGENTS
Another poorly understood question relates to the impact of specific glucose-lowering agents on CV events, regardless of the glucose control. Table 1 lists the studies of the currently available agents. Studies such as the UKPDS have investigated the question of intensive hyperglycemia control compared with standard, less intensive control.
The question of CV safety with glucose-lowering agents was highlighted in a 2007 meta-analysis of 42 short-term studies with rosiglitazone that reported significantly worse myocardial infarction (MI) risks along with increased mortality from all CV causes that was borderline significant (P = .06).12 This finding, however, was not confirmed in the only randomized controlled trial (RCT) completed with rosiglitazone.13 The controversy led the US Food and Drug Administration (FDA) to issue a 2008 guidance statement recommending that all new diabetes drugs undergo a long-term, noninferiority RCT to prove their CV safety vs an active comparator.14 Before the FDA mandate, few clinical trials had addressed the long-term effects of glucose-lowering drugs on CV outcomes in patients with type 2 diabetes.
In the UKPDS, the only primary prevention trial thus far, investigators used first-generation sulfonylureas (glyburide and chlorpropamide) with or without insulin as the intensive control strategy in 3,867 patients newly diagnosed with type 2 diabetes.2 After a median follow-up of 10 years, the active treatment group had a borderline benefit in fatal and nonfatal MI (16% reduction in relative risk for MI; P = .052) compared with the nondrug treatment.
Additionally, a small subgroup of overweight patients in the UKPDS who were randomized to metformin (N = 342) had 36% (P = .010) lower risk of all-cause mortality and 39% (P = .011) lower risk of MI compared with conventional treatment.15 This benefit occurred despite a more modest hemoglobin A1c (HbA1c) reduction (0.6%) in the metformin group than in the entire UKPDS trial (0.9%).
The mechanism underlying these impressive CV benefits remains unclear in view of the nonglucose effects of metformin, such as lack of weight gain. Metformin also has been reported to reduce generation of advanced glycosylation end products and oxidative damage to apolipoprotein B100 in patients with type 2 diabetes.16
One curious but unexplained finding in the UKPDS was an increase in both diabetes-related deaths (relative risk [RR], 1.96; P = .039) and total deaths (RR, 1.60; P = .04) in a subgroup of 268 patients in whom metformin was added to sulfonylurea therapy; however, the number of total deaths was relatively small, 47 deaths in the metformin added group and 31 deaths in the sulfonylurea group.15 Because of the absence of proven CV benefits with any other diabetes drug thus far, metformin is generally the preferred initial drug in all treatment guidelines.
The relative effects of metformin and sulfonylureas when used as the initial monotherapy regimen have been studied in several large observational studies.17–20 In general, there appears to be a consistent pattern of significantly increased CV events and total mortality—by 20% to 50%—in those treated with sulfonylureas, with or without prior CV disease. However, these analyses were not based on RCTs.
In two RCTs—NAVIGATOR Study Group21 and STOP-NIDDM Trial22—patients with impaired glucose tolerance were recruited with the primary aim of preventing progression to diabetes. In the NAVIGATOR trial, the short-acting insulin secretagogue nateglinide did not reduce CV events or the progression to diabetes.21 In the other study, acarbose, an alpha-glucosidase inhibitor, significantly reduced CV events (hazard ratio [HR], 0.51; 95% confidence interval [CI], 0.28–0.85; P = .03)22 and also prevented progression to diabetes. (P < .002).23 Although the number of CV events in that 3-year study was small, a meta-analyses of seven studies using acarbose therapy in patients with diabetes also found a significant reduction in composite CV events (HR, 0.65; P < .007), including MIs.24 A long-term, much larger RCT with acarbose is in progress.25
Following the demonstration of strikingly protective effects of metformin on CV events in the UKPDS,16 two major trials of thiazolidinediones (TZDs) investigated the effects of insulin sensitization on CV events.13,26 Pioglitazone in patients with long duration type 2 diabetes mellitus and pre-existing CV disease was reported to marginally reduce major CV outcomes (HR, 0.84; 95% CI, 0.72–98; P < .03),26 whereas rosiglitazone in patients with a shorter duration of diabetes was found to be noninferior to the control group.13 In both trials, however, there was a twofold increased risk for hospitalization for heart failure and increased risk for bone fractures in women,13,26 but without an increased risk for mortality.27 Furthermore, in the BARI 2D trial in patients with diabetes and established CV disease, adding rosiglitazone did not significantly reduce propensity-matched CV outcomes, compared with insulin secretagogues or insulin.28 Thus, while TZDs appear to have no major adverse effects on CV outcomes, the other associated adverse effects limit their use.
In a 1-year study of the efficacy and CV safety of the dopaminergic agent quick-release bromocriptine, an FDA-approved drug for diabetes, there was a marked decrease in incidence of composite CV end points (HR, 0.60; 95% CI, 0.37–0.96).29 However, there also was a 47% dropout rate and a small number of total events; thus, the implications remain inconclusive.
Incretin-mimetic agents and CV outcomes
Following the FDA guidance,14 all newer agents, including incretin-mimetic agents (dipeptidyl peptidase-4 [DPP-4] inhibitors and glucagon-like peptide-1 [GLP-1] receptor agonists) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been undergoing well-designed, long-term, noninferiority trials with the comparison group receiving the standard of diabetes and CV care. The goal of these trials, unlike that of most of the studies discussed in this article, was to investigate the safety of individual agents rather than different levels of glycemic control.
Since 2013, such trials with three of the available DPP-4 inhibitors have been completed (Table 2).30–34 Each trial was conducted in patients with pre-existing CV disease or high risk of it. The mean duration of follow-up in these trials was 1.5 to 3.0 years. There were significant, but only marginal, differences in HbA1c compared with the control groups receiving standard care. In each trial, the primary CV end points showed noninferiority, thus documenting their CV safety. One important difference in secondary end points was a significant increase in hospitalization rates for heart failure with saxagliptin31,33 that was not observed in the trials with alogliptin30,34 or sitagliptin.32 Another secondary end point—hospitalization for heart failure plus CV mortality—also was not increased in the alogliptin34 and sitagliptin32 trials (rates not reported for saxagliptin); however, there was no increase in total deaths from any cause in these trials.
The mechanisms underlying the increased rates of heart failure with saxagliptin are unclear. The baseline characteristics of patients in these three trials were similar (Table 2). Patients with type 2 diabetes have higher rates of heart failure in general, but the effects of concomitant drug therapy on risk of heart failure, other than with TZDs, have not been well studied. In an extensive meta-analysis of 84 RTCs of various durations, the overall risk (OR) of heart failure was higher in patients treated with DPP-4 inhibitors than in those treated with placebo or active comparators (OR, 1.19; 95% CI, 1.03–1.37; P = .015), suggesting that DPP-4 inhibitors as a class could be associated with an increased risk of heart failure.35 A case-control study, however, found no increase in rates of heart failure with DPP-4 inhibitors, although there were very few patients on saxagliptin.36 Yet another large retrospective, propensity-adjusted observational analysis of more than 112,000 patients, which compared those on saxagliptin and sitagliptin, reported no difference in rates of heart failure; however, the median follow-up period was less than 6 months.37
In comparative observational analyses,18,37,38 the risks of heart failure with TZDs and sulfonylureas were increased, compared with DPP-4 inhibitors, particularly with TZDs. On the other hand, a large population-based analysis from Italy found that DPP-4 inhibitors were associated with a propensity-matched 36% lower rate of hospitalization for heart failure compared with sulfonylureas.39 These data point to a need for more well-designed comparative studies to investigate valid differences between drugs in this class.
In the only GLP-1 receptor agonist trial completed thus far, ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome), there were no differences in primary and major secondary CV outcomes in 6,068 very high-risk patients randomized to lixisenatide or placebo after a 25-month follow-up (HR, 1.02; 95%, CI, 0.89–1.17).40 Moreover, the hospitalization rates for heart failure were not increased (HR, 0.96; 95% CI, 0.75–1.23). The earlier meta-analyses of short-term studies with DPP-4 inhibitors reporting significant reductions in CV events41,42 also underscore the need for well-designed long-term RCTs to accurately interpret drug effects.
SGLT-2 inhibitors and CV outcomes
The first CV outcome RCT with the SGLT-2 inhibitor empagliflozin, the EMPA-REG OUTCOME trial,43 was recently reported. Of great importance in this 7,020-patient trial comparing empagliflozin with placebo were the following results:
- 14% reduction in the primary end point (composite of death from CV causes, nonfatal MI, or nonfatal stroke) (P = .04)
- 32% reduction in all-cause deaths (P < .001)
- 35% reduction in hospitalization for heart failure (P = .002).
The mechanism underlying these impressive benefits is not known, although there were modest reductions in HbA1c levels, body weight (~2 kg), waist circumference (~2 cm), and systolic blood pressure (~4 mm Hg) with empagliflozin. The main adverse effects were related to a 3 to 4 times increased incidence of genital infections. Trials with other agents in this class are currently ongoing.
Insulin and CV outcomes
The UKPDS trial is the only primary prevention trial that provided evidence of significant benefits from intensive glucose control (with insulin, with or without sulfonylurea therapy) on CV outcomes and mortality, but only after 10 additional years of follow-up after the end of the trial.4 A few other trials have investigated the long-term effects of insulin compared with conventional therapy in patients with CV disease.
The DIGAMI-1 (Diabetes Insulin-Glucose in Acute Myocardial Infarction) was a RCT conducted between 1990 and 1993 in 620 patients with type 2 diabetes and acute MI randomized to short-term, intensive insulin-based glucose therapy or to conventional glucose-lowering therapy.44 Results showed the intensive treatment group had an 11% decrease in mortality rate at 3.4 years. A 20-year follow-up reassessment showed the overall survival was improved by a mean of 2.3 years at 8 years, particularly in those at lower risk at baseline.45 However, none of these patients were on statin therapy at baseline; thus, the implications of that study with current standards of care are quite uncertain. Subsequent studies—DIGAMI-2 (N = 1,253)46 and the HI-5 (Hyperglycemia: Intensive Insulin Infusion in Infarction) study (N = 240),47 both investigating the effects of intensive insulin therapy in patients with type 2 diabetes and MI—showed no significant effects on mortality in patients at 1 year (DIGAMI-2) and 6 months (HI-5).
The HEART2D trial (Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus), an RCT of 1,115 post-MI patients, investigated the effects of targeting prandial insulin compared with basal insulin. During a mean follow-up of 2.7 years, there were no between-group differences in CV outcomes (HR, 0.98; 95% CI, 0.8–1.21) or glycemic control.48 Also, there was no impact of glycemic variability.49 Finally, the ORIGIN trial (Outcome Reduction With an Initial Glargine Intervention), an RCT of more than 12,000 patients at high risk for CV disease but with relatively recent onset of either type 2 diabetes or prediabetes, randomized patients to basal insulin glargine or noninsulin treatments.50 The baseline HbA1c was relatively low at 6.4%, but it significantly declined by 0.3% by the end of trial, compared with the control group. There was no effect on CV outcomes (HR, 1.02; 95% CI, 0.94–1.11) after a median follow-up of 6.2 years.
However, it remains a perplexing question regarding whether long-term treatment with increasing insulin dosages in a subset of obese patients with poorly controlled type 2 diabetes and increasing insulin resistance could be potentially harmful to the CV system.51
CONCLUSION
The long-term RCTs with antihyperglycemic agents, including DCCT/EDIC in type 1 diabetes and UKPDS, ACCORD, and VADT in type 2 diabetes, with the exception of ADVANCE, have established the value of intensive glycemic control in reducing CV outcomes but only after many years of follow-up. However, the effects of intensive glycemic control on CV disease in type 2 diabetes are inconsistent, with only the primary prevention cohorts of UKPDS showing significant effects on mortality after prolonged follow-up. This is in contrast to the positive effects of statins in relatively short-term trials.
While it is difficult to interpret the CV results of specific drugs from the degree of glycemic control, it is reassuring that the large RCTs with several individual agents, including TZDs (both pioglitazone and rosiglitazone), several DPP-4 inhibitors, and one GLP-1 receptor agonist, have demonstrated no appreciable harm. The increase in the secondary outcome of heart failure but with no increase in mortality observed with saxagliptin requires further mechanistic studies while awaiting the results of other ongoing trials with newer agents including other incretin-based drugs and SGLT-2 inhibitors.
With SGLT-2 inhibitors, the recently published results of the empagliflozin trail (EMPA-REG OUTCOME trial) with type 2 diabetes revealed a significant reduction in CV end points and mortality. Before those data were published, metformin was the only antihyperglycemic drug that had shown a significant effect on CV events and mortality, but it was studied in only a small subgroup of the UKPDS cohort, and there are no RCTs of the relative impact of metformin or other agents as compared to sulfonylureas. The results of ongoing CV trials with SGLT-2 inhibitors are eagerly awaited.
The essential value of glycemic control in preventing microvascular and neuropathic complications was established in the Diabetes Control and Complications Trial (DCCT)1 and the United Kingdom Prospective Diabetes Study (UKPDS),2 conducted in patients with type 1 and type 2 diabetes, respectively. However, it took another 10 or more years of observational follow-up of those cohorts to demonstrate statistically significant atherosclerotic cardiovascular (CV) disease benefits resulting from the intensive glycemic control achieved during those trials, as reported in the DCCT-Epidemiology of Diabetes Interventions and Complications (EDIC)3 and the UKPDS follow-up studies.4 Overall, it took more than 20 years of observational follow-up of the original intensive glucose-treatment cohort of DCCT/EDIC to show a significant decline in total deaths compared with the conventional treatment cohort.5
In patients with type 2 diabetes, the relationship between intensive glycemic control and CV benefits is somewhat controversial, particularly in view of negative CV outcomes from several long-term clinical trials in subjects older than the UKPDS subjects and with longer duration of diabetes:
- ACCORD trial (Action to Control Cardiovascular Risk in Diabetes)6,7
- ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation)8,9
- VADT: (Veteran Affairs Diabetes Trial).10,11
The controversy was spurred by an unexplained increase in total deaths in ACCORD,6,7 despite a reduction in ischemic coronary events. In the VADT,10,11 a significant decline was reported for major CV events, but not total deaths, after a median of 9.8 years of observational follow-up.11 In the ADVANCE cohort,8,9 a reduction in total deaths or CV events was not seen after 5.4 years of additional follow-up.9 Some of these differences in outcomes between the UKPDS and these other long-term trials may well reflect the younger aged and the newly diagnosed patients in the UKPDS population, and differences in specific glucose-lowering strategies. Nevertheless, this remains an unsettled issue.
CV OUTCOMES WITH SPECIFIC ANTIHYPERGLYCEMIC AGENTS
Another poorly understood question relates to the impact of specific glucose-lowering agents on CV events, regardless of the glucose control. Table 1 lists the studies of the currently available agents. Studies such as the UKPDS have investigated the question of intensive hyperglycemia control compared with standard, less intensive control.
The question of CV safety with glucose-lowering agents was highlighted in a 2007 meta-analysis of 42 short-term studies with rosiglitazone that reported significantly worse myocardial infarction (MI) risks along with increased mortality from all CV causes that was borderline significant (P = .06).12 This finding, however, was not confirmed in the only randomized controlled trial (RCT) completed with rosiglitazone.13 The controversy led the US Food and Drug Administration (FDA) to issue a 2008 guidance statement recommending that all new diabetes drugs undergo a long-term, noninferiority RCT to prove their CV safety vs an active comparator.14 Before the FDA mandate, few clinical trials had addressed the long-term effects of glucose-lowering drugs on CV outcomes in patients with type 2 diabetes.
In the UKPDS, the only primary prevention trial thus far, investigators used first-generation sulfonylureas (glyburide and chlorpropamide) with or without insulin as the intensive control strategy in 3,867 patients newly diagnosed with type 2 diabetes.2 After a median follow-up of 10 years, the active treatment group had a borderline benefit in fatal and nonfatal MI (16% reduction in relative risk for MI; P = .052) compared with the nondrug treatment.
Additionally, a small subgroup of overweight patients in the UKPDS who were randomized to metformin (N = 342) had 36% (P = .010) lower risk of all-cause mortality and 39% (P = .011) lower risk of MI compared with conventional treatment.15 This benefit occurred despite a more modest hemoglobin A1c (HbA1c) reduction (0.6%) in the metformin group than in the entire UKPDS trial (0.9%).
The mechanism underlying these impressive CV benefits remains unclear in view of the nonglucose effects of metformin, such as lack of weight gain. Metformin also has been reported to reduce generation of advanced glycosylation end products and oxidative damage to apolipoprotein B100 in patients with type 2 diabetes.16
One curious but unexplained finding in the UKPDS was an increase in both diabetes-related deaths (relative risk [RR], 1.96; P = .039) and total deaths (RR, 1.60; P = .04) in a subgroup of 268 patients in whom metformin was added to sulfonylurea therapy; however, the number of total deaths was relatively small, 47 deaths in the metformin added group and 31 deaths in the sulfonylurea group.15 Because of the absence of proven CV benefits with any other diabetes drug thus far, metformin is generally the preferred initial drug in all treatment guidelines.
The relative effects of metformin and sulfonylureas when used as the initial monotherapy regimen have been studied in several large observational studies.17–20 In general, there appears to be a consistent pattern of significantly increased CV events and total mortality—by 20% to 50%—in those treated with sulfonylureas, with or without prior CV disease. However, these analyses were not based on RCTs.
In two RCTs—NAVIGATOR Study Group21 and STOP-NIDDM Trial22—patients with impaired glucose tolerance were recruited with the primary aim of preventing progression to diabetes. In the NAVIGATOR trial, the short-acting insulin secretagogue nateglinide did not reduce CV events or the progression to diabetes.21 In the other study, acarbose, an alpha-glucosidase inhibitor, significantly reduced CV events (hazard ratio [HR], 0.51; 95% confidence interval [CI], 0.28–0.85; P = .03)22 and also prevented progression to diabetes. (P < .002).23 Although the number of CV events in that 3-year study was small, a meta-analyses of seven studies using acarbose therapy in patients with diabetes also found a significant reduction in composite CV events (HR, 0.65; P < .007), including MIs.24 A long-term, much larger RCT with acarbose is in progress.25
Following the demonstration of strikingly protective effects of metformin on CV events in the UKPDS,16 two major trials of thiazolidinediones (TZDs) investigated the effects of insulin sensitization on CV events.13,26 Pioglitazone in patients with long duration type 2 diabetes mellitus and pre-existing CV disease was reported to marginally reduce major CV outcomes (HR, 0.84; 95% CI, 0.72–98; P < .03),26 whereas rosiglitazone in patients with a shorter duration of diabetes was found to be noninferior to the control group.13 In both trials, however, there was a twofold increased risk for hospitalization for heart failure and increased risk for bone fractures in women,13,26 but without an increased risk for mortality.27 Furthermore, in the BARI 2D trial in patients with diabetes and established CV disease, adding rosiglitazone did not significantly reduce propensity-matched CV outcomes, compared with insulin secretagogues or insulin.28 Thus, while TZDs appear to have no major adverse effects on CV outcomes, the other associated adverse effects limit their use.
In a 1-year study of the efficacy and CV safety of the dopaminergic agent quick-release bromocriptine, an FDA-approved drug for diabetes, there was a marked decrease in incidence of composite CV end points (HR, 0.60; 95% CI, 0.37–0.96).29 However, there also was a 47% dropout rate and a small number of total events; thus, the implications remain inconclusive.
Incretin-mimetic agents and CV outcomes
Following the FDA guidance,14 all newer agents, including incretin-mimetic agents (dipeptidyl peptidase-4 [DPP-4] inhibitors and glucagon-like peptide-1 [GLP-1] receptor agonists) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been undergoing well-designed, long-term, noninferiority trials with the comparison group receiving the standard of diabetes and CV care. The goal of these trials, unlike that of most of the studies discussed in this article, was to investigate the safety of individual agents rather than different levels of glycemic control.
Since 2013, such trials with three of the available DPP-4 inhibitors have been completed (Table 2).30–34 Each trial was conducted in patients with pre-existing CV disease or high risk of it. The mean duration of follow-up in these trials was 1.5 to 3.0 years. There were significant, but only marginal, differences in HbA1c compared with the control groups receiving standard care. In each trial, the primary CV end points showed noninferiority, thus documenting their CV safety. One important difference in secondary end points was a significant increase in hospitalization rates for heart failure with saxagliptin31,33 that was not observed in the trials with alogliptin30,34 or sitagliptin.32 Another secondary end point—hospitalization for heart failure plus CV mortality—also was not increased in the alogliptin34 and sitagliptin32 trials (rates not reported for saxagliptin); however, there was no increase in total deaths from any cause in these trials.
The mechanisms underlying the increased rates of heart failure with saxagliptin are unclear. The baseline characteristics of patients in these three trials were similar (Table 2). Patients with type 2 diabetes have higher rates of heart failure in general, but the effects of concomitant drug therapy on risk of heart failure, other than with TZDs, have not been well studied. In an extensive meta-analysis of 84 RTCs of various durations, the overall risk (OR) of heart failure was higher in patients treated with DPP-4 inhibitors than in those treated with placebo or active comparators (OR, 1.19; 95% CI, 1.03–1.37; P = .015), suggesting that DPP-4 inhibitors as a class could be associated with an increased risk of heart failure.35 A case-control study, however, found no increase in rates of heart failure with DPP-4 inhibitors, although there were very few patients on saxagliptin.36 Yet another large retrospective, propensity-adjusted observational analysis of more than 112,000 patients, which compared those on saxagliptin and sitagliptin, reported no difference in rates of heart failure; however, the median follow-up period was less than 6 months.37
In comparative observational analyses,18,37,38 the risks of heart failure with TZDs and sulfonylureas were increased, compared with DPP-4 inhibitors, particularly with TZDs. On the other hand, a large population-based analysis from Italy found that DPP-4 inhibitors were associated with a propensity-matched 36% lower rate of hospitalization for heart failure compared with sulfonylureas.39 These data point to a need for more well-designed comparative studies to investigate valid differences between drugs in this class.
In the only GLP-1 receptor agonist trial completed thus far, ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome), there were no differences in primary and major secondary CV outcomes in 6,068 very high-risk patients randomized to lixisenatide or placebo after a 25-month follow-up (HR, 1.02; 95%, CI, 0.89–1.17).40 Moreover, the hospitalization rates for heart failure were not increased (HR, 0.96; 95% CI, 0.75–1.23). The earlier meta-analyses of short-term studies with DPP-4 inhibitors reporting significant reductions in CV events41,42 also underscore the need for well-designed long-term RCTs to accurately interpret drug effects.
SGLT-2 inhibitors and CV outcomes
The first CV outcome RCT with the SGLT-2 inhibitor empagliflozin, the EMPA-REG OUTCOME trial,43 was recently reported. Of great importance in this 7,020-patient trial comparing empagliflozin with placebo were the following results:
- 14% reduction in the primary end point (composite of death from CV causes, nonfatal MI, or nonfatal stroke) (P = .04)
- 32% reduction in all-cause deaths (P < .001)
- 35% reduction in hospitalization for heart failure (P = .002).
The mechanism underlying these impressive benefits is not known, although there were modest reductions in HbA1c levels, body weight (~2 kg), waist circumference (~2 cm), and systolic blood pressure (~4 mm Hg) with empagliflozin. The main adverse effects were related to a 3 to 4 times increased incidence of genital infections. Trials with other agents in this class are currently ongoing.
Insulin and CV outcomes
The UKPDS trial is the only primary prevention trial that provided evidence of significant benefits from intensive glucose control (with insulin, with or without sulfonylurea therapy) on CV outcomes and mortality, but only after 10 additional years of follow-up after the end of the trial.4 A few other trials have investigated the long-term effects of insulin compared with conventional therapy in patients with CV disease.
The DIGAMI-1 (Diabetes Insulin-Glucose in Acute Myocardial Infarction) was a RCT conducted between 1990 and 1993 in 620 patients with type 2 diabetes and acute MI randomized to short-term, intensive insulin-based glucose therapy or to conventional glucose-lowering therapy.44 Results showed the intensive treatment group had an 11% decrease in mortality rate at 3.4 years. A 20-year follow-up reassessment showed the overall survival was improved by a mean of 2.3 years at 8 years, particularly in those at lower risk at baseline.45 However, none of these patients were on statin therapy at baseline; thus, the implications of that study with current standards of care are quite uncertain. Subsequent studies—DIGAMI-2 (N = 1,253)46 and the HI-5 (Hyperglycemia: Intensive Insulin Infusion in Infarction) study (N = 240),47 both investigating the effects of intensive insulin therapy in patients with type 2 diabetes and MI—showed no significant effects on mortality in patients at 1 year (DIGAMI-2) and 6 months (HI-5).
The HEART2D trial (Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus), an RCT of 1,115 post-MI patients, investigated the effects of targeting prandial insulin compared with basal insulin. During a mean follow-up of 2.7 years, there were no between-group differences in CV outcomes (HR, 0.98; 95% CI, 0.8–1.21) or glycemic control.48 Also, there was no impact of glycemic variability.49 Finally, the ORIGIN trial (Outcome Reduction With an Initial Glargine Intervention), an RCT of more than 12,000 patients at high risk for CV disease but with relatively recent onset of either type 2 diabetes or prediabetes, randomized patients to basal insulin glargine or noninsulin treatments.50 The baseline HbA1c was relatively low at 6.4%, but it significantly declined by 0.3% by the end of trial, compared with the control group. There was no effect on CV outcomes (HR, 1.02; 95% CI, 0.94–1.11) after a median follow-up of 6.2 years.
However, it remains a perplexing question regarding whether long-term treatment with increasing insulin dosages in a subset of obese patients with poorly controlled type 2 diabetes and increasing insulin resistance could be potentially harmful to the CV system.51
CONCLUSION
The long-term RCTs with antihyperglycemic agents, including DCCT/EDIC in type 1 diabetes and UKPDS, ACCORD, and VADT in type 2 diabetes, with the exception of ADVANCE, have established the value of intensive glycemic control in reducing CV outcomes but only after many years of follow-up. However, the effects of intensive glycemic control on CV disease in type 2 diabetes are inconsistent, with only the primary prevention cohorts of UKPDS showing significant effects on mortality after prolonged follow-up. This is in contrast to the positive effects of statins in relatively short-term trials.
While it is difficult to interpret the CV results of specific drugs from the degree of glycemic control, it is reassuring that the large RCTs with several individual agents, including TZDs (both pioglitazone and rosiglitazone), several DPP-4 inhibitors, and one GLP-1 receptor agonist, have demonstrated no appreciable harm. The increase in the secondary outcome of heart failure but with no increase in mortality observed with saxagliptin requires further mechanistic studies while awaiting the results of other ongoing trials with newer agents including other incretin-based drugs and SGLT-2 inhibitors.
With SGLT-2 inhibitors, the recently published results of the empagliflozin trail (EMPA-REG OUTCOME trial) with type 2 diabetes revealed a significant reduction in CV end points and mortality. Before those data were published, metformin was the only antihyperglycemic drug that had shown a significant effect on CV events and mortality, but it was studied in only a small subgroup of the UKPDS cohort, and there are no RCTs of the relative impact of metformin or other agents as compared to sulfonylureas. The results of ongoing CV trials with SGLT-2 inhibitors are eagerly awaited.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313:45–53.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Gerstein HC, Miller ME, Ismail-Beigi F, et al. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet 2014; 384: 1936–1941.
- The ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Zoungas S, Chalmers J, Neal B, et al; ADAVNCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014; 371:1392–1406.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hayward RA, Reaven PD, Wiitala WL et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372:2197–2206.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009; 373:2125–2135.
- US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. December 2008. Available at: http://www.fda.gov/
downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed March 15, 2016. - UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010; 59:1038–1045.
- Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all-cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009; 339:b4731.
- Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 2012; 157:601–610.
- Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab 2012; 97:4605–4612.
- Li Y, Hu Y, Ley SH, Rajpathak S, Hu FB. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014; 37:3106–3113.
- The NAVIGATOR Study Group, Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362:1463–1476.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003; 290:486–494.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002; 359:2072–2077.
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004; 25:10–16.
- Holman RR, Bethel MA, Chan JC, et al; ACE Study Group. Rationale for the design of the Acarbose Cardiovascular Event (ACE) trial. Am Heart J 2014; 168:23–29. Epub ahead of print April 5, 2014. doi:10.1016/j.ahj.2014.03.021
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitazone clinical trial in macrovascular events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomized clinical trials. Lancet 2007; 370:1129–1136.
- Bach RG, Brooks MM, Lombardero M, et al; BARI 2D Investigators. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 2013; 128:785–794.
- Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- White WB, Cannon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Scirica BM, Braunwald E, Raz I, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014; 130:1579–1588.
- Zannad F, Cannon CP, Cushman WC, EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015; 385:2067–2076.
- Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014; 24:689–697.
- Yu OH, Filion KB, Azoulay L, Patenaude V, Majdan A, Suissa S. Incretin-based drugs and the risk of congestive heart failure. Diabetes Care 2015; 38:277–284.
- Fu AZ, Johnston SS, Ghannam A, et al. Association between hospitalization for heart failure and dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care 2016 [epub ahead of print].
- Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015; 385:2107–2117.
- Fadini GP, Avagaro A, Degli Esposti L, et al; OsMed Health-DB Network. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J 2015; 36:2454–2462.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–2257.
- Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol 2012; 110:826–833.
- Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013; 15:112–120.
- Zinamn B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Malmberg K, Ryden L, Efendic S, et al. Randomised trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57–65.
- Ritsinger V, Malmberg K, Martensson A, Ryden L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol 2014; 2:627–633.
- Malmberg K, Ryden L, Wedel H, et al; DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26:650–661.
- Cheung NW, Wong VW, McLean M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006; 29:765–770.
- Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009; 32:381–386.
- Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care 2011; 34:855–857.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Nolan CJ, Ruderman NB, Kahn SE, et al. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015; 64:673–686.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313:45–53.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Gerstein HC, Miller ME, Ismail-Beigi F, et al. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet 2014; 384: 1936–1941.
- The ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Zoungas S, Chalmers J, Neal B, et al; ADAVNCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014; 371:1392–1406.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hayward RA, Reaven PD, Wiitala WL et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372:2197–2206.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009; 373:2125–2135.
- US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. December 2008. Available at: http://www.fda.gov/
downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed March 15, 2016. - UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010; 59:1038–1045.
- Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all-cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009; 339:b4731.
- Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 2012; 157:601–610.
- Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab 2012; 97:4605–4612.
- Li Y, Hu Y, Ley SH, Rajpathak S, Hu FB. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014; 37:3106–3113.
- The NAVIGATOR Study Group, Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362:1463–1476.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003; 290:486–494.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002; 359:2072–2077.
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004; 25:10–16.
- Holman RR, Bethel MA, Chan JC, et al; ACE Study Group. Rationale for the design of the Acarbose Cardiovascular Event (ACE) trial. Am Heart J 2014; 168:23–29. Epub ahead of print April 5, 2014. doi:10.1016/j.ahj.2014.03.021
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitazone clinical trial in macrovascular events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomized clinical trials. Lancet 2007; 370:1129–1136.
- Bach RG, Brooks MM, Lombardero M, et al; BARI 2D Investigators. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 2013; 128:785–794.
- Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- White WB, Cannon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Scirica BM, Braunwald E, Raz I, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014; 130:1579–1588.
- Zannad F, Cannon CP, Cushman WC, EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015; 385:2067–2076.
- Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014; 24:689–697.
- Yu OH, Filion KB, Azoulay L, Patenaude V, Majdan A, Suissa S. Incretin-based drugs and the risk of congestive heart failure. Diabetes Care 2015; 38:277–284.
- Fu AZ, Johnston SS, Ghannam A, et al. Association between hospitalization for heart failure and dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care 2016 [epub ahead of print].
- Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015; 385:2107–2117.
- Fadini GP, Avagaro A, Degli Esposti L, et al; OsMed Health-DB Network. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J 2015; 36:2454–2462.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–2257.
- Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol 2012; 110:826–833.
- Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013; 15:112–120.
- Zinamn B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Malmberg K, Ryden L, Efendic S, et al. Randomised trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57–65.
- Ritsinger V, Malmberg K, Martensson A, Ryden L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol 2014; 2:627–633.
- Malmberg K, Ryden L, Wedel H, et al; DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26:650–661.
- Cheung NW, Wong VW, McLean M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006; 29:765–770.
- Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009; 32:381–386.
- Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care 2011; 34:855–857.
- ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367:319–328.
- Nolan CJ, Ruderman NB, Kahn SE, et al. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015; 64:673–686.
KEY POINTS
- Long-term randomized controlled trials have established the value of intensive glycemic control in reducing CV outcomes in patients with type 2 but only after many years of follow-up.
- Despite reductions in ischemic coronary events, some clinical trials have reported unexplained increases in CV-associated mortality and total deaths in patients receiving intensive glycemic control.
- Trials reporting the impact of specific glucose-lowering agents on CV events have reported perplexing, sometimes contradictory results, underscoring the need for additional trials.