User login
Unusual Presentation of Ectopic Extramammary Paget Disease
Extramammary Paget disease (EMPD) is a malignant epithelial tumor that most commonly affects the anogenital region and less frequently arises in the axillae. Most cases occur in locations where apocrine glands predominate.1 Few cases of EMPD arising in nonapocrine-bearing regions, or ectopic EMPD, have been reported.2 We describe a case of primary ectopic EMPD with an infiltrative growth pattern arising on the back of a 67-year-old Thai man.
Case Report
A 67-year-old Thai man presented to the dermatology clinic for evaluation of a persistent rash on the right lower back of approximately 30 years’ duration. He reported that the eruption had started out as a small coin-shaped area but had slowly increased in size. Over the last 2 years, the area had grown more rapidly and became pruritic. His medical history was remarkable for hypertension treated with losartan, but he was otherwise healthy. He had no history of cancer or gastrointestinal tract or genitourinary symptoms, and he had no recent fever, weight loss, or night sweats.
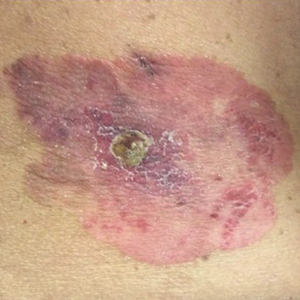
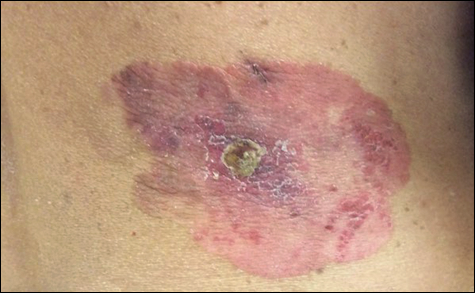
On physical examination a well-demarcated, asymmetric, erythematous to brown plaque was noted on the right lower back. The plaque was surfaced by scale and contained a central hyperkeratotic papule (Figure 1). The skin examination was otherwise unremarkable. The patient had no lymphadenopathy.
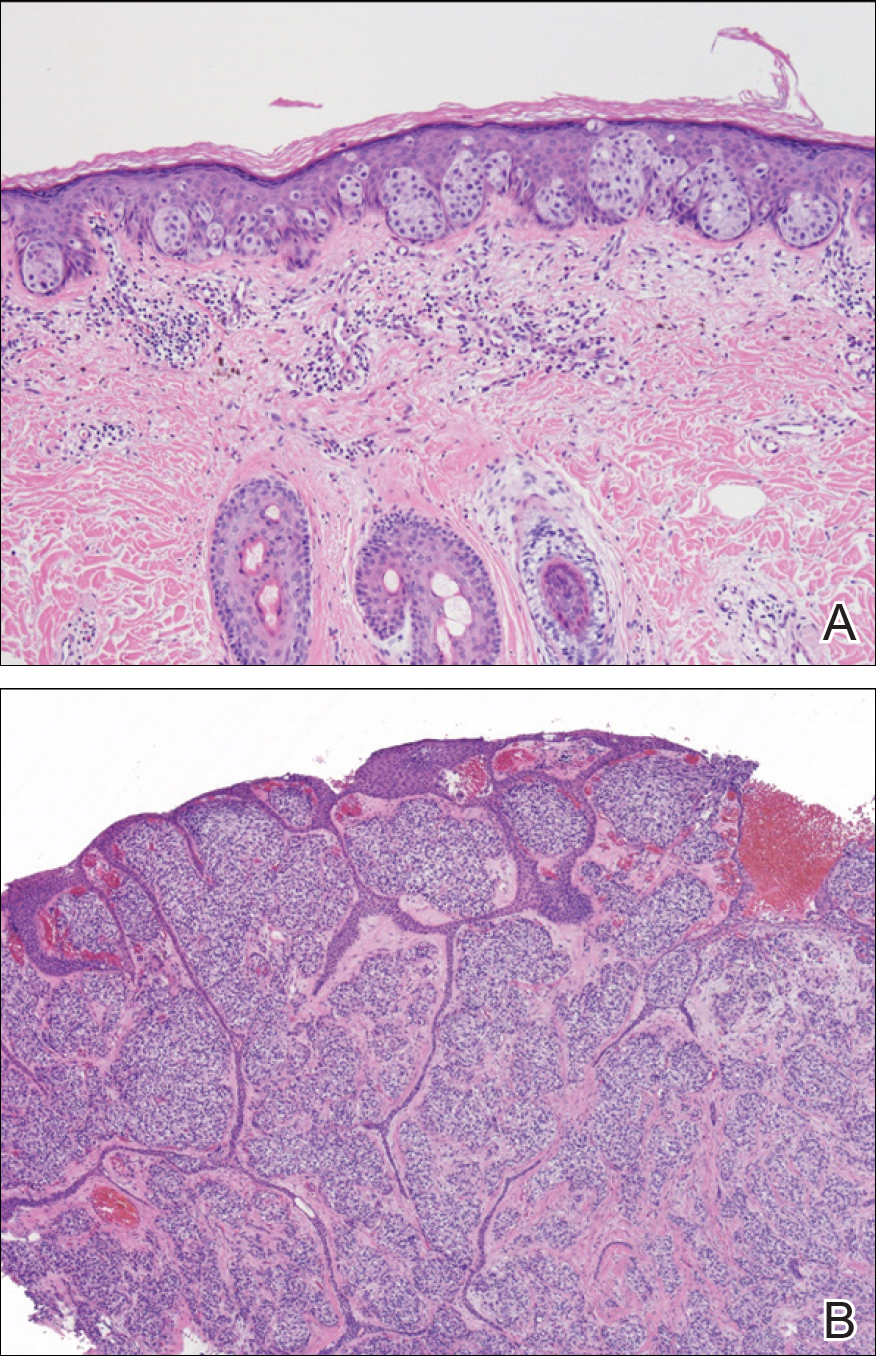
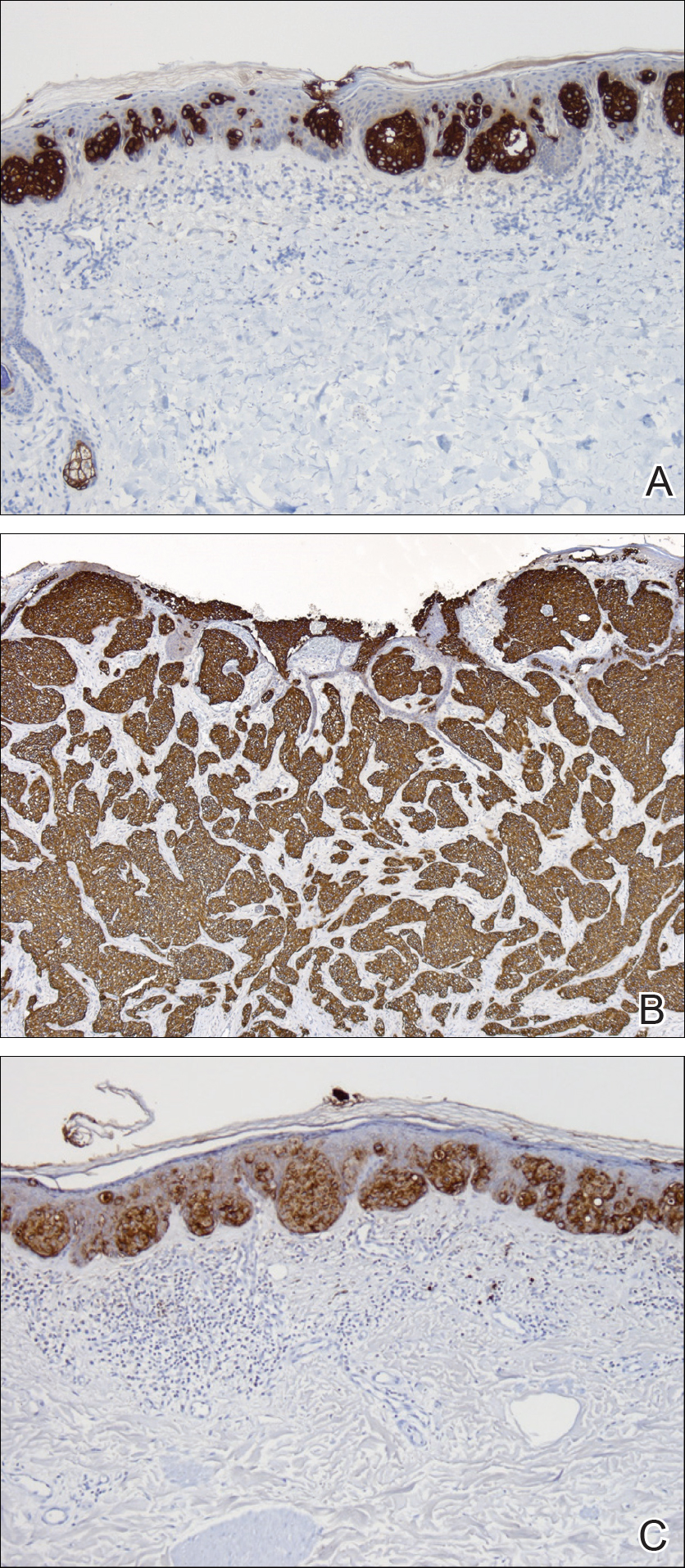
Two punch biopsies were performed. On low power, acanthosis and hyperkeratosis of the epidermis were noted. The epidermis contained a proliferation of large (tumor) cells with pleomorphic nuclei, prominent nucleoli, and abundant pale to clear cytoplasm. The cells were present singularly as well as in clusters and were most prominent along the basal layer but many were also seen extending to more superficial levels of the epidermis (Figure 2A). In one biopsy, the tumor cells were found in the dermis with an infiltrative growth pattern (Figure 2B). Immunohistochemistry (IHC) studies for cytokeratin 7 (Figure 3A and 3B) and carcinoembryonic antigen (Figure 3C) labeled the tumor cells. An IHC study for gross cystic disease fluid protein 15 labeled some of the tumor cells. Immunohistochemistry studies for S-100, human melanoma black 45 (HMB-45), p16, and renal cell carcinoma did not label the tumor cells. An IHC study for MIB-1 labeled many of the tumor cells, indicating a notably increased mitotic index. The patient was diagnosed with ectopic EMPD. He underwent an endoscopy, colonoscopy, and cystoscopy, all of which were normal.
Comment
Extramammary Paget disease is a malignant tumor typically found in apocrine-rich areas of the skin, particularly the anogenital skin. It is categorized as primary or secondary EMPD. Primary EMPD arises as an intraepithelial adenocarcinoma, with the Toker cell as the cell of origin.3 Secondary EMPD represents a cutaneous extension of an underlying malignancy (eg, colorectal, urothelial, prostatic, gynecologic).4
Ectopic EMPD arises in nonapocrine-bearing areas, specifically the nongerminative milk line. A review of the literature using Google Scholar and the search term ectopic extramammary Paget disease showed that there have been at least 30 cases of ectopic EMPD reported. Older men are more commonly affected, with a mean age at diagnosis of approximately 68 years. Although the tumor is most commonly seen on the trunk, cases on the head, arms, and legs have been reported.5
This tumor is most frequently seen in Asian individuals, as in our patient, with a ratio of approximately 3:1.5 Interestingly, triple or quadruple EMPD was reported in 68 Japanese patients but rarely has been reported outside of Japan.6 It is thought that some germinative apocrine-differentiating cells might preferentially exist on the trunk of Asians, leading to an increased incidence of EMPD in this population5; however, the exact reason for this racial preference is not completely understood, and more studies are needed to investigate this association.
Diagnosis of ectopic EMPD is made histologically. Tumor cells have abundant pale cytoplasm and large pleomorphic nuclei with prominent nucleoli. The cells are arranged in small groups or singly within the basal regions of the epidermis. In longstanding lesions, the entire thickness of the epidermis may be involved. Uncommonly, the tumor cells may invade the dermis, such as in our patient. On immunohistochemistry, the tumor cells stain positive for carcinoembryonic antigen, epithelial membrane antigen, and low-molecular-weight cytokeratins (eg, cytokeratin 7). Many of the tumor cells also express gross cystic disease fluid protein 15, which helps exclude cutaneous invasion of secondary EMPD.7-9 Cases of primary cutaneous apocrine carcinoma can have similar histologic and immunohistochemical findings to invasive EMPD, which further supports the possible apocrine derivation of Paget disease. In our patient, we considered the diagnosis of primary cutaneous apocrine adenocarcinoma with epidermotropism; however, we favored the diagnosis of ectopic EMPD with dermal invasion given the extensive epidermal-only involvement seen in one of the biopsies, which would be unusual for primary cutaneous apocrine adenocarcinoma.
Our patient had no identified underlying malignancy upon further workup; however, many cases of EMPD have been associated with an underlying malignancy.9-15 Several authors have reported a range of underlying malignancies associated with EMPD, with the incidence ranging from 11% to 45%.9-15 The location of the underlying internal malignancy appears to be closely related to the location of the EMPD.11 It is recommended that a thorough workup for internal malignancies be performed, including a full skin examination, lymph node examination, colonoscopy, cystoscopy, and gynecologic/prostate examination, among others.
No known differences in the prognosis or associated underlying malignancies between ectopic and ordinary EMPD have been reported; however, it has been noted that EMPD with invasion into the dermis does correlate with a more aggressive course and worse prognosis.8 Treatment includes surgical removal by Mohs micrographic surgery or wide local excision. Long-term follow-up is required since recurrences can be frequent.11-15
- Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget’s disease—evidence for an apocrine origin: an immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984;8:43-50.
- Saida T, Iwata M. “Ectopic” extramammary Paget’s disease affecting the lower anterior aspect of the chest. J Am Acad Dermatol. 1987;17(5, pt 2):910-913.
- Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease.J Clin Pathol. 2000;53:742-749.
- Sawada Y, Bito T, Kabashima R, et al. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502-505.
- Abe S, Kabashima K, Nishio D, et al. Quadruple extramammary Paget’s disease. Acta Derm Venereol. 2007;87:80-81.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Shepherd V, Davidson EJ, Davies‐Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009-1014.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73-78.
- Fanning J, Lambert HC, Hale TM, et al. Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24-27.
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183-189.
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol. 1997;4:475-480.
Extramammary Paget disease (EMPD) is a malignant epithelial tumor that most commonly affects the anogenital region and less frequently arises in the axillae. Most cases occur in locations where apocrine glands predominate.1 Few cases of EMPD arising in nonapocrine-bearing regions, or ectopic EMPD, have been reported.2 We describe a case of primary ectopic EMPD with an infiltrative growth pattern arising on the back of a 67-year-old Thai man.
Case Report
A 67-year-old Thai man presented to the dermatology clinic for evaluation of a persistent rash on the right lower back of approximately 30 years’ duration. He reported that the eruption had started out as a small coin-shaped area but had slowly increased in size. Over the last 2 years, the area had grown more rapidly and became pruritic. His medical history was remarkable for hypertension treated with losartan, but he was otherwise healthy. He had no history of cancer or gastrointestinal tract or genitourinary symptoms, and he had no recent fever, weight loss, or night sweats.
On physical examination a well-demarcated, asymmetric, erythematous to brown plaque was noted on the right lower back. The plaque was surfaced by scale and contained a central hyperkeratotic papule (Figure 1). The skin examination was otherwise unremarkable. The patient had no lymphadenopathy.

Two punch biopsies were performed. On low power, acanthosis and hyperkeratosis of the epidermis were noted. The epidermis contained a proliferation of large (tumor) cells with pleomorphic nuclei, prominent nucleoli, and abundant pale to clear cytoplasm. The cells were present singularly as well as in clusters and were most prominent along the basal layer but many were also seen extending to more superficial levels of the epidermis (Figure 2A). In one biopsy, the tumor cells were found in the dermis with an infiltrative growth pattern (Figure 2B). Immunohistochemistry (IHC) studies for cytokeratin 7 (Figure 3A and 3B) and carcinoembryonic antigen (Figure 3C) labeled the tumor cells. An IHC study for gross cystic disease fluid protein 15 labeled some of the tumor cells. Immunohistochemistry studies for S-100, human melanoma black 45 (HMB-45), p16, and renal cell carcinoma did not label the tumor cells. An IHC study for MIB-1 labeled many of the tumor cells, indicating a notably increased mitotic index. The patient was diagnosed with ectopic EMPD. He underwent an endoscopy, colonoscopy, and cystoscopy, all of which were normal.


Comment
Extramammary Paget disease is a malignant tumor typically found in apocrine-rich areas of the skin, particularly the anogenital skin. It is categorized as primary or secondary EMPD. Primary EMPD arises as an intraepithelial adenocarcinoma, with the Toker cell as the cell of origin.3 Secondary EMPD represents a cutaneous extension of an underlying malignancy (eg, colorectal, urothelial, prostatic, gynecologic).4
Ectopic EMPD arises in nonapocrine-bearing areas, specifically the nongerminative milk line. A review of the literature using Google Scholar and the search term ectopic extramammary Paget disease showed that there have been at least 30 cases of ectopic EMPD reported. Older men are more commonly affected, with a mean age at diagnosis of approximately 68 years. Although the tumor is most commonly seen on the trunk, cases on the head, arms, and legs have been reported.5
This tumor is most frequently seen in Asian individuals, as in our patient, with a ratio of approximately 3:1.5 Interestingly, triple or quadruple EMPD was reported in 68 Japanese patients but rarely has been reported outside of Japan.6 It is thought that some germinative apocrine-differentiating cells might preferentially exist on the trunk of Asians, leading to an increased incidence of EMPD in this population5; however, the exact reason for this racial preference is not completely understood, and more studies are needed to investigate this association.
Diagnosis of ectopic EMPD is made histologically. Tumor cells have abundant pale cytoplasm and large pleomorphic nuclei with prominent nucleoli. The cells are arranged in small groups or singly within the basal regions of the epidermis. In longstanding lesions, the entire thickness of the epidermis may be involved. Uncommonly, the tumor cells may invade the dermis, such as in our patient. On immunohistochemistry, the tumor cells stain positive for carcinoembryonic antigen, epithelial membrane antigen, and low-molecular-weight cytokeratins (eg, cytokeratin 7). Many of the tumor cells also express gross cystic disease fluid protein 15, which helps exclude cutaneous invasion of secondary EMPD.7-9 Cases of primary cutaneous apocrine carcinoma can have similar histologic and immunohistochemical findings to invasive EMPD, which further supports the possible apocrine derivation of Paget disease. In our patient, we considered the diagnosis of primary cutaneous apocrine adenocarcinoma with epidermotropism; however, we favored the diagnosis of ectopic EMPD with dermal invasion given the extensive epidermal-only involvement seen in one of the biopsies, which would be unusual for primary cutaneous apocrine adenocarcinoma.
Our patient had no identified underlying malignancy upon further workup; however, many cases of EMPD have been associated with an underlying malignancy.9-15 Several authors have reported a range of underlying malignancies associated with EMPD, with the incidence ranging from 11% to 45%.9-15 The location of the underlying internal malignancy appears to be closely related to the location of the EMPD.11 It is recommended that a thorough workup for internal malignancies be performed, including a full skin examination, lymph node examination, colonoscopy, cystoscopy, and gynecologic/prostate examination, among others.
No known differences in the prognosis or associated underlying malignancies between ectopic and ordinary EMPD have been reported; however, it has been noted that EMPD with invasion into the dermis does correlate with a more aggressive course and worse prognosis.8 Treatment includes surgical removal by Mohs micrographic surgery or wide local excision. Long-term follow-up is required since recurrences can be frequent.11-15
Extramammary Paget disease (EMPD) is a malignant epithelial tumor that most commonly affects the anogenital region and less frequently arises in the axillae. Most cases occur in locations where apocrine glands predominate.1 Few cases of EMPD arising in nonapocrine-bearing regions, or ectopic EMPD, have been reported.2 We describe a case of primary ectopic EMPD with an infiltrative growth pattern arising on the back of a 67-year-old Thai man.
Case Report
A 67-year-old Thai man presented to the dermatology clinic for evaluation of a persistent rash on the right lower back of approximately 30 years’ duration. He reported that the eruption had started out as a small coin-shaped area but had slowly increased in size. Over the last 2 years, the area had grown more rapidly and became pruritic. His medical history was remarkable for hypertension treated with losartan, but he was otherwise healthy. He had no history of cancer or gastrointestinal tract or genitourinary symptoms, and he had no recent fever, weight loss, or night sweats.
On physical examination a well-demarcated, asymmetric, erythematous to brown plaque was noted on the right lower back. The plaque was surfaced by scale and contained a central hyperkeratotic papule (Figure 1). The skin examination was otherwise unremarkable. The patient had no lymphadenopathy.

Two punch biopsies were performed. On low power, acanthosis and hyperkeratosis of the epidermis were noted. The epidermis contained a proliferation of large (tumor) cells with pleomorphic nuclei, prominent nucleoli, and abundant pale to clear cytoplasm. The cells were present singularly as well as in clusters and were most prominent along the basal layer but many were also seen extending to more superficial levels of the epidermis (Figure 2A). In one biopsy, the tumor cells were found in the dermis with an infiltrative growth pattern (Figure 2B). Immunohistochemistry (IHC) studies for cytokeratin 7 (Figure 3A and 3B) and carcinoembryonic antigen (Figure 3C) labeled the tumor cells. An IHC study for gross cystic disease fluid protein 15 labeled some of the tumor cells. Immunohistochemistry studies for S-100, human melanoma black 45 (HMB-45), p16, and renal cell carcinoma did not label the tumor cells. An IHC study for MIB-1 labeled many of the tumor cells, indicating a notably increased mitotic index. The patient was diagnosed with ectopic EMPD. He underwent an endoscopy, colonoscopy, and cystoscopy, all of which were normal.


Comment
Extramammary Paget disease is a malignant tumor typically found in apocrine-rich areas of the skin, particularly the anogenital skin. It is categorized as primary or secondary EMPD. Primary EMPD arises as an intraepithelial adenocarcinoma, with the Toker cell as the cell of origin.3 Secondary EMPD represents a cutaneous extension of an underlying malignancy (eg, colorectal, urothelial, prostatic, gynecologic).4
Ectopic EMPD arises in nonapocrine-bearing areas, specifically the nongerminative milk line. A review of the literature using Google Scholar and the search term ectopic extramammary Paget disease showed that there have been at least 30 cases of ectopic EMPD reported. Older men are more commonly affected, with a mean age at diagnosis of approximately 68 years. Although the tumor is most commonly seen on the trunk, cases on the head, arms, and legs have been reported.5
This tumor is most frequently seen in Asian individuals, as in our patient, with a ratio of approximately 3:1.5 Interestingly, triple or quadruple EMPD was reported in 68 Japanese patients but rarely has been reported outside of Japan.6 It is thought that some germinative apocrine-differentiating cells might preferentially exist on the trunk of Asians, leading to an increased incidence of EMPD in this population5; however, the exact reason for this racial preference is not completely understood, and more studies are needed to investigate this association.
Diagnosis of ectopic EMPD is made histologically. Tumor cells have abundant pale cytoplasm and large pleomorphic nuclei with prominent nucleoli. The cells are arranged in small groups or singly within the basal regions of the epidermis. In longstanding lesions, the entire thickness of the epidermis may be involved. Uncommonly, the tumor cells may invade the dermis, such as in our patient. On immunohistochemistry, the tumor cells stain positive for carcinoembryonic antigen, epithelial membrane antigen, and low-molecular-weight cytokeratins (eg, cytokeratin 7). Many of the tumor cells also express gross cystic disease fluid protein 15, which helps exclude cutaneous invasion of secondary EMPD.7-9 Cases of primary cutaneous apocrine carcinoma can have similar histologic and immunohistochemical findings to invasive EMPD, which further supports the possible apocrine derivation of Paget disease. In our patient, we considered the diagnosis of primary cutaneous apocrine adenocarcinoma with epidermotropism; however, we favored the diagnosis of ectopic EMPD with dermal invasion given the extensive epidermal-only involvement seen in one of the biopsies, which would be unusual for primary cutaneous apocrine adenocarcinoma.
Our patient had no identified underlying malignancy upon further workup; however, many cases of EMPD have been associated with an underlying malignancy.9-15 Several authors have reported a range of underlying malignancies associated with EMPD, with the incidence ranging from 11% to 45%.9-15 The location of the underlying internal malignancy appears to be closely related to the location of the EMPD.11 It is recommended that a thorough workup for internal malignancies be performed, including a full skin examination, lymph node examination, colonoscopy, cystoscopy, and gynecologic/prostate examination, among others.
No known differences in the prognosis or associated underlying malignancies between ectopic and ordinary EMPD have been reported; however, it has been noted that EMPD with invasion into the dermis does correlate with a more aggressive course and worse prognosis.8 Treatment includes surgical removal by Mohs micrographic surgery or wide local excision. Long-term follow-up is required since recurrences can be frequent.11-15
- Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget’s disease—evidence for an apocrine origin: an immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984;8:43-50.
- Saida T, Iwata M. “Ectopic” extramammary Paget’s disease affecting the lower anterior aspect of the chest. J Am Acad Dermatol. 1987;17(5, pt 2):910-913.
- Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease.J Clin Pathol. 2000;53:742-749.
- Sawada Y, Bito T, Kabashima R, et al. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502-505.
- Abe S, Kabashima K, Nishio D, et al. Quadruple extramammary Paget’s disease. Acta Derm Venereol. 2007;87:80-81.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Shepherd V, Davidson EJ, Davies‐Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009-1014.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73-78.
- Fanning J, Lambert HC, Hale TM, et al. Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24-27.
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183-189.
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol. 1997;4:475-480.
- Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget’s disease—evidence for an apocrine origin: an immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984;8:43-50.
- Saida T, Iwata M. “Ectopic” extramammary Paget’s disease affecting the lower anterior aspect of the chest. J Am Acad Dermatol. 1987;17(5, pt 2):910-913.
- Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease.J Clin Pathol. 2000;53:742-749.
- Sawada Y, Bito T, Kabashima R, et al. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502-505.
- Abe S, Kabashima K, Nishio D, et al. Quadruple extramammary Paget’s disease. Acta Derm Venereol. 2007;87:80-81.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Shepherd V, Davidson EJ, Davies‐Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009-1014.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73-78.
- Fanning J, Lambert HC, Hale TM, et al. Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24-27.
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183-189.
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol. 1997;4:475-480.
Practice Points
- Ectopic extramammary Paget disease (EMPD) is a rare presentation of EMPD that is histologically identical to EMPD.
- Ectopic EMPD can be associated with underlying malignancy and therefore warrants a thorough workup.