User login
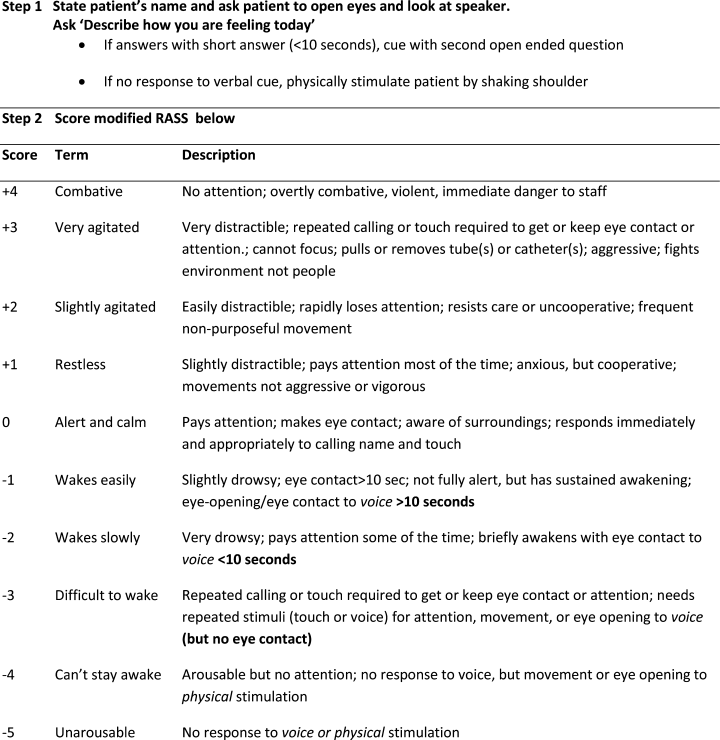
Modified RASS for Identifying Delirium
Vital signs constitute a fundamental component of the physical examination and serve key diagnostic and monitoring purposes. The brain is as vital to life as the cardiovascular, respiratory, and immune/thermoregulatory systems, yet currently no vital sign exists that would allow rapid, reliable, and easily reproducible assessment of cognition.1 As a result, acute mental status changes frequently go undetected and untreated.24 Delirium is defined as an acute change in attention with fluctuations in cognition, thought, and/or consciousness throughout the course of the day.5 Because delirium in older patients is common and is associated with increased morbidity, mortality, functional decline, and costs,69 development and validation of a rapid, objective screening assessment could be used by nursing staff to identify patients at high risk for delirium.
Current recommendations for inpatient delirium monitoring usually involve daily cognitive screening with a standardized screening instrument.6 Because this process is often time‐consuming (8‐12 minutes), most patients do not undergo routine screening. To facilitate clinical implementation, we focused on developing a brief (<30‐second) inpatient screening measure of a feature of mental status that could be administered serially. The purpose of this study was to (1) develop a brief screening tool for a core feature of mental status and (2) validate this screening tool for delirium in an older inpatient population.
METHODS
Consensus Panel
In June 2009, the Veterans Administration sponsored an interdisciplinary conference that solicited input on identifying the most targetable components of delirium and discussing potential clinical instruments. Following this, a consensus panel of 8 representatives from medicine, geriatrics, nursing, psychiatry, and psychology used a modified Delphi method to target characteristic features of delirium and identify instruments that could best capture mental status change. While inattention was agreed upon as the core cognitive feature of delirium, the group came to consensus that capturing the acute onset and fluctuating nature of delirium was better suited as a vital sign. To meet these criteria, the group modified the Richmond Agitation Sedation Scale (RASS).10
The RASS is an observational instrument that has been validated in the intesive care unit setting for objectively determining level of sedation. It has been shown to be highly reliable and associated with delirium.11 The RASS is a quick, objective scale of consciousness with a scoring system that captures both hyperactive and hypoactive levels of consciousness. A disadvantage of using the RASS includes its limited attention assessment. The Consensus panel modified the RASS to improve its assessment of attention, using a brief open‐ended question that was asked before scoring (Figure 1).
Participants
For this prospective validation study, we recruited 95 medical patients 65 years of age who had been admitted to a VA hospital. The study was approved by the institutional review board, and participants provided written informed consent. Patients were excluded if they refused (n = 64), anticipated leaving the hospital within 1 day (n = 42), or had vision or cognition impairments that would prevent their ability to complete informed consent forms and cognitive screening tools (n = 19). Five participants were discharged between enrollment and expert assessment.
Mental Status Assessments
After enrollment, 3 study staff members visited each participant independently. First, the trained research assistant obtained informed consent and demographic, cognitive, and functional assessments. The mini‐mental state examination was then administered to provide a baseline measure of cognitive function at the time of admission.12 A nurse‐interviewer later administered the modified RASS (mRASS) separately. Finally, a delirium expert performed an independent comprehensive mental status interview including assessments of attention, executive function, memory, and mood. Delirium was diagnosed by the delirium expert according to DSM‐IV criteria.5 Each investigator was blinded to the others' ratings. After the initial assessments, each participant was visited daily throughout the hospitalization by an mRASS assessor and, independently, by the delirium expert.
To determine inter‐rater reliability, 60 participants were evaluated with the mRASS by the trained research assistant and the nurse‐interviewer simultaneously. The mRASS was scored independently and the assessors were blinded to each others' ratings.
Statistics
The paired mRASS‐delirium assessments were analyzed in 3 ways: (1) as single‐day independent assessments; (2) longitudinally as a change from baseline including prevalent delirium; and (3) longitudinally as a change from baseline, excluding prevalent delirium cases. We examined 1‐point and 2‐point changes on the mRASS from baseline, which allowed determination of the most appropriate cut‐point for clinical use. Sensitivity, specificity, and likelihood ratios were calculated. The C‐statistic was calculated using absolute mRASS score for the single‐time assessments, and as a difference between minimum and maximum mRASS for the longitudinal analyses.
RESULTS
Characteristics of the study population are presented in Table 1. Because this was a VA population, the vast majority (94%) of participants were men, with a mean age of 81 years (range, 66‐96 years), and 89% were white. This population had a high Charlson Comorbidity Index (mean SD, 4.0 2.4), which was reflected in functional assessments, with 37% reporting difficulty with activities of daily living and 58% reporting difficulty with instrumental activities of daily living. Despite the age and comorbidity, delirium prevalence was 11% (n = 10) and incidence was 14% (n = 13). Interrater reliability of the mRASS yielded 98% agreement with a weighted kappa of 0.48 (P < 0.001).
| Characteristics | Values |
|---|---|
| |
| Age, years, mean (SD) | 81.0 (7.3) |
| Gender, male, no. (%) | 89 (94) |
| Race, white, no. (%) | 85 (89) |
| Charlson Comorbidity Index, mean (SD) | 4.0 (2.4) |
| BMI, kg/m2, mean (SD) | 27.2 (6.3) |
| Mini‐mental state examination, mean (SD) | 24.4 (4.1) |
| AUDIT, mean (SD) | 2.4 (2.9) |
| Tobacco use, pack‐years, no. (%) | 54 (56) |
| Current | 8 (8) |
| Never | 16 (17) |
| Prior | 68 (72) |
| Functional impairment, no. (%) | |
| Difficulty with 1 ADL | 35 (37) |
| Difficulty with 1 IADL | 55 (58) |
| Length of hospital stay | |
| Mean (SD), days | 6.3 (5.4) |
| Median, days | 5 |
| mRASS per patient, mean (SD) | 3.8 (3.3) |
When the mRASS was analyzed as a single‐day independent assessment, any abnormal score (ie, a score 0) had a sensitivity of 64% and a specificity of 93% for delirium relative to the expert evaluation (Table 2). With an abnormal mRASS as 2 or 2, the sensitivity fell to 34%, while the specificity increased to 99.6%.
| Criteria | mRASS | Sensitivity* (95% CI) | Specificity* (95% CI) | LR+ | LR |
|---|---|---|---|---|---|
| |||||
| Single‐day independent assessments | |||||
| Any abnormal | 63.9% (51.976.0) | 93.2% (90.396.4) | 9.4 | 0.4 | |
| RASS 2 or 2 | 34.4% (22.546.3) | 99.6% (98.8100) | 86 | 0.7 | |
| Longitudinal assessments | |||||
| Any delirium | Any change | 73.9% (56.091.9) | 91.7% (85.398.1) | 8.9 | 0.3 |
| Change in 2 points | 21.7% | 100% | 0.8 | ||
| Incident delirium | Any change | 84.6% (65.0100.0) | 91.7% (85.398.1) | 10.2 | 0.2 |
| Change in 2 points | 23.1% | 100% | 0.8 | ||
When the mRASS was used longitudinally to detect change in delirium during the hospital stay among all participants, it had a sensitivity of 74% and specificity of 92% for any change. Increasing the stringency of the criteria by looking at a change of 2 mRASS points decreased the sensitivity (22%) and increased the specificity (100%).
To capture the potential of the mRASS administered on a longitudinal basis as a diagnostic aid, the prevalent cases of delirium were excluded. In this analysis, any change in the mRASS had a sensitivity of 85% and a specificity of 92% for incident delirium. With more stringent criteria of a change of 2 points, the sensitivity was 23% and the specificity was 100%.
DISCUSSION
In this study, we developed a modified RASS (mRASS) for serial mental status assessment. Whereas a single measurement of the mRASS had modest sensitivity and good specificity for delirium, longitudinal measurement increased the sensitivity with no loss in specificity. Importantly, the <30 seconds required for the mRASS could be incorporated into daily workflow and provides an objective measure of consciousness. As such, we believe the mRASS can potentially serve as a longitudinal measure of consciousnessmuch like a vital sign for mental status.
Altered consciousness is a clinical and diagnostic feature of delirium,5, 13 and fluctuation in mental status is a diagnostic feature of delirium. As such, a screening instrument able to quantify the level of consciousness longitudinally and allow comparison to prior and subsequent determinations has face validity as a delirium screening instrument.
The mRASS has other features that make it appropriate for serial measurement in a manner similar to a vital sign. First, it objectively described consciousness on a scale, which is an improvement relative to many of the subjective descriptions clinically used. Consistent with other studies of the RASS,10, 11 the mRASS has good interrater reliability, allowing a common language to be used to describe level of consciousness across health care settings that can become the basis for a systematic and standardized monitor of cognitive change, improving continuity of care and communication between providers. It can be further used to objectively establish a patient's baseline and monitor change longitudinally.
The current study is limited by the lack of diversity and small size of the study population, which limits external validity (generalizability). Additional studies evaluating the utility of the mRASS by a variety of health care team members in a larger, more ethnically/racially diverse and heterogeneous population should be completed before we can determine if it can perform as a mental status vital sign, and if it is associated with better patient outcomes. Additionally, this study selected patients who were physically and cognitively capable of enrolling and excluded patients with severe cognitive and sensory impairment who were unable to provide consent to participate. Thus, some of the sickest, frailest, and most cognitively impaired patients were excluded. Unfortunately, this study therefore excluded a population significantly more vulnerable to the development of delirium.
Because a change in mental status (such as delirium) is common, morbid, and costly, a brief tool that can reliably and effectively assess mental status is needed. The mRASS used in this study provided an objective measurement of consciousness, a key component of mental status, and was demonstrated to reliably screen for presence or absence of delirium when administered longitudinally. Further study in diverse populations with administration by a variety of health care team members is needed to determine whether the mRASS can accurately serve as a mental status vital sign. If adopted widely, the mRASS could be used alongside the traditional vital signs to establish patient baselines, monitor change, improve provider communication, and potentially improve patient outcomes.
Acknowledgements
The authors are indebted to all of the veterans who willingly participated in this project. The VA Delirium Working Group Consensus Panel Consisted of Kenneth Boockvar, Joseph Flaherty, Sharon Gordon, Barbara Kamholz, James Rudolph, Marianne Shaughnessy, Kenneth Shay, and Joan Stein.
The authors maintained independence in the development, execution, and reporting of this study.
This article was presented in abstract form at the American Geriatrics Society Annual Meeting, May 12, 2011.
Funding: Jennifer G. Chester was funded by an Einstein Research Fellowship. James L. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Additional support was provided by the American Federation for Aging Research, the Boston MSTAR, and National Institutes of Health grants AG 026781‐05 and AG 038027. James L. Rudolph and Mary Beth Harrington and the VA Delirium Working Group Consensus Panel are VA employees. The authors have no additional disclosures to report.
- ,.Vital signs in older patients: age‐related changes.J Am Med Dir Assoc.2011;12:337–343.
- ,,.Acute confusional states (delirium) in the hospitalized elderly.Annu Rev Gerontol Geriatr.1986;6:1–26.
- ,,,,.Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients.J Am Geriatr Soc.1991;39:760–765.
- ,,,,.Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings.Arch Intern Med.2001;161:2467–2473.
- Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.One‐year health care costs associated with delirium in the elderly population.Arch Intern Med.2008;168:27–32.
- ,,,,.Delirium predicts 12‐month mortality.Arch Intern Med.2002;162:457–463.
- ,,, et al.Delirium: an independent predictor of functional decline after cardiac surgery.J Am Geriatr Soc.2010;58:643–649.
- ,,, et al.The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients.Am J Respir Crit Care Med.2002;166:1338–1344.
- ,,, et al.Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS).JAMA.2003;289:2983–2991.
- ,,.“Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
Vital signs constitute a fundamental component of the physical examination and serve key diagnostic and monitoring purposes. The brain is as vital to life as the cardiovascular, respiratory, and immune/thermoregulatory systems, yet currently no vital sign exists that would allow rapid, reliable, and easily reproducible assessment of cognition.1 As a result, acute mental status changes frequently go undetected and untreated.24 Delirium is defined as an acute change in attention with fluctuations in cognition, thought, and/or consciousness throughout the course of the day.5 Because delirium in older patients is common and is associated with increased morbidity, mortality, functional decline, and costs,69 development and validation of a rapid, objective screening assessment could be used by nursing staff to identify patients at high risk for delirium.
Current recommendations for inpatient delirium monitoring usually involve daily cognitive screening with a standardized screening instrument.6 Because this process is often time‐consuming (8‐12 minutes), most patients do not undergo routine screening. To facilitate clinical implementation, we focused on developing a brief (<30‐second) inpatient screening measure of a feature of mental status that could be administered serially. The purpose of this study was to (1) develop a brief screening tool for a core feature of mental status and (2) validate this screening tool for delirium in an older inpatient population.
METHODS
Consensus Panel
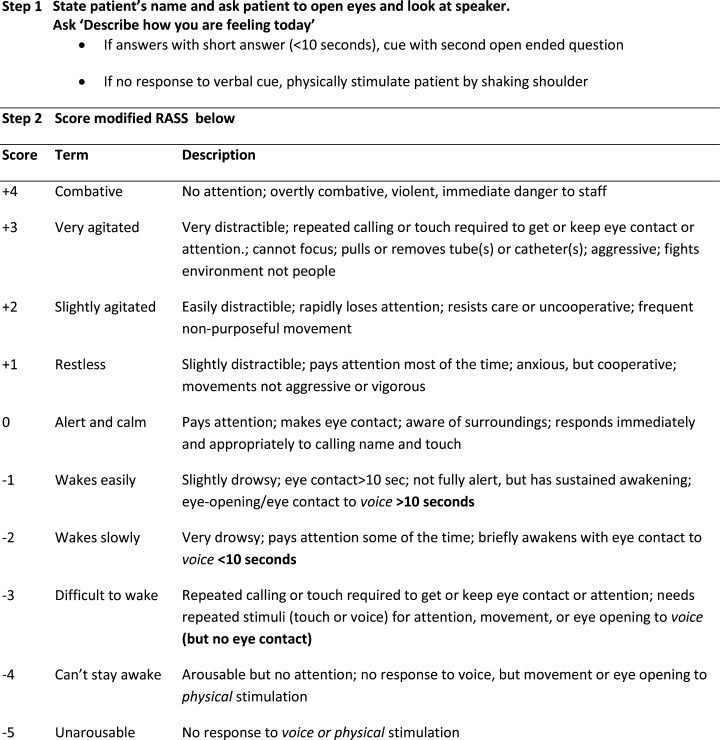
In June 2009, the Veterans Administration sponsored an interdisciplinary conference that solicited input on identifying the most targetable components of delirium and discussing potential clinical instruments. Following this, a consensus panel of 8 representatives from medicine, geriatrics, nursing, psychiatry, and psychology used a modified Delphi method to target characteristic features of delirium and identify instruments that could best capture mental status change. While inattention was agreed upon as the core cognitive feature of delirium, the group came to consensus that capturing the acute onset and fluctuating nature of delirium was better suited as a vital sign. To meet these criteria, the group modified the Richmond Agitation Sedation Scale (RASS).10
The RASS is an observational instrument that has been validated in the intesive care unit setting for objectively determining level of sedation. It has been shown to be highly reliable and associated with delirium.11 The RASS is a quick, objective scale of consciousness with a scoring system that captures both hyperactive and hypoactive levels of consciousness. A disadvantage of using the RASS includes its limited attention assessment. The Consensus panel modified the RASS to improve its assessment of attention, using a brief open‐ended question that was asked before scoring (Figure 1).

Participants
For this prospective validation study, we recruited 95 medical patients 65 years of age who had been admitted to a VA hospital. The study was approved by the institutional review board, and participants provided written informed consent. Patients were excluded if they refused (n = 64), anticipated leaving the hospital within 1 day (n = 42), or had vision or cognition impairments that would prevent their ability to complete informed consent forms and cognitive screening tools (n = 19). Five participants were discharged between enrollment and expert assessment.
Mental Status Assessments
After enrollment, 3 study staff members visited each participant independently. First, the trained research assistant obtained informed consent and demographic, cognitive, and functional assessments. The mini‐mental state examination was then administered to provide a baseline measure of cognitive function at the time of admission.12 A nurse‐interviewer later administered the modified RASS (mRASS) separately. Finally, a delirium expert performed an independent comprehensive mental status interview including assessments of attention, executive function, memory, and mood. Delirium was diagnosed by the delirium expert according to DSM‐IV criteria.5 Each investigator was blinded to the others' ratings. After the initial assessments, each participant was visited daily throughout the hospitalization by an mRASS assessor and, independently, by the delirium expert.
To determine inter‐rater reliability, 60 participants were evaluated with the mRASS by the trained research assistant and the nurse‐interviewer simultaneously. The mRASS was scored independently and the assessors were blinded to each others' ratings.
Statistics
The paired mRASS‐delirium assessments were analyzed in 3 ways: (1) as single‐day independent assessments; (2) longitudinally as a change from baseline including prevalent delirium; and (3) longitudinally as a change from baseline, excluding prevalent delirium cases. We examined 1‐point and 2‐point changes on the mRASS from baseline, which allowed determination of the most appropriate cut‐point for clinical use. Sensitivity, specificity, and likelihood ratios were calculated. The C‐statistic was calculated using absolute mRASS score for the single‐time assessments, and as a difference between minimum and maximum mRASS for the longitudinal analyses.
RESULTS
Characteristics of the study population are presented in Table 1. Because this was a VA population, the vast majority (94%) of participants were men, with a mean age of 81 years (range, 66‐96 years), and 89% were white. This population had a high Charlson Comorbidity Index (mean SD, 4.0 2.4), which was reflected in functional assessments, with 37% reporting difficulty with activities of daily living and 58% reporting difficulty with instrumental activities of daily living. Despite the age and comorbidity, delirium prevalence was 11% (n = 10) and incidence was 14% (n = 13). Interrater reliability of the mRASS yielded 98% agreement with a weighted kappa of 0.48 (P < 0.001).
| Characteristics | Values |
|---|---|
| |
| Age, years, mean (SD) | 81.0 (7.3) |
| Gender, male, no. (%) | 89 (94) |
| Race, white, no. (%) | 85 (89) |
| Charlson Comorbidity Index, mean (SD) | 4.0 (2.4) |
| BMI, kg/m2, mean (SD) | 27.2 (6.3) |
| Mini‐mental state examination, mean (SD) | 24.4 (4.1) |
| AUDIT, mean (SD) | 2.4 (2.9) |
| Tobacco use, pack‐years, no. (%) | 54 (56) |
| Current | 8 (8) |
| Never | 16 (17) |
| Prior | 68 (72) |
| Functional impairment, no. (%) | |
| Difficulty with 1 ADL | 35 (37) |
| Difficulty with 1 IADL | 55 (58) |
| Length of hospital stay | |
| Mean (SD), days | 6.3 (5.4) |
| Median, days | 5 |
| mRASS per patient, mean (SD) | 3.8 (3.3) |
When the mRASS was analyzed as a single‐day independent assessment, any abnormal score (ie, a score 0) had a sensitivity of 64% and a specificity of 93% for delirium relative to the expert evaluation (Table 2). With an abnormal mRASS as 2 or 2, the sensitivity fell to 34%, while the specificity increased to 99.6%.
| Criteria | mRASS | Sensitivity* (95% CI) | Specificity* (95% CI) | LR+ | LR |
|---|---|---|---|---|---|
| |||||
| Single‐day independent assessments | |||||
| Any abnormal | 63.9% (51.976.0) | 93.2% (90.396.4) | 9.4 | 0.4 | |
| RASS 2 or 2 | 34.4% (22.546.3) | 99.6% (98.8100) | 86 | 0.7 | |
| Longitudinal assessments | |||||
| Any delirium | Any change | 73.9% (56.091.9) | 91.7% (85.398.1) | 8.9 | 0.3 |
| Change in 2 points | 21.7% | 100% | 0.8 | ||
| Incident delirium | Any change | 84.6% (65.0100.0) | 91.7% (85.398.1) | 10.2 | 0.2 |
| Change in 2 points | 23.1% | 100% | 0.8 | ||
When the mRASS was used longitudinally to detect change in delirium during the hospital stay among all participants, it had a sensitivity of 74% and specificity of 92% for any change. Increasing the stringency of the criteria by looking at a change of 2 mRASS points decreased the sensitivity (22%) and increased the specificity (100%).
To capture the potential of the mRASS administered on a longitudinal basis as a diagnostic aid, the prevalent cases of delirium were excluded. In this analysis, any change in the mRASS had a sensitivity of 85% and a specificity of 92% for incident delirium. With more stringent criteria of a change of 2 points, the sensitivity was 23% and the specificity was 100%.
DISCUSSION
In this study, we developed a modified RASS (mRASS) for serial mental status assessment. Whereas a single measurement of the mRASS had modest sensitivity and good specificity for delirium, longitudinal measurement increased the sensitivity with no loss in specificity. Importantly, the <30 seconds required for the mRASS could be incorporated into daily workflow and provides an objective measure of consciousness. As such, we believe the mRASS can potentially serve as a longitudinal measure of consciousnessmuch like a vital sign for mental status.
Altered consciousness is a clinical and diagnostic feature of delirium,5, 13 and fluctuation in mental status is a diagnostic feature of delirium. As such, a screening instrument able to quantify the level of consciousness longitudinally and allow comparison to prior and subsequent determinations has face validity as a delirium screening instrument.
The mRASS has other features that make it appropriate for serial measurement in a manner similar to a vital sign. First, it objectively described consciousness on a scale, which is an improvement relative to many of the subjective descriptions clinically used. Consistent with other studies of the RASS,10, 11 the mRASS has good interrater reliability, allowing a common language to be used to describe level of consciousness across health care settings that can become the basis for a systematic and standardized monitor of cognitive change, improving continuity of care and communication between providers. It can be further used to objectively establish a patient's baseline and monitor change longitudinally.
The current study is limited by the lack of diversity and small size of the study population, which limits external validity (generalizability). Additional studies evaluating the utility of the mRASS by a variety of health care team members in a larger, more ethnically/racially diverse and heterogeneous population should be completed before we can determine if it can perform as a mental status vital sign, and if it is associated with better patient outcomes. Additionally, this study selected patients who were physically and cognitively capable of enrolling and excluded patients with severe cognitive and sensory impairment who were unable to provide consent to participate. Thus, some of the sickest, frailest, and most cognitively impaired patients were excluded. Unfortunately, this study therefore excluded a population significantly more vulnerable to the development of delirium.
Because a change in mental status (such as delirium) is common, morbid, and costly, a brief tool that can reliably and effectively assess mental status is needed. The mRASS used in this study provided an objective measurement of consciousness, a key component of mental status, and was demonstrated to reliably screen for presence or absence of delirium when administered longitudinally. Further study in diverse populations with administration by a variety of health care team members is needed to determine whether the mRASS can accurately serve as a mental status vital sign. If adopted widely, the mRASS could be used alongside the traditional vital signs to establish patient baselines, monitor change, improve provider communication, and potentially improve patient outcomes.
Acknowledgements
The authors are indebted to all of the veterans who willingly participated in this project. The VA Delirium Working Group Consensus Panel Consisted of Kenneth Boockvar, Joseph Flaherty, Sharon Gordon, Barbara Kamholz, James Rudolph, Marianne Shaughnessy, Kenneth Shay, and Joan Stein.
The authors maintained independence in the development, execution, and reporting of this study.
This article was presented in abstract form at the American Geriatrics Society Annual Meeting, May 12, 2011.
Funding: Jennifer G. Chester was funded by an Einstein Research Fellowship. James L. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Additional support was provided by the American Federation for Aging Research, the Boston MSTAR, and National Institutes of Health grants AG 026781‐05 and AG 038027. James L. Rudolph and Mary Beth Harrington and the VA Delirium Working Group Consensus Panel are VA employees. The authors have no additional disclosures to report.
Vital signs constitute a fundamental component of the physical examination and serve key diagnostic and monitoring purposes. The brain is as vital to life as the cardiovascular, respiratory, and immune/thermoregulatory systems, yet currently no vital sign exists that would allow rapid, reliable, and easily reproducible assessment of cognition.1 As a result, acute mental status changes frequently go undetected and untreated.24 Delirium is defined as an acute change in attention with fluctuations in cognition, thought, and/or consciousness throughout the course of the day.5 Because delirium in older patients is common and is associated with increased morbidity, mortality, functional decline, and costs,69 development and validation of a rapid, objective screening assessment could be used by nursing staff to identify patients at high risk for delirium.
Current recommendations for inpatient delirium monitoring usually involve daily cognitive screening with a standardized screening instrument.6 Because this process is often time‐consuming (8‐12 minutes), most patients do not undergo routine screening. To facilitate clinical implementation, we focused on developing a brief (<30‐second) inpatient screening measure of a feature of mental status that could be administered serially. The purpose of this study was to (1) develop a brief screening tool for a core feature of mental status and (2) validate this screening tool for delirium in an older inpatient population.
METHODS
Consensus Panel
In June 2009, the Veterans Administration sponsored an interdisciplinary conference that solicited input on identifying the most targetable components of delirium and discussing potential clinical instruments. Following this, a consensus panel of 8 representatives from medicine, geriatrics, nursing, psychiatry, and psychology used a modified Delphi method to target characteristic features of delirium and identify instruments that could best capture mental status change. While inattention was agreed upon as the core cognitive feature of delirium, the group came to consensus that capturing the acute onset and fluctuating nature of delirium was better suited as a vital sign. To meet these criteria, the group modified the Richmond Agitation Sedation Scale (RASS).10
The RASS is an observational instrument that has been validated in the intesive care unit setting for objectively determining level of sedation. It has been shown to be highly reliable and associated with delirium.11 The RASS is a quick, objective scale of consciousness with a scoring system that captures both hyperactive and hypoactive levels of consciousness. A disadvantage of using the RASS includes its limited attention assessment. The Consensus panel modified the RASS to improve its assessment of attention, using a brief open‐ended question that was asked before scoring (Figure 1).

Participants
For this prospective validation study, we recruited 95 medical patients 65 years of age who had been admitted to a VA hospital. The study was approved by the institutional review board, and participants provided written informed consent. Patients were excluded if they refused (n = 64), anticipated leaving the hospital within 1 day (n = 42), or had vision or cognition impairments that would prevent their ability to complete informed consent forms and cognitive screening tools (n = 19). Five participants were discharged between enrollment and expert assessment.
Mental Status Assessments
After enrollment, 3 study staff members visited each participant independently. First, the trained research assistant obtained informed consent and demographic, cognitive, and functional assessments. The mini‐mental state examination was then administered to provide a baseline measure of cognitive function at the time of admission.12 A nurse‐interviewer later administered the modified RASS (mRASS) separately. Finally, a delirium expert performed an independent comprehensive mental status interview including assessments of attention, executive function, memory, and mood. Delirium was diagnosed by the delirium expert according to DSM‐IV criteria.5 Each investigator was blinded to the others' ratings. After the initial assessments, each participant was visited daily throughout the hospitalization by an mRASS assessor and, independently, by the delirium expert.
To determine inter‐rater reliability, 60 participants were evaluated with the mRASS by the trained research assistant and the nurse‐interviewer simultaneously. The mRASS was scored independently and the assessors were blinded to each others' ratings.
Statistics
The paired mRASS‐delirium assessments were analyzed in 3 ways: (1) as single‐day independent assessments; (2) longitudinally as a change from baseline including prevalent delirium; and (3) longitudinally as a change from baseline, excluding prevalent delirium cases. We examined 1‐point and 2‐point changes on the mRASS from baseline, which allowed determination of the most appropriate cut‐point for clinical use. Sensitivity, specificity, and likelihood ratios were calculated. The C‐statistic was calculated using absolute mRASS score for the single‐time assessments, and as a difference between minimum and maximum mRASS for the longitudinal analyses.
RESULTS
Characteristics of the study population are presented in Table 1. Because this was a VA population, the vast majority (94%) of participants were men, with a mean age of 81 years (range, 66‐96 years), and 89% were white. This population had a high Charlson Comorbidity Index (mean SD, 4.0 2.4), which was reflected in functional assessments, with 37% reporting difficulty with activities of daily living and 58% reporting difficulty with instrumental activities of daily living. Despite the age and comorbidity, delirium prevalence was 11% (n = 10) and incidence was 14% (n = 13). Interrater reliability of the mRASS yielded 98% agreement with a weighted kappa of 0.48 (P < 0.001).
| Characteristics | Values |
|---|---|
| |
| Age, years, mean (SD) | 81.0 (7.3) |
| Gender, male, no. (%) | 89 (94) |
| Race, white, no. (%) | 85 (89) |
| Charlson Comorbidity Index, mean (SD) | 4.0 (2.4) |
| BMI, kg/m2, mean (SD) | 27.2 (6.3) |
| Mini‐mental state examination, mean (SD) | 24.4 (4.1) |
| AUDIT, mean (SD) | 2.4 (2.9) |
| Tobacco use, pack‐years, no. (%) | 54 (56) |
| Current | 8 (8) |
| Never | 16 (17) |
| Prior | 68 (72) |
| Functional impairment, no. (%) | |
| Difficulty with 1 ADL | 35 (37) |
| Difficulty with 1 IADL | 55 (58) |
| Length of hospital stay | |
| Mean (SD), days | 6.3 (5.4) |
| Median, days | 5 |
| mRASS per patient, mean (SD) | 3.8 (3.3) |
When the mRASS was analyzed as a single‐day independent assessment, any abnormal score (ie, a score 0) had a sensitivity of 64% and a specificity of 93% for delirium relative to the expert evaluation (Table 2). With an abnormal mRASS as 2 or 2, the sensitivity fell to 34%, while the specificity increased to 99.6%.
| Criteria | mRASS | Sensitivity* (95% CI) | Specificity* (95% CI) | LR+ | LR |
|---|---|---|---|---|---|
| |||||
| Single‐day independent assessments | |||||
| Any abnormal | 63.9% (51.976.0) | 93.2% (90.396.4) | 9.4 | 0.4 | |
| RASS 2 or 2 | 34.4% (22.546.3) | 99.6% (98.8100) | 86 | 0.7 | |
| Longitudinal assessments | |||||
| Any delirium | Any change | 73.9% (56.091.9) | 91.7% (85.398.1) | 8.9 | 0.3 |
| Change in 2 points | 21.7% | 100% | 0.8 | ||
| Incident delirium | Any change | 84.6% (65.0100.0) | 91.7% (85.398.1) | 10.2 | 0.2 |
| Change in 2 points | 23.1% | 100% | 0.8 | ||
When the mRASS was used longitudinally to detect change in delirium during the hospital stay among all participants, it had a sensitivity of 74% and specificity of 92% for any change. Increasing the stringency of the criteria by looking at a change of 2 mRASS points decreased the sensitivity (22%) and increased the specificity (100%).
To capture the potential of the mRASS administered on a longitudinal basis as a diagnostic aid, the prevalent cases of delirium were excluded. In this analysis, any change in the mRASS had a sensitivity of 85% and a specificity of 92% for incident delirium. With more stringent criteria of a change of 2 points, the sensitivity was 23% and the specificity was 100%.
DISCUSSION
In this study, we developed a modified RASS (mRASS) for serial mental status assessment. Whereas a single measurement of the mRASS had modest sensitivity and good specificity for delirium, longitudinal measurement increased the sensitivity with no loss in specificity. Importantly, the <30 seconds required for the mRASS could be incorporated into daily workflow and provides an objective measure of consciousness. As such, we believe the mRASS can potentially serve as a longitudinal measure of consciousnessmuch like a vital sign for mental status.
Altered consciousness is a clinical and diagnostic feature of delirium,5, 13 and fluctuation in mental status is a diagnostic feature of delirium. As such, a screening instrument able to quantify the level of consciousness longitudinally and allow comparison to prior and subsequent determinations has face validity as a delirium screening instrument.
The mRASS has other features that make it appropriate for serial measurement in a manner similar to a vital sign. First, it objectively described consciousness on a scale, which is an improvement relative to many of the subjective descriptions clinically used. Consistent with other studies of the RASS,10, 11 the mRASS has good interrater reliability, allowing a common language to be used to describe level of consciousness across health care settings that can become the basis for a systematic and standardized monitor of cognitive change, improving continuity of care and communication between providers. It can be further used to objectively establish a patient's baseline and monitor change longitudinally.
The current study is limited by the lack of diversity and small size of the study population, which limits external validity (generalizability). Additional studies evaluating the utility of the mRASS by a variety of health care team members in a larger, more ethnically/racially diverse and heterogeneous population should be completed before we can determine if it can perform as a mental status vital sign, and if it is associated with better patient outcomes. Additionally, this study selected patients who were physically and cognitively capable of enrolling and excluded patients with severe cognitive and sensory impairment who were unable to provide consent to participate. Thus, some of the sickest, frailest, and most cognitively impaired patients were excluded. Unfortunately, this study therefore excluded a population significantly more vulnerable to the development of delirium.
Because a change in mental status (such as delirium) is common, morbid, and costly, a brief tool that can reliably and effectively assess mental status is needed. The mRASS used in this study provided an objective measurement of consciousness, a key component of mental status, and was demonstrated to reliably screen for presence or absence of delirium when administered longitudinally. Further study in diverse populations with administration by a variety of health care team members is needed to determine whether the mRASS can accurately serve as a mental status vital sign. If adopted widely, the mRASS could be used alongside the traditional vital signs to establish patient baselines, monitor change, improve provider communication, and potentially improve patient outcomes.
Acknowledgements
The authors are indebted to all of the veterans who willingly participated in this project. The VA Delirium Working Group Consensus Panel Consisted of Kenneth Boockvar, Joseph Flaherty, Sharon Gordon, Barbara Kamholz, James Rudolph, Marianne Shaughnessy, Kenneth Shay, and Joan Stein.
The authors maintained independence in the development, execution, and reporting of this study.
This article was presented in abstract form at the American Geriatrics Society Annual Meeting, May 12, 2011.
Funding: Jennifer G. Chester was funded by an Einstein Research Fellowship. James L. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Additional support was provided by the American Federation for Aging Research, the Boston MSTAR, and National Institutes of Health grants AG 026781‐05 and AG 038027. James L. Rudolph and Mary Beth Harrington and the VA Delirium Working Group Consensus Panel are VA employees. The authors have no additional disclosures to report.
- ,.Vital signs in older patients: age‐related changes.J Am Med Dir Assoc.2011;12:337–343.
- ,,.Acute confusional states (delirium) in the hospitalized elderly.Annu Rev Gerontol Geriatr.1986;6:1–26.
- ,,,,.Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients.J Am Geriatr Soc.1991;39:760–765.
- ,,,,.Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings.Arch Intern Med.2001;161:2467–2473.
- Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.One‐year health care costs associated with delirium in the elderly population.Arch Intern Med.2008;168:27–32.
- ,,,,.Delirium predicts 12‐month mortality.Arch Intern Med.2002;162:457–463.
- ,,, et al.Delirium: an independent predictor of functional decline after cardiac surgery.J Am Geriatr Soc.2010;58:643–649.
- ,,, et al.The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients.Am J Respir Crit Care Med.2002;166:1338–1344.
- ,,, et al.Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS).JAMA.2003;289:2983–2991.
- ,,.“Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,.Vital signs in older patients: age‐related changes.J Am Med Dir Assoc.2011;12:337–343.
- ,,.Acute confusional states (delirium) in the hospitalized elderly.Annu Rev Gerontol Geriatr.1986;6:1–26.
- ,,,,.Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients.J Am Geriatr Soc.1991;39:760–765.
- ,,,,.Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings.Arch Intern Med.2001;161:2467–2473.
- Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.One‐year health care costs associated with delirium in the elderly population.Arch Intern Med.2008;168:27–32.
- ,,,,.Delirium predicts 12‐month mortality.Arch Intern Med.2002;162:457–463.
- ,,, et al.Delirium: an independent predictor of functional decline after cardiac surgery.J Am Geriatr Soc.2010;58:643–649.
- ,,, et al.The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients.Am J Respir Crit Care Med.2002;166:1338–1344.
- ,,, et al.Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS).JAMA.2003;289:2983–2991.
- ,,.“Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.