User login
Squamoid Eccrine Ductal Carcinoma
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
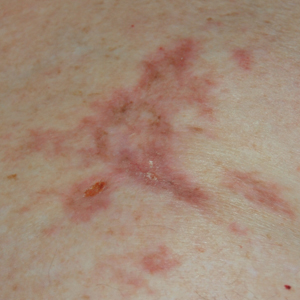
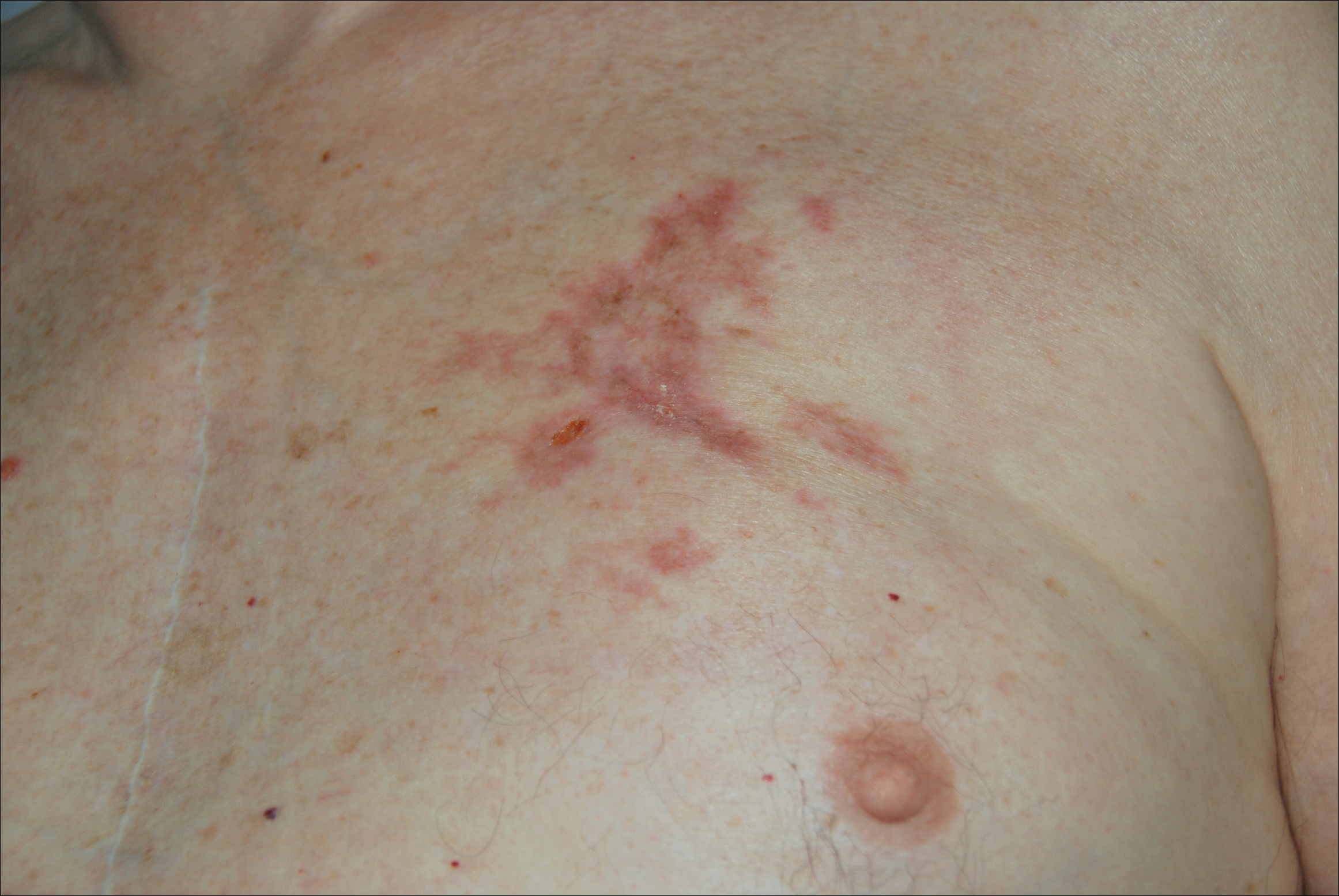
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.
Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.


Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.


Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Practice Points
- Squamoid eccrine ductal carcinoma (SEDC) is an extremely rare cutaneous tumor of unknown etiology.
- A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Development of a second or even a third primary malignancy in patients with SEDC is increasingly being reported in CLL patients.
Recalcitrant Ulcer on the Lower Leg
The Diagnosis: Nonuremic Calciphylaxis
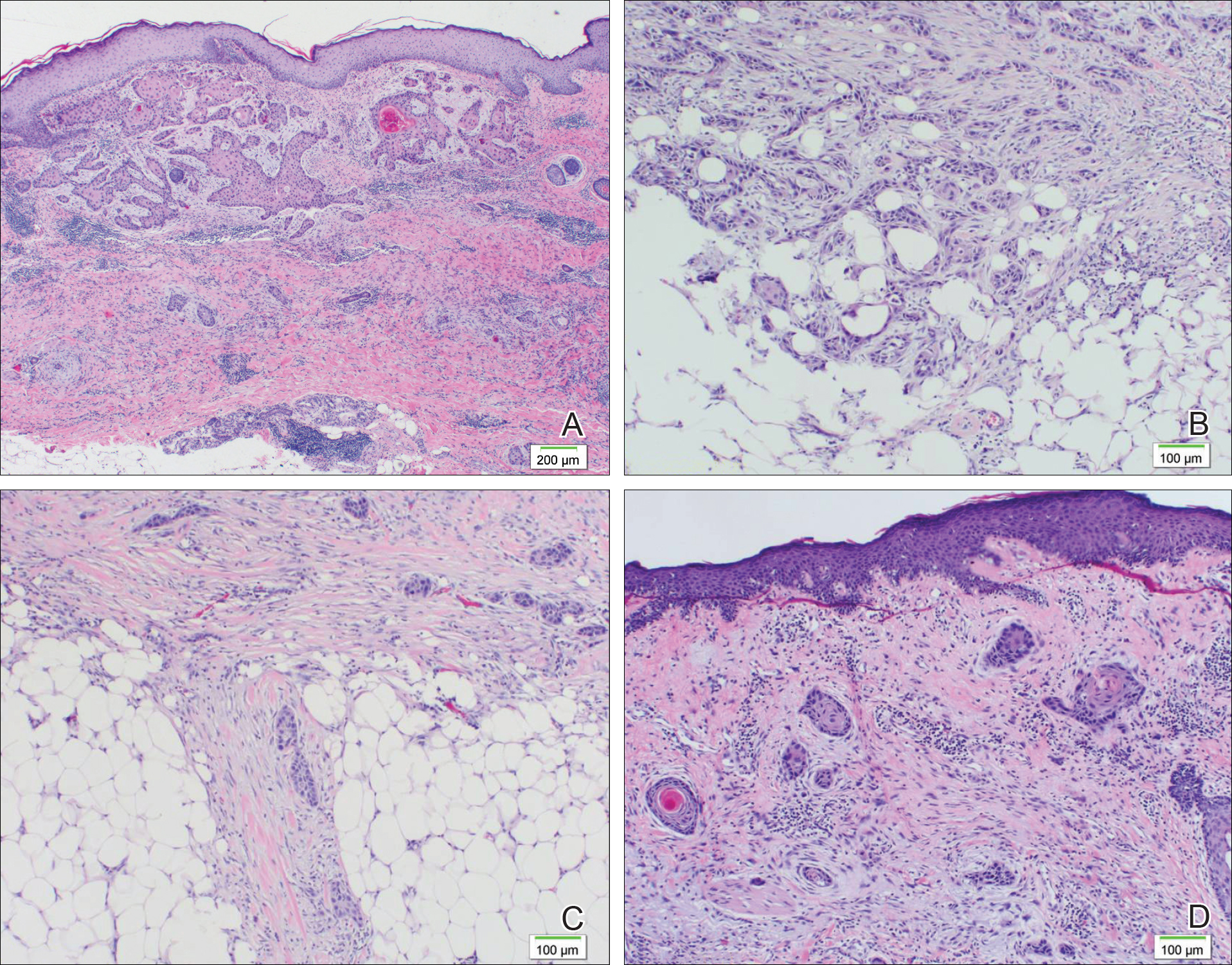
Histopathologic findings revealed ischemic necrosis and a subepidermal blister (Figure 1) with arteriosclerotic changes and fat necrosis. Foci of calcification were noted within the fat lobules. Arterioles within the deeper dermis and subcutis showed thickened hyalinized walls, narrowed lumina, and medial calcification (Figure 2). Multiple sections did not reveal any granulomatous inflammation. Periodic acid-Schiff and Gram stains were negative for fungal and bacterial elements, respectively. No dense neutrophilic infiltrate was seen. Multifocal calcific deposits within fat lobules and vessel walls (endothelium highlighted by the CD31 stain) suggested calciphylaxis.
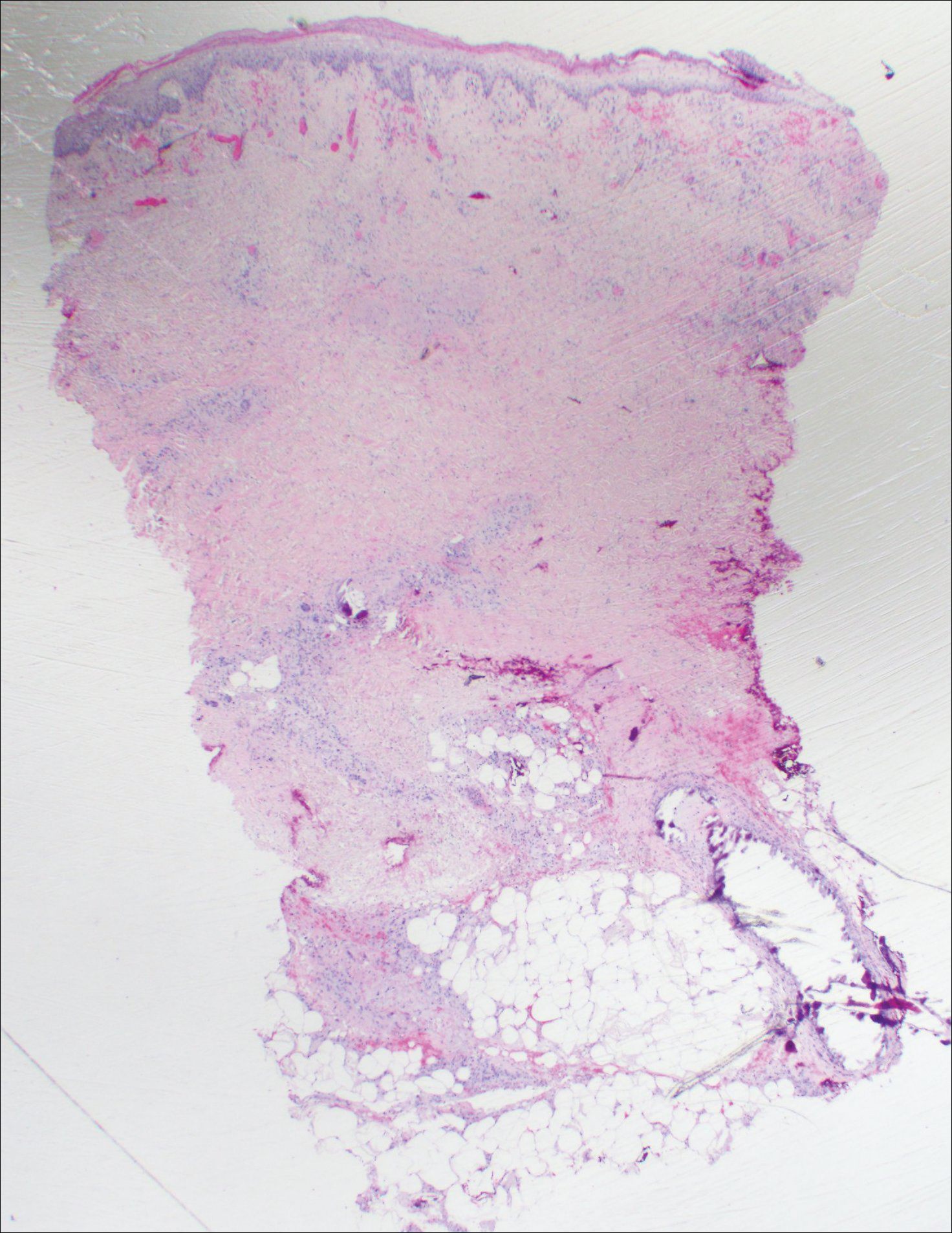
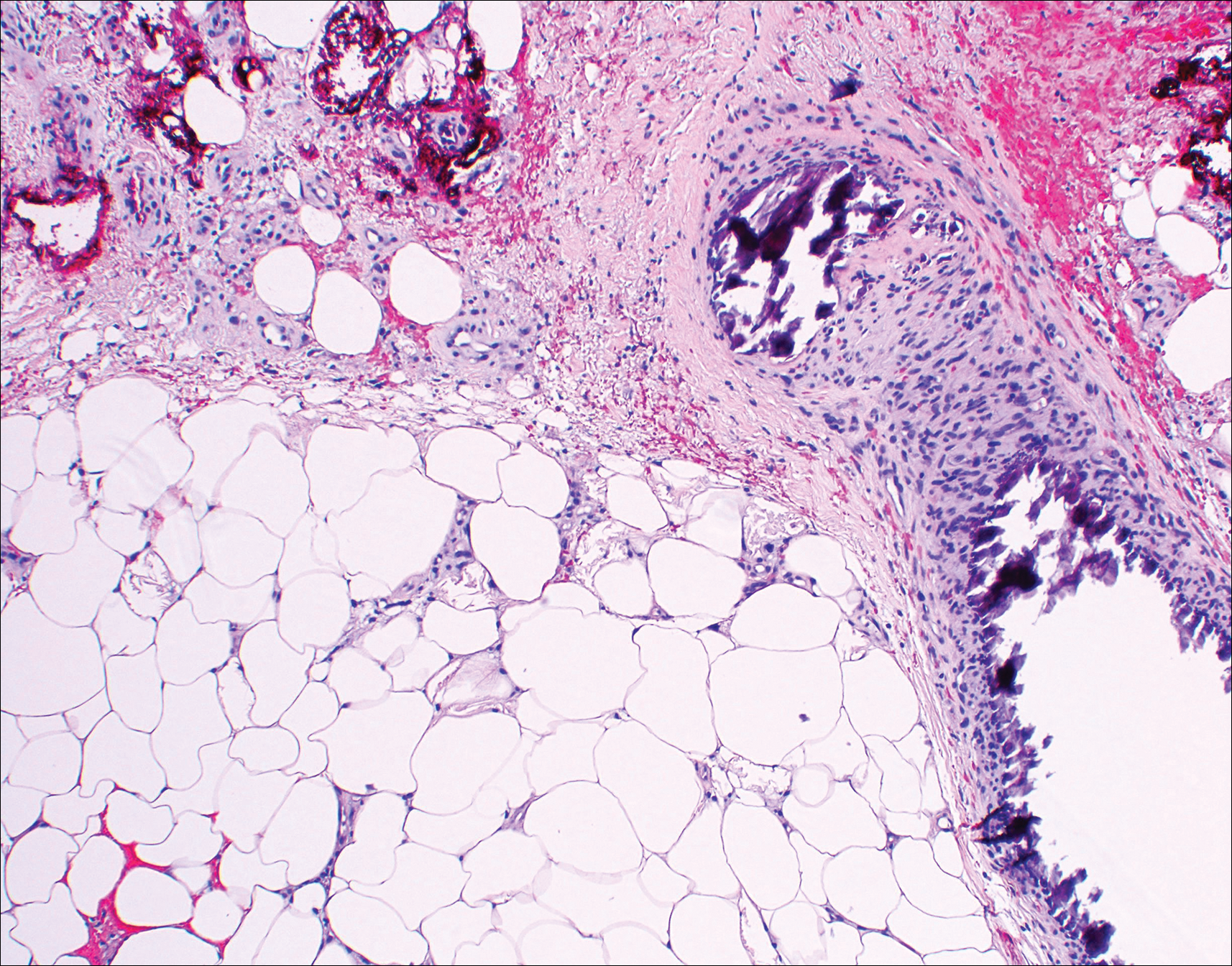
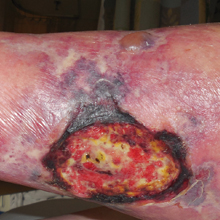
Laboratory test results revealed a normal white blood cell count, international normalized ratio level of 4 (on warfarin), and an elevated sedimentation rate at 72 mm/h (reference range, 0-20 mm/h). Serum creatinine was 1.1 mg/dL (reference range, 0.6-1.2 mg/dL) and the calcium-phosphorous product was 40.8 mg2/dL (reference range, <55 mg2/dL). Hemoglobin A1C (glycated hemoglobin) was 8.2% (reference range, 4%-7%). Wound cultures grew Proteus mirabilis sensitive to cefazolin. Acid-fast bacilli and fungal cultures were negative. Computed tomography of the left lower leg without contrast showed no evidence of osteomyelitis. Of note, the popliteal arteries and distal vessels showed moderate vascular calcification.
Histopathology findings as well as a clinical picture of painful ulceration on the distal extremities and uncontrolled diabetes with normal renal function favored a diagnosis of nonuremic calciphylaxis (NUC). The patient was treated with intravenous infusions of sodium thiosulfate 25 mg 3 times weekly and oral cefazolin for superadded bacterial infection. Local wound care included collagenase dressings with light compression. Warfarin was discontinued, as it can worsen calciphylaxis. Complete reepithelialization of the ulcer along with substantial reduction in pain was noted within 4 weeks.
Ulceration of the lower legs is a relatively common condition in the Western world, the prevalence of which increases up to 5% in patients older than 65 years.1 Of the myriad of causes that lead to ulceration of the distal aspect of the leg, NUC is a rare but known phenomenon. The pathogenesis of NUC is complicated based on theories of derangement of receptor activator of nuclear factor κβ, receptor activator of nuclear factor κβ ligand, and osteoprotegerin, leading to calcium deposits in the media of the arteries.2 This deposition precipitates vascular occlusion coupled with ischemic necrosis of the subcutaneous tissue and skin.3 Some of the more common causes of NUC are primary hyperparathyroidism, malignancy, and rheumatoid arthritis. Type 2 diabetes mellitus is a less common cause but often is found in association with NUC, as noted by Nigwekar et al.2 According to their study, the laboratory parameters commonly found in NUC included a calcium-phosphorous product greater than 50 mg2/dL and serum creatinine of 1.2 mg/dL or less.2
Our patient displayed these laboratory findings. However, distinguishing NUC from other atypical lower extremity ulcers such as Martorell hypertensive ischemic ulcer, pyoderma gangrenosum, and warfarin necrosis can pose a challenge to the dermatologist. Martorell hypertensive ischemic ulcer is excruciatingly painful and occurs more frequently near the Achilles tendon, responding well to surgical debridement. Histopathologically, medial calcinosis and arteriosclerosis are seen.4
Pyoderma gangrenosum is a neutrophilic dermatosis wherein the classical ulcerative variant is painful. It occurs mostly on the pretibial area and worsens after debridement.5 Clinically and histopathologically, it is a diagnosis of exclusion in which a dense neutrophilic to mixed lymphocytic infiltrate is seen with necrosis of dermal vessels.6
Warfarin necrosis is extremely rare, affecting 0.01% to 0.1% of patients on warfarin-derived anticoagulant therapy.7 Necrosis occurs mostly on fat-bearing areas such as the breasts, abdomen, and thighs 3 to 5 days after initiating treatment. Histologically, fibrin deposits occlude dermal vessels without perivascular inflammation.8
Necrobiosis lipoidica is a rare cutaneous entity seen in 0.3% of diabetic patients.9 The exact pathogenesis is unknown; however, microangiopathy in collaboration with cross-linking of abnormal collagen fibers play a role. These lesions appear as erythematous plaques with a slightly depressed to atrophic center, ultimately taking on a waxy porcelain appearance. Although most of these lesions either resolve or become chronically persistent, approximately 15% undergo ulceration, which can be painful. Histologically, with hematoxylin and eosin staining, areas of necrobiosis are seen surrounded by an inflammatory infiltrate comprised mainly of histiocytes along with lymphocytes and plasma cells.9
Nonuremic calciphylaxis can mimic the aforementioned conditions to a greater extent in female patients with obesity, diabetes mellitus, and hypertension. However, microscopic calcium deposition in the media of dermal arterioles, extravascular calcification within fat lobules, and cutaneous necrosis, along with remarkable response to intravenous sodium thiosulfate, confirmed a diagnosis of NUC in our patient. Sodium thiosulfate scavenges reactive oxygen species and promotes nitric oxygen generation, thereby reducing endothelial damage.10 Although there are no randomized controlled trials to support its use, sodium thiosulfate has been successfully used to treat established cases of NUC.11
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48-54.
- Hafner J, Nobbe S, Partsch H, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961-968.
- Sedda S, Caruso R, Marafini I, et al. Pyoderma gangrenosum in refractory celiac disease: a case report. BMC Gastroenterol. 2013;13:162.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Breakey W, Hall C, Vann Jones S, et al. Warfarin-induced skin necrosis progressing to calciphylaxis. J Plast Reconstr Aesthet Surg. 2014;67:244-246.
- Kakagia DD, Papanas N, Karadimas E, et al. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258-262.
- Ning MS, Dahir KM, Castellanos EH, et al. Sodium thiosulfate in the treatment of non-uremic calciphylaxis. J Dermatol. 2013;40:649-652.
The Diagnosis: Nonuremic Calciphylaxis
Histopathologic findings revealed ischemic necrosis and a subepidermal blister (Figure 1) with arteriosclerotic changes and fat necrosis. Foci of calcification were noted within the fat lobules. Arterioles within the deeper dermis and subcutis showed thickened hyalinized walls, narrowed lumina, and medial calcification (Figure 2). Multiple sections did not reveal any granulomatous inflammation. Periodic acid-Schiff and Gram stains were negative for fungal and bacterial elements, respectively. No dense neutrophilic infiltrate was seen. Multifocal calcific deposits within fat lobules and vessel walls (endothelium highlighted by the CD31 stain) suggested calciphylaxis.


Laboratory test results revealed a normal white blood cell count, international normalized ratio level of 4 (on warfarin), and an elevated sedimentation rate at 72 mm/h (reference range, 0-20 mm/h). Serum creatinine was 1.1 mg/dL (reference range, 0.6-1.2 mg/dL) and the calcium-phosphorous product was 40.8 mg2/dL (reference range, <55 mg2/dL). Hemoglobin A1C (glycated hemoglobin) was 8.2% (reference range, 4%-7%). Wound cultures grew Proteus mirabilis sensitive to cefazolin. Acid-fast bacilli and fungal cultures were negative. Computed tomography of the left lower leg without contrast showed no evidence of osteomyelitis. Of note, the popliteal arteries and distal vessels showed moderate vascular calcification.
Histopathology findings as well as a clinical picture of painful ulceration on the distal extremities and uncontrolled diabetes with normal renal function favored a diagnosis of nonuremic calciphylaxis (NUC). The patient was treated with intravenous infusions of sodium thiosulfate 25 mg 3 times weekly and oral cefazolin for superadded bacterial infection. Local wound care included collagenase dressings with light compression. Warfarin was discontinued, as it can worsen calciphylaxis. Complete reepithelialization of the ulcer along with substantial reduction in pain was noted within 4 weeks.
Ulceration of the lower legs is a relatively common condition in the Western world, the prevalence of which increases up to 5% in patients older than 65 years.1 Of the myriad of causes that lead to ulceration of the distal aspect of the leg, NUC is a rare but known phenomenon. The pathogenesis of NUC is complicated based on theories of derangement of receptor activator of nuclear factor κβ, receptor activator of nuclear factor κβ ligand, and osteoprotegerin, leading to calcium deposits in the media of the arteries.2 This deposition precipitates vascular occlusion coupled with ischemic necrosis of the subcutaneous tissue and skin.3 Some of the more common causes of NUC are primary hyperparathyroidism, malignancy, and rheumatoid arthritis. Type 2 diabetes mellitus is a less common cause but often is found in association with NUC, as noted by Nigwekar et al.2 According to their study, the laboratory parameters commonly found in NUC included a calcium-phosphorous product greater than 50 mg2/dL and serum creatinine of 1.2 mg/dL or less.2
Our patient displayed these laboratory findings. However, distinguishing NUC from other atypical lower extremity ulcers such as Martorell hypertensive ischemic ulcer, pyoderma gangrenosum, and warfarin necrosis can pose a challenge to the dermatologist. Martorell hypertensive ischemic ulcer is excruciatingly painful and occurs more frequently near the Achilles tendon, responding well to surgical debridement. Histopathologically, medial calcinosis and arteriosclerosis are seen.4
Pyoderma gangrenosum is a neutrophilic dermatosis wherein the classical ulcerative variant is painful. It occurs mostly on the pretibial area and worsens after debridement.5 Clinically and histopathologically, it is a diagnosis of exclusion in which a dense neutrophilic to mixed lymphocytic infiltrate is seen with necrosis of dermal vessels.6
Warfarin necrosis is extremely rare, affecting 0.01% to 0.1% of patients on warfarin-derived anticoagulant therapy.7 Necrosis occurs mostly on fat-bearing areas such as the breasts, abdomen, and thighs 3 to 5 days after initiating treatment. Histologically, fibrin deposits occlude dermal vessels without perivascular inflammation.8
Necrobiosis lipoidica is a rare cutaneous entity seen in 0.3% of diabetic patients.9 The exact pathogenesis is unknown; however, microangiopathy in collaboration with cross-linking of abnormal collagen fibers play a role. These lesions appear as erythematous plaques with a slightly depressed to atrophic center, ultimately taking on a waxy porcelain appearance. Although most of these lesions either resolve or become chronically persistent, approximately 15% undergo ulceration, which can be painful. Histologically, with hematoxylin and eosin staining, areas of necrobiosis are seen surrounded by an inflammatory infiltrate comprised mainly of histiocytes along with lymphocytes and plasma cells.9
Nonuremic calciphylaxis can mimic the aforementioned conditions to a greater extent in female patients with obesity, diabetes mellitus, and hypertension. However, microscopic calcium deposition in the media of dermal arterioles, extravascular calcification within fat lobules, and cutaneous necrosis, along with remarkable response to intravenous sodium thiosulfate, confirmed a diagnosis of NUC in our patient. Sodium thiosulfate scavenges reactive oxygen species and promotes nitric oxygen generation, thereby reducing endothelial damage.10 Although there are no randomized controlled trials to support its use, sodium thiosulfate has been successfully used to treat established cases of NUC.11
The Diagnosis: Nonuremic Calciphylaxis
Histopathologic findings revealed ischemic necrosis and a subepidermal blister (Figure 1) with arteriosclerotic changes and fat necrosis. Foci of calcification were noted within the fat lobules. Arterioles within the deeper dermis and subcutis showed thickened hyalinized walls, narrowed lumina, and medial calcification (Figure 2). Multiple sections did not reveal any granulomatous inflammation. Periodic acid-Schiff and Gram stains were negative for fungal and bacterial elements, respectively. No dense neutrophilic infiltrate was seen. Multifocal calcific deposits within fat lobules and vessel walls (endothelium highlighted by the CD31 stain) suggested calciphylaxis.


Laboratory test results revealed a normal white blood cell count, international normalized ratio level of 4 (on warfarin), and an elevated sedimentation rate at 72 mm/h (reference range, 0-20 mm/h). Serum creatinine was 1.1 mg/dL (reference range, 0.6-1.2 mg/dL) and the calcium-phosphorous product was 40.8 mg2/dL (reference range, <55 mg2/dL). Hemoglobin A1C (glycated hemoglobin) was 8.2% (reference range, 4%-7%). Wound cultures grew Proteus mirabilis sensitive to cefazolin. Acid-fast bacilli and fungal cultures were negative. Computed tomography of the left lower leg without contrast showed no evidence of osteomyelitis. Of note, the popliteal arteries and distal vessels showed moderate vascular calcification.
Histopathology findings as well as a clinical picture of painful ulceration on the distal extremities and uncontrolled diabetes with normal renal function favored a diagnosis of nonuremic calciphylaxis (NUC). The patient was treated with intravenous infusions of sodium thiosulfate 25 mg 3 times weekly and oral cefazolin for superadded bacterial infection. Local wound care included collagenase dressings with light compression. Warfarin was discontinued, as it can worsen calciphylaxis. Complete reepithelialization of the ulcer along with substantial reduction in pain was noted within 4 weeks.
Ulceration of the lower legs is a relatively common condition in the Western world, the prevalence of which increases up to 5% in patients older than 65 years.1 Of the myriad of causes that lead to ulceration of the distal aspect of the leg, NUC is a rare but known phenomenon. The pathogenesis of NUC is complicated based on theories of derangement of receptor activator of nuclear factor κβ, receptor activator of nuclear factor κβ ligand, and osteoprotegerin, leading to calcium deposits in the media of the arteries.2 This deposition precipitates vascular occlusion coupled with ischemic necrosis of the subcutaneous tissue and skin.3 Some of the more common causes of NUC are primary hyperparathyroidism, malignancy, and rheumatoid arthritis. Type 2 diabetes mellitus is a less common cause but often is found in association with NUC, as noted by Nigwekar et al.2 According to their study, the laboratory parameters commonly found in NUC included a calcium-phosphorous product greater than 50 mg2/dL and serum creatinine of 1.2 mg/dL or less.2
Our patient displayed these laboratory findings. However, distinguishing NUC from other atypical lower extremity ulcers such as Martorell hypertensive ischemic ulcer, pyoderma gangrenosum, and warfarin necrosis can pose a challenge to the dermatologist. Martorell hypertensive ischemic ulcer is excruciatingly painful and occurs more frequently near the Achilles tendon, responding well to surgical debridement. Histopathologically, medial calcinosis and arteriosclerosis are seen.4
Pyoderma gangrenosum is a neutrophilic dermatosis wherein the classical ulcerative variant is painful. It occurs mostly on the pretibial area and worsens after debridement.5 Clinically and histopathologically, it is a diagnosis of exclusion in which a dense neutrophilic to mixed lymphocytic infiltrate is seen with necrosis of dermal vessels.6
Warfarin necrosis is extremely rare, affecting 0.01% to 0.1% of patients on warfarin-derived anticoagulant therapy.7 Necrosis occurs mostly on fat-bearing areas such as the breasts, abdomen, and thighs 3 to 5 days after initiating treatment. Histologically, fibrin deposits occlude dermal vessels without perivascular inflammation.8
Necrobiosis lipoidica is a rare cutaneous entity seen in 0.3% of diabetic patients.9 The exact pathogenesis is unknown; however, microangiopathy in collaboration with cross-linking of abnormal collagen fibers play a role. These lesions appear as erythematous plaques with a slightly depressed to atrophic center, ultimately taking on a waxy porcelain appearance. Although most of these lesions either resolve or become chronically persistent, approximately 15% undergo ulceration, which can be painful. Histologically, with hematoxylin and eosin staining, areas of necrobiosis are seen surrounded by an inflammatory infiltrate comprised mainly of histiocytes along with lymphocytes and plasma cells.9
Nonuremic calciphylaxis can mimic the aforementioned conditions to a greater extent in female patients with obesity, diabetes mellitus, and hypertension. However, microscopic calcium deposition in the media of dermal arterioles, extravascular calcification within fat lobules, and cutaneous necrosis, along with remarkable response to intravenous sodium thiosulfate, confirmed a diagnosis of NUC in our patient. Sodium thiosulfate scavenges reactive oxygen species and promotes nitric oxygen generation, thereby reducing endothelial damage.10 Although there are no randomized controlled trials to support its use, sodium thiosulfate has been successfully used to treat established cases of NUC.11
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48-54.
- Hafner J, Nobbe S, Partsch H, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961-968.
- Sedda S, Caruso R, Marafini I, et al. Pyoderma gangrenosum in refractory celiac disease: a case report. BMC Gastroenterol. 2013;13:162.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Breakey W, Hall C, Vann Jones S, et al. Warfarin-induced skin necrosis progressing to calciphylaxis. J Plast Reconstr Aesthet Surg. 2014;67:244-246.
- Kakagia DD, Papanas N, Karadimas E, et al. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258-262.
- Ning MS, Dahir KM, Castellanos EH, et al. Sodium thiosulfate in the treatment of non-uremic calciphylaxis. J Dermatol. 2013;40:649-652.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48-54.
- Hafner J, Nobbe S, Partsch H, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961-968.
- Sedda S, Caruso R, Marafini I, et al. Pyoderma gangrenosum in refractory celiac disease: a case report. BMC Gastroenterol. 2013;13:162.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Breakey W, Hall C, Vann Jones S, et al. Warfarin-induced skin necrosis progressing to calciphylaxis. J Plast Reconstr Aesthet Surg. 2014;67:244-246.
- Kakagia DD, Papanas N, Karadimas E, et al. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258-262.
- Ning MS, Dahir KM, Castellanos EH, et al. Sodium thiosulfate in the treatment of non-uremic calciphylaxis. J Dermatol. 2013;40:649-652.
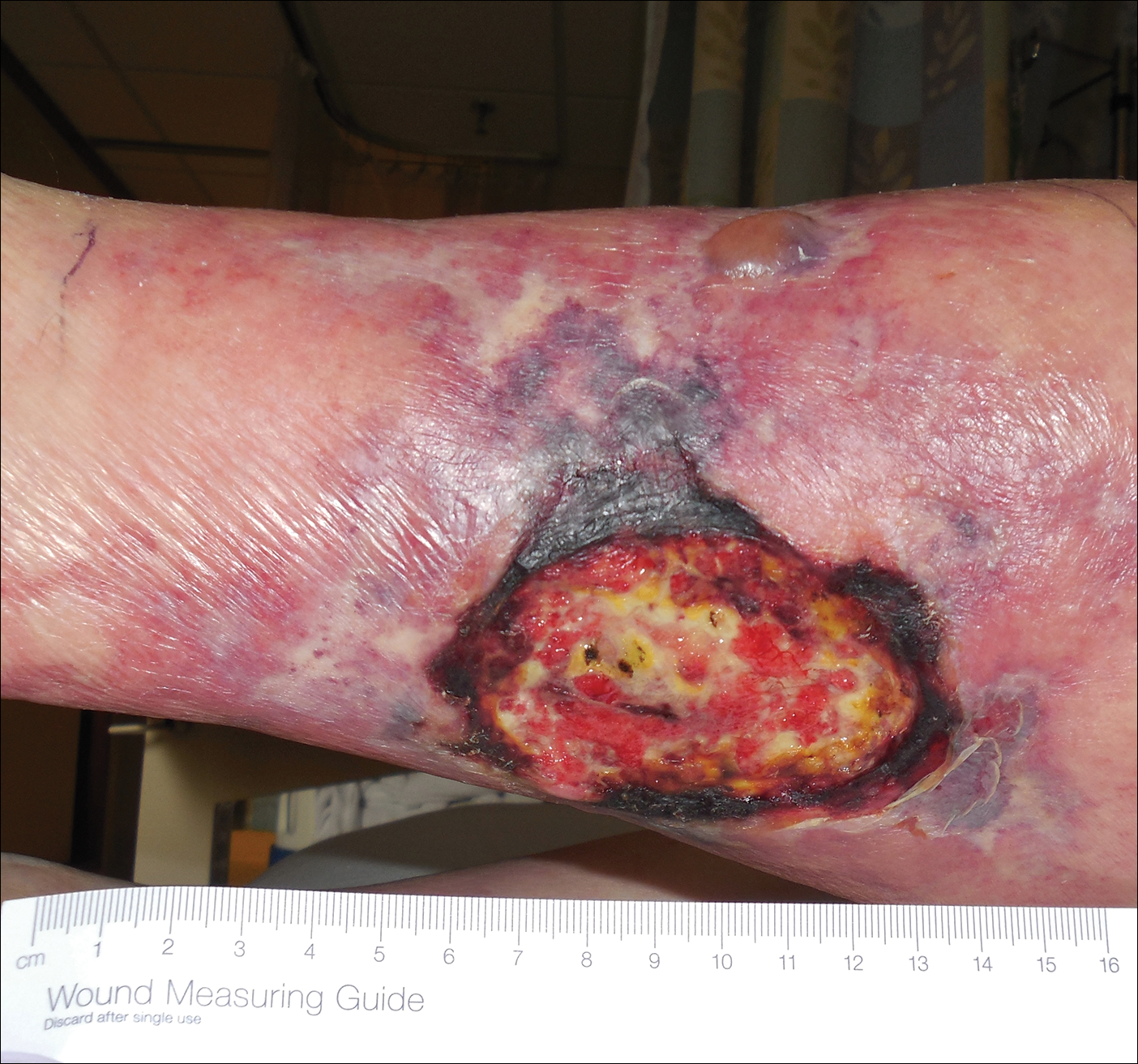
An 80-year-old woman with a medical history notable for obesity (body mass index, 31.2), type 2 diabetes mellitus, hypertension, and chronic atrial fibrillation treated with warfarin presented with a chronic painful wound on the left lower calf of 1 month's duration. A 7×7-cm ulcer on the posterior aspect of the left calf with necrotic debris was seen surrounded by skin of mottled purple discoloration. The edge of the ulcer was not undermined. There were tense nonhemorrhagic bullae on the medial aspect of the left leg and on bilateral anterior tibial areas. Two punch biopsy specimens were obtained from the anterior tibial bulla and the edge of the ulcer.