User login
Tickborne diseases other than Lyme in the United States
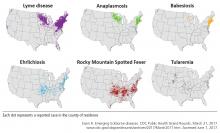
Ticks are responsible for most vector-borne infections in the United States. Most infections occur between April and October, when tick populations peak.1 However, infections can occur year-round.2,3
Tick bites are often unnoticed because the ticks are small when they are at the infective stage of their life cycle, and their attachment is characteristically painless and often in intertriginous body sites.1 Therefore, absence of a known tick bite never precludes the diagnosis of a tickborne infection.1,4,5
Although rural outdoor activities are recognized risk factors, tickborne infections also occur in urban areas.6 Thus, the lack of classic epidemiologic clues does not rule out a diagnosis of tickborne infection.
In most cases, tickborne illnesses present with nonspecific symptoms such as fever, malaise, headache, nausea, and myalgia. Accurate diagnosis of tickborne diseases can be challenging due to the similar clinical manifestations and overlapping geographic distributions of potential tick vectors.1
This review summarizes the epidemiology, clinical features, treatment, and prevention of the most prevalent non-Lyme tickborne diseases of the United States: Rocky Mountain spotted fever (RMSF), other spotted fever group rickettsial (SFGR) infections, ehrlichiosis, babesiosis, tickborne relapsing fever, Borrelia miyamotoi infection, southern tick-associated rash illness (STARI), tularemia, and tickborne viral infections.
ROCKY MOUNTAIN SPOTTED FEVER
Dermacentor variabilis, the American dog tick, is the major vector in the southern and eastern United States, and D andersoni, the Rocky Mountain wood tick, is the most common vector in the western United States.4,7,8Rhipicephalus sanguineus, the brown dog tick, has also been found to transmit RMSF in Arizona.9,10
While most infections in humans are transmitted by tick bite, rare cases of RMSF are contracted through exposure to infective tick hemolymph during tick removal, parenteral inoculation or infectious aerosols in laboratory settings, and blood transfusion.7,8
RMSF is both the most common and the most likely cause of death among rickettsial infections in the United States.4,7,8 Most cases occur in children ages 5 to 9.10,11 The case-fatality rate is over 20% without antimicrobial therapy but less than 1% with timely and appropriate antibiotic treatment.7,8
Clinical manifestations of Rocky Mountain spotted fever
RMSF is transmitted after only 2 to 20 hours of tick attachment, and symptoms begin 3 to 12 days after inoculation.1,7,8 Unlike many other species that cause SFGR infection, R rickettsii does not cause an eschar at the site of inoculation.7,12
The classic triad of RMSF is fever, headache, and a rash. This triad is present in only 3% of early infections, but the prevalence increases to 60% to 70% by 2 weeks after the tick bite.1,7 Other common initial symptoms include generalized malaise, weakness, and myalgia.7,8,12 Gastrointestinal symptoms are common, and RMSF can be misdiagnosed as gastroenteritis, particularly in children.8
A rash usually occurs. It is due to systemic vasculitis and endothelial injury and often presents 2 to 5 days after the onset of fever, which can delay diagnosis.7,12,13 It usually progresses from macular to petechial and begins on the ankles, forearms, and wrists, spreading centripetally to the trunk and face and often including the palms and soles.7 Large areas of ecchymosis, ulceration, and (uncommonly) gangrene may occur as lesions coalesce.7,8 The 10% of patients who do not develop a rash (“spotless” fever) tend to have a poorer prognosis due to delayed diagnosis.8
Risk factors for severe disease include delay or lack of appropriate treatment, extremes of age, Native American descent, glucose-6-phosphate dehydrogenase deficiency, and immunocompromised states.1,10,11,13 Complications from the widespread Rickettsia-induced vasculitis may include a septic or toxic shock-like syndrome and neurovascular, cardiac, respiratory, and renal damage.7,11 Without appropriate therapy, death occurs 7 to 15 days after symptom onset.8
Laboratory evaluation may reveal thrombocytopenia and anemia.7 Leukocytosis or leukopenia may be present.8 Hyponatremia, elevated aminotransferase levels, elevated creatine kinase levels, prolonged coagulation times, and decreased fibrinogen may also be present.7,8
Diagnosis of Rocky Mountain spotted fever
No diagnostic studies are available for the acute phase of RMSF. Therefore, a high suspicion of RMSF is essential, and treatment should be started as soon as RMSF is suspected. Confirmatory testing can retrospectively validate a clinical diagnosis.4,7,11
Serologic testing with an immunofluorescence antibody assay remains the principal diagnostic test for RMSF, and paired testing (during the acute and convalescent phases) has a sensitivity of 94%.4 A 4-fold or greater increase in antibody titer (with a minimum titer of 1:64) between acute and convalescent samples is considered diagnostic of acute infection.4,7,8 Serology is often negative early in the disease course.4,7,8 The assay cross-reacts with other SFGR species, however.4,8
Amplification of R rickettsii DNA by polymerase chain reaction (PCR) from blood or biopsy sites can be done in some research settings, but its utility is limited because of low sensitivity early in the course of the infection.4,7
Immunohistochemical staining of a skin biopsy or autopsy specimen is a highly specific diagnostic test performed at a limited number of laboratories, though it has a sensitivity of only 60% to 92%.4,7,8
Cell culture can also be performed, but only in biosafety level 3 (scale of 1 to 4) laboratories.1
Treatment of Rocky Mountain spotted fever
Prompt initiation of antibiotic therapy greatly improves prognosis.1,13,14
Doxycycline for 7 days is the treatment of choice for RMSF, including in pregnant patients with life-threatening disease and in children.4,7,8,15,16
Tetracycline can also be used.
Chloramphenicol is an alternative treatment for pregnant patients with mild to moderate disease or those patients with a severe hypersensitivity reaction to doxycycline.1,4,7,9,15,16 In the United States, chloramphenicol is currently available only in an intravenous formulation.
Fever typically subsides within 24 to 48 hours of starting treatment.4,8 Failure to clinically improve within 48 hours suggests an alternative diagnosis.1,4 Long-term complications of severe infection may include hearing loss, blindness, and amputation of digits or extremities due to gangrene.1,8 Persistence of disease beyond acute infection has not been observed.1
OTHER SPOTTED FEVER GROUP RICKETTSIAl INFECTIONS
Both infections are characterized by an inoculation eschar. Symptoms include fever, headache, myalgia, and regional lymphadenopathy.1 Rash (most often maculopapular or vesicopustular) is characteristic of R parkeri, but it is not common in Rickettsia species 364D rickettsiosis.17,18 Mild thrombocytopenia, leukopenia, and elevated aminotransferase levels are common in R parkeri infection.1 Both infections appear to be milder than RMSF.
EHRLICHIOSES: EHRLICHIOSIS AND ANAPLASMOSIS
“Ehrlichiosis” is the generic name for infections caused by both the Ehrlichia and Anaplasma genera,19,20 which are small, gram-negative obligate intracellular bacterial pathogens.21 In the United States, infections are most commonly caused by A phagocytophilum, the causative organism of human granulocytic anaplasmosis (HGA) (Table 4), and E chaffeensis, the causative organism of human monocytic ehrlichiosis (HME) (Table 5). The incidence rates of these 2 infections have increased over the past decade, in part due to increased clinical awareness and improved diagnostic capabilities.3,22,23
E ewingii (Table 6) and E muris-like agent (Table 7) are lesser known causes of human ehrlichiosis in the United States.20,23–25 Initially, E ewingii was believed to primarily affect immunocompromised patients, but it was later recognized in immunocompetent hosts.23E muris-like agent was first discovered as a cause of infection in 2009, and cases have been limited to Wisconsin and Minnesota.24,25
Human granulocytic anaplasmosis. A phagocytophilum is transmitted by Ixodes scapularis (the deer tick or blacklegged tick) in the northeastern and upper-midwestern regions of the United States, and I pacificus (the western blacklegged tick) along the northern Pacific coast.1,19,20,26 The 6 states accounting for most cases are New York, Connecticut, Massachusetts, Rhode Island, Minnesota, and Wisconsin.27 The white-footed mouse serves as the primary reservoir for A phagocytophilum, and humans are an accidental, “dead-end” host.21 Cases have also been reported to be transmitted via blood transfusion and transplacentally.20,26,28,29
Clinical manifestations of ehrlichiosis
After an incubation time of 5 to 21 days, ehrlichiosis typically presents as a febrile viral-like illness with nonspecific symptoms that include fever, chills, sweats, myalgia, headache, malaise, and cough.1,26,27,31
Gastrointestinal symptoms, arthralgia, photophobia, and nervous system involvement may also occur.1,20,29,32 Gastrointestinal symptoms tend to be more common in HME than HGA.20
Rash occurs in up to one-third of patients with HME, but it is rare in HGA.4,19,20,27 HME presents with more central nervous system involvement (such as meningitis or seizures) than HGA, in which central nervous system involvement is rare.
Severe complications of HME and HGA occur in a minority of cases and may include acute respiratory distress syndrome, renal failure, disseminated intravascular coagulopathy, and spontaneous hemorrhage.19 In general, HME is more severe than HGA and is more likely to progress to fulminant toxic or septic shocklike syndrome in rare instances.19
Laboratory tests may reveal leukopenia, lymphopenia, thrombocytopenia, and elevated liver-associated enzyme levels.1,19,20,26 Anemia and hyponatremia may also be present.4,30
Diagnosis of ehrlichiosis
The most rapid diagnostic method is examination of Wright- or Giemsa-stained peripheral blood smears for morulae, which are cytoplasmic intravacuolar inclusions of bacteria within leukocytes.20 However, its sensitivity is as low as 20% and declines even further after the first week of infection.4,20
PCR testing is the most sensitive and rapid tool available during acute infection.1,20,26,30,31 However, due to waning of the bacteremic phase, its sensitivity decreases after the first week of infection and after treatment is started.19,20
Serologic detection of antibodies with an indirect immunofluorescence assay is the most frequently used test for diagnosis of ehrlichiosis, and paired serology demonstrating seroconversion (at least a 4-fold increase in titer, with a minimal titer of 1:64) is most sensitive (82% to 100%).4,19,20,26 Cross-reactivity can occur, so testing for antibodies to both A phagocytophilum and E chaffeensis might assist in a more accurate diagnosis in areas where tick vectors overlap.4,19,20,26
HGA and HME can be isolated through cell culture in blood or cerebrospinal fluid. However, this is labor-intensive and performed in only a few specialized laboratories.4,19,20,27,31
Treatment of ehrlichiosis
If ehrlichiosis is suspected, treatment should not be delayed; the disease can be life-threatening and the ability to diagnose acute infection is often limited.20,26,32
Doxycycline is the treatment of choice, even in pregnant patients with severe infection and in children.1,19,26,27 Antibiotics are given for 5 to 10 days and continued for at least 3 days after the fever subsides.19,20,26,27,30 In HGA, a 10-day course of doxycycline is recommended to also provide the appropriate length of treatment for Borrelia burgdorferi.1,31
Rifampin is an alternative for those with severe tetracycline allergy, as well as those with mild to moderate infection during pregnancy.1,20,26,29–32
Fever typically resolves within 24 to 48 hours of starting treatment, and persistence of fever over 48 hours after starting antibiotics suggests an alternative diagnosis or possible coinfection.1,4,19,20,26,27,30,32
Persistence of chronic A phagocytophilum or E chaffeensis infection in humans beyond 2 months has not been demonstrated.20,26,30,33 Therefore, antibiotic treatment beyond the acute stage of infection is not indicated.30 Long-term prognosis is favorable, and patients are expected to make a full recovery.26,30
BABESIOSIS
Babesiosis occurs in the northeastern and upper midwestern states, with most cases reported in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin.31,32,34–36 Outbreaks have also been documented in Washington, California, and Missouri.31,32,35 The spread mimics that of Lyme disease, though it can be slower.34,36–39
Most cases in the Northeast and upper Midwest are caused by Babesia microti, while Babesia duncani has sporadically caused disease along the Pacific coast and Babesia divergens has been found in the Midwest and Northwest.34,36,39
Though babesiosis is usually a tickborne illness, it can also be transmitted through blood transfusion and, rarely, transplacental spread.31,32,34,36,39–41 The I scapularis tick is the host vector for Babesia microti, and transmission of disease requires 24 to 72 hours of attachment to a host.34,35 The primary reservoir for Babesia microti is the white-footed mouse, and humans are accidental hosts.32,34–36,39
Clinical manifestations of babesiosis
Babesia species cause illness by lysing erythrocytes, with resultant cytokine release.34
Symptoms typically appear 1 to 4 weeks after inoculation, after which most cases present as a viral-like illness with gradual onset of fever, chills, sweats, fatigue, malaise, headache, arthralgia, myalgia, nausea, anorexia, and nonproductive cough.32,34–36,39
Physical findings may include splenomegaly, hepatomegaly, jaundice, petechiae, and ecchymosis.32,34–36,39 Rash is seldom present and is not a characteristic feature of babesiosis.35,36
Laboratory features may include thrombocytopenia, hemolytic anemia, and elevated liver enzyme levels.32,34,36,39
Severe disease can occur in elderly, immunocompromised, or splenectomized individuals and can be life-threatening.34,39 Complications of severe infection can include acute respiratory distress syndrome, diffuse intravascular coagulation, and liver or renal failure.31,32,34–36,39 Splenic infarction or rupture may occur at lower levels of parasitemia in those without other manifestations of severe disease.31 The course can be prolonged and relapsing despite standard antibiotic therapy, typically in the setting of severe immunocompromise.32,34,42,43 Death occurs in up to 10% of severe cases.34
Diagnosis of babesiosis
Babesiosis should be considered if a patient presents with a febrile illness and nonspecific symptoms and comes from an endemic area or has received a blood transfusion within 6 months.34,35
The diagnosis of babesiosis is most commonly made by finding the intraerythrocytic ring form of the organism (trophozoite) on Giemsa- or Wright-stained thin blood smears.34,36,39Babesia can be distinguished from Plasmodia (the agent of malaria) by the rare presence of tetrads of merozoites arranged in a cross-like pattern (the Maltese cross); the absence of hemozoin (brownish deposits) in the ring form; and the occasional presence of extracellular ring forms.34,36
The level of parasitemia (representing the number of parasites per microliter of blood) is generally between 1% and 10%, although it can be as high as 80%.36,39 Because parasitemia is often low early in disease (< 1%), multiple blood smears should be examined.34–36,39
Several real-time PCR assays are available to detect low-grade Babesia microti parasitemia in patients with negative blood smears during early infection.31 These assays have high diagnostic sensitivity and specificity and do not cross-react with other Babesia or Plasmodium species.34–36,39
Paired serology (immunoglobulin G) can confirm infection, although antibody may be absent early in the course of illness.31,34–36,39
Treatment of babesiosis
Current guidelines recommend antimicrobial therapy only for patients with symptoms and positive test results for Babesia.32 Treatment of asymptomatic patients should additionally be considered if parasitemia (not positive PCR or serology) persists for 3 months or longer.32,34–36,39
For mild to moderate babesiosis, the combination of oral atovaquone and azithromycin for 7 to 10 days has similar efficacy and a lower incidence of adverse effects than clindamycin plus quinine.31,32,34,44 For immunocompromised patients, higher doses of azithromycin can be used.31,32
For severe babesiosis or those with risk factors for severe disease, intravenous clindamycin and oral quinine are recommended for 7 to 10 days based on expert opinion.31,32,34–36,39,43 Adverse effects of this regimen include diarrhea, tinnitus, and hearing deficits.35,39 If necessary, intravenous quinidine can be used, but the patient should receive cardiac monitoring for possible prolongation of the QT interval.34,39 As quinine therapy is often interrupted due to the above side effects, alternative regimens such as intravenous azithromycin or clindamycin in combination with oral atovaquone should be considered for severe cases.31 However, these regimens are not well studied.31
Partial or complete exchange transfusion of whole blood or packed red blood cells should be considered in patients with a high level of parasitemia (≥ 10%), severe anemia (hemoglobin < 10 g/dL), or renal, hepatic, or pulmonary compromise.31,32,34–36,39 In critically ill patients, parasitemia should be monitored daily until it has decreased to less than 5%.32,34,39
Generally, symptoms improve within 48 hours of antimicrobial therapy initiation; however, parasitemia may take up to 3 months to resolve.32,34,39 In severely immunocompromised patients, babesiosis may persist or relapse despite appropriate therapy.34,39,42,43 In these cases, at least 6 weeks of antimicrobial therapy is recommended, including 2 weeks of therapy after Babesia organisms are no longer seen on blood smear.31,33,36,39,42
TICKBORNE RELAPSING FEVER
The illness is transmitted by either ticks or body lice. The tick-borne illness is caused by spirochetes of the genus Borrelia and transmitted to humans by the bite of an infected Ornithodoros soft tick.45 Approximately 70% of reported cases in the United States occur in California, Washington, and Colorado.46 Most cases are caused by Borrelia hermsii and are linked to sleeping in rodent-infested cabins in mountainous areas.46 Remarkably, tick-borne borreliae are transmitted within about 30 seconds of tick attachment.47,48
The hallmark of tickborne relapsing fever is febrile episodes lasting 3 to 5 days, with relapses after 5 to 7 days of apparent recovery.49 If untreated, several episodes of fever and nonspecific symptoms will occur before illness resolves spontaneously. Overall mortality rates are very low (< 5%).50
Laboratory confirmation of tickborne relapsing fever is made by detecting spirochetes in a blood smear during a febrile episode or serologic antibody confirmation. However, serologic testing is unhelpful in the acute setting and can yield false-positive results with prior exposure to other Borrelia species (eg, Lyme disease) or other spirochetes. Serologic antibody testing with a 4-fold increase between acute and convalescent samples or PCR can aid in diagnosis, though the latter is available only in research settings.47
The preferred treatment regimen for adults is an oral tetracycline for 10 days. Erythromycin is recommended when tetracyclines are contraindicated.51
When starting treatment, all patients should be monitored closely for the Jarisch-Herxheimer reaction (rigors, hypotension, and high fevers), which develops in over 50% of cases as a result of rapid spirochetal killing and massive cytokine release.52
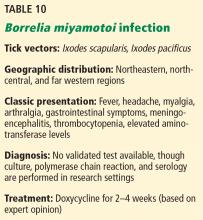
BORRELIA MIYAMOTOI INFECTION
The most common clinical manifestations are similar to other tickborne relapsing fever infections, although a true “relapsing fever” itself is not usually present.53 The characteristic erythema migrans rash often found in Lyme disease is typically absent in B miyamotoi infection; however, when present, it should prompt investigation into coinfection.54 Cases of meningoencephalitis have been reported in immunosuppressed hosts.55
There is currently no validated test available for diagnosis of B miyamotoi; however, PCR and serology are available in a few specialized laboratories.31,53
The treatment of choice is doxycycline for 2 to 4 weeks. Amoxicillin and ceftriaxone also appear effective.53
SOUTHERN TICK-ASSOCIATED RASH ILLNESS
Infection can present similarly to Lyme disease with an erythema migrans-like rash and associated flulike symptoms, although systemic symptoms and multiple erythema migrans lesions are less likely with STARI. Also, the erythema migrans-like lesions tend to be smaller and more likely to have central clearing than those in Lyme disease.57 Nevertheless, it is difficult to distinguish the 2 illnesses, especially in mid-Atlantic states such as Maryland or Virginia, where both diseases coexist. The most reliable method of distinguishing STARI from Lyme disease is demonstrating that the patient was bitten by a Lone Star tick rather than an Ixodes tick. Numerous questions remain unanswered about the causative organism, pathophysiology, definitive diagnosis, geographic range of illness, and most effective treatment for STARI.
Most reported cases have responded promptly to doxycycline, though it is not known whether antibiotic treatment is necessary.58
TULAREMIA
Ticks are thought to be the most important vectors, and most cases occur in the south-central United States.59 The geographic distribution of disease is gradually shifting northward due to spread of the major tick vectors, A americanum, D variabilis, and D andersoni. Approximately 100 to 200 cases of tularemia are diagnosed each year in the United States, with most concentrated in Kansas, Oklahoma, Missouri, and Arkansas.60
Humans can acquire F tularensis by several routes, and the route of infection ultimately dictates the clinical syndrome. Ulceroglandular and glandular forms of the disease are the most common in the United States, and both frequently result from a tick bite. A few days after tick exposure, an erythematous, often painful papuloulcerative lesion with a central eschar manifests at the site of the tick bite. Additional symptoms may include fever, chills, headache, myalgia, malaise, and suppurative lymphadenitis.61
Diagnosis can be made by identifying F tularensis in blood, fluid, or tissue culture performed under biosafety level 3 conditions; however, serology is used in most cases.62
Streptomycin and gentamicin are considered drugs of choice and should be continued for at least 10 days. For relatively mild disease, oral doxycycline or ciprofloxacin can be considered for at least 14 days, although the latter is not approved for treatment.59,63
TICKBORNE VIRAL INFECTIONS
Powassan virus, an uncommon flavivirus, is found in the Great Lakes region and northeast United States. In the Great Lakes region, I cookei ticks transmit the traditional lineage of this virus. However, more recent cases have been identified in the Northeast and Midwest, where Powassan virus lineage II (or deer tick virus) is transmitted by I scapularis.31,64
The classic presentation is a viral encephalitis. Rash (most often maculopapular) and gastrointestinal symptoms have been reported as well. A high index of suspicion is needed for diagnosis because clinical features and laboratory findings resemble those of other arboviral infections.
Treatment for Powassan viral encephalitis is supportive, although corticosteroids have been used with some success.64 While asymptomatic infection has been documented, the reported mortality rate of Powassan virus encephalitis is 10% to 15%, and focal neurologic deficits can persist among survivors.65
Clinical and laboratory features appear to be very similar to those of the ehrlichioses.1 A clinical diagnosis should be considered in patients with A americanum exposure, fever, and cytopenias who lack PCR or serologic evidence for ehrlichiosis infection or who fail to respond to doxycycline therapy.24
COINFECTION
Some tick vectors transmit more than 1 type of infection, and therefore, coinfection with multiple pathogens may occur. For example, I scapularis transmits Borrelia burgdorferi (Lyme disease), HGA, Babesia microti, B miyamotoi, E muris-like agent, and Powassan virus lineage II, while A americanum transmits HME and Heartland virus.24,26,31,34,36,67 Coinfection may increase the severity of disease, often due to a delay in diagnosis, though more research is needed to understand the clinical manifestations of coinfection.31,35,67
PREVENTION
Unfortunately, there are no available human vaccines for tickborne illnesses in the United States, and the effectiveness of single-dose prophylaxis with doxycycline for non-Lyme infections has not been evaluated.4,7,26
Illness is best prevented by minimizing skin exposure to ticks, use of tick repellents containing DEET, use of long-legged and long-sleeved clothing impregnated with an acaricide such as permethrin, and conducting timely body checks for ticks after potential exposure.1,31,32 Light-colored clothing is suggested, since it allows for better visibility of crawling ticks.4,32 Bathing or showering within 2 hours of tick exposure helps prevent attachment of ticks.4,31,68 If camping outside, use of a bed net is recommended.68
Ticks are most easily removed by grasping the head of the tick as close to the skin surface as possible with fine-tipped tweezers.32,68 Removing or crushing ticks with bare hands should be avoided to prevent potential contamination, and hands should be washed thoroughly after tick removal.1,4
Blood donors are screened for a history of symptomatic tickborne disease; however, asymptomatic donors who are not identified at screening pose the greatest risk to the blood supply. Babesia microti is the most common reported transfusion-transmitted parasite in the United States, and transmission of R rickettsii, A phagocytophilum, and E ewingii have also been reported infrequently.28,40,69 Currently, no test is approved to screen blood for tickborne illnesses, though such a test would help prevent transmission of tickborne illnesses by blood transfusion in areas where these diseases are endemic.40,41
TAKE-HOME POINTS
Tickborne illnesses are increasing throughout the United States as a result of vector expansion and changes in human ecology.
It is essential that primary care clinicians consider tickborne illnesses in the differential diagnosis for any patient presenting with a fever and constitutional symptoms when the cause of symptoms is unclear and tick exposure is possible or known.
All the diseases discussed are nationally notifiable conditions, and confirmed cases should be reported.
Knowledge of the geographic locations of potential exposure is paramount to determining which tickborne infections to consider, and the absence of a tick bite history should not exclude the diagnosis in the correct clinical presentation.
In addition, it is important to recognize the limitations of diagnostic testing for many tickborne infections; empiric treatment is most often warranted before confirming the diagnosis.
Tick avoidance is the most effective way to prevent these often severe infections.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44.
- Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182.
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg 2011; 85:124–131.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55:1–27.
- Mukkada S, Buckingham SC. Recognition of and prompt treatment for tick-borne infections in children. Infect Dis Clin North Am 2015; 29:539–555.
- Schutze GE, Buckingham SC, Marshall GS, et al; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J 2007; 26:475–479.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis 2007; 7:724–732.
- Lin L, Decker C. Rocky Mountain spotted fever. Dis Mon 2012; 58:361–369.
- Demma LJ, Traeger MS, Nicholson WL, et al. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594.
- Traeger MS, Regan JJ, Humpherys D, et al. Rocky mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1650–1658.
- Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 2012; 86:713–719.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702.
- Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1659–1666.
- Nelson R. Rocky Mountain spotted fever in Native Americans. Lancet Infect Dis 2015; 15:1013–1014.
- Botelho-Nevers E, Socolovschi C, Raoult D, Parola P. Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 2012; 10:1425–1437.
- Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv 2013; 68:635–649.
- Paddock CD, Finley RW, Wright CS, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 2008; 47:1188–1196.
- Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 2010; 50:541–548.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 2007; 45(suppl 1):S45–S51.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 2009; 7:709–722.
- Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol 2012; 7:719–731.
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg 2015; 93:66–72.
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med 2016; 94:52–60.
- Wormser GP, Pritt B. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 2015; 29:371–381.
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011; 365:422–429.
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2015; 29:341–355.
- St Clair K, Decker CF. Ehrlichioses: anaplasmosis and human ehrlichiosis. Dis Mon 2012; 58:346–354.
- Centers for Disease Control and Prevention (CDC). Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1145–1148.
- Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 2007; 45:589–593.
- Stone JH, Dierberg K, Aram G, Dumler JS. Human monocytic ehrlichiosis. JAMA 2004; 292:2263–2270.
- Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. JAMA 2016; 315:1767–1777.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134.
- Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005; 11:1828–1834.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–370.
- Kavanaugh MJ, Decker CF. Babesiosis. Dis Mon 2012; 58:355–360.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–488.
- Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis 2014; 20:225–231.
- Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014; 9:e115494.
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–2407.
- Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–519.
- Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 2011; 124:800–805.
- Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–376.
- Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–386.
- Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–1458.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): distribution. www.cdc.gov/relapsing-fever/distribution/index.html. Accessed June 7, 2017.
- Forrester JD, Kjemtrup AM, Fritz CL, et al; Centers for Disease Control and Prevention (CDC). Tickborne relapsing fever—United States, 1990-2011. MMWR Morb Mortal Wkly Rep 2015; 64:58–60.
- Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am 2008; 22:449–468.
- Anderson JF. The natural history of ticks. Med Clin North Am 2002; 86:205–218.
- Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 1990; 44:155–171.
- Centers for Disease Control and Prevention (CDC). Acute respiratory distress syndrome in persons with tickborne relapsing fever—three states, 2004-2005. MMWR Morb Mortal Wkly Rep 2007; 56:1073–1076.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): information for clinicians. www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed June 7, 2017.
- Dworkin MS, Anderson DE Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis 1998; 26:122–131.
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 2015; 31:260–269.
- Krause PJ, Narasimhan S, Wormser GP, et al; Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014; 20:1183–1190.
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 2013; 368:240–245.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376.
- Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–965.
- Feder HM Jr, Hoss DM, Zemel L, Telford SR 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146.
- Carvalho CL, Lopes de Carvalho I, Ze-Ze L, Nuncio MS, Duarte EL. Tularaemia: a challenging zoonosis. Comp Immunol Microbiol Infect Dis 2014; 37):85–96.
- Centers for Disease Control and Prevention (CDC). Tularemia: statistics. www.cdc.gov/tularemia/statistics/index.html. Accessed June 7, 2017.
- Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–1290.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22:489–504.
- Johansson A, Berglund L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–268.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–713.
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110.
- Pastula DM, Turabelidze G, Yates KF, et al; Centers for Disease Control and Prevention (CDC). Notes from the field: heartland virus disease—United States, 2012-–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–271.
- Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015:587131.
- Pujalte GG, Chua JV. Tick-borne infections in the United States. Prim Care 2013; 40:619–635.
- Regan J, Matthias J, Green-Murphy A, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013; 56:e105–e107.
Ticks are responsible for most vector-borne infections in the United States. Most infections occur between April and October, when tick populations peak.1 However, infections can occur year-round.2,3
Tick bites are often unnoticed because the ticks are small when they are at the infective stage of their life cycle, and their attachment is characteristically painless and often in intertriginous body sites.1 Therefore, absence of a known tick bite never precludes the diagnosis of a tickborne infection.1,4,5
Although rural outdoor activities are recognized risk factors, tickborne infections also occur in urban areas.6 Thus, the lack of classic epidemiologic clues does not rule out a diagnosis of tickborne infection.
In most cases, tickborne illnesses present with nonspecific symptoms such as fever, malaise, headache, nausea, and myalgia. Accurate diagnosis of tickborne diseases can be challenging due to the similar clinical manifestations and overlapping geographic distributions of potential tick vectors.1
This review summarizes the epidemiology, clinical features, treatment, and prevention of the most prevalent non-Lyme tickborne diseases of the United States: Rocky Mountain spotted fever (RMSF), other spotted fever group rickettsial (SFGR) infections, ehrlichiosis, babesiosis, tickborne relapsing fever, Borrelia miyamotoi infection, southern tick-associated rash illness (STARI), tularemia, and tickborne viral infections.
ROCKY MOUNTAIN SPOTTED FEVER
Dermacentor variabilis, the American dog tick, is the major vector in the southern and eastern United States, and D andersoni, the Rocky Mountain wood tick, is the most common vector in the western United States.4,7,8Rhipicephalus sanguineus, the brown dog tick, has also been found to transmit RMSF in Arizona.9,10
While most infections in humans are transmitted by tick bite, rare cases of RMSF are contracted through exposure to infective tick hemolymph during tick removal, parenteral inoculation or infectious aerosols in laboratory settings, and blood transfusion.7,8
RMSF is both the most common and the most likely cause of death among rickettsial infections in the United States.4,7,8 Most cases occur in children ages 5 to 9.10,11 The case-fatality rate is over 20% without antimicrobial therapy but less than 1% with timely and appropriate antibiotic treatment.7,8
Clinical manifestations of Rocky Mountain spotted fever
RMSF is transmitted after only 2 to 20 hours of tick attachment, and symptoms begin 3 to 12 days after inoculation.1,7,8 Unlike many other species that cause SFGR infection, R rickettsii does not cause an eschar at the site of inoculation.7,12
The classic triad of RMSF is fever, headache, and a rash. This triad is present in only 3% of early infections, but the prevalence increases to 60% to 70% by 2 weeks after the tick bite.1,7 Other common initial symptoms include generalized malaise, weakness, and myalgia.7,8,12 Gastrointestinal symptoms are common, and RMSF can be misdiagnosed as gastroenteritis, particularly in children.8
A rash usually occurs. It is due to systemic vasculitis and endothelial injury and often presents 2 to 5 days after the onset of fever, which can delay diagnosis.7,12,13 It usually progresses from macular to petechial and begins on the ankles, forearms, and wrists, spreading centripetally to the trunk and face and often including the palms and soles.7 Large areas of ecchymosis, ulceration, and (uncommonly) gangrene may occur as lesions coalesce.7,8 The 10% of patients who do not develop a rash (“spotless” fever) tend to have a poorer prognosis due to delayed diagnosis.8
Risk factors for severe disease include delay or lack of appropriate treatment, extremes of age, Native American descent, glucose-6-phosphate dehydrogenase deficiency, and immunocompromised states.1,10,11,13 Complications from the widespread Rickettsia-induced vasculitis may include a septic or toxic shock-like syndrome and neurovascular, cardiac, respiratory, and renal damage.7,11 Without appropriate therapy, death occurs 7 to 15 days after symptom onset.8
Laboratory evaluation may reveal thrombocytopenia and anemia.7 Leukocytosis or leukopenia may be present.8 Hyponatremia, elevated aminotransferase levels, elevated creatine kinase levels, prolonged coagulation times, and decreased fibrinogen may also be present.7,8
Diagnosis of Rocky Mountain spotted fever
No diagnostic studies are available for the acute phase of RMSF. Therefore, a high suspicion of RMSF is essential, and treatment should be started as soon as RMSF is suspected. Confirmatory testing can retrospectively validate a clinical diagnosis.4,7,11
Serologic testing with an immunofluorescence antibody assay remains the principal diagnostic test for RMSF, and paired testing (during the acute and convalescent phases) has a sensitivity of 94%.4 A 4-fold or greater increase in antibody titer (with a minimum titer of 1:64) between acute and convalescent samples is considered diagnostic of acute infection.4,7,8 Serology is often negative early in the disease course.4,7,8 The assay cross-reacts with other SFGR species, however.4,8
Amplification of R rickettsii DNA by polymerase chain reaction (PCR) from blood or biopsy sites can be done in some research settings, but its utility is limited because of low sensitivity early in the course of the infection.4,7
Immunohistochemical staining of a skin biopsy or autopsy specimen is a highly specific diagnostic test performed at a limited number of laboratories, though it has a sensitivity of only 60% to 92%.4,7,8
Cell culture can also be performed, but only in biosafety level 3 (scale of 1 to 4) laboratories.1
Treatment of Rocky Mountain spotted fever
Prompt initiation of antibiotic therapy greatly improves prognosis.1,13,14
Doxycycline for 7 days is the treatment of choice for RMSF, including in pregnant patients with life-threatening disease and in children.4,7,8,15,16
Tetracycline can also be used.
Chloramphenicol is an alternative treatment for pregnant patients with mild to moderate disease or those patients with a severe hypersensitivity reaction to doxycycline.1,4,7,9,15,16 In the United States, chloramphenicol is currently available only in an intravenous formulation.
Fever typically subsides within 24 to 48 hours of starting treatment.4,8 Failure to clinically improve within 48 hours suggests an alternative diagnosis.1,4 Long-term complications of severe infection may include hearing loss, blindness, and amputation of digits or extremities due to gangrene.1,8 Persistence of disease beyond acute infection has not been observed.1
OTHER SPOTTED FEVER GROUP RICKETTSIAl INFECTIONS
Both infections are characterized by an inoculation eschar. Symptoms include fever, headache, myalgia, and regional lymphadenopathy.1 Rash (most often maculopapular or vesicopustular) is characteristic of R parkeri, but it is not common in Rickettsia species 364D rickettsiosis.17,18 Mild thrombocytopenia, leukopenia, and elevated aminotransferase levels are common in R parkeri infection.1 Both infections appear to be milder than RMSF.
EHRLICHIOSES: EHRLICHIOSIS AND ANAPLASMOSIS
“Ehrlichiosis” is the generic name for infections caused by both the Ehrlichia and Anaplasma genera,19,20 which are small, gram-negative obligate intracellular bacterial pathogens.21 In the United States, infections are most commonly caused by A phagocytophilum, the causative organism of human granulocytic anaplasmosis (HGA) (Table 4), and E chaffeensis, the causative organism of human monocytic ehrlichiosis (HME) (Table 5). The incidence rates of these 2 infections have increased over the past decade, in part due to increased clinical awareness and improved diagnostic capabilities.3,22,23
E ewingii (Table 6) and E muris-like agent (Table 7) are lesser known causes of human ehrlichiosis in the United States.20,23–25 Initially, E ewingii was believed to primarily affect immunocompromised patients, but it was later recognized in immunocompetent hosts.23E muris-like agent was first discovered as a cause of infection in 2009, and cases have been limited to Wisconsin and Minnesota.24,25
Human granulocytic anaplasmosis. A phagocytophilum is transmitted by Ixodes scapularis (the deer tick or blacklegged tick) in the northeastern and upper-midwestern regions of the United States, and I pacificus (the western blacklegged tick) along the northern Pacific coast.1,19,20,26 The 6 states accounting for most cases are New York, Connecticut, Massachusetts, Rhode Island, Minnesota, and Wisconsin.27 The white-footed mouse serves as the primary reservoir for A phagocytophilum, and humans are an accidental, “dead-end” host.21 Cases have also been reported to be transmitted via blood transfusion and transplacentally.20,26,28,29
Clinical manifestations of ehrlichiosis
After an incubation time of 5 to 21 days, ehrlichiosis typically presents as a febrile viral-like illness with nonspecific symptoms that include fever, chills, sweats, myalgia, headache, malaise, and cough.1,26,27,31
Gastrointestinal symptoms, arthralgia, photophobia, and nervous system involvement may also occur.1,20,29,32 Gastrointestinal symptoms tend to be more common in HME than HGA.20
Rash occurs in up to one-third of patients with HME, but it is rare in HGA.4,19,20,27 HME presents with more central nervous system involvement (such as meningitis or seizures) than HGA, in which central nervous system involvement is rare.
Severe complications of HME and HGA occur in a minority of cases and may include acute respiratory distress syndrome, renal failure, disseminated intravascular coagulopathy, and spontaneous hemorrhage.19 In general, HME is more severe than HGA and is more likely to progress to fulminant toxic or septic shocklike syndrome in rare instances.19
Laboratory tests may reveal leukopenia, lymphopenia, thrombocytopenia, and elevated liver-associated enzyme levels.1,19,20,26 Anemia and hyponatremia may also be present.4,30
Diagnosis of ehrlichiosis
The most rapid diagnostic method is examination of Wright- or Giemsa-stained peripheral blood smears for morulae, which are cytoplasmic intravacuolar inclusions of bacteria within leukocytes.20 However, its sensitivity is as low as 20% and declines even further after the first week of infection.4,20
PCR testing is the most sensitive and rapid tool available during acute infection.1,20,26,30,31 However, due to waning of the bacteremic phase, its sensitivity decreases after the first week of infection and after treatment is started.19,20
Serologic detection of antibodies with an indirect immunofluorescence assay is the most frequently used test for diagnosis of ehrlichiosis, and paired serology demonstrating seroconversion (at least a 4-fold increase in titer, with a minimal titer of 1:64) is most sensitive (82% to 100%).4,19,20,26 Cross-reactivity can occur, so testing for antibodies to both A phagocytophilum and E chaffeensis might assist in a more accurate diagnosis in areas where tick vectors overlap.4,19,20,26
HGA and HME can be isolated through cell culture in blood or cerebrospinal fluid. However, this is labor-intensive and performed in only a few specialized laboratories.4,19,20,27,31
Treatment of ehrlichiosis
If ehrlichiosis is suspected, treatment should not be delayed; the disease can be life-threatening and the ability to diagnose acute infection is often limited.20,26,32
Doxycycline is the treatment of choice, even in pregnant patients with severe infection and in children.1,19,26,27 Antibiotics are given for 5 to 10 days and continued for at least 3 days after the fever subsides.19,20,26,27,30 In HGA, a 10-day course of doxycycline is recommended to also provide the appropriate length of treatment for Borrelia burgdorferi.1,31
Rifampin is an alternative for those with severe tetracycline allergy, as well as those with mild to moderate infection during pregnancy.1,20,26,29–32
Fever typically resolves within 24 to 48 hours of starting treatment, and persistence of fever over 48 hours after starting antibiotics suggests an alternative diagnosis or possible coinfection.1,4,19,20,26,27,30,32
Persistence of chronic A phagocytophilum or E chaffeensis infection in humans beyond 2 months has not been demonstrated.20,26,30,33 Therefore, antibiotic treatment beyond the acute stage of infection is not indicated.30 Long-term prognosis is favorable, and patients are expected to make a full recovery.26,30
BABESIOSIS
Babesiosis occurs in the northeastern and upper midwestern states, with most cases reported in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin.31,32,34–36 Outbreaks have also been documented in Washington, California, and Missouri.31,32,35 The spread mimics that of Lyme disease, though it can be slower.34,36–39
Most cases in the Northeast and upper Midwest are caused by Babesia microti, while Babesia duncani has sporadically caused disease along the Pacific coast and Babesia divergens has been found in the Midwest and Northwest.34,36,39
Though babesiosis is usually a tickborne illness, it can also be transmitted through blood transfusion and, rarely, transplacental spread.31,32,34,36,39–41 The I scapularis tick is the host vector for Babesia microti, and transmission of disease requires 24 to 72 hours of attachment to a host.34,35 The primary reservoir for Babesia microti is the white-footed mouse, and humans are accidental hosts.32,34–36,39
Clinical manifestations of babesiosis
Babesia species cause illness by lysing erythrocytes, with resultant cytokine release.34
Symptoms typically appear 1 to 4 weeks after inoculation, after which most cases present as a viral-like illness with gradual onset of fever, chills, sweats, fatigue, malaise, headache, arthralgia, myalgia, nausea, anorexia, and nonproductive cough.32,34–36,39
Physical findings may include splenomegaly, hepatomegaly, jaundice, petechiae, and ecchymosis.32,34–36,39 Rash is seldom present and is not a characteristic feature of babesiosis.35,36
Laboratory features may include thrombocytopenia, hemolytic anemia, and elevated liver enzyme levels.32,34,36,39
Severe disease can occur in elderly, immunocompromised, or splenectomized individuals and can be life-threatening.34,39 Complications of severe infection can include acute respiratory distress syndrome, diffuse intravascular coagulation, and liver or renal failure.31,32,34–36,39 Splenic infarction or rupture may occur at lower levels of parasitemia in those without other manifestations of severe disease.31 The course can be prolonged and relapsing despite standard antibiotic therapy, typically in the setting of severe immunocompromise.32,34,42,43 Death occurs in up to 10% of severe cases.34
Diagnosis of babesiosis
Babesiosis should be considered if a patient presents with a febrile illness and nonspecific symptoms and comes from an endemic area or has received a blood transfusion within 6 months.34,35
The diagnosis of babesiosis is most commonly made by finding the intraerythrocytic ring form of the organism (trophozoite) on Giemsa- or Wright-stained thin blood smears.34,36,39Babesia can be distinguished from Plasmodia (the agent of malaria) by the rare presence of tetrads of merozoites arranged in a cross-like pattern (the Maltese cross); the absence of hemozoin (brownish deposits) in the ring form; and the occasional presence of extracellular ring forms.34,36
The level of parasitemia (representing the number of parasites per microliter of blood) is generally between 1% and 10%, although it can be as high as 80%.36,39 Because parasitemia is often low early in disease (< 1%), multiple blood smears should be examined.34–36,39
Several real-time PCR assays are available to detect low-grade Babesia microti parasitemia in patients with negative blood smears during early infection.31 These assays have high diagnostic sensitivity and specificity and do not cross-react with other Babesia or Plasmodium species.34–36,39
Paired serology (immunoglobulin G) can confirm infection, although antibody may be absent early in the course of illness.31,34–36,39
Treatment of babesiosis
Current guidelines recommend antimicrobial therapy only for patients with symptoms and positive test results for Babesia.32 Treatment of asymptomatic patients should additionally be considered if parasitemia (not positive PCR or serology) persists for 3 months or longer.32,34–36,39
For mild to moderate babesiosis, the combination of oral atovaquone and azithromycin for 7 to 10 days has similar efficacy and a lower incidence of adverse effects than clindamycin plus quinine.31,32,34,44 For immunocompromised patients, higher doses of azithromycin can be used.31,32
For severe babesiosis or those with risk factors for severe disease, intravenous clindamycin and oral quinine are recommended for 7 to 10 days based on expert opinion.31,32,34–36,39,43 Adverse effects of this regimen include diarrhea, tinnitus, and hearing deficits.35,39 If necessary, intravenous quinidine can be used, but the patient should receive cardiac monitoring for possible prolongation of the QT interval.34,39 As quinine therapy is often interrupted due to the above side effects, alternative regimens such as intravenous azithromycin or clindamycin in combination with oral atovaquone should be considered for severe cases.31 However, these regimens are not well studied.31
Partial or complete exchange transfusion of whole blood or packed red blood cells should be considered in patients with a high level of parasitemia (≥ 10%), severe anemia (hemoglobin < 10 g/dL), or renal, hepatic, or pulmonary compromise.31,32,34–36,39 In critically ill patients, parasitemia should be monitored daily until it has decreased to less than 5%.32,34,39
Generally, symptoms improve within 48 hours of antimicrobial therapy initiation; however, parasitemia may take up to 3 months to resolve.32,34,39 In severely immunocompromised patients, babesiosis may persist or relapse despite appropriate therapy.34,39,42,43 In these cases, at least 6 weeks of antimicrobial therapy is recommended, including 2 weeks of therapy after Babesia organisms are no longer seen on blood smear.31,33,36,39,42
TICKBORNE RELAPSING FEVER
The illness is transmitted by either ticks or body lice. The tick-borne illness is caused by spirochetes of the genus Borrelia and transmitted to humans by the bite of an infected Ornithodoros soft tick.45 Approximately 70% of reported cases in the United States occur in California, Washington, and Colorado.46 Most cases are caused by Borrelia hermsii and are linked to sleeping in rodent-infested cabins in mountainous areas.46 Remarkably, tick-borne borreliae are transmitted within about 30 seconds of tick attachment.47,48
The hallmark of tickborne relapsing fever is febrile episodes lasting 3 to 5 days, with relapses after 5 to 7 days of apparent recovery.49 If untreated, several episodes of fever and nonspecific symptoms will occur before illness resolves spontaneously. Overall mortality rates are very low (< 5%).50
Laboratory confirmation of tickborne relapsing fever is made by detecting spirochetes in a blood smear during a febrile episode or serologic antibody confirmation. However, serologic testing is unhelpful in the acute setting and can yield false-positive results with prior exposure to other Borrelia species (eg, Lyme disease) or other spirochetes. Serologic antibody testing with a 4-fold increase between acute and convalescent samples or PCR can aid in diagnosis, though the latter is available only in research settings.47
The preferred treatment regimen for adults is an oral tetracycline for 10 days. Erythromycin is recommended when tetracyclines are contraindicated.51
When starting treatment, all patients should be monitored closely for the Jarisch-Herxheimer reaction (rigors, hypotension, and high fevers), which develops in over 50% of cases as a result of rapid spirochetal killing and massive cytokine release.52
BORRELIA MIYAMOTOI INFECTION
The most common clinical manifestations are similar to other tickborne relapsing fever infections, although a true “relapsing fever” itself is not usually present.53 The characteristic erythema migrans rash often found in Lyme disease is typically absent in B miyamotoi infection; however, when present, it should prompt investigation into coinfection.54 Cases of meningoencephalitis have been reported in immunosuppressed hosts.55
There is currently no validated test available for diagnosis of B miyamotoi; however, PCR and serology are available in a few specialized laboratories.31,53
The treatment of choice is doxycycline for 2 to 4 weeks. Amoxicillin and ceftriaxone also appear effective.53
SOUTHERN TICK-ASSOCIATED RASH ILLNESS
Infection can present similarly to Lyme disease with an erythema migrans-like rash and associated flulike symptoms, although systemic symptoms and multiple erythema migrans lesions are less likely with STARI. Also, the erythema migrans-like lesions tend to be smaller and more likely to have central clearing than those in Lyme disease.57 Nevertheless, it is difficult to distinguish the 2 illnesses, especially in mid-Atlantic states such as Maryland or Virginia, where both diseases coexist. The most reliable method of distinguishing STARI from Lyme disease is demonstrating that the patient was bitten by a Lone Star tick rather than an Ixodes tick. Numerous questions remain unanswered about the causative organism, pathophysiology, definitive diagnosis, geographic range of illness, and most effective treatment for STARI.
Most reported cases have responded promptly to doxycycline, though it is not known whether antibiotic treatment is necessary.58
TULAREMIA
Ticks are thought to be the most important vectors, and most cases occur in the south-central United States.59 The geographic distribution of disease is gradually shifting northward due to spread of the major tick vectors, A americanum, D variabilis, and D andersoni. Approximately 100 to 200 cases of tularemia are diagnosed each year in the United States, with most concentrated in Kansas, Oklahoma, Missouri, and Arkansas.60
Humans can acquire F tularensis by several routes, and the route of infection ultimately dictates the clinical syndrome. Ulceroglandular and glandular forms of the disease are the most common in the United States, and both frequently result from a tick bite. A few days after tick exposure, an erythematous, often painful papuloulcerative lesion with a central eschar manifests at the site of the tick bite. Additional symptoms may include fever, chills, headache, myalgia, malaise, and suppurative lymphadenitis.61
Diagnosis can be made by identifying F tularensis in blood, fluid, or tissue culture performed under biosafety level 3 conditions; however, serology is used in most cases.62
Streptomycin and gentamicin are considered drugs of choice and should be continued for at least 10 days. For relatively mild disease, oral doxycycline or ciprofloxacin can be considered for at least 14 days, although the latter is not approved for treatment.59,63
TICKBORNE VIRAL INFECTIONS
Powassan virus, an uncommon flavivirus, is found in the Great Lakes region and northeast United States. In the Great Lakes region, I cookei ticks transmit the traditional lineage of this virus. However, more recent cases have been identified in the Northeast and Midwest, where Powassan virus lineage II (or deer tick virus) is transmitted by I scapularis.31,64
The classic presentation is a viral encephalitis. Rash (most often maculopapular) and gastrointestinal symptoms have been reported as well. A high index of suspicion is needed for diagnosis because clinical features and laboratory findings resemble those of other arboviral infections.
Treatment for Powassan viral encephalitis is supportive, although corticosteroids have been used with some success.64 While asymptomatic infection has been documented, the reported mortality rate of Powassan virus encephalitis is 10% to 15%, and focal neurologic deficits can persist among survivors.65
Clinical and laboratory features appear to be very similar to those of the ehrlichioses.1 A clinical diagnosis should be considered in patients with A americanum exposure, fever, and cytopenias who lack PCR or serologic evidence for ehrlichiosis infection or who fail to respond to doxycycline therapy.24
COINFECTION
Some tick vectors transmit more than 1 type of infection, and therefore, coinfection with multiple pathogens may occur. For example, I scapularis transmits Borrelia burgdorferi (Lyme disease), HGA, Babesia microti, B miyamotoi, E muris-like agent, and Powassan virus lineage II, while A americanum transmits HME and Heartland virus.24,26,31,34,36,67 Coinfection may increase the severity of disease, often due to a delay in diagnosis, though more research is needed to understand the clinical manifestations of coinfection.31,35,67
PREVENTION
Unfortunately, there are no available human vaccines for tickborne illnesses in the United States, and the effectiveness of single-dose prophylaxis with doxycycline for non-Lyme infections has not been evaluated.4,7,26
Illness is best prevented by minimizing skin exposure to ticks, use of tick repellents containing DEET, use of long-legged and long-sleeved clothing impregnated with an acaricide such as permethrin, and conducting timely body checks for ticks after potential exposure.1,31,32 Light-colored clothing is suggested, since it allows for better visibility of crawling ticks.4,32 Bathing or showering within 2 hours of tick exposure helps prevent attachment of ticks.4,31,68 If camping outside, use of a bed net is recommended.68
Ticks are most easily removed by grasping the head of the tick as close to the skin surface as possible with fine-tipped tweezers.32,68 Removing or crushing ticks with bare hands should be avoided to prevent potential contamination, and hands should be washed thoroughly after tick removal.1,4
Blood donors are screened for a history of symptomatic tickborne disease; however, asymptomatic donors who are not identified at screening pose the greatest risk to the blood supply. Babesia microti is the most common reported transfusion-transmitted parasite in the United States, and transmission of R rickettsii, A phagocytophilum, and E ewingii have also been reported infrequently.28,40,69 Currently, no test is approved to screen blood for tickborne illnesses, though such a test would help prevent transmission of tickborne illnesses by blood transfusion in areas where these diseases are endemic.40,41
TAKE-HOME POINTS
Tickborne illnesses are increasing throughout the United States as a result of vector expansion and changes in human ecology.
It is essential that primary care clinicians consider tickborne illnesses in the differential diagnosis for any patient presenting with a fever and constitutional symptoms when the cause of symptoms is unclear and tick exposure is possible or known.
All the diseases discussed are nationally notifiable conditions, and confirmed cases should be reported.
Knowledge of the geographic locations of potential exposure is paramount to determining which tickborne infections to consider, and the absence of a tick bite history should not exclude the diagnosis in the correct clinical presentation.
In addition, it is important to recognize the limitations of diagnostic testing for many tickborne infections; empiric treatment is most often warranted before confirming the diagnosis.
Tick avoidance is the most effective way to prevent these often severe infections.
Ticks are responsible for most vector-borne infections in the United States. Most infections occur between April and October, when tick populations peak.1 However, infections can occur year-round.2,3
Tick bites are often unnoticed because the ticks are small when they are at the infective stage of their life cycle, and their attachment is characteristically painless and often in intertriginous body sites.1 Therefore, absence of a known tick bite never precludes the diagnosis of a tickborne infection.1,4,5
Although rural outdoor activities are recognized risk factors, tickborne infections also occur in urban areas.6 Thus, the lack of classic epidemiologic clues does not rule out a diagnosis of tickborne infection.
In most cases, tickborne illnesses present with nonspecific symptoms such as fever, malaise, headache, nausea, and myalgia. Accurate diagnosis of tickborne diseases can be challenging due to the similar clinical manifestations and overlapping geographic distributions of potential tick vectors.1
This review summarizes the epidemiology, clinical features, treatment, and prevention of the most prevalent non-Lyme tickborne diseases of the United States: Rocky Mountain spotted fever (RMSF), other spotted fever group rickettsial (SFGR) infections, ehrlichiosis, babesiosis, tickborne relapsing fever, Borrelia miyamotoi infection, southern tick-associated rash illness (STARI), tularemia, and tickborne viral infections.
ROCKY MOUNTAIN SPOTTED FEVER
Dermacentor variabilis, the American dog tick, is the major vector in the southern and eastern United States, and D andersoni, the Rocky Mountain wood tick, is the most common vector in the western United States.4,7,8Rhipicephalus sanguineus, the brown dog tick, has also been found to transmit RMSF in Arizona.9,10
While most infections in humans are transmitted by tick bite, rare cases of RMSF are contracted through exposure to infective tick hemolymph during tick removal, parenteral inoculation or infectious aerosols in laboratory settings, and blood transfusion.7,8
RMSF is both the most common and the most likely cause of death among rickettsial infections in the United States.4,7,8 Most cases occur in children ages 5 to 9.10,11 The case-fatality rate is over 20% without antimicrobial therapy but less than 1% with timely and appropriate antibiotic treatment.7,8
Clinical manifestations of Rocky Mountain spotted fever
RMSF is transmitted after only 2 to 20 hours of tick attachment, and symptoms begin 3 to 12 days after inoculation.1,7,8 Unlike many other species that cause SFGR infection, R rickettsii does not cause an eschar at the site of inoculation.7,12
The classic triad of RMSF is fever, headache, and a rash. This triad is present in only 3% of early infections, but the prevalence increases to 60% to 70% by 2 weeks after the tick bite.1,7 Other common initial symptoms include generalized malaise, weakness, and myalgia.7,8,12 Gastrointestinal symptoms are common, and RMSF can be misdiagnosed as gastroenteritis, particularly in children.8
A rash usually occurs. It is due to systemic vasculitis and endothelial injury and often presents 2 to 5 days after the onset of fever, which can delay diagnosis.7,12,13 It usually progresses from macular to petechial and begins on the ankles, forearms, and wrists, spreading centripetally to the trunk and face and often including the palms and soles.7 Large areas of ecchymosis, ulceration, and (uncommonly) gangrene may occur as lesions coalesce.7,8 The 10% of patients who do not develop a rash (“spotless” fever) tend to have a poorer prognosis due to delayed diagnosis.8
Risk factors for severe disease include delay or lack of appropriate treatment, extremes of age, Native American descent, glucose-6-phosphate dehydrogenase deficiency, and immunocompromised states.1,10,11,13 Complications from the widespread Rickettsia-induced vasculitis may include a septic or toxic shock-like syndrome and neurovascular, cardiac, respiratory, and renal damage.7,11 Without appropriate therapy, death occurs 7 to 15 days after symptom onset.8
Laboratory evaluation may reveal thrombocytopenia and anemia.7 Leukocytosis or leukopenia may be present.8 Hyponatremia, elevated aminotransferase levels, elevated creatine kinase levels, prolonged coagulation times, and decreased fibrinogen may also be present.7,8
Diagnosis of Rocky Mountain spotted fever
No diagnostic studies are available for the acute phase of RMSF. Therefore, a high suspicion of RMSF is essential, and treatment should be started as soon as RMSF is suspected. Confirmatory testing can retrospectively validate a clinical diagnosis.4,7,11
Serologic testing with an immunofluorescence antibody assay remains the principal diagnostic test for RMSF, and paired testing (during the acute and convalescent phases) has a sensitivity of 94%.4 A 4-fold or greater increase in antibody titer (with a minimum titer of 1:64) between acute and convalescent samples is considered diagnostic of acute infection.4,7,8 Serology is often negative early in the disease course.4,7,8 The assay cross-reacts with other SFGR species, however.4,8
Amplification of R rickettsii DNA by polymerase chain reaction (PCR) from blood or biopsy sites can be done in some research settings, but its utility is limited because of low sensitivity early in the course of the infection.4,7
Immunohistochemical staining of a skin biopsy or autopsy specimen is a highly specific diagnostic test performed at a limited number of laboratories, though it has a sensitivity of only 60% to 92%.4,7,8
Cell culture can also be performed, but only in biosafety level 3 (scale of 1 to 4) laboratories.1
Treatment of Rocky Mountain spotted fever
Prompt initiation of antibiotic therapy greatly improves prognosis.1,13,14
Doxycycline for 7 days is the treatment of choice for RMSF, including in pregnant patients with life-threatening disease and in children.4,7,8,15,16
Tetracycline can also be used.
Chloramphenicol is an alternative treatment for pregnant patients with mild to moderate disease or those patients with a severe hypersensitivity reaction to doxycycline.1,4,7,9,15,16 In the United States, chloramphenicol is currently available only in an intravenous formulation.
Fever typically subsides within 24 to 48 hours of starting treatment.4,8 Failure to clinically improve within 48 hours suggests an alternative diagnosis.1,4 Long-term complications of severe infection may include hearing loss, blindness, and amputation of digits or extremities due to gangrene.1,8 Persistence of disease beyond acute infection has not been observed.1
OTHER SPOTTED FEVER GROUP RICKETTSIAl INFECTIONS
Both infections are characterized by an inoculation eschar. Symptoms include fever, headache, myalgia, and regional lymphadenopathy.1 Rash (most often maculopapular or vesicopustular) is characteristic of R parkeri, but it is not common in Rickettsia species 364D rickettsiosis.17,18 Mild thrombocytopenia, leukopenia, and elevated aminotransferase levels are common in R parkeri infection.1 Both infections appear to be milder than RMSF.
EHRLICHIOSES: EHRLICHIOSIS AND ANAPLASMOSIS
“Ehrlichiosis” is the generic name for infections caused by both the Ehrlichia and Anaplasma genera,19,20 which are small, gram-negative obligate intracellular bacterial pathogens.21 In the United States, infections are most commonly caused by A phagocytophilum, the causative organism of human granulocytic anaplasmosis (HGA) (Table 4), and E chaffeensis, the causative organism of human monocytic ehrlichiosis (HME) (Table 5). The incidence rates of these 2 infections have increased over the past decade, in part due to increased clinical awareness and improved diagnostic capabilities.3,22,23
E ewingii (Table 6) and E muris-like agent (Table 7) are lesser known causes of human ehrlichiosis in the United States.20,23–25 Initially, E ewingii was believed to primarily affect immunocompromised patients, but it was later recognized in immunocompetent hosts.23E muris-like agent was first discovered as a cause of infection in 2009, and cases have been limited to Wisconsin and Minnesota.24,25
Human granulocytic anaplasmosis. A phagocytophilum is transmitted by Ixodes scapularis (the deer tick or blacklegged tick) in the northeastern and upper-midwestern regions of the United States, and I pacificus (the western blacklegged tick) along the northern Pacific coast.1,19,20,26 The 6 states accounting for most cases are New York, Connecticut, Massachusetts, Rhode Island, Minnesota, and Wisconsin.27 The white-footed mouse serves as the primary reservoir for A phagocytophilum, and humans are an accidental, “dead-end” host.21 Cases have also been reported to be transmitted via blood transfusion and transplacentally.20,26,28,29
Clinical manifestations of ehrlichiosis
After an incubation time of 5 to 21 days, ehrlichiosis typically presents as a febrile viral-like illness with nonspecific symptoms that include fever, chills, sweats, myalgia, headache, malaise, and cough.1,26,27,31
Gastrointestinal symptoms, arthralgia, photophobia, and nervous system involvement may also occur.1,20,29,32 Gastrointestinal symptoms tend to be more common in HME than HGA.20
Rash occurs in up to one-third of patients with HME, but it is rare in HGA.4,19,20,27 HME presents with more central nervous system involvement (such as meningitis or seizures) than HGA, in which central nervous system involvement is rare.
Severe complications of HME and HGA occur in a minority of cases and may include acute respiratory distress syndrome, renal failure, disseminated intravascular coagulopathy, and spontaneous hemorrhage.19 In general, HME is more severe than HGA and is more likely to progress to fulminant toxic or septic shocklike syndrome in rare instances.19
Laboratory tests may reveal leukopenia, lymphopenia, thrombocytopenia, and elevated liver-associated enzyme levels.1,19,20,26 Anemia and hyponatremia may also be present.4,30
Diagnosis of ehrlichiosis
The most rapid diagnostic method is examination of Wright- or Giemsa-stained peripheral blood smears for morulae, which are cytoplasmic intravacuolar inclusions of bacteria within leukocytes.20 However, its sensitivity is as low as 20% and declines even further after the first week of infection.4,20
PCR testing is the most sensitive and rapid tool available during acute infection.1,20,26,30,31 However, due to waning of the bacteremic phase, its sensitivity decreases after the first week of infection and after treatment is started.19,20
Serologic detection of antibodies with an indirect immunofluorescence assay is the most frequently used test for diagnosis of ehrlichiosis, and paired serology demonstrating seroconversion (at least a 4-fold increase in titer, with a minimal titer of 1:64) is most sensitive (82% to 100%).4,19,20,26 Cross-reactivity can occur, so testing for antibodies to both A phagocytophilum and E chaffeensis might assist in a more accurate diagnosis in areas where tick vectors overlap.4,19,20,26
HGA and HME can be isolated through cell culture in blood or cerebrospinal fluid. However, this is labor-intensive and performed in only a few specialized laboratories.4,19,20,27,31
Treatment of ehrlichiosis
If ehrlichiosis is suspected, treatment should not be delayed; the disease can be life-threatening and the ability to diagnose acute infection is often limited.20,26,32
Doxycycline is the treatment of choice, even in pregnant patients with severe infection and in children.1,19,26,27 Antibiotics are given for 5 to 10 days and continued for at least 3 days after the fever subsides.19,20,26,27,30 In HGA, a 10-day course of doxycycline is recommended to also provide the appropriate length of treatment for Borrelia burgdorferi.1,31
Rifampin is an alternative for those with severe tetracycline allergy, as well as those with mild to moderate infection during pregnancy.1,20,26,29–32
Fever typically resolves within 24 to 48 hours of starting treatment, and persistence of fever over 48 hours after starting antibiotics suggests an alternative diagnosis or possible coinfection.1,4,19,20,26,27,30,32
Persistence of chronic A phagocytophilum or E chaffeensis infection in humans beyond 2 months has not been demonstrated.20,26,30,33 Therefore, antibiotic treatment beyond the acute stage of infection is not indicated.30 Long-term prognosis is favorable, and patients are expected to make a full recovery.26,30
BABESIOSIS
Babesiosis occurs in the northeastern and upper midwestern states, with most cases reported in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin.31,32,34–36 Outbreaks have also been documented in Washington, California, and Missouri.31,32,35 The spread mimics that of Lyme disease, though it can be slower.34,36–39
Most cases in the Northeast and upper Midwest are caused by Babesia microti, while Babesia duncani has sporadically caused disease along the Pacific coast and Babesia divergens has been found in the Midwest and Northwest.34,36,39
Though babesiosis is usually a tickborne illness, it can also be transmitted through blood transfusion and, rarely, transplacental spread.31,32,34,36,39–41 The I scapularis tick is the host vector for Babesia microti, and transmission of disease requires 24 to 72 hours of attachment to a host.34,35 The primary reservoir for Babesia microti is the white-footed mouse, and humans are accidental hosts.32,34–36,39
Clinical manifestations of babesiosis
Babesia species cause illness by lysing erythrocytes, with resultant cytokine release.34
Symptoms typically appear 1 to 4 weeks after inoculation, after which most cases present as a viral-like illness with gradual onset of fever, chills, sweats, fatigue, malaise, headache, arthralgia, myalgia, nausea, anorexia, and nonproductive cough.32,34–36,39
Physical findings may include splenomegaly, hepatomegaly, jaundice, petechiae, and ecchymosis.32,34–36,39 Rash is seldom present and is not a characteristic feature of babesiosis.35,36
Laboratory features may include thrombocytopenia, hemolytic anemia, and elevated liver enzyme levels.32,34,36,39
Severe disease can occur in elderly, immunocompromised, or splenectomized individuals and can be life-threatening.34,39 Complications of severe infection can include acute respiratory distress syndrome, diffuse intravascular coagulation, and liver or renal failure.31,32,34–36,39 Splenic infarction or rupture may occur at lower levels of parasitemia in those without other manifestations of severe disease.31 The course can be prolonged and relapsing despite standard antibiotic therapy, typically in the setting of severe immunocompromise.32,34,42,43 Death occurs in up to 10% of severe cases.34
Diagnosis of babesiosis
Babesiosis should be considered if a patient presents with a febrile illness and nonspecific symptoms and comes from an endemic area or has received a blood transfusion within 6 months.34,35
The diagnosis of babesiosis is most commonly made by finding the intraerythrocytic ring form of the organism (trophozoite) on Giemsa- or Wright-stained thin blood smears.34,36,39Babesia can be distinguished from Plasmodia (the agent of malaria) by the rare presence of tetrads of merozoites arranged in a cross-like pattern (the Maltese cross); the absence of hemozoin (brownish deposits) in the ring form; and the occasional presence of extracellular ring forms.34,36
The level of parasitemia (representing the number of parasites per microliter of blood) is generally between 1% and 10%, although it can be as high as 80%.36,39 Because parasitemia is often low early in disease (< 1%), multiple blood smears should be examined.34–36,39
Several real-time PCR assays are available to detect low-grade Babesia microti parasitemia in patients with negative blood smears during early infection.31 These assays have high diagnostic sensitivity and specificity and do not cross-react with other Babesia or Plasmodium species.34–36,39
Paired serology (immunoglobulin G) can confirm infection, although antibody may be absent early in the course of illness.31,34–36,39
Treatment of babesiosis
Current guidelines recommend antimicrobial therapy only for patients with symptoms and positive test results for Babesia.32 Treatment of asymptomatic patients should additionally be considered if parasitemia (not positive PCR or serology) persists for 3 months or longer.32,34–36,39
For mild to moderate babesiosis, the combination of oral atovaquone and azithromycin for 7 to 10 days has similar efficacy and a lower incidence of adverse effects than clindamycin plus quinine.31,32,34,44 For immunocompromised patients, higher doses of azithromycin can be used.31,32
For severe babesiosis or those with risk factors for severe disease, intravenous clindamycin and oral quinine are recommended for 7 to 10 days based on expert opinion.31,32,34–36,39,43 Adverse effects of this regimen include diarrhea, tinnitus, and hearing deficits.35,39 If necessary, intravenous quinidine can be used, but the patient should receive cardiac monitoring for possible prolongation of the QT interval.34,39 As quinine therapy is often interrupted due to the above side effects, alternative regimens such as intravenous azithromycin or clindamycin in combination with oral atovaquone should be considered for severe cases.31 However, these regimens are not well studied.31
Partial or complete exchange transfusion of whole blood or packed red blood cells should be considered in patients with a high level of parasitemia (≥ 10%), severe anemia (hemoglobin < 10 g/dL), or renal, hepatic, or pulmonary compromise.31,32,34–36,39 In critically ill patients, parasitemia should be monitored daily until it has decreased to less than 5%.32,34,39
Generally, symptoms improve within 48 hours of antimicrobial therapy initiation; however, parasitemia may take up to 3 months to resolve.32,34,39 In severely immunocompromised patients, babesiosis may persist or relapse despite appropriate therapy.34,39,42,43 In these cases, at least 6 weeks of antimicrobial therapy is recommended, including 2 weeks of therapy after Babesia organisms are no longer seen on blood smear.31,33,36,39,42
TICKBORNE RELAPSING FEVER
The illness is transmitted by either ticks or body lice. The tick-borne illness is caused by spirochetes of the genus Borrelia and transmitted to humans by the bite of an infected Ornithodoros soft tick.45 Approximately 70% of reported cases in the United States occur in California, Washington, and Colorado.46 Most cases are caused by Borrelia hermsii and are linked to sleeping in rodent-infested cabins in mountainous areas.46 Remarkably, tick-borne borreliae are transmitted within about 30 seconds of tick attachment.47,48
The hallmark of tickborne relapsing fever is febrile episodes lasting 3 to 5 days, with relapses after 5 to 7 days of apparent recovery.49 If untreated, several episodes of fever and nonspecific symptoms will occur before illness resolves spontaneously. Overall mortality rates are very low (< 5%).50
Laboratory confirmation of tickborne relapsing fever is made by detecting spirochetes in a blood smear during a febrile episode or serologic antibody confirmation. However, serologic testing is unhelpful in the acute setting and can yield false-positive results with prior exposure to other Borrelia species (eg, Lyme disease) or other spirochetes. Serologic antibody testing with a 4-fold increase between acute and convalescent samples or PCR can aid in diagnosis, though the latter is available only in research settings.47
The preferred treatment regimen for adults is an oral tetracycline for 10 days. Erythromycin is recommended when tetracyclines are contraindicated.51
When starting treatment, all patients should be monitored closely for the Jarisch-Herxheimer reaction (rigors, hypotension, and high fevers), which develops in over 50% of cases as a result of rapid spirochetal killing and massive cytokine release.52
BORRELIA MIYAMOTOI INFECTION
The most common clinical manifestations are similar to other tickborne relapsing fever infections, although a true “relapsing fever” itself is not usually present.53 The characteristic erythema migrans rash often found in Lyme disease is typically absent in B miyamotoi infection; however, when present, it should prompt investigation into coinfection.54 Cases of meningoencephalitis have been reported in immunosuppressed hosts.55
There is currently no validated test available for diagnosis of B miyamotoi; however, PCR and serology are available in a few specialized laboratories.31,53
The treatment of choice is doxycycline for 2 to 4 weeks. Amoxicillin and ceftriaxone also appear effective.53
SOUTHERN TICK-ASSOCIATED RASH ILLNESS
Infection can present similarly to Lyme disease with an erythema migrans-like rash and associated flulike symptoms, although systemic symptoms and multiple erythema migrans lesions are less likely with STARI. Also, the erythema migrans-like lesions tend to be smaller and more likely to have central clearing than those in Lyme disease.57 Nevertheless, it is difficult to distinguish the 2 illnesses, especially in mid-Atlantic states such as Maryland or Virginia, where both diseases coexist. The most reliable method of distinguishing STARI from Lyme disease is demonstrating that the patient was bitten by a Lone Star tick rather than an Ixodes tick. Numerous questions remain unanswered about the causative organism, pathophysiology, definitive diagnosis, geographic range of illness, and most effective treatment for STARI.
Most reported cases have responded promptly to doxycycline, though it is not known whether antibiotic treatment is necessary.58
TULAREMIA
Ticks are thought to be the most important vectors, and most cases occur in the south-central United States.59 The geographic distribution of disease is gradually shifting northward due to spread of the major tick vectors, A americanum, D variabilis, and D andersoni. Approximately 100 to 200 cases of tularemia are diagnosed each year in the United States, with most concentrated in Kansas, Oklahoma, Missouri, and Arkansas.60
Humans can acquire F tularensis by several routes, and the route of infection ultimately dictates the clinical syndrome. Ulceroglandular and glandular forms of the disease are the most common in the United States, and both frequently result from a tick bite. A few days after tick exposure, an erythematous, often painful papuloulcerative lesion with a central eschar manifests at the site of the tick bite. Additional symptoms may include fever, chills, headache, myalgia, malaise, and suppurative lymphadenitis.61
Diagnosis can be made by identifying F tularensis in blood, fluid, or tissue culture performed under biosafety level 3 conditions; however, serology is used in most cases.62
Streptomycin and gentamicin are considered drugs of choice and should be continued for at least 10 days. For relatively mild disease, oral doxycycline or ciprofloxacin can be considered for at least 14 days, although the latter is not approved for treatment.59,63
TICKBORNE VIRAL INFECTIONS
Powassan virus, an uncommon flavivirus, is found in the Great Lakes region and northeast United States. In the Great Lakes region, I cookei ticks transmit the traditional lineage of this virus. However, more recent cases have been identified in the Northeast and Midwest, where Powassan virus lineage II (or deer tick virus) is transmitted by I scapularis.31,64
The classic presentation is a viral encephalitis. Rash (most often maculopapular) and gastrointestinal symptoms have been reported as well. A high index of suspicion is needed for diagnosis because clinical features and laboratory findings resemble those of other arboviral infections.
Treatment for Powassan viral encephalitis is supportive, although corticosteroids have been used with some success.64 While asymptomatic infection has been documented, the reported mortality rate of Powassan virus encephalitis is 10% to 15%, and focal neurologic deficits can persist among survivors.65
Clinical and laboratory features appear to be very similar to those of the ehrlichioses.1 A clinical diagnosis should be considered in patients with A americanum exposure, fever, and cytopenias who lack PCR or serologic evidence for ehrlichiosis infection or who fail to respond to doxycycline therapy.24
COINFECTION
Some tick vectors transmit more than 1 type of infection, and therefore, coinfection with multiple pathogens may occur. For example, I scapularis transmits Borrelia burgdorferi (Lyme disease), HGA, Babesia microti, B miyamotoi, E muris-like agent, and Powassan virus lineage II, while A americanum transmits HME and Heartland virus.24,26,31,34,36,67 Coinfection may increase the severity of disease, often due to a delay in diagnosis, though more research is needed to understand the clinical manifestations of coinfection.31,35,67
PREVENTION
Unfortunately, there are no available human vaccines for tickborne illnesses in the United States, and the effectiveness of single-dose prophylaxis with doxycycline for non-Lyme infections has not been evaluated.4,7,26
Illness is best prevented by minimizing skin exposure to ticks, use of tick repellents containing DEET, use of long-legged and long-sleeved clothing impregnated with an acaricide such as permethrin, and conducting timely body checks for ticks after potential exposure.1,31,32 Light-colored clothing is suggested, since it allows for better visibility of crawling ticks.4,32 Bathing or showering within 2 hours of tick exposure helps prevent attachment of ticks.4,31,68 If camping outside, use of a bed net is recommended.68
Ticks are most easily removed by grasping the head of the tick as close to the skin surface as possible with fine-tipped tweezers.32,68 Removing or crushing ticks with bare hands should be avoided to prevent potential contamination, and hands should be washed thoroughly after tick removal.1,4
Blood donors are screened for a history of symptomatic tickborne disease; however, asymptomatic donors who are not identified at screening pose the greatest risk to the blood supply. Babesia microti is the most common reported transfusion-transmitted parasite in the United States, and transmission of R rickettsii, A phagocytophilum, and E ewingii have also been reported infrequently.28,40,69 Currently, no test is approved to screen blood for tickborne illnesses, though such a test would help prevent transmission of tickborne illnesses by blood transfusion in areas where these diseases are endemic.40,41
TAKE-HOME POINTS
Tickborne illnesses are increasing throughout the United States as a result of vector expansion and changes in human ecology.
It is essential that primary care clinicians consider tickborne illnesses in the differential diagnosis for any patient presenting with a fever and constitutional symptoms when the cause of symptoms is unclear and tick exposure is possible or known.
All the diseases discussed are nationally notifiable conditions, and confirmed cases should be reported.
Knowledge of the geographic locations of potential exposure is paramount to determining which tickborne infections to consider, and the absence of a tick bite history should not exclude the diagnosis in the correct clinical presentation.
In addition, it is important to recognize the limitations of diagnostic testing for many tickborne infections; empiric treatment is most often warranted before confirming the diagnosis.
Tick avoidance is the most effective way to prevent these often severe infections.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44.
- Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182.
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg 2011; 85:124–131.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55:1–27.
- Mukkada S, Buckingham SC. Recognition of and prompt treatment for tick-borne infections in children. Infect Dis Clin North Am 2015; 29:539–555.
- Schutze GE, Buckingham SC, Marshall GS, et al; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J 2007; 26:475–479.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis 2007; 7:724–732.
- Lin L, Decker C. Rocky Mountain spotted fever. Dis Mon 2012; 58:361–369.
- Demma LJ, Traeger MS, Nicholson WL, et al. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594.
- Traeger MS, Regan JJ, Humpherys D, et al. Rocky mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1650–1658.
- Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 2012; 86:713–719.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702.
- Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1659–1666.
- Nelson R. Rocky Mountain spotted fever in Native Americans. Lancet Infect Dis 2015; 15:1013–1014.
- Botelho-Nevers E, Socolovschi C, Raoult D, Parola P. Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 2012; 10:1425–1437.
- Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv 2013; 68:635–649.
- Paddock CD, Finley RW, Wright CS, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 2008; 47:1188–1196.
- Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 2010; 50:541–548.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 2007; 45(suppl 1):S45–S51.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 2009; 7:709–722.
- Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol 2012; 7:719–731.
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg 2015; 93:66–72.
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med 2016; 94:52–60.
- Wormser GP, Pritt B. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 2015; 29:371–381.
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011; 365:422–429.
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2015; 29:341–355.
- St Clair K, Decker CF. Ehrlichioses: anaplasmosis and human ehrlichiosis. Dis Mon 2012; 58:346–354.
- Centers for Disease Control and Prevention (CDC). Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1145–1148.
- Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 2007; 45:589–593.
- Stone JH, Dierberg K, Aram G, Dumler JS. Human monocytic ehrlichiosis. JAMA 2004; 292:2263–2270.
- Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. JAMA 2016; 315:1767–1777.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134.
- Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005; 11:1828–1834.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–370.
- Kavanaugh MJ, Decker CF. Babesiosis. Dis Mon 2012; 58:355–360.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–488.
- Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis 2014; 20:225–231.
- Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014; 9:e115494.
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–2407.
- Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–519.
- Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 2011; 124:800–805.
- Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–376.
- Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–386.
- Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–1458.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): distribution. www.cdc.gov/relapsing-fever/distribution/index.html. Accessed June 7, 2017.
- Forrester JD, Kjemtrup AM, Fritz CL, et al; Centers for Disease Control and Prevention (CDC). Tickborne relapsing fever—United States, 1990-2011. MMWR Morb Mortal Wkly Rep 2015; 64:58–60.
- Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am 2008; 22:449–468.
- Anderson JF. The natural history of ticks. Med Clin North Am 2002; 86:205–218.
- Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 1990; 44:155–171.
- Centers for Disease Control and Prevention (CDC). Acute respiratory distress syndrome in persons with tickborne relapsing fever—three states, 2004-2005. MMWR Morb Mortal Wkly Rep 2007; 56:1073–1076.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): information for clinicians. www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed June 7, 2017.
- Dworkin MS, Anderson DE Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis 1998; 26:122–131.
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 2015; 31:260–269.
- Krause PJ, Narasimhan S, Wormser GP, et al; Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014; 20:1183–1190.
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 2013; 368:240–245.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376.
- Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–965.
- Feder HM Jr, Hoss DM, Zemel L, Telford SR 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146.
- Carvalho CL, Lopes de Carvalho I, Ze-Ze L, Nuncio MS, Duarte EL. Tularaemia: a challenging zoonosis. Comp Immunol Microbiol Infect Dis 2014; 37):85–96.
- Centers for Disease Control and Prevention (CDC). Tularemia: statistics. www.cdc.gov/tularemia/statistics/index.html. Accessed June 7, 2017.
- Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–1290.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22:489–504.
- Johansson A, Berglund L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–268.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–713.
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110.
- Pastula DM, Turabelidze G, Yates KF, et al; Centers for Disease Control and Prevention (CDC). Notes from the field: heartland virus disease—United States, 2012-–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–271.
- Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015:587131.
- Pujalte GG, Chua JV. Tick-borne infections in the United States. Prim Care 2013; 40:619–635.
- Regan J, Matthias J, Green-Murphy A, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013; 56:e105–e107.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44.
- Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182.
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg 2011; 85:124–131.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55:1–27.
- Mukkada S, Buckingham SC. Recognition of and prompt treatment for tick-borne infections in children. Infect Dis Clin North Am 2015; 29:539–555.
- Schutze GE, Buckingham SC, Marshall GS, et al; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J 2007; 26:475–479.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis 2007; 7:724–732.
- Lin L, Decker C. Rocky Mountain spotted fever. Dis Mon 2012; 58:361–369.
- Demma LJ, Traeger MS, Nicholson WL, et al. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594.
- Traeger MS, Regan JJ, Humpherys D, et al. Rocky mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1650–1658.
- Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 2012; 86:713–719.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702.
- Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1659–1666.
- Nelson R. Rocky Mountain spotted fever in Native Americans. Lancet Infect Dis 2015; 15:1013–1014.
- Botelho-Nevers E, Socolovschi C, Raoult D, Parola P. Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 2012; 10:1425–1437.
- Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv 2013; 68:635–649.
- Paddock CD, Finley RW, Wright CS, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 2008; 47:1188–1196.
- Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 2010; 50:541–548.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 2007; 45(suppl 1):S45–S51.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 2009; 7:709–722.
- Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol 2012; 7:719–731.
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg 2015; 93:66–72.
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med 2016; 94:52–60.
- Wormser GP, Pritt B. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 2015; 29:371–381.
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011; 365:422–429.
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2015; 29:341–355.
- St Clair K, Decker CF. Ehrlichioses: anaplasmosis and human ehrlichiosis. Dis Mon 2012; 58:346–354.
- Centers for Disease Control and Prevention (CDC). Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1145–1148.
- Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 2007; 45:589–593.
- Stone JH, Dierberg K, Aram G, Dumler JS. Human monocytic ehrlichiosis. JAMA 2004; 292:2263–2270.
- Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. JAMA 2016; 315:1767–1777.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134.
- Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005; 11:1828–1834.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–370.
- Kavanaugh MJ, Decker CF. Babesiosis. Dis Mon 2012; 58:355–360.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–488.
- Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis 2014; 20:225–231.
- Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014; 9:e115494.
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–2407.
- Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–519.
- Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 2011; 124:800–805.
- Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–376.
- Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–386.
- Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–1458.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): distribution. www.cdc.gov/relapsing-fever/distribution/index.html. Accessed June 7, 2017.
- Forrester JD, Kjemtrup AM, Fritz CL, et al; Centers for Disease Control and Prevention (CDC). Tickborne relapsing fever—United States, 1990-2011. MMWR Morb Mortal Wkly Rep 2015; 64:58–60.
- Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am 2008; 22:449–468.
- Anderson JF. The natural history of ticks. Med Clin North Am 2002; 86:205–218.
- Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 1990; 44:155–171.
- Centers for Disease Control and Prevention (CDC). Acute respiratory distress syndrome in persons with tickborne relapsing fever—three states, 2004-2005. MMWR Morb Mortal Wkly Rep 2007; 56:1073–1076.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): information for clinicians. www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed June 7, 2017.
- Dworkin MS, Anderson DE Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis 1998; 26:122–131.
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 2015; 31:260–269.
- Krause PJ, Narasimhan S, Wormser GP, et al; Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014; 20:1183–1190.
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 2013; 368:240–245.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376.
- Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–965.
- Feder HM Jr, Hoss DM, Zemel L, Telford SR 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146.
- Carvalho CL, Lopes de Carvalho I, Ze-Ze L, Nuncio MS, Duarte EL. Tularaemia: a challenging zoonosis. Comp Immunol Microbiol Infect Dis 2014; 37):85–96.
- Centers for Disease Control and Prevention (CDC). Tularemia: statistics. www.cdc.gov/tularemia/statistics/index.html. Accessed June 7, 2017.
- Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–1290.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22:489–504.
- Johansson A, Berglund L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–268.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–713.
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110.
- Pastula DM, Turabelidze G, Yates KF, et al; Centers for Disease Control and Prevention (CDC). Notes from the field: heartland virus disease—United States, 2012-–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–271.
- Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015:587131.
- Pujalte GG, Chua JV. Tick-borne infections in the United States. Prim Care 2013; 40:619–635.
- Regan J, Matthias J, Green-Murphy A, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013; 56:e105–e107.
KEY POINTS
- Tickborne illnesses should be considered in patients with known or potential tick exposure presenting with fever or vague constitutional symptoms in tick-endemic regions.
- Given that tick-bite history is commonly unknown, absence of a known tick bite does not exclude the diagnosis of a tick-borne illness.
- Starting empiric treatment is usually warranted before the diagnosis of tickborne illness is confirmed.
- Tick avoidance is the most effective measure for preventing tickborne infections.