User login
Leveraging the Million Veteran Program Infrastructure and Data for a Rapid Research Response to COVID-19
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
Preventing cardiovascular disease in older adults: One size does not fit all
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
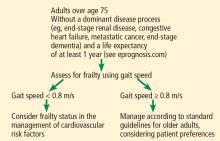
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
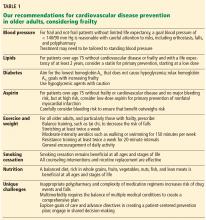
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
KEY POINTS
- With the aging of the population, individualized prevention strategies must incorporate geriatric syndromes such as frailty.
- However, current guidelines and available evidence for cardiovascular disease prevention strategies have not incorporated frailty or make no recommendation at all for those over age 75.
- Four-meter gait speed, a simple measure of physical function and a proxy for frailty, can be used clinically to diagnose frailty.