User login
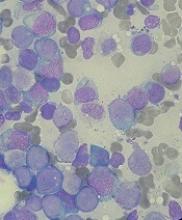
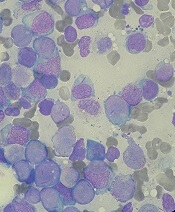
Older people with CHIP are safe donor source for HSCT
New research suggests older individuals with clonal hematopoiesis of indeterminate potential (CHIP) are a safe donor source for allogeneic hematopoietic stem cell transplant (HSCT).
Researchers found that transplants from older donors with CHIP resulted in similar survival as transplants from older donors without CHIP.
HSCT recipients with CHIP donors had a lower risk of relapse or progression but a higher risk of chronic graft-versus-host disease (GHVD), and they were more likely to develop donor-cell malignancies.
Frederik Damm, MD, of Charité – University Medical Center Berlin in Germany, and his colleagues reported these findings in the Journal of Clinical Oncology.
Little is known about the influence of donor CHIP in allogeneic HSCT, according to the researchers.
However, an increasing number of older patients are receiving donations from older, mostly sibling, donors who may have CHIP. CHIP increases with age and is associated with a greater risk of hematologic cancers, cardiovascular disease, and death from coronary heart disease.
With this in mind, Dr. Damm and his colleagues set out to determine how donor CHIP affects the outcome of HSCT.
Between 1993 and 2017, the researchers collected blood samples from 500 healthy, related HSCT donors, age 55 or older, at 10 transplant centers in Germany and France. The researchers sequenced the samples with a 66-gene panel.
The team also assessed overall survival (OS), non-relapse mortality (NRM), cumulative incidence of relapse/progression (CIR/P), cytomegalovirus (CMV) reactivation, and acute and chronic GVHD in the HSCT recipients.
Mutation analysis
The researchers identified 92 clonal mutations in 80 (16.0%) of the 500 donors. The variant allele frequency was 5.9% (range, 2% to 43%). In 20 patients, 25 variant alleles were present at a frequency of 10% or greater.
The researchers detected a single gene mutation in 70 donors, nine donors had two mutations, and one donor had four mutations. The most frequently mutated genes were DNMT3A (40/500, 8%), TET2 (11/500, 2.2%), and ASXL1 (7/500, 1.4%).
Baseline characteristics such as age, sex, or stem cells harvested per kilogram of body weight were similar between donors with and without CHIP.
Recipient characteristics were also evenly distributed regarding donor CHIP status and baseline characteristics.
However, the researchers noted that donor CHIP was present significantly more often in donors for recipients with myeloid compared to lymphoid neoplasms—19.2% and 6.3%, respectively (P≤0.001).
The prevalence of donor CHIP increased with age, from 10.3% (ages 60 to 64) to 20.3% (ages 65 to 69), 22.5% (ages 70 to 74), and 28.6% (ages 75 to 79).
The researchers observed no statistically significant association between CHIP and age, most likely, they wrote, “because 82% of all donors in our cohort were age 60 to 69 years.”
Transplant outcome
Donor CHIP led to slightly faster leukocyte engraftment, although it did not impact thrombocyte engraftment time.
The cumulative incidence of leukocyte engraftment after 15 days was 64.1% for HSCT recipients with CHIP donors and 51.4% for those with non-CHIP donors (P=0.023).
Chronic GVHD after HSCT was more likely for recipients with CHIP donors, with a 5-year cumulative incidence of 52.9%, compared to 35.7% for patients with non-CHIP donors (P=0.008).
Multivariate analysis showed donor CHIP to be an independent risk factor for chronic GVHD when adjusted for antithymocyte globulin application and donor age.
The researchers identified donor DNMT3A mutation as the predominant CHIP factor for developing chronic GVHD. The incidence of chronic GVHD was 58.5% in patients with DNMT3A-mutated donors and 36.6% in patients with wild-type donors (P=0.006).
The researchers noted that no other CHIP mutations or characteristics affected the development of chronic GVHD.
Donor CHIP status did not impact the incidence of acute GVHD or CMV reactivation.
Transplant recipients with CHIP donors had a lower CIR/P (P=0.027) than recipients with non-CHIP donors. Patients whose donors had CHIP with a DNMT3A mutation also had a lower CIR/P (P=0.029).
Donor CHIP had no effect on NRM.
Neither donor CHIP nor donor DNMT3A mutational status impacted CIR/P or NRM in patients who underwent HSCT while they were in complete response (CR).
However, patients not in CR at the time of HSCT had a lower CIR/P when the donor was CHIP-positive (hazard ratio [HR]=0.49; 95% confidence interval [CI], 0.27 to 0.88; P=0.019) or had a DNMT3A mutation (HR=0.34; 95% CI, 0.15 to 0.81; P=0.015).
Two of 82 patients with CHIP donors developed donor-cell malignancies, but there were no donor-cell malignancies in recipients with non-CHIP donors (P=0.026).
Survival
“To our knowledge, we showed, for the first time, that donor CHIP did not affect the survival of 500 HSCT recipients,” the researchers wrote.
At a median follow-up of 3.3 years, the median OS was 2 years, and the 5-year OS was 37.6%.
The researchers observed no survival differences in patients with CHIP donors or non-CHIP donors (HR=0.88; 95% CI, 0.65 to 1.321; P=0.434). And the same was true for DNMT3A mutation (HR=0.89; 95% CI, 0.58 to 1.35; P=0.573).
The researchers pointed out that patients with myelodysplastic syndromes or acute myeloid leukemia who were not in CR when they underwent HSCT had a survival benefit with donor CHIP (HR=0.49; 95% CI, 0.28 to 0.86; P=0.011).
“This finding could implicate an important clinical potential in this difficult-to-treat subgroup,” the researchers noted.
However, this was not the case for patients with myeloproliferative neoplasia, for whom donor CHIP tended to reduce survival (P=0.077).
The research team recommended future studies be conducted in younger and unrelated donors to confirm the results of this study.
The research was funded by numerous grants and fellowships, and the researchers reported no relevant conflicts of interest.
New research suggests older individuals with clonal hematopoiesis of indeterminate potential (CHIP) are a safe donor source for allogeneic hematopoietic stem cell transplant (HSCT).
Researchers found that transplants from older donors with CHIP resulted in similar survival as transplants from older donors without CHIP.
HSCT recipients with CHIP donors had a lower risk of relapse or progression but a higher risk of chronic graft-versus-host disease (GHVD), and they were more likely to develop donor-cell malignancies.
Frederik Damm, MD, of Charité – University Medical Center Berlin in Germany, and his colleagues reported these findings in the Journal of Clinical Oncology.
Little is known about the influence of donor CHIP in allogeneic HSCT, according to the researchers.
However, an increasing number of older patients are receiving donations from older, mostly sibling, donors who may have CHIP. CHIP increases with age and is associated with a greater risk of hematologic cancers, cardiovascular disease, and death from coronary heart disease.
With this in mind, Dr. Damm and his colleagues set out to determine how donor CHIP affects the outcome of HSCT.
Between 1993 and 2017, the researchers collected blood samples from 500 healthy, related HSCT donors, age 55 or older, at 10 transplant centers in Germany and France. The researchers sequenced the samples with a 66-gene panel.
The team also assessed overall survival (OS), non-relapse mortality (NRM), cumulative incidence of relapse/progression (CIR/P), cytomegalovirus (CMV) reactivation, and acute and chronic GVHD in the HSCT recipients.
Mutation analysis
The researchers identified 92 clonal mutations in 80 (16.0%) of the 500 donors. The variant allele frequency was 5.9% (range, 2% to 43%). In 20 patients, 25 variant alleles were present at a frequency of 10% or greater.
The researchers detected a single gene mutation in 70 donors, nine donors had two mutations, and one donor had four mutations. The most frequently mutated genes were DNMT3A (40/500, 8%), TET2 (11/500, 2.2%), and ASXL1 (7/500, 1.4%).
Baseline characteristics such as age, sex, or stem cells harvested per kilogram of body weight were similar between donors with and without CHIP.
Recipient characteristics were also evenly distributed regarding donor CHIP status and baseline characteristics.
However, the researchers noted that donor CHIP was present significantly more often in donors for recipients with myeloid compared to lymphoid neoplasms—19.2% and 6.3%, respectively (P≤0.001).
The prevalence of donor CHIP increased with age, from 10.3% (ages 60 to 64) to 20.3% (ages 65 to 69), 22.5% (ages 70 to 74), and 28.6% (ages 75 to 79).
The researchers observed no statistically significant association between CHIP and age, most likely, they wrote, “because 82% of all donors in our cohort were age 60 to 69 years.”
Transplant outcome
Donor CHIP led to slightly faster leukocyte engraftment, although it did not impact thrombocyte engraftment time.
The cumulative incidence of leukocyte engraftment after 15 days was 64.1% for HSCT recipients with CHIP donors and 51.4% for those with non-CHIP donors (P=0.023).
Chronic GVHD after HSCT was more likely for recipients with CHIP donors, with a 5-year cumulative incidence of 52.9%, compared to 35.7% for patients with non-CHIP donors (P=0.008).
Multivariate analysis showed donor CHIP to be an independent risk factor for chronic GVHD when adjusted for antithymocyte globulin application and donor age.
The researchers identified donor DNMT3A mutation as the predominant CHIP factor for developing chronic GVHD. The incidence of chronic GVHD was 58.5% in patients with DNMT3A-mutated donors and 36.6% in patients with wild-type donors (P=0.006).
The researchers noted that no other CHIP mutations or characteristics affected the development of chronic GVHD.
Donor CHIP status did not impact the incidence of acute GVHD or CMV reactivation.
Transplant recipients with CHIP donors had a lower CIR/P (P=0.027) than recipients with non-CHIP donors. Patients whose donors had CHIP with a DNMT3A mutation also had a lower CIR/P (P=0.029).
Donor CHIP had no effect on NRM.
Neither donor CHIP nor donor DNMT3A mutational status impacted CIR/P or NRM in patients who underwent HSCT while they were in complete response (CR).
However, patients not in CR at the time of HSCT had a lower CIR/P when the donor was CHIP-positive (hazard ratio [HR]=0.49; 95% confidence interval [CI], 0.27 to 0.88; P=0.019) or had a DNMT3A mutation (HR=0.34; 95% CI, 0.15 to 0.81; P=0.015).
Two of 82 patients with CHIP donors developed donor-cell malignancies, but there were no donor-cell malignancies in recipients with non-CHIP donors (P=0.026).
Survival
“To our knowledge, we showed, for the first time, that donor CHIP did not affect the survival of 500 HSCT recipients,” the researchers wrote.
At a median follow-up of 3.3 years, the median OS was 2 years, and the 5-year OS was 37.6%.
The researchers observed no survival differences in patients with CHIP donors or non-CHIP donors (HR=0.88; 95% CI, 0.65 to 1.321; P=0.434). And the same was true for DNMT3A mutation (HR=0.89; 95% CI, 0.58 to 1.35; P=0.573).
The researchers pointed out that patients with myelodysplastic syndromes or acute myeloid leukemia who were not in CR when they underwent HSCT had a survival benefit with donor CHIP (HR=0.49; 95% CI, 0.28 to 0.86; P=0.011).
“This finding could implicate an important clinical potential in this difficult-to-treat subgroup,” the researchers noted.
However, this was not the case for patients with myeloproliferative neoplasia, for whom donor CHIP tended to reduce survival (P=0.077).
The research team recommended future studies be conducted in younger and unrelated donors to confirm the results of this study.
The research was funded by numerous grants and fellowships, and the researchers reported no relevant conflicts of interest.
New research suggests older individuals with clonal hematopoiesis of indeterminate potential (CHIP) are a safe donor source for allogeneic hematopoietic stem cell transplant (HSCT).
Researchers found that transplants from older donors with CHIP resulted in similar survival as transplants from older donors without CHIP.
HSCT recipients with CHIP donors had a lower risk of relapse or progression but a higher risk of chronic graft-versus-host disease (GHVD), and they were more likely to develop donor-cell malignancies.
Frederik Damm, MD, of Charité – University Medical Center Berlin in Germany, and his colleagues reported these findings in the Journal of Clinical Oncology.
Little is known about the influence of donor CHIP in allogeneic HSCT, according to the researchers.
However, an increasing number of older patients are receiving donations from older, mostly sibling, donors who may have CHIP. CHIP increases with age and is associated with a greater risk of hematologic cancers, cardiovascular disease, and death from coronary heart disease.
With this in mind, Dr. Damm and his colleagues set out to determine how donor CHIP affects the outcome of HSCT.
Between 1993 and 2017, the researchers collected blood samples from 500 healthy, related HSCT donors, age 55 or older, at 10 transplant centers in Germany and France. The researchers sequenced the samples with a 66-gene panel.
The team also assessed overall survival (OS), non-relapse mortality (NRM), cumulative incidence of relapse/progression (CIR/P), cytomegalovirus (CMV) reactivation, and acute and chronic GVHD in the HSCT recipients.
Mutation analysis
The researchers identified 92 clonal mutations in 80 (16.0%) of the 500 donors. The variant allele frequency was 5.9% (range, 2% to 43%). In 20 patients, 25 variant alleles were present at a frequency of 10% or greater.
The researchers detected a single gene mutation in 70 donors, nine donors had two mutations, and one donor had four mutations. The most frequently mutated genes were DNMT3A (40/500, 8%), TET2 (11/500, 2.2%), and ASXL1 (7/500, 1.4%).
Baseline characteristics such as age, sex, or stem cells harvested per kilogram of body weight were similar between donors with and without CHIP.
Recipient characteristics were also evenly distributed regarding donor CHIP status and baseline characteristics.
However, the researchers noted that donor CHIP was present significantly more often in donors for recipients with myeloid compared to lymphoid neoplasms—19.2% and 6.3%, respectively (P≤0.001).
The prevalence of donor CHIP increased with age, from 10.3% (ages 60 to 64) to 20.3% (ages 65 to 69), 22.5% (ages 70 to 74), and 28.6% (ages 75 to 79).
The researchers observed no statistically significant association between CHIP and age, most likely, they wrote, “because 82% of all donors in our cohort were age 60 to 69 years.”
Transplant outcome
Donor CHIP led to slightly faster leukocyte engraftment, although it did not impact thrombocyte engraftment time.
The cumulative incidence of leukocyte engraftment after 15 days was 64.1% for HSCT recipients with CHIP donors and 51.4% for those with non-CHIP donors (P=0.023).
Chronic GVHD after HSCT was more likely for recipients with CHIP donors, with a 5-year cumulative incidence of 52.9%, compared to 35.7% for patients with non-CHIP donors (P=0.008).
Multivariate analysis showed donor CHIP to be an independent risk factor for chronic GVHD when adjusted for antithymocyte globulin application and donor age.
The researchers identified donor DNMT3A mutation as the predominant CHIP factor for developing chronic GVHD. The incidence of chronic GVHD was 58.5% in patients with DNMT3A-mutated donors and 36.6% in patients with wild-type donors (P=0.006).
The researchers noted that no other CHIP mutations or characteristics affected the development of chronic GVHD.
Donor CHIP status did not impact the incidence of acute GVHD or CMV reactivation.
Transplant recipients with CHIP donors had a lower CIR/P (P=0.027) than recipients with non-CHIP donors. Patients whose donors had CHIP with a DNMT3A mutation also had a lower CIR/P (P=0.029).
Donor CHIP had no effect on NRM.
Neither donor CHIP nor donor DNMT3A mutational status impacted CIR/P or NRM in patients who underwent HSCT while they were in complete response (CR).
However, patients not in CR at the time of HSCT had a lower CIR/P when the donor was CHIP-positive (hazard ratio [HR]=0.49; 95% confidence interval [CI], 0.27 to 0.88; P=0.019) or had a DNMT3A mutation (HR=0.34; 95% CI, 0.15 to 0.81; P=0.015).
Two of 82 patients with CHIP donors developed donor-cell malignancies, but there were no donor-cell malignancies in recipients with non-CHIP donors (P=0.026).
Survival
“To our knowledge, we showed, for the first time, that donor CHIP did not affect the survival of 500 HSCT recipients,” the researchers wrote.
At a median follow-up of 3.3 years, the median OS was 2 years, and the 5-year OS was 37.6%.
The researchers observed no survival differences in patients with CHIP donors or non-CHIP donors (HR=0.88; 95% CI, 0.65 to 1.321; P=0.434). And the same was true for DNMT3A mutation (HR=0.89; 95% CI, 0.58 to 1.35; P=0.573).
The researchers pointed out that patients with myelodysplastic syndromes or acute myeloid leukemia who were not in CR when they underwent HSCT had a survival benefit with donor CHIP (HR=0.49; 95% CI, 0.28 to 0.86; P=0.011).
“This finding could implicate an important clinical potential in this difficult-to-treat subgroup,” the researchers noted.
However, this was not the case for patients with myeloproliferative neoplasia, for whom donor CHIP tended to reduce survival (P=0.077).
The research team recommended future studies be conducted in younger and unrelated donors to confirm the results of this study.
The research was funded by numerous grants and fellowships, and the researchers reported no relevant conflicts of interest.
EC approves pegfilgrastim biosimilar
The European Commission (EC) has approved Mundipharma’s pegfilgrastim product Pelmeg, a biosimilar of Amgen’s Neulasta.
Pelmeg is approved for use in reducing the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy for malignancies, with the exceptions of chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of Pelmeg was supported by research showing pharmacokinetic comparability between Pelmeg and Neulasta at a dose of 6 mg, pharmacodynamic comparability at doses of 6 mg and 3 mg, and no clinically meaningful differences in the safety and immunogenicity profiles of Pelmeg and Neulasta.1,2,3
1. Roth K. et al. Demonstration of pharmacokinetic and pharmacodynamic comparability in healthy volunteers for B12019, a proposed pegfilgrastim biosimilar. ECCO 2017, abstract 241.
2. Roth K. et al. Comparability of pharmacodynamics and immunogenicity of B12019, a proposed pegfilgrastim biosimilar to Neulasta®. ASH 2017, abstract 1002.
3. Roth K. et al. Pharmacokinetic and pharmacodynamic comparability of B12019, a proposed pegfilgrastim biosimilar. ESMO 2017, poster 1573.
The European Commission (EC) has approved Mundipharma’s pegfilgrastim product Pelmeg, a biosimilar of Amgen’s Neulasta.
Pelmeg is approved for use in reducing the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy for malignancies, with the exceptions of chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of Pelmeg was supported by research showing pharmacokinetic comparability between Pelmeg and Neulasta at a dose of 6 mg, pharmacodynamic comparability at doses of 6 mg and 3 mg, and no clinically meaningful differences in the safety and immunogenicity profiles of Pelmeg and Neulasta.1,2,3
1. Roth K. et al. Demonstration of pharmacokinetic and pharmacodynamic comparability in healthy volunteers for B12019, a proposed pegfilgrastim biosimilar. ECCO 2017, abstract 241.
2. Roth K. et al. Comparability of pharmacodynamics and immunogenicity of B12019, a proposed pegfilgrastim biosimilar to Neulasta®. ASH 2017, abstract 1002.
3. Roth K. et al. Pharmacokinetic and pharmacodynamic comparability of B12019, a proposed pegfilgrastim biosimilar. ESMO 2017, poster 1573.
The European Commission (EC) has approved Mundipharma’s pegfilgrastim product Pelmeg, a biosimilar of Amgen’s Neulasta.
Pelmeg is approved for use in reducing the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy for malignancies, with the exceptions of chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of Pelmeg was supported by research showing pharmacokinetic comparability between Pelmeg and Neulasta at a dose of 6 mg, pharmacodynamic comparability at doses of 6 mg and 3 mg, and no clinically meaningful differences in the safety and immunogenicity profiles of Pelmeg and Neulasta.1,2,3
1. Roth K. et al. Demonstration of pharmacokinetic and pharmacodynamic comparability in healthy volunteers for B12019, a proposed pegfilgrastim biosimilar. ECCO 2017, abstract 241.
2. Roth K. et al. Comparability of pharmacodynamics and immunogenicity of B12019, a proposed pegfilgrastim biosimilar to Neulasta®. ASH 2017, abstract 1002.
3. Roth K. et al. Pharmacokinetic and pharmacodynamic comparability of B12019, a proposed pegfilgrastim biosimilar. ESMO 2017, poster 1573.
FDA grants priority review to quizartinib
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
ASH expands late-breaking abstract session
An additional presentation has been added to the late-breaking abstract session of the 2018 ASH Annual Meeting.
The session was expanded from six abstracts to seven this year because of a record number of “exciting” submissions, according to ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland.
“We received 98 late-breaking abstracts, which is a record,” Dr. Brodsky said.
“They were so exciting this year that we added a seventh, and, quite frankly, we could have added several more, but we just didn’t have time in the meeting.”
Dr. Brodsky discussed this year’s late-breaking abstracts during a recent press briefing.
Abstract LBA-1 reports results of rivaroxaban thromboprophylaxis in cancer patients with an increased risk of venous thromboembolism (VTE). Compared to placebo, rivaroxaban significantly reduced VTE and VTE-related death during treatment but not over the entire study period.
Abstract LBA-2 describes a phase 3, randomized trial comparing daratumumab plus lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who are ineligible for transplant. The addition of daratumumab reduced the risk of disease progression or death by 45%.
Abstract LBA-3 details results with HemoTypeSC, a test used to detect sickle cell trait and sickle cell disease. The test correctly identified all phenotypes in the 1,000 children studied.
Abstract LBA-4 describes a randomized, phase 3 study comparing ibrutinib plus rituximab to fludarabine, cyclophosphamide, and rituximab in younger patients with untreated chronic lymphocytic leukemia (CLL). Investigators found that ibrutinib plus rituximab provided significantly better progression-free and overall survival than the three-drug combination.
Abstract LBA-5 details a strategy for direct oral anticoagulant use in patients with atrial fibrillation undergoing surgery. Investigators say the strategy is likely to be “practice-changing” and incorporated into guidelines.
Abstract LBA-6 covers a trial of emapalumab in patients with primary hemophagocytic lymphohistiocytosis. Emapalumab, which was recently approved by the U.S. Food and Drug Administration, produced responses in most of the 34 patients studied and had a favorable safety profile, according to investigators.
Abstract LBA-7 reports the discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax in patients with progressive CLL. Investigators say this mutation could be a biomarker of CLL relapse.
An additional presentation has been added to the late-breaking abstract session of the 2018 ASH Annual Meeting.
The session was expanded from six abstracts to seven this year because of a record number of “exciting” submissions, according to ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland.
“We received 98 late-breaking abstracts, which is a record,” Dr. Brodsky said.
“They were so exciting this year that we added a seventh, and, quite frankly, we could have added several more, but we just didn’t have time in the meeting.”
Dr. Brodsky discussed this year’s late-breaking abstracts during a recent press briefing.
Abstract LBA-1 reports results of rivaroxaban thromboprophylaxis in cancer patients with an increased risk of venous thromboembolism (VTE). Compared to placebo, rivaroxaban significantly reduced VTE and VTE-related death during treatment but not over the entire study period.
Abstract LBA-2 describes a phase 3, randomized trial comparing daratumumab plus lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who are ineligible for transplant. The addition of daratumumab reduced the risk of disease progression or death by 45%.
Abstract LBA-3 details results with HemoTypeSC, a test used to detect sickle cell trait and sickle cell disease. The test correctly identified all phenotypes in the 1,000 children studied.
Abstract LBA-4 describes a randomized, phase 3 study comparing ibrutinib plus rituximab to fludarabine, cyclophosphamide, and rituximab in younger patients with untreated chronic lymphocytic leukemia (CLL). Investigators found that ibrutinib plus rituximab provided significantly better progression-free and overall survival than the three-drug combination.
Abstract LBA-5 details a strategy for direct oral anticoagulant use in patients with atrial fibrillation undergoing surgery. Investigators say the strategy is likely to be “practice-changing” and incorporated into guidelines.
Abstract LBA-6 covers a trial of emapalumab in patients with primary hemophagocytic lymphohistiocytosis. Emapalumab, which was recently approved by the U.S. Food and Drug Administration, produced responses in most of the 34 patients studied and had a favorable safety profile, according to investigators.
Abstract LBA-7 reports the discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax in patients with progressive CLL. Investigators say this mutation could be a biomarker of CLL relapse.
An additional presentation has been added to the late-breaking abstract session of the 2018 ASH Annual Meeting.
The session was expanded from six abstracts to seven this year because of a record number of “exciting” submissions, according to ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland.
“We received 98 late-breaking abstracts, which is a record,” Dr. Brodsky said.
“They were so exciting this year that we added a seventh, and, quite frankly, we could have added several more, but we just didn’t have time in the meeting.”
Dr. Brodsky discussed this year’s late-breaking abstracts during a recent press briefing.
Abstract LBA-1 reports results of rivaroxaban thromboprophylaxis in cancer patients with an increased risk of venous thromboembolism (VTE). Compared to placebo, rivaroxaban significantly reduced VTE and VTE-related death during treatment but not over the entire study period.
Abstract LBA-2 describes a phase 3, randomized trial comparing daratumumab plus lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who are ineligible for transplant. The addition of daratumumab reduced the risk of disease progression or death by 45%.
Abstract LBA-3 details results with HemoTypeSC, a test used to detect sickle cell trait and sickle cell disease. The test correctly identified all phenotypes in the 1,000 children studied.
Abstract LBA-4 describes a randomized, phase 3 study comparing ibrutinib plus rituximab to fludarabine, cyclophosphamide, and rituximab in younger patients with untreated chronic lymphocytic leukemia (CLL). Investigators found that ibrutinib plus rituximab provided significantly better progression-free and overall survival than the three-drug combination.
Abstract LBA-5 details a strategy for direct oral anticoagulant use in patients with atrial fibrillation undergoing surgery. Investigators say the strategy is likely to be “practice-changing” and incorporated into guidelines.
Abstract LBA-6 covers a trial of emapalumab in patients with primary hemophagocytic lymphohistiocytosis. Emapalumab, which was recently approved by the U.S. Food and Drug Administration, produced responses in most of the 34 patients studied and had a favorable safety profile, according to investigators.
Abstract LBA-7 reports the discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax in patients with progressive CLL. Investigators say this mutation could be a biomarker of CLL relapse.
Americans concerned about cost of cancer care
A recent survey suggests Americans are nearly as worried about the cost of a cancer diagnosis as they are about dying from cancer.
The cost of cancer care was a top concern even among people who had no prior experience with cancer.
At the same time, cancer patients/survivors admitted to delaying or forgoing care due to costs, and caregivers reported taking “dramatic” actions to pay for their loved one’s care.
These are findings from the American Society of Clinical Oncology (ASCO)’s second annual National Cancer Opinion Survey.
The survey was conducted online by The Harris Poll from July 10, 2018, to August 10, 2018. It included 4,887 U.S. adults age 18 and older—1,001 of whom have or had cancer.
Cost among top concerns
Death and pain/suffering were the top concerns related to a cancer diagnosis. Fifty-four percent of respondents said death would be one of their greatest concerns if they were diagnosed with cancer, and the same percentage rated pain/suffering a top concern.
Forty-four percent of respondents said paying for cancer treatment would be a top concern, and 45% said the same about the financial impact of a cancer diagnosis on their family. When combined, financial issues were a top concern for 57% of respondents.
Paying for treatment was a top concern for:
- 36% of respondents who had/have cancer
- 51% of caregivers
- 43% of people with no prior cancer experience.
The financial impact on family was a top concern for:
- 39% of respondents who had/have cancer
- 55% of caregivers
- 42% of people with no prior cancer experience.
Cutting costs
Sixty-one percent of caregivers surveyed said they or another relative have taken a “dramatic” step to help pay for their loved one’s care, including:
- Dipping into savings accounts (35%)
- Working extra hours (23%)
- Taking an early withdrawal from a retirement account or college fund (14%)
- Postponing retirement (14%)
- Taking out a second mortgage or other type of loan (13%)
- Taking an additional job (13%)
- Selling family heirlooms (9%).
Twenty percent of cancer patients/survivors said they have taken actions to reduce treatment costs, including:
- Delaying scans (7%)
- Skipping or delaying appointments (7%)
- Skipping doses of prescribed treatment (6%)
- Postponing or not filling prescriptions (5%)
- Refusing treatment (3%).
“Patients are right to be concerned about the financial impact of a cancer diagnosis on their families,” said Richard L. Schilsky, MD, ASCO’s chief medical officer.
“It’s clear that high treatment costs are taking a serious toll not only on patients, but also on the people who care for them. If a family member has been diagnosed with cancer, the sole focus should be helping them get well. Instead, Americans are worrying about affording treatment, and, in many cases, they’re making serious personal sacrifices to help pay for their loved ones’ care.”
A recent survey suggests Americans are nearly as worried about the cost of a cancer diagnosis as they are about dying from cancer.
The cost of cancer care was a top concern even among people who had no prior experience with cancer.
At the same time, cancer patients/survivors admitted to delaying or forgoing care due to costs, and caregivers reported taking “dramatic” actions to pay for their loved one’s care.
These are findings from the American Society of Clinical Oncology (ASCO)’s second annual National Cancer Opinion Survey.
The survey was conducted online by The Harris Poll from July 10, 2018, to August 10, 2018. It included 4,887 U.S. adults age 18 and older—1,001 of whom have or had cancer.
Cost among top concerns
Death and pain/suffering were the top concerns related to a cancer diagnosis. Fifty-four percent of respondents said death would be one of their greatest concerns if they were diagnosed with cancer, and the same percentage rated pain/suffering a top concern.
Forty-four percent of respondents said paying for cancer treatment would be a top concern, and 45% said the same about the financial impact of a cancer diagnosis on their family. When combined, financial issues were a top concern for 57% of respondents.
Paying for treatment was a top concern for:
- 36% of respondents who had/have cancer
- 51% of caregivers
- 43% of people with no prior cancer experience.
The financial impact on family was a top concern for:
- 39% of respondents who had/have cancer
- 55% of caregivers
- 42% of people with no prior cancer experience.
Cutting costs
Sixty-one percent of caregivers surveyed said they or another relative have taken a “dramatic” step to help pay for their loved one’s care, including:
- Dipping into savings accounts (35%)
- Working extra hours (23%)
- Taking an early withdrawal from a retirement account or college fund (14%)
- Postponing retirement (14%)
- Taking out a second mortgage or other type of loan (13%)
- Taking an additional job (13%)
- Selling family heirlooms (9%).
Twenty percent of cancer patients/survivors said they have taken actions to reduce treatment costs, including:
- Delaying scans (7%)
- Skipping or delaying appointments (7%)
- Skipping doses of prescribed treatment (6%)
- Postponing or not filling prescriptions (5%)
- Refusing treatment (3%).
“Patients are right to be concerned about the financial impact of a cancer diagnosis on their families,” said Richard L. Schilsky, MD, ASCO’s chief medical officer.
“It’s clear that high treatment costs are taking a serious toll not only on patients, but also on the people who care for them. If a family member has been diagnosed with cancer, the sole focus should be helping them get well. Instead, Americans are worrying about affording treatment, and, in many cases, they’re making serious personal sacrifices to help pay for their loved ones’ care.”
A recent survey suggests Americans are nearly as worried about the cost of a cancer diagnosis as they are about dying from cancer.
The cost of cancer care was a top concern even among people who had no prior experience with cancer.
At the same time, cancer patients/survivors admitted to delaying or forgoing care due to costs, and caregivers reported taking “dramatic” actions to pay for their loved one’s care.
These are findings from the American Society of Clinical Oncology (ASCO)’s second annual National Cancer Opinion Survey.
The survey was conducted online by The Harris Poll from July 10, 2018, to August 10, 2018. It included 4,887 U.S. adults age 18 and older—1,001 of whom have or had cancer.
Cost among top concerns
Death and pain/suffering were the top concerns related to a cancer diagnosis. Fifty-four percent of respondents said death would be one of their greatest concerns if they were diagnosed with cancer, and the same percentage rated pain/suffering a top concern.
Forty-four percent of respondents said paying for cancer treatment would be a top concern, and 45% said the same about the financial impact of a cancer diagnosis on their family. When combined, financial issues were a top concern for 57% of respondents.
Paying for treatment was a top concern for:
- 36% of respondents who had/have cancer
- 51% of caregivers
- 43% of people with no prior cancer experience.
The financial impact on family was a top concern for:
- 39% of respondents who had/have cancer
- 55% of caregivers
- 42% of people with no prior cancer experience.
Cutting costs
Sixty-one percent of caregivers surveyed said they or another relative have taken a “dramatic” step to help pay for their loved one’s care, including:
- Dipping into savings accounts (35%)
- Working extra hours (23%)
- Taking an early withdrawal from a retirement account or college fund (14%)
- Postponing retirement (14%)
- Taking out a second mortgage or other type of loan (13%)
- Taking an additional job (13%)
- Selling family heirlooms (9%).
Twenty percent of cancer patients/survivors said they have taken actions to reduce treatment costs, including:
- Delaying scans (7%)
- Skipping or delaying appointments (7%)
- Skipping doses of prescribed treatment (6%)
- Postponing or not filling prescriptions (5%)
- Refusing treatment (3%).
“Patients are right to be concerned about the financial impact of a cancer diagnosis on their families,” said Richard L. Schilsky, MD, ASCO’s chief medical officer.
“It’s clear that high treatment costs are taking a serious toll not only on patients, but also on the people who care for them. If a family member has been diagnosed with cancer, the sole focus should be helping them get well. Instead, Americans are worrying about affording treatment, and, in many cases, they’re making serious personal sacrifices to help pay for their loved ones’ care.”
CHMP backs blinatumomab for MRD
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
Eltrombopag approved as first-line SAA therapy
The U.S. Food and Drug Administration (FDA) has expanded the approved use of eltrombopag (Promacta®) in severe aplastic anemia (SAA).
Eltrombopag is now approved for use in combination with standard immunosuppressive therapy as first-line treatment for adults and pediatric patients age 2 and older with SAA.
Eltrombopag received breakthrough therapy designation and priority review for this indication.
Eltrombopag is also FDA-approved for SAA patients who have had an insufficient response to immunosuppressive therapy, for patients with chronic immune thrombocytopenia who have had an insufficient response to other treatments, and to treat thrombocytopenia in patients with chronic hepatitis C virus infection.
The FDA’s latest approval of eltrombopag is based on results from a phase 1/2 trial (NCT01623167), which were published in The New England Journal of Medicine in April 2017.
Updated results from the trial are available in the prescribing information for eltrombopag.
The trial included 153 previously untreated SAA patients age 2 and older. The patients received eltrombopag in combination with horse antithymocyte globulin and cyclosporine.
The starting dose of eltrombopag was:
- 150 mg once daily for patients age 12 and older (75 mg for East and Southeast Asians)
- 75 mg once daily for patients ages 6 to 11 (37.5 mg for East and Southeast Asians)
- 5 mg/kg once daily for patients ages 2 to 5 (1.25 mg/kg for East and Southeast Asians).
Patients were divided into three cohorts with different dosing schedules.
The recommended schedule, from the third cohort (n=92), was eltrombopag from day 1 to month 6, plus horse antithymocyte globulin and cyclosporine. All patients in this cohort were eligible to receive a low dose of cyclosporine for an additional 18 months if they achieved a hematologic response at 6 months.
Among the patients treated at the recommended dosing schedule, the 6-month overall response rate was 79%, and the complete response rate was 44%.
The median duration of both overall and complete response was 24.3 months.
The most common adverse events in these patients were ALT increase (29%), AST increase (17%), blood bilirubin increase (17%), rash (8%), and skin discoloration including hyperpigmentation (5%).
Eltrombopag is a product of Novartis.
The U.S. Food and Drug Administration (FDA) has expanded the approved use of eltrombopag (Promacta®) in severe aplastic anemia (SAA).
Eltrombopag is now approved for use in combination with standard immunosuppressive therapy as first-line treatment for adults and pediatric patients age 2 and older with SAA.
Eltrombopag received breakthrough therapy designation and priority review for this indication.
Eltrombopag is also FDA-approved for SAA patients who have had an insufficient response to immunosuppressive therapy, for patients with chronic immune thrombocytopenia who have had an insufficient response to other treatments, and to treat thrombocytopenia in patients with chronic hepatitis C virus infection.
The FDA’s latest approval of eltrombopag is based on results from a phase 1/2 trial (NCT01623167), which were published in The New England Journal of Medicine in April 2017.
Updated results from the trial are available in the prescribing information for eltrombopag.
The trial included 153 previously untreated SAA patients age 2 and older. The patients received eltrombopag in combination with horse antithymocyte globulin and cyclosporine.
The starting dose of eltrombopag was:
- 150 mg once daily for patients age 12 and older (75 mg for East and Southeast Asians)
- 75 mg once daily for patients ages 6 to 11 (37.5 mg for East and Southeast Asians)
- 5 mg/kg once daily for patients ages 2 to 5 (1.25 mg/kg for East and Southeast Asians).
Patients were divided into three cohorts with different dosing schedules.
The recommended schedule, from the third cohort (n=92), was eltrombopag from day 1 to month 6, plus horse antithymocyte globulin and cyclosporine. All patients in this cohort were eligible to receive a low dose of cyclosporine for an additional 18 months if they achieved a hematologic response at 6 months.
Among the patients treated at the recommended dosing schedule, the 6-month overall response rate was 79%, and the complete response rate was 44%.
The median duration of both overall and complete response was 24.3 months.
The most common adverse events in these patients were ALT increase (29%), AST increase (17%), blood bilirubin increase (17%), rash (8%), and skin discoloration including hyperpigmentation (5%).
Eltrombopag is a product of Novartis.
The U.S. Food and Drug Administration (FDA) has expanded the approved use of eltrombopag (Promacta®) in severe aplastic anemia (SAA).
Eltrombopag is now approved for use in combination with standard immunosuppressive therapy as first-line treatment for adults and pediatric patients age 2 and older with SAA.
Eltrombopag received breakthrough therapy designation and priority review for this indication.
Eltrombopag is also FDA-approved for SAA patients who have had an insufficient response to immunosuppressive therapy, for patients with chronic immune thrombocytopenia who have had an insufficient response to other treatments, and to treat thrombocytopenia in patients with chronic hepatitis C virus infection.
The FDA’s latest approval of eltrombopag is based on results from a phase 1/2 trial (NCT01623167), which were published in The New England Journal of Medicine in April 2017.
Updated results from the trial are available in the prescribing information for eltrombopag.
The trial included 153 previously untreated SAA patients age 2 and older. The patients received eltrombopag in combination with horse antithymocyte globulin and cyclosporine.
The starting dose of eltrombopag was:
- 150 mg once daily for patients age 12 and older (75 mg for East and Southeast Asians)
- 75 mg once daily for patients ages 6 to 11 (37.5 mg for East and Southeast Asians)
- 5 mg/kg once daily for patients ages 2 to 5 (1.25 mg/kg for East and Southeast Asians).
Patients were divided into three cohorts with different dosing schedules.
The recommended schedule, from the third cohort (n=92), was eltrombopag from day 1 to month 6, plus horse antithymocyte globulin and cyclosporine. All patients in this cohort were eligible to receive a low dose of cyclosporine for an additional 18 months if they achieved a hematologic response at 6 months.
Among the patients treated at the recommended dosing schedule, the 6-month overall response rate was 79%, and the complete response rate was 44%.
The median duration of both overall and complete response was 24.3 months.
The most common adverse events in these patients were ALT increase (29%), AST increase (17%), blood bilirubin increase (17%), rash (8%), and skin discoloration including hyperpigmentation (5%).
Eltrombopag is a product of Novartis.
Quick FDA approval for brentuximab vedotin in PTCL
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
FDA approves generic drugs for APL
The U.S. Food and Drug Administration (FDA) has now approved three generic arsenic trioxide products for use in patients with acute promyelocytic leukemia (APL).
Two of the products—from Zydus Cadila and Amring Pharmaceuticals—were approved on November 13.
The third—from Fresenius Kabi—was approved in August and launched in the United States last month.
All three injectable arsenic trioxide products (1 mg/mL) are generic versions of Teva’s Trisenox.
Since 2000, Trisenox has been FDA-approved to induce remission and as consolidation therapy for patients with APL who are refractory to, or have relapsed after, retinoid and anthracycline chemotherapy, and whose APL is characterized by presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
In January, the FDA approved Trisenox for use in combination with tretinoin to treat adults with newly diagnosed, low-risk APL with the t(15;17) translocation or PML/RAR-alpha gene expression.
The U.S. Food and Drug Administration (FDA) has now approved three generic arsenic trioxide products for use in patients with acute promyelocytic leukemia (APL).
Two of the products—from Zydus Cadila and Amring Pharmaceuticals—were approved on November 13.
The third—from Fresenius Kabi—was approved in August and launched in the United States last month.
All three injectable arsenic trioxide products (1 mg/mL) are generic versions of Teva’s Trisenox.
Since 2000, Trisenox has been FDA-approved to induce remission and as consolidation therapy for patients with APL who are refractory to, or have relapsed after, retinoid and anthracycline chemotherapy, and whose APL is characterized by presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
In January, the FDA approved Trisenox for use in combination with tretinoin to treat adults with newly diagnosed, low-risk APL with the t(15;17) translocation or PML/RAR-alpha gene expression.
The U.S. Food and Drug Administration (FDA) has now approved three generic arsenic trioxide products for use in patients with acute promyelocytic leukemia (APL).
Two of the products—from Zydus Cadila and Amring Pharmaceuticals—were approved on November 13.
The third—from Fresenius Kabi—was approved in August and launched in the United States last month.
All three injectable arsenic trioxide products (1 mg/mL) are generic versions of Teva’s Trisenox.
Since 2000, Trisenox has been FDA-approved to induce remission and as consolidation therapy for patients with APL who are refractory to, or have relapsed after, retinoid and anthracycline chemotherapy, and whose APL is characterized by presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
In January, the FDA approved Trisenox for use in combination with tretinoin to treat adults with newly diagnosed, low-risk APL with the t(15;17) translocation or PML/RAR-alpha gene expression.
FDA approves generic decitabine for MDS
The U.S. Food and Drug Administration has approved Lupin’s decitabine product, a generic version of Otsuka Pharmaceutical Co. Ltd.’s Dacogen, to treat patients with myelodysplastic syndromes (MDS).
Lupin’s decitabine for injection (50 mg, single-dose vial) is approved to treat patients with intermediate-1, intermediate-2, and high-risk MDS.
This includes previously treated, untreated, de novo, and secondary MDS of all French-American-British subtypes—refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia.
The U.S. Food and Drug Administration has approved Lupin’s decitabine product, a generic version of Otsuka Pharmaceutical Co. Ltd.’s Dacogen, to treat patients with myelodysplastic syndromes (MDS).
Lupin’s decitabine for injection (50 mg, single-dose vial) is approved to treat patients with intermediate-1, intermediate-2, and high-risk MDS.
This includes previously treated, untreated, de novo, and secondary MDS of all French-American-British subtypes—refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia.
The U.S. Food and Drug Administration has approved Lupin’s decitabine product, a generic version of Otsuka Pharmaceutical Co. Ltd.’s Dacogen, to treat patients with myelodysplastic syndromes (MDS).
Lupin’s decitabine for injection (50 mg, single-dose vial) is approved to treat patients with intermediate-1, intermediate-2, and high-risk MDS.
This includes previously treated, untreated, de novo, and secondary MDS of all French-American-British subtypes—refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia.