User login
Does giving a sweet-tasting solution before vaccine injection reduce infant crying?
EVIDENCE SUMMARY
A 2010 meta-analysis evaluated 14 RCTs investigating the effectiveness of giving sweet solutions before immunization in 1707 healthy term infants from beyond the neonatal period to 12 months of age.1 Intervention groups received 0.25 to 10 mL (median, 2 mL) of 12% to 75% sucrose or 30% to 40% glucose orally 2 minutes before one to 4 injections (one study used 3 oral doses every 30 seconds, and one study added topical EMLA cream). Control groups received water or nothing (plus topical placebo in one study).
Pooled outcome data for crying duration from 6 studies (5 sucrose, one glucose; 716 injections) showed no significant difference between groups. When 2 studies with widely differing results using 12% sucrose were removed, however, a statistically significant weighted mean difference of 12 seconds less crying favored sweet solutions (3 sucrose, one glucose; 568 injections; 95% confidence interval, −23 to −0.78).
Differences among studies in volumes and concentrations of sweet solutions used prevented investigators from ascertaining optimal dosing.
Sucrose solution significantly reduces crying time compared with placebo
A 2014 double-blind RCT evaluated sucrose solutions compared with sterile water in older infants.2 One nurse gave 2 mL of a 75% sucrose solution, a 25% sucrose solution, or sterile water orally over 15 seconds immediately before administering diphtheria, tetanus, acellular pertussis/Haemophilus influenzae type b/inactivated poliovirus (DTaP/Hib/IPV), pneumococcal, and hepatitis A vaccines to 537 healthy 16- to 19-month-old infants simultaneously in the right and left deltoids. Parents cuddled the infant over one shoulder while a distracting noise was made. Pacifiers (5 infants) and pretreatment paracetamol (8 infants) were permitted.
Infants receiving sucrose solutions showed significantly reduced total crying times compared with controls (75% sucrose, 43 seconds; 25% sucrose, 62 seconds; placebo, 120 seconds; P<.001 for 75% sucrose compared with other solutions; P<.001 for 25% sucrose compared with placebo).
Glucose also shortens crying
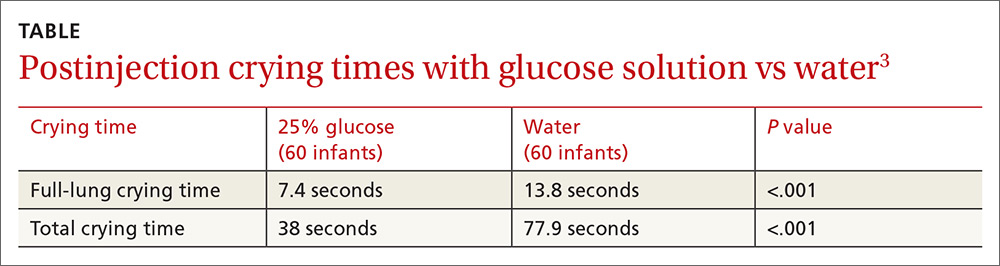
A 2012 double-blind RCT compared glucose solution with sterile water before vaccination in 120 healthy infants 2 months of age.3 Parents used a syringe to apply 2 mL of a 25% glucose solution or sterile water over 30 seconds to the lateral side of the infant’s tongue immediately before injection of DTaP/Hib/IPV vaccine into the right thigh followed by injection of hepatitis B vaccine into the left thigh.
Infants lay on the examination table in the supine position with the head elevated. Parents weren’t permitted to use a pacifier or bottle, or swaddle, cuddle, or restrain the infant during the procedure, but they were allowed to lift and calm the infant 15 seconds after the injections. Mean full-lung crying time and mean total crying time were significantly shorter in the treatment group (TABLE3).
1. Harrison D, Stevens B, Bueno M, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406-413.
2. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16-19 month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-1532.
3. Kassab M, Sheehy A, King M, et al. A double-blind randomised controlled trial of 25% oral glucose for pain relief in 2-month-old infants undergoing immunisation. Int J Nurs Stud. 2012;49:249-256.
EVIDENCE SUMMARY
A 2010 meta-analysis evaluated 14 RCTs investigating the effectiveness of giving sweet solutions before immunization in 1707 healthy term infants from beyond the neonatal period to 12 months of age.1 Intervention groups received 0.25 to 10 mL (median, 2 mL) of 12% to 75% sucrose or 30% to 40% glucose orally 2 minutes before one to 4 injections (one study used 3 oral doses every 30 seconds, and one study added topical EMLA cream). Control groups received water or nothing (plus topical placebo in one study).
Pooled outcome data for crying duration from 6 studies (5 sucrose, one glucose; 716 injections) showed no significant difference between groups. When 2 studies with widely differing results using 12% sucrose were removed, however, a statistically significant weighted mean difference of 12 seconds less crying favored sweet solutions (3 sucrose, one glucose; 568 injections; 95% confidence interval, −23 to −0.78).
Differences among studies in volumes and concentrations of sweet solutions used prevented investigators from ascertaining optimal dosing.
Sucrose solution significantly reduces crying time compared with placebo
A 2014 double-blind RCT evaluated sucrose solutions compared with sterile water in older infants.2 One nurse gave 2 mL of a 75% sucrose solution, a 25% sucrose solution, or sterile water orally over 15 seconds immediately before administering diphtheria, tetanus, acellular pertussis/Haemophilus influenzae type b/inactivated poliovirus (DTaP/Hib/IPV), pneumococcal, and hepatitis A vaccines to 537 healthy 16- to 19-month-old infants simultaneously in the right and left deltoids. Parents cuddled the infant over one shoulder while a distracting noise was made. Pacifiers (5 infants) and pretreatment paracetamol (8 infants) were permitted.
Infants receiving sucrose solutions showed significantly reduced total crying times compared with controls (75% sucrose, 43 seconds; 25% sucrose, 62 seconds; placebo, 120 seconds; P<.001 for 75% sucrose compared with other solutions; P<.001 for 25% sucrose compared with placebo).
Glucose also shortens crying
A 2012 double-blind RCT compared glucose solution with sterile water before vaccination in 120 healthy infants 2 months of age.3 Parents used a syringe to apply 2 mL of a 25% glucose solution or sterile water over 30 seconds to the lateral side of the infant’s tongue immediately before injection of DTaP/Hib/IPV vaccine into the right thigh followed by injection of hepatitis B vaccine into the left thigh.
Infants lay on the examination table in the supine position with the head elevated. Parents weren’t permitted to use a pacifier or bottle, or swaddle, cuddle, or restrain the infant during the procedure, but they were allowed to lift and calm the infant 15 seconds after the injections. Mean full-lung crying time and mean total crying time were significantly shorter in the treatment group (TABLE3).
EVIDENCE SUMMARY
A 2010 meta-analysis evaluated 14 RCTs investigating the effectiveness of giving sweet solutions before immunization in 1707 healthy term infants from beyond the neonatal period to 12 months of age.1 Intervention groups received 0.25 to 10 mL (median, 2 mL) of 12% to 75% sucrose or 30% to 40% glucose orally 2 minutes before one to 4 injections (one study used 3 oral doses every 30 seconds, and one study added topical EMLA cream). Control groups received water or nothing (plus topical placebo in one study).
Pooled outcome data for crying duration from 6 studies (5 sucrose, one glucose; 716 injections) showed no significant difference between groups. When 2 studies with widely differing results using 12% sucrose were removed, however, a statistically significant weighted mean difference of 12 seconds less crying favored sweet solutions (3 sucrose, one glucose; 568 injections; 95% confidence interval, −23 to −0.78).
Differences among studies in volumes and concentrations of sweet solutions used prevented investigators from ascertaining optimal dosing.
Sucrose solution significantly reduces crying time compared with placebo
A 2014 double-blind RCT evaluated sucrose solutions compared with sterile water in older infants.2 One nurse gave 2 mL of a 75% sucrose solution, a 25% sucrose solution, or sterile water orally over 15 seconds immediately before administering diphtheria, tetanus, acellular pertussis/Haemophilus influenzae type b/inactivated poliovirus (DTaP/Hib/IPV), pneumococcal, and hepatitis A vaccines to 537 healthy 16- to 19-month-old infants simultaneously in the right and left deltoids. Parents cuddled the infant over one shoulder while a distracting noise was made. Pacifiers (5 infants) and pretreatment paracetamol (8 infants) were permitted.
Infants receiving sucrose solutions showed significantly reduced total crying times compared with controls (75% sucrose, 43 seconds; 25% sucrose, 62 seconds; placebo, 120 seconds; P<.001 for 75% sucrose compared with other solutions; P<.001 for 25% sucrose compared with placebo).
Glucose also shortens crying
A 2012 double-blind RCT compared glucose solution with sterile water before vaccination in 120 healthy infants 2 months of age.3 Parents used a syringe to apply 2 mL of a 25% glucose solution or sterile water over 30 seconds to the lateral side of the infant’s tongue immediately before injection of DTaP/Hib/IPV vaccine into the right thigh followed by injection of hepatitis B vaccine into the left thigh.
Infants lay on the examination table in the supine position with the head elevated. Parents weren’t permitted to use a pacifier or bottle, or swaddle, cuddle, or restrain the infant during the procedure, but they were allowed to lift and calm the infant 15 seconds after the injections. Mean full-lung crying time and mean total crying time were significantly shorter in the treatment group (TABLE3).
1. Harrison D, Stevens B, Bueno M, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406-413.
2. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16-19 month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-1532.
3. Kassab M, Sheehy A, King M, et al. A double-blind randomised controlled trial of 25% oral glucose for pain relief in 2-month-old infants undergoing immunisation. Int J Nurs Stud. 2012;49:249-256.
1. Harrison D, Stevens B, Bueno M, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406-413.
2. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16-19 month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-1532.
3. Kassab M, Sheehy A, King M, et al. A double-blind randomised controlled trial of 25% oral glucose for pain relief in 2-month-old infants undergoing immunisation. Int J Nurs Stud. 2012;49:249-256.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. Oral administration of a sucrose or glucose solution before intramuscular vaccine injection reduces expected crying duration by 12 to 77 seconds following the shot (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs] and 2 RCTs).