User login
Papillary Transitional Cell Bladder Carcinoma and Systematized Epidermal Nevus Syndrome
Epidermal nevi can occur in isolation or in association with internal abnormalities. Epidermal nevus syndrome (ENS) is a heterogeneous group of neurocutaneous disorders characterized by mosaicism and epidermal nevi found in association with various systemic abnormalities.1-4 There are many possible associated systemic findings, including abnormalities of the central nervous, musculoskeletal, renal, and hematologic systems. Epidermal nevi have been associated with internal malignancies. We present the case of a patient with epidermal nevi associated with papillary transitional cell bladder carcinoma. According to a PubMed search of articles indexed for MEDLINE using the search terms transitional cell bladder carcinoma and epidermal nevus, there have only been 4 other cases of transitional cell bladder carcinoma and ENS reported in the literature,5-8 2 of which were reports of papillary transitional cell bladder carcinoma.5,6
Case Report
A 29-year-old woman presented to our clinic with a rash that had been present since 3 years of age. The emergency department consulted dermatology for evaluation of what was believed to be contact dermatitis; however, upon questioning the patient, it was revealed that the rash was chronic and persistent.
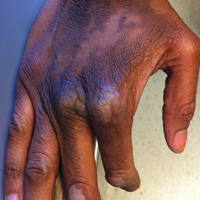
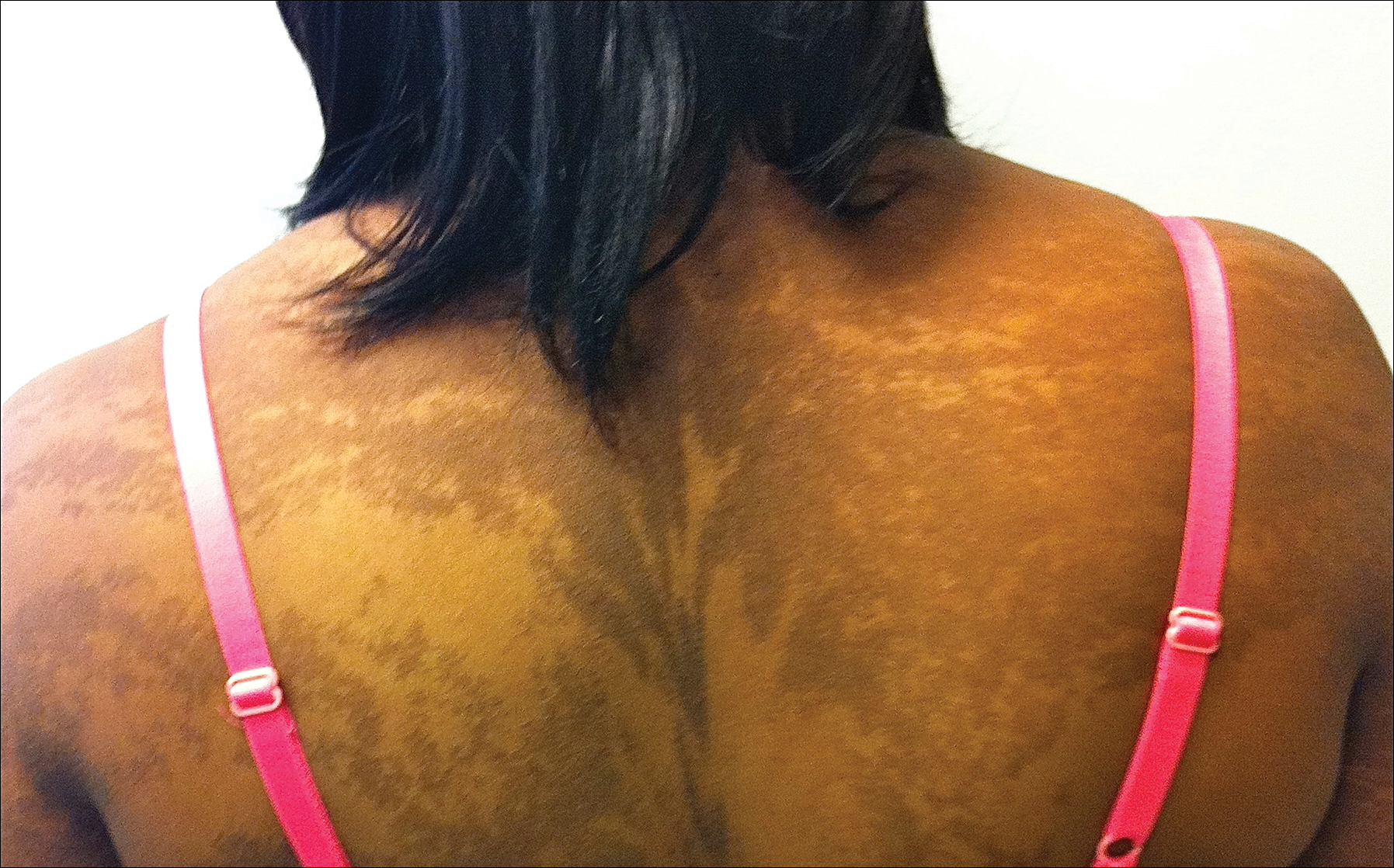
The rash was nonpruritic and was located on the face, hands (Figure 1), chest, buttocks, thighs, legs, and back (Figure 2). Although asymptomatic, the appearance of the skin caused the patient some emotional distress. As a child she had been evaluated by a dermatologist and a biopsy was performed, but she did not recall the results or have any records. She had been prescribed an oral medication by the dermatologist, but treatment was terminated early due to nausea. The skin lesions did not improve with the short course of treatment.
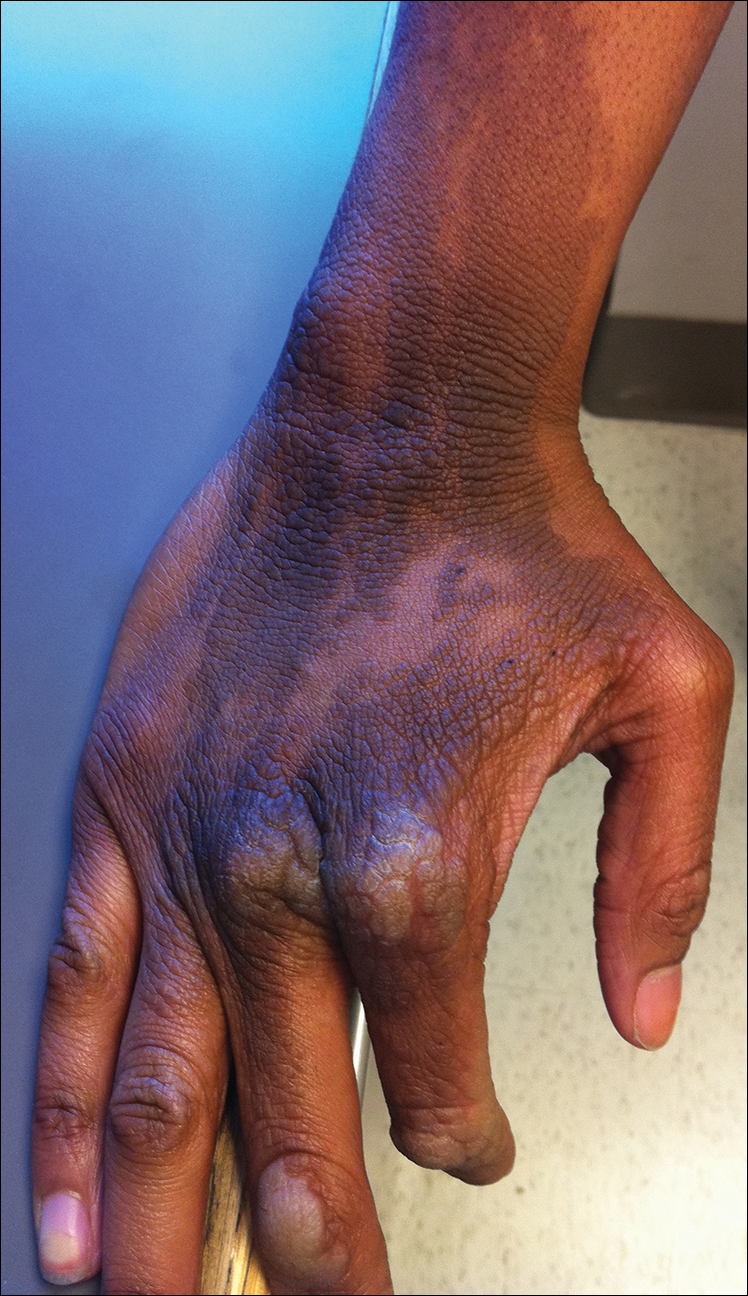
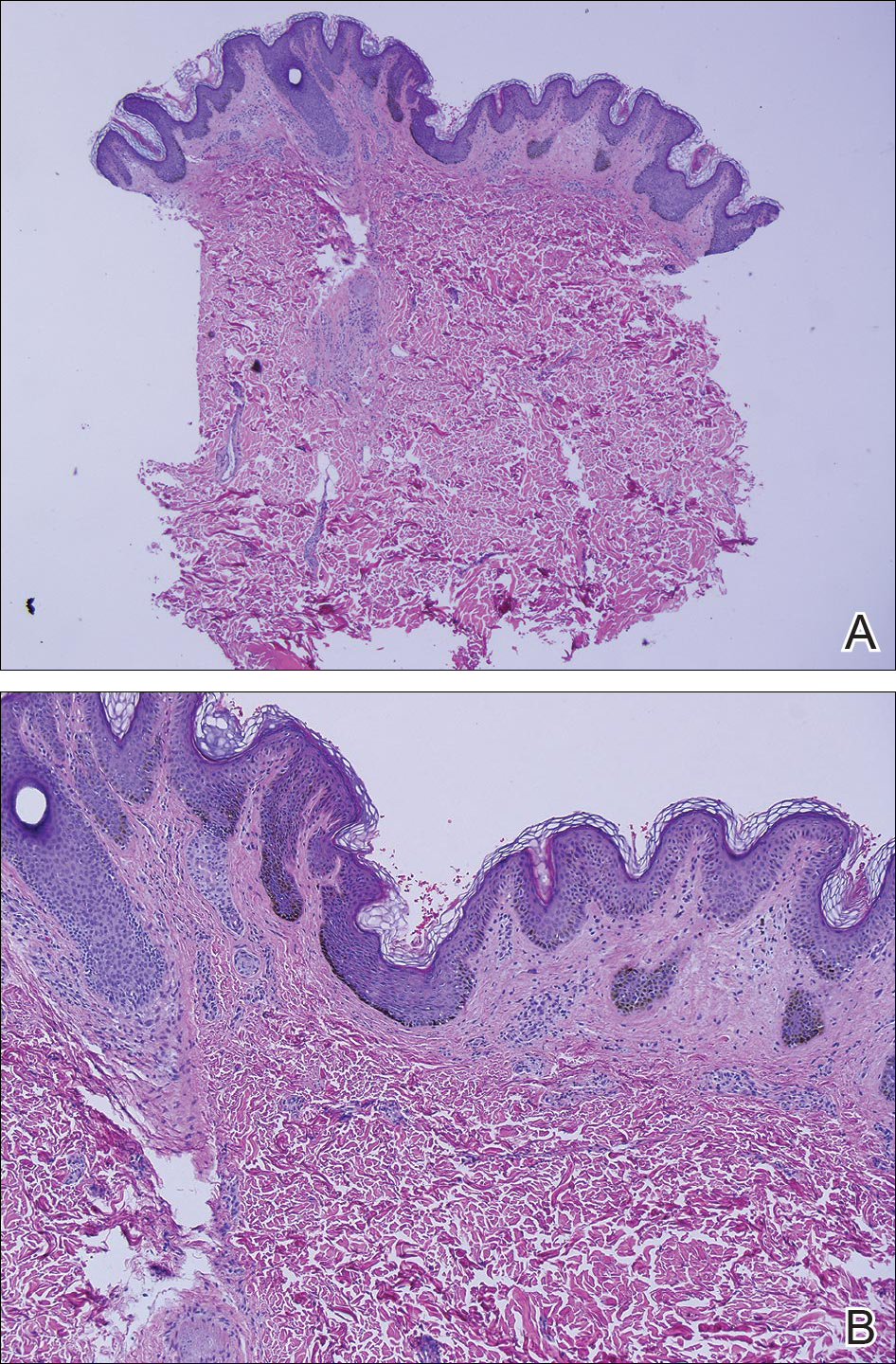
Eighteen months prior to presentation to our clinic, the patient was discovered to have hematuria on routine examination by her primary care physician. At that time, the patient underwent a workup for hematuria and a mass was discovered in the bladder via cystoscopy. A diagnosis of low-grade papillary transitional cell bladder carcinoma was made, and she underwent a partial cystectomy. No radiation or chemotherapy was required. The remainder of her medical history was only remarkable for asthma, which was well controlled with albuterol. On examination, generalized, hyperpigmented, reticulated patches, macules, and hyperpigmented verrucous plaques were distributed along the Blaschko lines, sparing the face. No limb abnormalities or dental or nail abnormalities were noted. Examination of the axillary and cervical lymph nodes was unremarkable, and no neurological abnormalities were noted. A 3-mm punch biopsy of the mid upper back was performed. Histopathology revealed papillomatous, nonorganoid, nonepidermolytic hyperplasia of the epidermis with elongated rete ridges (Figure 3), which was diagnosed as a nonorganoid nonepidermolytic epidermal nevus.
Comment
Epidermal nevus syndrome is a group of disorders characterized by both local or systematized epidermal nevi and systemic findings. Solomon et al4 first coined the term epidermal nevus syndrome more than 40 years ago; however, since then there has been confusion about how to define ENS. Epidermal nevus syndrome has been considered an umbrella term that includes more specific syndromes involving epidermal nevi, such as Proteus syndrome and Schimmelpenning syndrome; conversely, it also has been considered a term for those who do not meet the criteria for more specific syndromes.1,9 Happle1 discussed that the genetic variations found in ENS warrant recognition. Simply put, ENS is a heterogeneous group of syndromes that are similar in that they involve epidermal nevi and internal abnormalities but are genetically distinct. The list of definitive ENSs, as suggested by Happle1 and others, will likely continue to grow.3,5
The exact pathomechanism of ENS is unknown, but the clinical presentation most likely represents a lethal disorder mitigated by mosaicism.2,9 Gene defects vary depending on the specific ENS. For instance, the phosphatase and tensin homolog gene, PTEN, mutations have been associated with type 2 segmental Cowden disease. Fibroblast growth factor receptor 3, FGFR3, mutations have been linked to Garcia-Hafner-Happle syndrome.3FGFR3 mutations have been found in nonepidermolytic epidermal nevi, and some suggest that the majority of epidermal nevi exhibit mutations in FGFR3.5,10,11 On the other hand, other gene defects have not been elucidated, such as in Schimmelpenning syndrome.3
Clinically, ENS may involve nonepidermolytic verrucous nevi, sebaceous nevi, organoid nevi, linear Cowden nevi, and woolly hair nevi. Lesions may be flesh-colored, pink, yellow, or hyperpigmented plaques in a blaschkoid distribution and may be localized or systematized. Nevi typically are present at birth or develop within the first year of life.9,12,13 Other cutaneous findings may be noted apart from epidermal nevi, including melanocytic nevi, aplasia cutis congenita, and hemangiomas.13,14
Extracutaneous findings include central nervous system, skeletal, ocular, cardiac, and genitourinary defects, which are often observed in these patients.3,9,13,14 Central nervous system findings are seen in 50% to 70% of cases, with seizures and mental retardation among the most common.13-15 Genitourinary abnormalities associated with epidermal nevi, including horseshoe kidney, cystic kidney, duplicated collecting system, testicular and paratesticular tumors, and hypospadias have been documented in the literature.16 Our patient had a history of papillary transitional cell bladder carcinoma, which is rare for a patient younger than 30 years. The overall median age of diagnosis of bladder cancer is 65 years, and it is more common in men than in women.17 Transitional cell carcinomas account for approximately 90% of all bladder cancers in the United States. Other common types of bladder cancer include squamous cell carcinoma, adenocarcinoma, and rhabdomyosarcoma.16 Typically, transitional cell carcinoma is associated with smoking, exposure to aniline dyes, cyclophosphamide, and living in industrialized areas.16,17 Individuals who work with textiles, dyes, leather, tires, rubber, and/or petroleum; painters; truck drivers; drill press operators; and hairdressers are at an increased risk for development of bladder cancer.16
Interestingly, it has been shown in some studies that papillary transitional cell bladder carcinoma frequently is associated with FGFR3 mutations, which may be the missing link in the rare finding of papillary transitional cell bladder carcinoma and epidermal nevi.5,18,19 In addition, PTEN mutations also have been identified in low-grade papillary transitional cell carcinomas of the bladder, another gene linked to an ENS with type 2 segmental Cowden disease.3,20
Histopathologically, epidermal nevi have 10 different descriptions. Our patient had a nonorganoid nonepidermolytic epidermal nevus characterized by hyperkeratosis, acanthosis, papillomatosis, and elongated rete ridges. Focal acantholysis and epidermolytic hyperkeratosis also is seen in some epidermal nevi but was not seen in this case.9,21
Simple epidermal nevi occur in approximately 1 in 1000 newborns; however, when a child presents with multiple or systematized epidermal nevi, investigation should be undertaken for other possible associations.13,14 Of note, there have been several cases of squamous cell, verrucous, basal cell, and adnexal carcinomas arising in linear epidermal nevi.22-24
Epidermal nevi can be difficult to treat. Some patients are troubled by the appearance of these nevi, especially those with systematized disease. Unfortunately, for patients with multiple nevi or systematized disease, there are no consistently effective treatment options; however, there are case reports25,26 in the literature citing improvement or cure of epidermal nevi with full-thickness excision, continuous and pulsed CO2 laser, pulsed dye laser, and erbium-doped YAG laser.25 Other therapies that have been purported to help improve epidermal nevi are topical and oral retinoids, corticosteroids, topical 5-fluorouracil, anthralin, and podophyllin.26
Conclusion
Transitional cell bladder carcinoma is rare in patients in the third decade of life and younger. Given the age of our patient and her concomitant lack of risk factors, such as older age, history of smoking, and exposure to certain chemicals (eg, aniline dyes) and medications (eg, cyclophosphamide), it is more likely that the finding of papillary transitional cell bladder carcinoma and ENS are related. A clear genetic link between ENS and transitional cell papillary bladder carcinoma has yet to be elucidated, but the FGFR3 gene is promising.
- Happle R. What is a nevus? a proposed definition of a common medical term. Dermatology. 1995;191:1-5.
- Gonzalez ME, Jabbari A, Tlougan BE, et al. Epidermal nevus. Dermatol Online J. 2010;16:12.
- Happle R. The group of epidermal nevus syndromes. part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273-285.
- Flosadottir E, Bjarnason B. A non-epidermolytic epidermal nevus of a soft, papillomatous type with transitional cell cancer of the bladder: a case report and review of non-cutaneous cancers associated with epidermal naevi. Acta Derm Venerol. 2008;88:173-175.
- Rosenthal D, Fretzin DF. Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder. Pediatr Dermatol. 1986;3:455-458.
- Garcia de Jalon A, Azua-Romea J, Trivez MA, et al. Epidermal naevus syndrome (Solomon’s syndrome) associated with bladder cancer in a 20-year-old female. Scand J Urol Nephrol. 2004;38:85-87.
- Rongioletti F, Rebora A. Epidermal nevus with transitional cell carcinomas of the urinary tract. J Am Acad Dermatol. 1991;25:856-858.
- Moss C. Mosacism and linear lesions. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:943-962.
- Hafner C, van Oers JM, Vogt T, et al. Mosaicisim of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201-2207.
- Bygum A, Fagerberg CR, Clemmensen OJ, et al. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- Happle R. Linear Cowden nevus: a new distinct epidermal nevus. Eur J Dermatol. 2007;17:133-136.
- Vujevich JJ, Mancini AJ. The epidermal nevus syndromes: multisystem disorders. J Am Acad Dermatol. 2004;50:957-961.
- Solomon L, Esterly N. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1-56.
- Grebe TA, Rimsa ME, Richter SF, et al. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24-30.
- Lamm DL, Torti FM. Bladder cancer, 1996. Ca Cancer J Clin. 1996;46:93-112.
- Metts MC, Metts JC, Milito SJ, et al. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc. 2000;92:285-294.
- Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555-2561.
- Cappellen D, DeOliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18-20.
- Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305-316.
- Luzar B, Calonje E, Bastian B. Tumors of the surface epithelium. In: Calonje JE, Breen T, McKee PH, eds. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012:1076-1149.
- Masood Q, Narayan D. Squamous cell carcinoma in a linear epidermal nevus. J Plast Reconstr Aesthet Surg. 2009;62:693-694.
- Cramer SF, Mandel MA, Hauler R, et al. Squamous cell carcinoma arising in a linear epidermal nevus. Arch Dermatol. 1981;117:222-224.
- Affleck AG, Leach IJ, Varma S. Two squamous cell carcinomas arising in a linear epidermal nevus in a 28-year-old female. Clin Exp Dermatol. 2005;30:382-384.
- Alam M, Arndt KA. A method for pulsed carbon dioxide laser treatment of epidermal nevi. J Am Acad Dermatol. 2002;46:554-556.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:1809-1810.
Epidermal nevi can occur in isolation or in association with internal abnormalities. Epidermal nevus syndrome (ENS) is a heterogeneous group of neurocutaneous disorders characterized by mosaicism and epidermal nevi found in association with various systemic abnormalities.1-4 There are many possible associated systemic findings, including abnormalities of the central nervous, musculoskeletal, renal, and hematologic systems. Epidermal nevi have been associated with internal malignancies. We present the case of a patient with epidermal nevi associated with papillary transitional cell bladder carcinoma. According to a PubMed search of articles indexed for MEDLINE using the search terms transitional cell bladder carcinoma and epidermal nevus, there have only been 4 other cases of transitional cell bladder carcinoma and ENS reported in the literature,5-8 2 of which were reports of papillary transitional cell bladder carcinoma.5,6
Case Report
A 29-year-old woman presented to our clinic with a rash that had been present since 3 years of age. The emergency department consulted dermatology for evaluation of what was believed to be contact dermatitis; however, upon questioning the patient, it was revealed that the rash was chronic and persistent.
The rash was nonpruritic and was located on the face, hands (Figure 1), chest, buttocks, thighs, legs, and back (Figure 2). Although asymptomatic, the appearance of the skin caused the patient some emotional distress. As a child she had been evaluated by a dermatologist and a biopsy was performed, but she did not recall the results or have any records. She had been prescribed an oral medication by the dermatologist, but treatment was terminated early due to nausea. The skin lesions did not improve with the short course of treatment.


Eighteen months prior to presentation to our clinic, the patient was discovered to have hematuria on routine examination by her primary care physician. At that time, the patient underwent a workup for hematuria and a mass was discovered in the bladder via cystoscopy. A diagnosis of low-grade papillary transitional cell bladder carcinoma was made, and she underwent a partial cystectomy. No radiation or chemotherapy was required. The remainder of her medical history was only remarkable for asthma, which was well controlled with albuterol. On examination, generalized, hyperpigmented, reticulated patches, macules, and hyperpigmented verrucous plaques were distributed along the Blaschko lines, sparing the face. No limb abnormalities or dental or nail abnormalities were noted. Examination of the axillary and cervical lymph nodes was unremarkable, and no neurological abnormalities were noted. A 3-mm punch biopsy of the mid upper back was performed. Histopathology revealed papillomatous, nonorganoid, nonepidermolytic hyperplasia of the epidermis with elongated rete ridges (Figure 3), which was diagnosed as a nonorganoid nonepidermolytic epidermal nevus.

Comment
Epidermal nevus syndrome is a group of disorders characterized by both local or systematized epidermal nevi and systemic findings. Solomon et al4 first coined the term epidermal nevus syndrome more than 40 years ago; however, since then there has been confusion about how to define ENS. Epidermal nevus syndrome has been considered an umbrella term that includes more specific syndromes involving epidermal nevi, such as Proteus syndrome and Schimmelpenning syndrome; conversely, it also has been considered a term for those who do not meet the criteria for more specific syndromes.1,9 Happle1 discussed that the genetic variations found in ENS warrant recognition. Simply put, ENS is a heterogeneous group of syndromes that are similar in that they involve epidermal nevi and internal abnormalities but are genetically distinct. The list of definitive ENSs, as suggested by Happle1 and others, will likely continue to grow.3,5
The exact pathomechanism of ENS is unknown, but the clinical presentation most likely represents a lethal disorder mitigated by mosaicism.2,9 Gene defects vary depending on the specific ENS. For instance, the phosphatase and tensin homolog gene, PTEN, mutations have been associated with type 2 segmental Cowden disease. Fibroblast growth factor receptor 3, FGFR3, mutations have been linked to Garcia-Hafner-Happle syndrome.3FGFR3 mutations have been found in nonepidermolytic epidermal nevi, and some suggest that the majority of epidermal nevi exhibit mutations in FGFR3.5,10,11 On the other hand, other gene defects have not been elucidated, such as in Schimmelpenning syndrome.3
Clinically, ENS may involve nonepidermolytic verrucous nevi, sebaceous nevi, organoid nevi, linear Cowden nevi, and woolly hair nevi. Lesions may be flesh-colored, pink, yellow, or hyperpigmented plaques in a blaschkoid distribution and may be localized or systematized. Nevi typically are present at birth or develop within the first year of life.9,12,13 Other cutaneous findings may be noted apart from epidermal nevi, including melanocytic nevi, aplasia cutis congenita, and hemangiomas.13,14
Extracutaneous findings include central nervous system, skeletal, ocular, cardiac, and genitourinary defects, which are often observed in these patients.3,9,13,14 Central nervous system findings are seen in 50% to 70% of cases, with seizures and mental retardation among the most common.13-15 Genitourinary abnormalities associated with epidermal nevi, including horseshoe kidney, cystic kidney, duplicated collecting system, testicular and paratesticular tumors, and hypospadias have been documented in the literature.16 Our patient had a history of papillary transitional cell bladder carcinoma, which is rare for a patient younger than 30 years. The overall median age of diagnosis of bladder cancer is 65 years, and it is more common in men than in women.17 Transitional cell carcinomas account for approximately 90% of all bladder cancers in the United States. Other common types of bladder cancer include squamous cell carcinoma, adenocarcinoma, and rhabdomyosarcoma.16 Typically, transitional cell carcinoma is associated with smoking, exposure to aniline dyes, cyclophosphamide, and living in industrialized areas.16,17 Individuals who work with textiles, dyes, leather, tires, rubber, and/or petroleum; painters; truck drivers; drill press operators; and hairdressers are at an increased risk for development of bladder cancer.16
Interestingly, it has been shown in some studies that papillary transitional cell bladder carcinoma frequently is associated with FGFR3 mutations, which may be the missing link in the rare finding of papillary transitional cell bladder carcinoma and epidermal nevi.5,18,19 In addition, PTEN mutations also have been identified in low-grade papillary transitional cell carcinomas of the bladder, another gene linked to an ENS with type 2 segmental Cowden disease.3,20
Histopathologically, epidermal nevi have 10 different descriptions. Our patient had a nonorganoid nonepidermolytic epidermal nevus characterized by hyperkeratosis, acanthosis, papillomatosis, and elongated rete ridges. Focal acantholysis and epidermolytic hyperkeratosis also is seen in some epidermal nevi but was not seen in this case.9,21
Simple epidermal nevi occur in approximately 1 in 1000 newborns; however, when a child presents with multiple or systematized epidermal nevi, investigation should be undertaken for other possible associations.13,14 Of note, there have been several cases of squamous cell, verrucous, basal cell, and adnexal carcinomas arising in linear epidermal nevi.22-24
Epidermal nevi can be difficult to treat. Some patients are troubled by the appearance of these nevi, especially those with systematized disease. Unfortunately, for patients with multiple nevi or systematized disease, there are no consistently effective treatment options; however, there are case reports25,26 in the literature citing improvement or cure of epidermal nevi with full-thickness excision, continuous and pulsed CO2 laser, pulsed dye laser, and erbium-doped YAG laser.25 Other therapies that have been purported to help improve epidermal nevi are topical and oral retinoids, corticosteroids, topical 5-fluorouracil, anthralin, and podophyllin.26
Conclusion
Transitional cell bladder carcinoma is rare in patients in the third decade of life and younger. Given the age of our patient and her concomitant lack of risk factors, such as older age, history of smoking, and exposure to certain chemicals (eg, aniline dyes) and medications (eg, cyclophosphamide), it is more likely that the finding of papillary transitional cell bladder carcinoma and ENS are related. A clear genetic link between ENS and transitional cell papillary bladder carcinoma has yet to be elucidated, but the FGFR3 gene is promising.
Epidermal nevi can occur in isolation or in association with internal abnormalities. Epidermal nevus syndrome (ENS) is a heterogeneous group of neurocutaneous disorders characterized by mosaicism and epidermal nevi found in association with various systemic abnormalities.1-4 There are many possible associated systemic findings, including abnormalities of the central nervous, musculoskeletal, renal, and hematologic systems. Epidermal nevi have been associated with internal malignancies. We present the case of a patient with epidermal nevi associated with papillary transitional cell bladder carcinoma. According to a PubMed search of articles indexed for MEDLINE using the search terms transitional cell bladder carcinoma and epidermal nevus, there have only been 4 other cases of transitional cell bladder carcinoma and ENS reported in the literature,5-8 2 of which were reports of papillary transitional cell bladder carcinoma.5,6
Case Report
A 29-year-old woman presented to our clinic with a rash that had been present since 3 years of age. The emergency department consulted dermatology for evaluation of what was believed to be contact dermatitis; however, upon questioning the patient, it was revealed that the rash was chronic and persistent.
The rash was nonpruritic and was located on the face, hands (Figure 1), chest, buttocks, thighs, legs, and back (Figure 2). Although asymptomatic, the appearance of the skin caused the patient some emotional distress. As a child she had been evaluated by a dermatologist and a biopsy was performed, but she did not recall the results or have any records. She had been prescribed an oral medication by the dermatologist, but treatment was terminated early due to nausea. The skin lesions did not improve with the short course of treatment.


Eighteen months prior to presentation to our clinic, the patient was discovered to have hematuria on routine examination by her primary care physician. At that time, the patient underwent a workup for hematuria and a mass was discovered in the bladder via cystoscopy. A diagnosis of low-grade papillary transitional cell bladder carcinoma was made, and she underwent a partial cystectomy. No radiation or chemotherapy was required. The remainder of her medical history was only remarkable for asthma, which was well controlled with albuterol. On examination, generalized, hyperpigmented, reticulated patches, macules, and hyperpigmented verrucous plaques were distributed along the Blaschko lines, sparing the face. No limb abnormalities or dental or nail abnormalities were noted. Examination of the axillary and cervical lymph nodes was unremarkable, and no neurological abnormalities were noted. A 3-mm punch biopsy of the mid upper back was performed. Histopathology revealed papillomatous, nonorganoid, nonepidermolytic hyperplasia of the epidermis with elongated rete ridges (Figure 3), which was diagnosed as a nonorganoid nonepidermolytic epidermal nevus.

Comment
Epidermal nevus syndrome is a group of disorders characterized by both local or systematized epidermal nevi and systemic findings. Solomon et al4 first coined the term epidermal nevus syndrome more than 40 years ago; however, since then there has been confusion about how to define ENS. Epidermal nevus syndrome has been considered an umbrella term that includes more specific syndromes involving epidermal nevi, such as Proteus syndrome and Schimmelpenning syndrome; conversely, it also has been considered a term for those who do not meet the criteria for more specific syndromes.1,9 Happle1 discussed that the genetic variations found in ENS warrant recognition. Simply put, ENS is a heterogeneous group of syndromes that are similar in that they involve epidermal nevi and internal abnormalities but are genetically distinct. The list of definitive ENSs, as suggested by Happle1 and others, will likely continue to grow.3,5
The exact pathomechanism of ENS is unknown, but the clinical presentation most likely represents a lethal disorder mitigated by mosaicism.2,9 Gene defects vary depending on the specific ENS. For instance, the phosphatase and tensin homolog gene, PTEN, mutations have been associated with type 2 segmental Cowden disease. Fibroblast growth factor receptor 3, FGFR3, mutations have been linked to Garcia-Hafner-Happle syndrome.3FGFR3 mutations have been found in nonepidermolytic epidermal nevi, and some suggest that the majority of epidermal nevi exhibit mutations in FGFR3.5,10,11 On the other hand, other gene defects have not been elucidated, such as in Schimmelpenning syndrome.3
Clinically, ENS may involve nonepidermolytic verrucous nevi, sebaceous nevi, organoid nevi, linear Cowden nevi, and woolly hair nevi. Lesions may be flesh-colored, pink, yellow, or hyperpigmented plaques in a blaschkoid distribution and may be localized or systematized. Nevi typically are present at birth or develop within the first year of life.9,12,13 Other cutaneous findings may be noted apart from epidermal nevi, including melanocytic nevi, aplasia cutis congenita, and hemangiomas.13,14
Extracutaneous findings include central nervous system, skeletal, ocular, cardiac, and genitourinary defects, which are often observed in these patients.3,9,13,14 Central nervous system findings are seen in 50% to 70% of cases, with seizures and mental retardation among the most common.13-15 Genitourinary abnormalities associated with epidermal nevi, including horseshoe kidney, cystic kidney, duplicated collecting system, testicular and paratesticular tumors, and hypospadias have been documented in the literature.16 Our patient had a history of papillary transitional cell bladder carcinoma, which is rare for a patient younger than 30 years. The overall median age of diagnosis of bladder cancer is 65 years, and it is more common in men than in women.17 Transitional cell carcinomas account for approximately 90% of all bladder cancers in the United States. Other common types of bladder cancer include squamous cell carcinoma, adenocarcinoma, and rhabdomyosarcoma.16 Typically, transitional cell carcinoma is associated with smoking, exposure to aniline dyes, cyclophosphamide, and living in industrialized areas.16,17 Individuals who work with textiles, dyes, leather, tires, rubber, and/or petroleum; painters; truck drivers; drill press operators; and hairdressers are at an increased risk for development of bladder cancer.16
Interestingly, it has been shown in some studies that papillary transitional cell bladder carcinoma frequently is associated with FGFR3 mutations, which may be the missing link in the rare finding of papillary transitional cell bladder carcinoma and epidermal nevi.5,18,19 In addition, PTEN mutations also have been identified in low-grade papillary transitional cell carcinomas of the bladder, another gene linked to an ENS with type 2 segmental Cowden disease.3,20
Histopathologically, epidermal nevi have 10 different descriptions. Our patient had a nonorganoid nonepidermolytic epidermal nevus characterized by hyperkeratosis, acanthosis, papillomatosis, and elongated rete ridges. Focal acantholysis and epidermolytic hyperkeratosis also is seen in some epidermal nevi but was not seen in this case.9,21
Simple epidermal nevi occur in approximately 1 in 1000 newborns; however, when a child presents with multiple or systematized epidermal nevi, investigation should be undertaken for other possible associations.13,14 Of note, there have been several cases of squamous cell, verrucous, basal cell, and adnexal carcinomas arising in linear epidermal nevi.22-24
Epidermal nevi can be difficult to treat. Some patients are troubled by the appearance of these nevi, especially those with systematized disease. Unfortunately, for patients with multiple nevi or systematized disease, there are no consistently effective treatment options; however, there are case reports25,26 in the literature citing improvement or cure of epidermal nevi with full-thickness excision, continuous and pulsed CO2 laser, pulsed dye laser, and erbium-doped YAG laser.25 Other therapies that have been purported to help improve epidermal nevi are topical and oral retinoids, corticosteroids, topical 5-fluorouracil, anthralin, and podophyllin.26
Conclusion
Transitional cell bladder carcinoma is rare in patients in the third decade of life and younger. Given the age of our patient and her concomitant lack of risk factors, such as older age, history of smoking, and exposure to certain chemicals (eg, aniline dyes) and medications (eg, cyclophosphamide), it is more likely that the finding of papillary transitional cell bladder carcinoma and ENS are related. A clear genetic link between ENS and transitional cell papillary bladder carcinoma has yet to be elucidated, but the FGFR3 gene is promising.
- Happle R. What is a nevus? a proposed definition of a common medical term. Dermatology. 1995;191:1-5.
- Gonzalez ME, Jabbari A, Tlougan BE, et al. Epidermal nevus. Dermatol Online J. 2010;16:12.
- Happle R. The group of epidermal nevus syndromes. part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273-285.
- Flosadottir E, Bjarnason B. A non-epidermolytic epidermal nevus of a soft, papillomatous type with transitional cell cancer of the bladder: a case report and review of non-cutaneous cancers associated with epidermal naevi. Acta Derm Venerol. 2008;88:173-175.
- Rosenthal D, Fretzin DF. Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder. Pediatr Dermatol. 1986;3:455-458.
- Garcia de Jalon A, Azua-Romea J, Trivez MA, et al. Epidermal naevus syndrome (Solomon’s syndrome) associated with bladder cancer in a 20-year-old female. Scand J Urol Nephrol. 2004;38:85-87.
- Rongioletti F, Rebora A. Epidermal nevus with transitional cell carcinomas of the urinary tract. J Am Acad Dermatol. 1991;25:856-858.
- Moss C. Mosacism and linear lesions. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:943-962.
- Hafner C, van Oers JM, Vogt T, et al. Mosaicisim of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201-2207.
- Bygum A, Fagerberg CR, Clemmensen OJ, et al. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- Happle R. Linear Cowden nevus: a new distinct epidermal nevus. Eur J Dermatol. 2007;17:133-136.
- Vujevich JJ, Mancini AJ. The epidermal nevus syndromes: multisystem disorders. J Am Acad Dermatol. 2004;50:957-961.
- Solomon L, Esterly N. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1-56.
- Grebe TA, Rimsa ME, Richter SF, et al. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24-30.
- Lamm DL, Torti FM. Bladder cancer, 1996. Ca Cancer J Clin. 1996;46:93-112.
- Metts MC, Metts JC, Milito SJ, et al. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc. 2000;92:285-294.
- Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555-2561.
- Cappellen D, DeOliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18-20.
- Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305-316.
- Luzar B, Calonje E, Bastian B. Tumors of the surface epithelium. In: Calonje JE, Breen T, McKee PH, eds. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012:1076-1149.
- Masood Q, Narayan D. Squamous cell carcinoma in a linear epidermal nevus. J Plast Reconstr Aesthet Surg. 2009;62:693-694.
- Cramer SF, Mandel MA, Hauler R, et al. Squamous cell carcinoma arising in a linear epidermal nevus. Arch Dermatol. 1981;117:222-224.
- Affleck AG, Leach IJ, Varma S. Two squamous cell carcinomas arising in a linear epidermal nevus in a 28-year-old female. Clin Exp Dermatol. 2005;30:382-384.
- Alam M, Arndt KA. A method for pulsed carbon dioxide laser treatment of epidermal nevi. J Am Acad Dermatol. 2002;46:554-556.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:1809-1810.
- Happle R. What is a nevus? a proposed definition of a common medical term. Dermatology. 1995;191:1-5.
- Gonzalez ME, Jabbari A, Tlougan BE, et al. Epidermal nevus. Dermatol Online J. 2010;16:12.
- Happle R. The group of epidermal nevus syndromes. part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273-285.
- Flosadottir E, Bjarnason B. A non-epidermolytic epidermal nevus of a soft, papillomatous type with transitional cell cancer of the bladder: a case report and review of non-cutaneous cancers associated with epidermal naevi. Acta Derm Venerol. 2008;88:173-175.
- Rosenthal D, Fretzin DF. Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder. Pediatr Dermatol. 1986;3:455-458.
- Garcia de Jalon A, Azua-Romea J, Trivez MA, et al. Epidermal naevus syndrome (Solomon’s syndrome) associated with bladder cancer in a 20-year-old female. Scand J Urol Nephrol. 2004;38:85-87.
- Rongioletti F, Rebora A. Epidermal nevus with transitional cell carcinomas of the urinary tract. J Am Acad Dermatol. 1991;25:856-858.
- Moss C. Mosacism and linear lesions. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:943-962.
- Hafner C, van Oers JM, Vogt T, et al. Mosaicisim of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201-2207.
- Bygum A, Fagerberg CR, Clemmensen OJ, et al. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- Happle R. Linear Cowden nevus: a new distinct epidermal nevus. Eur J Dermatol. 2007;17:133-136.
- Vujevich JJ, Mancini AJ. The epidermal nevus syndromes: multisystem disorders. J Am Acad Dermatol. 2004;50:957-961.
- Solomon L, Esterly N. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1-56.
- Grebe TA, Rimsa ME, Richter SF, et al. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24-30.
- Lamm DL, Torti FM. Bladder cancer, 1996. Ca Cancer J Clin. 1996;46:93-112.
- Metts MC, Metts JC, Milito SJ, et al. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc. 2000;92:285-294.
- Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555-2561.
- Cappellen D, DeOliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18-20.
- Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305-316.
- Luzar B, Calonje E, Bastian B. Tumors of the surface epithelium. In: Calonje JE, Breen T, McKee PH, eds. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012:1076-1149.
- Masood Q, Narayan D. Squamous cell carcinoma in a linear epidermal nevus. J Plast Reconstr Aesthet Surg. 2009;62:693-694.
- Cramer SF, Mandel MA, Hauler R, et al. Squamous cell carcinoma arising in a linear epidermal nevus. Arch Dermatol. 1981;117:222-224.
- Affleck AG, Leach IJ, Varma S. Two squamous cell carcinomas arising in a linear epidermal nevus in a 28-year-old female. Clin Exp Dermatol. 2005;30:382-384.
- Alam M, Arndt KA. A method for pulsed carbon dioxide laser treatment of epidermal nevi. J Am Acad Dermatol. 2002;46:554-556.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012:1809-1810.
Practice Points
- Epidermal nevi are common benign cutaneous neoplasms.
- Extensive systematized epidermal nevi can be a sign of internal disease.