User login
Intensive Care Unit Utilization After Adoption of a Ward-Based High-Flow Nasal Cannula Protocol
Children hospitalized for bronchiolitis frequently require admission to the intensive care unit (ICU), with estimates as high as 18%1,2 and 35%3 in two prospective, multicenter studies. The indication for ICU admission is nearly always a need for advanced respiratory support, which historically consisted of continuous or bilevel positive airway pressure (CPAP and BiPAP, respectively) or mechanical ventilation. High-flow nasal cannula (HFNC) is a recent addition to the respiratory support armamentarium, delivering heated and humidified oxygen at rates of up to 60 L/min and allowing for clinicians to titrate both flow rate and fraction of inspired oxygen (FiO2).4
Several studies have demonstrated that HFNC is capable of decreasing a child’s work of breathing,5-8 and it has the potential advantage of being better tolerated than other forms of advanced respiratory support.9,10 These case-series physiologic studies informed early ward-based HFNC protocols for bronchiolitis, which were adopted to decrease ICU utilization. Since then, single center observational studies examining the association between ward-based HFNC protocols and subsequent ICU utilization have come to discordant conclusions.11-14 Studying the effect of employing HFNC outside of the ICU is challenging in the context of a randomized, controlled trial (RCT) because it is difficult to blind healthcare providers to the intervention and because crossover from the control group to HFNC is frequent. Two unblinded RCTs published in 2017 and 2018 found that children randomized to conventional nasal cannula were frequently escalated to HFNC (flow rates of 1-2 L/kg per minute), but neither trial found a difference in ICU admission.15,16 Sample sizes substantially larger than those present in currently published or registered RCTs would be required to evaluate the impact of ward-based HFNC protocols on the outcome that inspired the protocols in the first place, namely ICU utilization.17
Children’s hospitals have adopted ward-based HFNC protocols at different time points over the last decade, which allows for a natural experiment—a promising alternative study design that avoids the challenges of blinding, crossover, and modest sample sizes. In order to have sufficient postadoption data for analyses, the present study is limited to ward-based HFNC protocols adopted prior to 2016, which we have termed “early” ward-based HFNC protocols. Among children with bronchiolitis, our objective was to measure the association between hospital-level adoption of a ward-based HFNC protocol and subsequent ICU utilization, using a multicenter network of children’s hospitals.
METHODS
We conducted a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database is operated by the Children’s Hospital Association (Lenexa, Kansas) and provides deidentified patient-level information for children who receive hospital care at 55 US children’s hospitals. Available data elements include patient demographic data, discharge diagnosis and procedure codes, and detailed billing information, such as laboratory, imaging, pharmacy, and supply charges. At the patient level, the use of HFNC vs standard oxygen therapy circuits cannot be discriminated.
Exposure
The study exposure was a hospital’s first ward-based HFNC protocol, with adoption measured at the hospital level at each PHIS site via direct communication with leaders in hospital medicine. In most cases, first contact was made with the pediatric hospital medicine division chief or fellowship program director, who then, if necessary, connected study investigators to local HFNC champions aware of site-specific historical HFNC protocol details. Contact with a hospital was made only if the hospital had contributed at least 6 consecutive years of data to PHIS. Hospitals were classified as “adopting” hospitals if their HFNC protocol met all of the following criteria: (a) allows initiation of HFNC outside of the ICU (on the floor or in the ED), (b) allows continued care outside of the ICU (on the floor), (c) not limited to a small unit like an intermediate care unit, and (d) adopted during a specific, known respiratory season. Hospitals for which ward-based HFNC protocols were adopted but did not meet these criteria were excluded from further analysis. Our intent was to identify large scale, programmatic protocol launches and exclude hospitals with exceptions that might preclude a sizable portion of our cohort from being eligible for the protocol. Hospitals for which inpatient use of HFNC remains limited to the ICU were defined as “nonadopting” hospitals. Respondents at adopting hospitals were asked to share details about their protocol, including patient eligibility criteria and maximum HFNC rates of flow permitted outside of the ICU.
Patient Characteristics
Patients aged 3 to 24 months who were hospitalized at adopting and nonadopting hospitals were included if an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code for bronchiolitis (466.XX) was present in any position (not limited to a primary diagnosis). The lower age limit of 3 months was chosen to match the most restrictive age eligibility criteria of provided HFNC protocols (Appendix 1). A crosswalk available from the Centers for Medicare & Medicaid Services18 was used to convert ICD-10 diagnosis and procedure codes from recent years to ICD-9 diagnosis and procedure codes. Patients were excluded if their encounter contained a diagnosis or procedure code signifying a complex chronic condition,19 if their hospitalization involved care in the neonatal ICU, or if their admission date occurred outside of the respiratory season. Respiratory season was defined as November 1 through April 30.
Outcomes
Outcomes were measured during three respiratory seasons leading up to adoption and during three respiratory seasons after adoption. The primary outcome was ICU utilization, including the proportion of patients admitted to the ICU and ICU length of stay, expressed as ICU days per 100 patients. Secondary outcomes included mean total length of stay and the proportion of patients who received mechanical ventilation. Lengths of stay were measured in days, the most granular unit of time provided in PHIS over the entire study period. As such, partial days of care are rounded up to 1 full day. A previously published strict definition for mechanical ventilation that limits false positives was used, requiring that patients have a procedure or supply code for mechanical ventilation and a pharmacy charge for a neuromuscular blocking agent.20
Primary Analysis
The primary analysis was restricted to adopting hospitals. An interrupted time series approach was used to measure two possible types of change associated with HFNC protocol adoption: an immediate intervention effect and a change in the slope of an outcome.21 The immediate intervention effect represents the change in the level of the outcome that occurs in the period immediately following the introduction of the protocol. The change in slope is the extent to which the outcome changes on a per season basis, attributable to the protocol. Interrupted time series estimates were adjusted for patient age, gender, race, ethnicity, and insurance type; linear regression was used for continuous outcomes and logistic regression for dichotomous outcomes. An ordinary least squares time series model was used to adjust for autocorrelation and Newey-West standard errors were employed.22 Analyses were performed using STATA version 14 (Stata-Corp, College Station, Texas).
Supplementary Analyses
Two preplanned supplementary analyses were conducted. Supplementary analysis 1 was identical to the primary analysis, with the exception that the first season after adoption was censored. The rationale for censoring the first adoption season was to account for a potential learning effect and/or delayed start to full protocol implementation. Supplementary analysis 2 used the nonadopting hospitals as a control group and subtracted the effects measured from an interrupted time series analysis among nonadopting hospitals from the effects measured among adopting hospitals. The rationale for this approach was to control for unmeasured secular (eg, availability of ICU beds) and temporal (eg, severity of a given bronchiolitis season) factors that may have coincidentally occurred with HFNC adoption seasons. The only modification to the interrupted time series approach for supplementary analysis 2 was to provide the nonadopting hospitals with an artificial interruption point because nonadopting hospitals, by definition, did not have an adoption season that could be used in an interrupted time series approach. The interruption point for nonadopting hospitals was set at the median adoption season for adopting hospitals.
RESULTS
Exposure
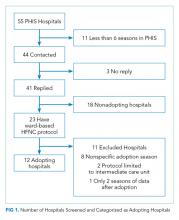
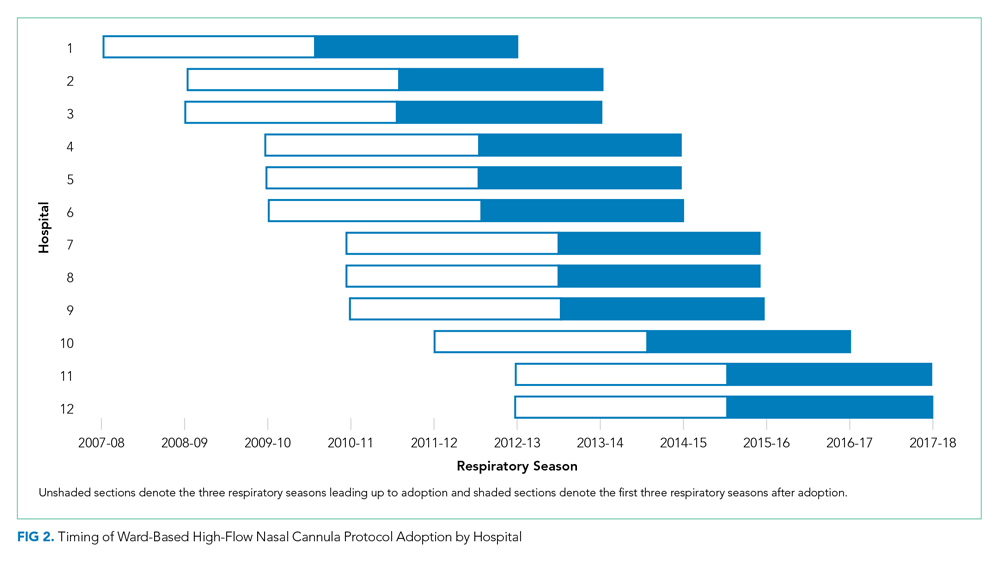
Leaders at 44 hospitals were contacted regarding their hospital’s use of HFNC outside of the ICU (Figure 1). Responses were obtained for 41 hospitals (93% response rate), 18 of which were classified as nonadopting hospitals. Of the 23 hospitals where the presence of ward-based HFNC protocols were reported, 12 met inclusion criteria and were classified as adopting hospitals. HFNC protocols were adopted at these hospitals in a staggered fashion between the 2010-2011 and 2015-2016 respiratory seasons (Figure 2). The median adoption season was the 2013-14 respiratory season.
Nine adopting hospitals were able to provide details about their first HFNC protocols (Appendix 1). No two protocols were identical, but they shared many similarities. Minimum age requirements ranged from birth to a few months of age. Exclusion criteria were particularly variable, with a history of chronic lung disease or apnea being the most common criteria. Maximum allowed rates of flow ranged from 4 to 10 liters per minute. Criteria for transfer to the ICU were consistently based on an elevated FiO2 and duration of HFNC exposure.
Patient Characteristics
A total of 32,809 bronchiolitis encounters occurred at adopting hospitals during qualifying respiratory seasons, of which 6,556 (20%) involved patients with a complex chronic condition and were excluded. Of the 26,253 included bronchiolitis encounters, 12,495 encounters occurred prior to ward-based HFNC protocol adoption and 13,758 encounters occurred after adoption. The median age of patients was 8 months (interquartile range, 5-14 months). Most patients were on government insurance (64%), male (58%), of white (56%) or black (18%) race, and of non-Hispanic ethnicity (72%). Pre- and postadoption patient demographics were similar (Appendix 2).
Primary Analysis
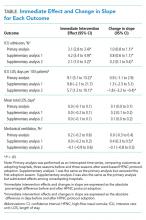
Shifts in the level of ICU use and ICU length of stay were observed at the time of adoption of a ward-based HFNC protocol (Figure 3). Specifically, ward-based HFNC protocol adoption was associated with an immediate 3.1% absolute increase (95% CI, 2.8%-3.4%) in the proportion of patients admitted to the ICU and a 9.1 days per 100 patients increase (95% CI, 5.1-13.2) in ICU length of stay (Table). The slope of ICU admissions per season was increasing after HFNC protocol adoption (1.0% increase per season; 95% CI, 0.8%-1.1%). When examined at the individual-hospital level (Appendix 3), seven hospitals were found to have significant increases in ICU admissions (immediate intervention effect or change in slope) after adoption, and one hospital was found to have a significant decrease in ICU admissions (change in slope only). Neither immediate intervention effects nor changes in the slopes of total length of stay and mechanical ventilation were observed, with mean total length of stay approximately 3 days and just over 1% of patients receiving mechanical ventilation (Figure 3).

Supplementary Analyses
Supplementary analyses were largely consistent with the primary analysis. Associations with increased ICU utilization were again observed, although the immediate change in ICU length of stay for supplementary analysis 1 was not significant and the slope for ICU length of stay in supplementary analysis 2 was down trending (Table). Changes in total length of stay and mechanical ventilation were not observed in either supplementary analysis, with the lone exception being an increase in the proportion of patients receiving mechanical ventilation per season (increase in slope) in supplementary analysis 1.
DISCUSSION
This is the largest multicenter study to date evaluating ICU utilization after adoption of a ward-based HFNC protocol for patients with bronchiolitis. While a principal goal of allowing HFNC use outside of the ICU is to reduce the time that patients with bronchiolitis spend in the ICU, we found that early protocols were, paradoxically, associated with increased ICU utilization. Ward-based HFNC protocols were not associated with changes in hospital length of stay or need for mechanical ventilation. Our findings are particularly relevant given that the majority of children’s hospitals in our sample have adopted ward-based HFNC protocols to care for patients with bronchiolitis.
The increase in ICU utilization measured in our study is a novel finding, seemingly in contradiction to existing literature. Early pilot studies inspired hope that employing HFNC on the general ward might prevent a portion of children from needing ICU care.11,12 Subsequent larger observational studies did not demonstrate decreases in ICU utilization after adoption of ward-based HFNC protocols.13,14 The two RCTs comparing low-flow and high-flow nasal cannula use outside of the ICU did not measure a statistically significant effect on ICU utilization, an exploratory outcome in both trials.15,16 However, the reported point estimates for absolute differences in ICU admission were 2% to 3% higher among patients randomized to HFNC, which is consistent with the 2% to 4% increase in ICU admission measured in the present study.
What might explain this surprising finding? While our observational study cannot speak to mechanism, the protocol details examined in the present study suggest that initial adoption of a ward-based HFNC protocol is often coupled with specific ICU transfer criteria that were unlikely in place prior to protocol initiation. For example, most protocols recommended consideration of ICU transfer for elevated FiO2 or prolonged duration of HFNC. Transfer to the ICU for prolonged HFNC duration is only possible in the setting of a ward-based HFNC protocol and transfer for elevated FiO2 was probably unnecessary prior to protocol adoption given that low-flow nasal cannula generally delivers 100% FiO2. It is also possible that with HFNC comes a perception of increased acuity. For example, medical providers may see patients on HFNC as sicker than patients with the same amount of work of breathing but off HFNC, which makes providers more likely to seek ICU admission for patients on HFNC. The combination of unchanged total length of stay and increased ICU utilization suggests that early ward-based HFNC protocols were an ineffective instrument to improve hospital bed availability during the peak census times that often occur in bronchiolitis season.
The large sample size afforded our study by its multicenter, retrospective design also allowed for a meaningful assessment of the association between a ward-based HFNC protocol and the need for mechanical ventilation. Early indications suggested a lack of substantial association between HFNC use outside of the ICU and rates of mechanical ventilation, but prior studies were limited by small numbers of patients receiving mechanical ventilation (<30 patients in each study).13,14,16 The present study, in which 783 patients received mechanical ventilation, supports the lack of association between early ward-based HFNC protocols and the need for mechanical ventilation. It should be noted that other studies have measured decreases in mechanical ventilation in association with ICU-based HFNC use.23-26 In addition to examining HFNC use in a different clinical context, decreases in mechanical ventilation measured after HFNC implementation in the ICU could be explained by preexisting practice trends to limit invasive ventilation and/or selection bias resulting from an increase in less severely ill patients being admitted to the ICU over time. The interrupted time series approach and the staggered adoption of HFNC protocols make the present study less susceptible to biases from preexisting trends and the inclusion of patients cared for both on the ward and within the ICU reduces selection bias.
Our study has several important limitations. First, all hospitals included in the analysis were US children’s hospitals and these findings may not generalize to other practice environments, including community hospitals and other countries. Second, our cohort and outcomes were defined using administrative billing data, which have been incompletely validated, making some degree of misclassification likely. Third, we measured HFNC exposure at the hospital level, but could not examine the extent to which individual patients were exposed to HFNC because such data are not present in PHIS. Even if we had access to patient-level HFNC exposure data, we would have still compared outcomes among all patients with bronchiolitis (not just those who received HFNC), to avoid selection bias. However, knowing HFNC exposure status at the patient level would have allowed for weighting of the effects measured at each hospital according to the extent of HFNC exposure. Fourth, there are likely other, unmeasured secular and temporal factors that could affect study outcomes. To some degree, the interrupted time series approach, observed staggered adoption of protocols, and nonadopting hospital supplementary analysis mitigate this risk of bias. Fifth, while the pre- and postadoption populations appeared demographically similar, it is possible that the populations might have differed by other unmeasured factors. Finally, early ward-based HFNC protocols have likely undergone iterative changes since adoption. We compared pre- and postadoption outcome slopes and censored the first adoption season in a supplementary analysis to attempt to account for this potential limitation.
In conclusion, our findings suggest that initial implementation of ward-based HFNC protocols were not successful at reducing ICU utilization for children with bronchiolitis. Future research should examine whether more evolved HFNC protocols that use higher flow rates, more generous ICU transfer criteria, and more rapid weaning criteria can reduce ICU utilization.
Acknowledgments
We thank Dr Vineeta Mittal (University of Texas Southwestern Medical Center) for providing feedback regarding the manuscript.
1. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. https://doi.org/10.1001/archpediatrics.2011.1669.
2. Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015;15(1):77-81. https://doi.org/10.1016/j.acap.2014.06.008.
3. Schroeder AR, Destino LA, Brooks R, Wang CJ, Coon ER. Outcomes of follow-up visits after bronchiolitis hospitalizations. JAMA Pediatr. 2018;172(3):296-297. https://doi.org/10.1001/jamapediatrics.2017.4002.
4. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145-155. https://doi.org/10.1513/AnnalsATS.201707-548FR.
5. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1-6. https://doi.org/10.1097/PCC.0000000000000011.
6. Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219. https://doi.org/10.1097/PCC.0000000000000112.
7. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713-720. https://doi.org/10.1002/ppul.23060.
8. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71.e63. https://doi.org/10.1016/j.jpeds.2017.06.006.
9. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014(3):CD009850. https://doi.org/10.1002/14651858.CD009850.pub2.
10. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413.
11. Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: a 3-year experience. Arch Dis Child. 2014;99(8):790-791. https://doi.org/10.1136/archdischild-2014-306637.
12. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
13. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
16. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
17. Coon ER, Mittal V, Brady PW. High flow nasal cannula-just expensive paracetamol? Lancet Child Adolesc Health. 2019;3(9):593-595. https://doi.org/10.1016/S2352-4642(19)30235-4.
18. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. 2012. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed November 19, 2016.
19. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
20. Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal changes in prescription of neuropharmacologic drugs and utilization of resources related to neurologic morbidity in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2017;18(12):e606-e614. https://doi.org/10.1097/PCC.0000000000001351.
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002.
22. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703-708.
23. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
24. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
25. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. 2017;18(2):112-119. https://doi.org/10.1097/PCC.0000000000000985.
26. Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(6):1601648. https://doi.org/10.1183/13993003.01648-2016.
Children hospitalized for bronchiolitis frequently require admission to the intensive care unit (ICU), with estimates as high as 18%1,2 and 35%3 in two prospective, multicenter studies. The indication for ICU admission is nearly always a need for advanced respiratory support, which historically consisted of continuous or bilevel positive airway pressure (CPAP and BiPAP, respectively) or mechanical ventilation. High-flow nasal cannula (HFNC) is a recent addition to the respiratory support armamentarium, delivering heated and humidified oxygen at rates of up to 60 L/min and allowing for clinicians to titrate both flow rate and fraction of inspired oxygen (FiO2).4
Several studies have demonstrated that HFNC is capable of decreasing a child’s work of breathing,5-8 and it has the potential advantage of being better tolerated than other forms of advanced respiratory support.9,10 These case-series physiologic studies informed early ward-based HFNC protocols for bronchiolitis, which were adopted to decrease ICU utilization. Since then, single center observational studies examining the association between ward-based HFNC protocols and subsequent ICU utilization have come to discordant conclusions.11-14 Studying the effect of employing HFNC outside of the ICU is challenging in the context of a randomized, controlled trial (RCT) because it is difficult to blind healthcare providers to the intervention and because crossover from the control group to HFNC is frequent. Two unblinded RCTs published in 2017 and 2018 found that children randomized to conventional nasal cannula were frequently escalated to HFNC (flow rates of 1-2 L/kg per minute), but neither trial found a difference in ICU admission.15,16 Sample sizes substantially larger than those present in currently published or registered RCTs would be required to evaluate the impact of ward-based HFNC protocols on the outcome that inspired the protocols in the first place, namely ICU utilization.17
Children’s hospitals have adopted ward-based HFNC protocols at different time points over the last decade, which allows for a natural experiment—a promising alternative study design that avoids the challenges of blinding, crossover, and modest sample sizes. In order to have sufficient postadoption data for analyses, the present study is limited to ward-based HFNC protocols adopted prior to 2016, which we have termed “early” ward-based HFNC protocols. Among children with bronchiolitis, our objective was to measure the association between hospital-level adoption of a ward-based HFNC protocol and subsequent ICU utilization, using a multicenter network of children’s hospitals.
METHODS
We conducted a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database is operated by the Children’s Hospital Association (Lenexa, Kansas) and provides deidentified patient-level information for children who receive hospital care at 55 US children’s hospitals. Available data elements include patient demographic data, discharge diagnosis and procedure codes, and detailed billing information, such as laboratory, imaging, pharmacy, and supply charges. At the patient level, the use of HFNC vs standard oxygen therapy circuits cannot be discriminated.
Exposure
The study exposure was a hospital’s first ward-based HFNC protocol, with adoption measured at the hospital level at each PHIS site via direct communication with leaders in hospital medicine. In most cases, first contact was made with the pediatric hospital medicine division chief or fellowship program director, who then, if necessary, connected study investigators to local HFNC champions aware of site-specific historical HFNC protocol details. Contact with a hospital was made only if the hospital had contributed at least 6 consecutive years of data to PHIS. Hospitals were classified as “adopting” hospitals if their HFNC protocol met all of the following criteria: (a) allows initiation of HFNC outside of the ICU (on the floor or in the ED), (b) allows continued care outside of the ICU (on the floor), (c) not limited to a small unit like an intermediate care unit, and (d) adopted during a specific, known respiratory season. Hospitals for which ward-based HFNC protocols were adopted but did not meet these criteria were excluded from further analysis. Our intent was to identify large scale, programmatic protocol launches and exclude hospitals with exceptions that might preclude a sizable portion of our cohort from being eligible for the protocol. Hospitals for which inpatient use of HFNC remains limited to the ICU were defined as “nonadopting” hospitals. Respondents at adopting hospitals were asked to share details about their protocol, including patient eligibility criteria and maximum HFNC rates of flow permitted outside of the ICU.
Patient Characteristics
Patients aged 3 to 24 months who were hospitalized at adopting and nonadopting hospitals were included if an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code for bronchiolitis (466.XX) was present in any position (not limited to a primary diagnosis). The lower age limit of 3 months was chosen to match the most restrictive age eligibility criteria of provided HFNC protocols (Appendix 1). A crosswalk available from the Centers for Medicare & Medicaid Services18 was used to convert ICD-10 diagnosis and procedure codes from recent years to ICD-9 diagnosis and procedure codes. Patients were excluded if their encounter contained a diagnosis or procedure code signifying a complex chronic condition,19 if their hospitalization involved care in the neonatal ICU, or if their admission date occurred outside of the respiratory season. Respiratory season was defined as November 1 through April 30.
Outcomes
Outcomes were measured during three respiratory seasons leading up to adoption and during three respiratory seasons after adoption. The primary outcome was ICU utilization, including the proportion of patients admitted to the ICU and ICU length of stay, expressed as ICU days per 100 patients. Secondary outcomes included mean total length of stay and the proportion of patients who received mechanical ventilation. Lengths of stay were measured in days, the most granular unit of time provided in PHIS over the entire study period. As such, partial days of care are rounded up to 1 full day. A previously published strict definition for mechanical ventilation that limits false positives was used, requiring that patients have a procedure or supply code for mechanical ventilation and a pharmacy charge for a neuromuscular blocking agent.20
Primary Analysis
The primary analysis was restricted to adopting hospitals. An interrupted time series approach was used to measure two possible types of change associated with HFNC protocol adoption: an immediate intervention effect and a change in the slope of an outcome.21 The immediate intervention effect represents the change in the level of the outcome that occurs in the period immediately following the introduction of the protocol. The change in slope is the extent to which the outcome changes on a per season basis, attributable to the protocol. Interrupted time series estimates were adjusted for patient age, gender, race, ethnicity, and insurance type; linear regression was used for continuous outcomes and logistic regression for dichotomous outcomes. An ordinary least squares time series model was used to adjust for autocorrelation and Newey-West standard errors were employed.22 Analyses were performed using STATA version 14 (Stata-Corp, College Station, Texas).
Supplementary Analyses
Two preplanned supplementary analyses were conducted. Supplementary analysis 1 was identical to the primary analysis, with the exception that the first season after adoption was censored. The rationale for censoring the first adoption season was to account for a potential learning effect and/or delayed start to full protocol implementation. Supplementary analysis 2 used the nonadopting hospitals as a control group and subtracted the effects measured from an interrupted time series analysis among nonadopting hospitals from the effects measured among adopting hospitals. The rationale for this approach was to control for unmeasured secular (eg, availability of ICU beds) and temporal (eg, severity of a given bronchiolitis season) factors that may have coincidentally occurred with HFNC adoption seasons. The only modification to the interrupted time series approach for supplementary analysis 2 was to provide the nonadopting hospitals with an artificial interruption point because nonadopting hospitals, by definition, did not have an adoption season that could be used in an interrupted time series approach. The interruption point for nonadopting hospitals was set at the median adoption season for adopting hospitals.
RESULTS
Exposure
Leaders at 44 hospitals were contacted regarding their hospital’s use of HFNC outside of the ICU (Figure 1). Responses were obtained for 41 hospitals (93% response rate), 18 of which were classified as nonadopting hospitals. Of the 23 hospitals where the presence of ward-based HFNC protocols were reported, 12 met inclusion criteria and were classified as adopting hospitals. HFNC protocols were adopted at these hospitals in a staggered fashion between the 2010-2011 and 2015-2016 respiratory seasons (Figure 2). The median adoption season was the 2013-14 respiratory season.
Nine adopting hospitals were able to provide details about their first HFNC protocols (Appendix 1). No two protocols were identical, but they shared many similarities. Minimum age requirements ranged from birth to a few months of age. Exclusion criteria were particularly variable, with a history of chronic lung disease or apnea being the most common criteria. Maximum allowed rates of flow ranged from 4 to 10 liters per minute. Criteria for transfer to the ICU were consistently based on an elevated FiO2 and duration of HFNC exposure.

Patient Characteristics
A total of 32,809 bronchiolitis encounters occurred at adopting hospitals during qualifying respiratory seasons, of which 6,556 (20%) involved patients with a complex chronic condition and were excluded. Of the 26,253 included bronchiolitis encounters, 12,495 encounters occurred prior to ward-based HFNC protocol adoption and 13,758 encounters occurred after adoption. The median age of patients was 8 months (interquartile range, 5-14 months). Most patients were on government insurance (64%), male (58%), of white (56%) or black (18%) race, and of non-Hispanic ethnicity (72%). Pre- and postadoption patient demographics were similar (Appendix 2).
Primary Analysis
Shifts in the level of ICU use and ICU length of stay were observed at the time of adoption of a ward-based HFNC protocol (Figure 3). Specifically, ward-based HFNC protocol adoption was associated with an immediate 3.1% absolute increase (95% CI, 2.8%-3.4%) in the proportion of patients admitted to the ICU and a 9.1 days per 100 patients increase (95% CI, 5.1-13.2) in ICU length of stay (Table). The slope of ICU admissions per season was increasing after HFNC protocol adoption (1.0% increase per season; 95% CI, 0.8%-1.1%). When examined at the individual-hospital level (Appendix 3), seven hospitals were found to have significant increases in ICU admissions (immediate intervention effect or change in slope) after adoption, and one hospital was found to have a significant decrease in ICU admissions (change in slope only). Neither immediate intervention effects nor changes in the slopes of total length of stay and mechanical ventilation were observed, with mean total length of stay approximately 3 days and just over 1% of patients receiving mechanical ventilation (Figure 3).

Supplementary Analyses
Supplementary analyses were largely consistent with the primary analysis. Associations with increased ICU utilization were again observed, although the immediate change in ICU length of stay for supplementary analysis 1 was not significant and the slope for ICU length of stay in supplementary analysis 2 was down trending (Table). Changes in total length of stay and mechanical ventilation were not observed in either supplementary analysis, with the lone exception being an increase in the proportion of patients receiving mechanical ventilation per season (increase in slope) in supplementary analysis 1.
DISCUSSION
This is the largest multicenter study to date evaluating ICU utilization after adoption of a ward-based HFNC protocol for patients with bronchiolitis. While a principal goal of allowing HFNC use outside of the ICU is to reduce the time that patients with bronchiolitis spend in the ICU, we found that early protocols were, paradoxically, associated with increased ICU utilization. Ward-based HFNC protocols were not associated with changes in hospital length of stay or need for mechanical ventilation. Our findings are particularly relevant given that the majority of children’s hospitals in our sample have adopted ward-based HFNC protocols to care for patients with bronchiolitis.
The increase in ICU utilization measured in our study is a novel finding, seemingly in contradiction to existing literature. Early pilot studies inspired hope that employing HFNC on the general ward might prevent a portion of children from needing ICU care.11,12 Subsequent larger observational studies did not demonstrate decreases in ICU utilization after adoption of ward-based HFNC protocols.13,14 The two RCTs comparing low-flow and high-flow nasal cannula use outside of the ICU did not measure a statistically significant effect on ICU utilization, an exploratory outcome in both trials.15,16 However, the reported point estimates for absolute differences in ICU admission were 2% to 3% higher among patients randomized to HFNC, which is consistent with the 2% to 4% increase in ICU admission measured in the present study.
What might explain this surprising finding? While our observational study cannot speak to mechanism, the protocol details examined in the present study suggest that initial adoption of a ward-based HFNC protocol is often coupled with specific ICU transfer criteria that were unlikely in place prior to protocol initiation. For example, most protocols recommended consideration of ICU transfer for elevated FiO2 or prolonged duration of HFNC. Transfer to the ICU for prolonged HFNC duration is only possible in the setting of a ward-based HFNC protocol and transfer for elevated FiO2 was probably unnecessary prior to protocol adoption given that low-flow nasal cannula generally delivers 100% FiO2. It is also possible that with HFNC comes a perception of increased acuity. For example, medical providers may see patients on HFNC as sicker than patients with the same amount of work of breathing but off HFNC, which makes providers more likely to seek ICU admission for patients on HFNC. The combination of unchanged total length of stay and increased ICU utilization suggests that early ward-based HFNC protocols were an ineffective instrument to improve hospital bed availability during the peak census times that often occur in bronchiolitis season.
The large sample size afforded our study by its multicenter, retrospective design also allowed for a meaningful assessment of the association between a ward-based HFNC protocol and the need for mechanical ventilation. Early indications suggested a lack of substantial association between HFNC use outside of the ICU and rates of mechanical ventilation, but prior studies were limited by small numbers of patients receiving mechanical ventilation (<30 patients in each study).13,14,16 The present study, in which 783 patients received mechanical ventilation, supports the lack of association between early ward-based HFNC protocols and the need for mechanical ventilation. It should be noted that other studies have measured decreases in mechanical ventilation in association with ICU-based HFNC use.23-26 In addition to examining HFNC use in a different clinical context, decreases in mechanical ventilation measured after HFNC implementation in the ICU could be explained by preexisting practice trends to limit invasive ventilation and/or selection bias resulting from an increase in less severely ill patients being admitted to the ICU over time. The interrupted time series approach and the staggered adoption of HFNC protocols make the present study less susceptible to biases from preexisting trends and the inclusion of patients cared for both on the ward and within the ICU reduces selection bias.
Our study has several important limitations. First, all hospitals included in the analysis were US children’s hospitals and these findings may not generalize to other practice environments, including community hospitals and other countries. Second, our cohort and outcomes were defined using administrative billing data, which have been incompletely validated, making some degree of misclassification likely. Third, we measured HFNC exposure at the hospital level, but could not examine the extent to which individual patients were exposed to HFNC because such data are not present in PHIS. Even if we had access to patient-level HFNC exposure data, we would have still compared outcomes among all patients with bronchiolitis (not just those who received HFNC), to avoid selection bias. However, knowing HFNC exposure status at the patient level would have allowed for weighting of the effects measured at each hospital according to the extent of HFNC exposure. Fourth, there are likely other, unmeasured secular and temporal factors that could affect study outcomes. To some degree, the interrupted time series approach, observed staggered adoption of protocols, and nonadopting hospital supplementary analysis mitigate this risk of bias. Fifth, while the pre- and postadoption populations appeared demographically similar, it is possible that the populations might have differed by other unmeasured factors. Finally, early ward-based HFNC protocols have likely undergone iterative changes since adoption. We compared pre- and postadoption outcome slopes and censored the first adoption season in a supplementary analysis to attempt to account for this potential limitation.
In conclusion, our findings suggest that initial implementation of ward-based HFNC protocols were not successful at reducing ICU utilization for children with bronchiolitis. Future research should examine whether more evolved HFNC protocols that use higher flow rates, more generous ICU transfer criteria, and more rapid weaning criteria can reduce ICU utilization.
Acknowledgments
We thank Dr Vineeta Mittal (University of Texas Southwestern Medical Center) for providing feedback regarding the manuscript.
Children hospitalized for bronchiolitis frequently require admission to the intensive care unit (ICU), with estimates as high as 18%1,2 and 35%3 in two prospective, multicenter studies. The indication for ICU admission is nearly always a need for advanced respiratory support, which historically consisted of continuous or bilevel positive airway pressure (CPAP and BiPAP, respectively) or mechanical ventilation. High-flow nasal cannula (HFNC) is a recent addition to the respiratory support armamentarium, delivering heated and humidified oxygen at rates of up to 60 L/min and allowing for clinicians to titrate both flow rate and fraction of inspired oxygen (FiO2).4
Several studies have demonstrated that HFNC is capable of decreasing a child’s work of breathing,5-8 and it has the potential advantage of being better tolerated than other forms of advanced respiratory support.9,10 These case-series physiologic studies informed early ward-based HFNC protocols for bronchiolitis, which were adopted to decrease ICU utilization. Since then, single center observational studies examining the association between ward-based HFNC protocols and subsequent ICU utilization have come to discordant conclusions.11-14 Studying the effect of employing HFNC outside of the ICU is challenging in the context of a randomized, controlled trial (RCT) because it is difficult to blind healthcare providers to the intervention and because crossover from the control group to HFNC is frequent. Two unblinded RCTs published in 2017 and 2018 found that children randomized to conventional nasal cannula were frequently escalated to HFNC (flow rates of 1-2 L/kg per minute), but neither trial found a difference in ICU admission.15,16 Sample sizes substantially larger than those present in currently published or registered RCTs would be required to evaluate the impact of ward-based HFNC protocols on the outcome that inspired the protocols in the first place, namely ICU utilization.17
Children’s hospitals have adopted ward-based HFNC protocols at different time points over the last decade, which allows for a natural experiment—a promising alternative study design that avoids the challenges of blinding, crossover, and modest sample sizes. In order to have sufficient postadoption data for analyses, the present study is limited to ward-based HFNC protocols adopted prior to 2016, which we have termed “early” ward-based HFNC protocols. Among children with bronchiolitis, our objective was to measure the association between hospital-level adoption of a ward-based HFNC protocol and subsequent ICU utilization, using a multicenter network of children’s hospitals.
METHODS
We conducted a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database is operated by the Children’s Hospital Association (Lenexa, Kansas) and provides deidentified patient-level information for children who receive hospital care at 55 US children’s hospitals. Available data elements include patient demographic data, discharge diagnosis and procedure codes, and detailed billing information, such as laboratory, imaging, pharmacy, and supply charges. At the patient level, the use of HFNC vs standard oxygen therapy circuits cannot be discriminated.
Exposure
The study exposure was a hospital’s first ward-based HFNC protocol, with adoption measured at the hospital level at each PHIS site via direct communication with leaders in hospital medicine. In most cases, first contact was made with the pediatric hospital medicine division chief or fellowship program director, who then, if necessary, connected study investigators to local HFNC champions aware of site-specific historical HFNC protocol details. Contact with a hospital was made only if the hospital had contributed at least 6 consecutive years of data to PHIS. Hospitals were classified as “adopting” hospitals if their HFNC protocol met all of the following criteria: (a) allows initiation of HFNC outside of the ICU (on the floor or in the ED), (b) allows continued care outside of the ICU (on the floor), (c) not limited to a small unit like an intermediate care unit, and (d) adopted during a specific, known respiratory season. Hospitals for which ward-based HFNC protocols were adopted but did not meet these criteria were excluded from further analysis. Our intent was to identify large scale, programmatic protocol launches and exclude hospitals with exceptions that might preclude a sizable portion of our cohort from being eligible for the protocol. Hospitals for which inpatient use of HFNC remains limited to the ICU were defined as “nonadopting” hospitals. Respondents at adopting hospitals were asked to share details about their protocol, including patient eligibility criteria and maximum HFNC rates of flow permitted outside of the ICU.
Patient Characteristics
Patients aged 3 to 24 months who were hospitalized at adopting and nonadopting hospitals were included if an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code for bronchiolitis (466.XX) was present in any position (not limited to a primary diagnosis). The lower age limit of 3 months was chosen to match the most restrictive age eligibility criteria of provided HFNC protocols (Appendix 1). A crosswalk available from the Centers for Medicare & Medicaid Services18 was used to convert ICD-10 diagnosis and procedure codes from recent years to ICD-9 diagnosis and procedure codes. Patients were excluded if their encounter contained a diagnosis or procedure code signifying a complex chronic condition,19 if their hospitalization involved care in the neonatal ICU, or if their admission date occurred outside of the respiratory season. Respiratory season was defined as November 1 through April 30.
Outcomes
Outcomes were measured during three respiratory seasons leading up to adoption and during three respiratory seasons after adoption. The primary outcome was ICU utilization, including the proportion of patients admitted to the ICU and ICU length of stay, expressed as ICU days per 100 patients. Secondary outcomes included mean total length of stay and the proportion of patients who received mechanical ventilation. Lengths of stay were measured in days, the most granular unit of time provided in PHIS over the entire study period. As such, partial days of care are rounded up to 1 full day. A previously published strict definition for mechanical ventilation that limits false positives was used, requiring that patients have a procedure or supply code for mechanical ventilation and a pharmacy charge for a neuromuscular blocking agent.20
Primary Analysis
The primary analysis was restricted to adopting hospitals. An interrupted time series approach was used to measure two possible types of change associated with HFNC protocol adoption: an immediate intervention effect and a change in the slope of an outcome.21 The immediate intervention effect represents the change in the level of the outcome that occurs in the period immediately following the introduction of the protocol. The change in slope is the extent to which the outcome changes on a per season basis, attributable to the protocol. Interrupted time series estimates were adjusted for patient age, gender, race, ethnicity, and insurance type; linear regression was used for continuous outcomes and logistic regression for dichotomous outcomes. An ordinary least squares time series model was used to adjust for autocorrelation and Newey-West standard errors were employed.22 Analyses were performed using STATA version 14 (Stata-Corp, College Station, Texas).
Supplementary Analyses
Two preplanned supplementary analyses were conducted. Supplementary analysis 1 was identical to the primary analysis, with the exception that the first season after adoption was censored. The rationale for censoring the first adoption season was to account for a potential learning effect and/or delayed start to full protocol implementation. Supplementary analysis 2 used the nonadopting hospitals as a control group and subtracted the effects measured from an interrupted time series analysis among nonadopting hospitals from the effects measured among adopting hospitals. The rationale for this approach was to control for unmeasured secular (eg, availability of ICU beds) and temporal (eg, severity of a given bronchiolitis season) factors that may have coincidentally occurred with HFNC adoption seasons. The only modification to the interrupted time series approach for supplementary analysis 2 was to provide the nonadopting hospitals with an artificial interruption point because nonadopting hospitals, by definition, did not have an adoption season that could be used in an interrupted time series approach. The interruption point for nonadopting hospitals was set at the median adoption season for adopting hospitals.
RESULTS
Exposure
Leaders at 44 hospitals were contacted regarding their hospital’s use of HFNC outside of the ICU (Figure 1). Responses were obtained for 41 hospitals (93% response rate), 18 of which were classified as nonadopting hospitals. Of the 23 hospitals where the presence of ward-based HFNC protocols were reported, 12 met inclusion criteria and were classified as adopting hospitals. HFNC protocols were adopted at these hospitals in a staggered fashion between the 2010-2011 and 2015-2016 respiratory seasons (Figure 2). The median adoption season was the 2013-14 respiratory season.
Nine adopting hospitals were able to provide details about their first HFNC protocols (Appendix 1). No two protocols were identical, but they shared many similarities. Minimum age requirements ranged from birth to a few months of age. Exclusion criteria were particularly variable, with a history of chronic lung disease or apnea being the most common criteria. Maximum allowed rates of flow ranged from 4 to 10 liters per minute. Criteria for transfer to the ICU were consistently based on an elevated FiO2 and duration of HFNC exposure.

Patient Characteristics
A total of 32,809 bronchiolitis encounters occurred at adopting hospitals during qualifying respiratory seasons, of which 6,556 (20%) involved patients with a complex chronic condition and were excluded. Of the 26,253 included bronchiolitis encounters, 12,495 encounters occurred prior to ward-based HFNC protocol adoption and 13,758 encounters occurred after adoption. The median age of patients was 8 months (interquartile range, 5-14 months). Most patients were on government insurance (64%), male (58%), of white (56%) or black (18%) race, and of non-Hispanic ethnicity (72%). Pre- and postadoption patient demographics were similar (Appendix 2).
Primary Analysis
Shifts in the level of ICU use and ICU length of stay were observed at the time of adoption of a ward-based HFNC protocol (Figure 3). Specifically, ward-based HFNC protocol adoption was associated with an immediate 3.1% absolute increase (95% CI, 2.8%-3.4%) in the proportion of patients admitted to the ICU and a 9.1 days per 100 patients increase (95% CI, 5.1-13.2) in ICU length of stay (Table). The slope of ICU admissions per season was increasing after HFNC protocol adoption (1.0% increase per season; 95% CI, 0.8%-1.1%). When examined at the individual-hospital level (Appendix 3), seven hospitals were found to have significant increases in ICU admissions (immediate intervention effect or change in slope) after adoption, and one hospital was found to have a significant decrease in ICU admissions (change in slope only). Neither immediate intervention effects nor changes in the slopes of total length of stay and mechanical ventilation were observed, with mean total length of stay approximately 3 days and just over 1% of patients receiving mechanical ventilation (Figure 3).

Supplementary Analyses
Supplementary analyses were largely consistent with the primary analysis. Associations with increased ICU utilization were again observed, although the immediate change in ICU length of stay for supplementary analysis 1 was not significant and the slope for ICU length of stay in supplementary analysis 2 was down trending (Table). Changes in total length of stay and mechanical ventilation were not observed in either supplementary analysis, with the lone exception being an increase in the proportion of patients receiving mechanical ventilation per season (increase in slope) in supplementary analysis 1.
DISCUSSION
This is the largest multicenter study to date evaluating ICU utilization after adoption of a ward-based HFNC protocol for patients with bronchiolitis. While a principal goal of allowing HFNC use outside of the ICU is to reduce the time that patients with bronchiolitis spend in the ICU, we found that early protocols were, paradoxically, associated with increased ICU utilization. Ward-based HFNC protocols were not associated with changes in hospital length of stay or need for mechanical ventilation. Our findings are particularly relevant given that the majority of children’s hospitals in our sample have adopted ward-based HFNC protocols to care for patients with bronchiolitis.
The increase in ICU utilization measured in our study is a novel finding, seemingly in contradiction to existing literature. Early pilot studies inspired hope that employing HFNC on the general ward might prevent a portion of children from needing ICU care.11,12 Subsequent larger observational studies did not demonstrate decreases in ICU utilization after adoption of ward-based HFNC protocols.13,14 The two RCTs comparing low-flow and high-flow nasal cannula use outside of the ICU did not measure a statistically significant effect on ICU utilization, an exploratory outcome in both trials.15,16 However, the reported point estimates for absolute differences in ICU admission were 2% to 3% higher among patients randomized to HFNC, which is consistent with the 2% to 4% increase in ICU admission measured in the present study.
What might explain this surprising finding? While our observational study cannot speak to mechanism, the protocol details examined in the present study suggest that initial adoption of a ward-based HFNC protocol is often coupled with specific ICU transfer criteria that were unlikely in place prior to protocol initiation. For example, most protocols recommended consideration of ICU transfer for elevated FiO2 or prolonged duration of HFNC. Transfer to the ICU for prolonged HFNC duration is only possible in the setting of a ward-based HFNC protocol and transfer for elevated FiO2 was probably unnecessary prior to protocol adoption given that low-flow nasal cannula generally delivers 100% FiO2. It is also possible that with HFNC comes a perception of increased acuity. For example, medical providers may see patients on HFNC as sicker than patients with the same amount of work of breathing but off HFNC, which makes providers more likely to seek ICU admission for patients on HFNC. The combination of unchanged total length of stay and increased ICU utilization suggests that early ward-based HFNC protocols were an ineffective instrument to improve hospital bed availability during the peak census times that often occur in bronchiolitis season.
The large sample size afforded our study by its multicenter, retrospective design also allowed for a meaningful assessment of the association between a ward-based HFNC protocol and the need for mechanical ventilation. Early indications suggested a lack of substantial association between HFNC use outside of the ICU and rates of mechanical ventilation, but prior studies were limited by small numbers of patients receiving mechanical ventilation (<30 patients in each study).13,14,16 The present study, in which 783 patients received mechanical ventilation, supports the lack of association between early ward-based HFNC protocols and the need for mechanical ventilation. It should be noted that other studies have measured decreases in mechanical ventilation in association with ICU-based HFNC use.23-26 In addition to examining HFNC use in a different clinical context, decreases in mechanical ventilation measured after HFNC implementation in the ICU could be explained by preexisting practice trends to limit invasive ventilation and/or selection bias resulting from an increase in less severely ill patients being admitted to the ICU over time. The interrupted time series approach and the staggered adoption of HFNC protocols make the present study less susceptible to biases from preexisting trends and the inclusion of patients cared for both on the ward and within the ICU reduces selection bias.
Our study has several important limitations. First, all hospitals included in the analysis were US children’s hospitals and these findings may not generalize to other practice environments, including community hospitals and other countries. Second, our cohort and outcomes were defined using administrative billing data, which have been incompletely validated, making some degree of misclassification likely. Third, we measured HFNC exposure at the hospital level, but could not examine the extent to which individual patients were exposed to HFNC because such data are not present in PHIS. Even if we had access to patient-level HFNC exposure data, we would have still compared outcomes among all patients with bronchiolitis (not just those who received HFNC), to avoid selection bias. However, knowing HFNC exposure status at the patient level would have allowed for weighting of the effects measured at each hospital according to the extent of HFNC exposure. Fourth, there are likely other, unmeasured secular and temporal factors that could affect study outcomes. To some degree, the interrupted time series approach, observed staggered adoption of protocols, and nonadopting hospital supplementary analysis mitigate this risk of bias. Fifth, while the pre- and postadoption populations appeared demographically similar, it is possible that the populations might have differed by other unmeasured factors. Finally, early ward-based HFNC protocols have likely undergone iterative changes since adoption. We compared pre- and postadoption outcome slopes and censored the first adoption season in a supplementary analysis to attempt to account for this potential limitation.
In conclusion, our findings suggest that initial implementation of ward-based HFNC protocols were not successful at reducing ICU utilization for children with bronchiolitis. Future research should examine whether more evolved HFNC protocols that use higher flow rates, more generous ICU transfer criteria, and more rapid weaning criteria can reduce ICU utilization.
Acknowledgments
We thank Dr Vineeta Mittal (University of Texas Southwestern Medical Center) for providing feedback regarding the manuscript.
1. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. https://doi.org/10.1001/archpediatrics.2011.1669.
2. Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015;15(1):77-81. https://doi.org/10.1016/j.acap.2014.06.008.
3. Schroeder AR, Destino LA, Brooks R, Wang CJ, Coon ER. Outcomes of follow-up visits after bronchiolitis hospitalizations. JAMA Pediatr. 2018;172(3):296-297. https://doi.org/10.1001/jamapediatrics.2017.4002.
4. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145-155. https://doi.org/10.1513/AnnalsATS.201707-548FR.
5. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1-6. https://doi.org/10.1097/PCC.0000000000000011.
6. Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219. https://doi.org/10.1097/PCC.0000000000000112.
7. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713-720. https://doi.org/10.1002/ppul.23060.
8. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71.e63. https://doi.org/10.1016/j.jpeds.2017.06.006.
9. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014(3):CD009850. https://doi.org/10.1002/14651858.CD009850.pub2.
10. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413.
11. Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: a 3-year experience. Arch Dis Child. 2014;99(8):790-791. https://doi.org/10.1136/archdischild-2014-306637.
12. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
13. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
16. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
17. Coon ER, Mittal V, Brady PW. High flow nasal cannula-just expensive paracetamol? Lancet Child Adolesc Health. 2019;3(9):593-595. https://doi.org/10.1016/S2352-4642(19)30235-4.
18. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. 2012. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed November 19, 2016.
19. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
20. Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal changes in prescription of neuropharmacologic drugs and utilization of resources related to neurologic morbidity in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2017;18(12):e606-e614. https://doi.org/10.1097/PCC.0000000000001351.
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002.
22. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703-708.
23. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
24. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
25. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. 2017;18(2):112-119. https://doi.org/10.1097/PCC.0000000000000985.
26. Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(6):1601648. https://doi.org/10.1183/13993003.01648-2016.
1. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. https://doi.org/10.1001/archpediatrics.2011.1669.
2. Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015;15(1):77-81. https://doi.org/10.1016/j.acap.2014.06.008.
3. Schroeder AR, Destino LA, Brooks R, Wang CJ, Coon ER. Outcomes of follow-up visits after bronchiolitis hospitalizations. JAMA Pediatr. 2018;172(3):296-297. https://doi.org/10.1001/jamapediatrics.2017.4002.
4. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145-155. https://doi.org/10.1513/AnnalsATS.201707-548FR.
5. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1-6. https://doi.org/10.1097/PCC.0000000000000011.
6. Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219. https://doi.org/10.1097/PCC.0000000000000112.
7. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713-720. https://doi.org/10.1002/ppul.23060.
8. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71.e63. https://doi.org/10.1016/j.jpeds.2017.06.006.
9. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014(3):CD009850. https://doi.org/10.1002/14651858.CD009850.pub2.
10. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413.
11. Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: a 3-year experience. Arch Dis Child. 2014;99(8):790-791. https://doi.org/10.1136/archdischild-2014-306637.
12. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
13. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
16. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
17. Coon ER, Mittal V, Brady PW. High flow nasal cannula-just expensive paracetamol? Lancet Child Adolesc Health. 2019;3(9):593-595. https://doi.org/10.1016/S2352-4642(19)30235-4.
18. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. 2012. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed November 19, 2016.
19. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
20. Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal changes in prescription of neuropharmacologic drugs and utilization of resources related to neurologic morbidity in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2017;18(12):e606-e614. https://doi.org/10.1097/PCC.0000000000001351.
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002.
22. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703-708.
23. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
24. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
25. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. 2017;18(2):112-119. https://doi.org/10.1097/PCC.0000000000000985.
26. Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(6):1601648. https://doi.org/10.1183/13993003.01648-2016.
© 2020 Society of Hospital Medicine