User login
Air travel and venous thromboembolism: Minimizing the risk
Editor’s Note: The views expressed in this article are solely those of the authors and do not reflect the official policy or position of the Department of State or the United States Government. This version of the article was peer-reviewed.
Venous thromboembolism (VTE) associated with travel has emerged as an important public health concern over the past decade. Numerous epidemiologic and case control studies have reported air travel as a risk factor for the development of VTE and have attempted to determine who is at risk and which precautions need to be taken to prevent this potentially fatal event.1–7 Often referred to as “traveler’s thrombosis” or “flight-related deep vein thrombosis,” VTE can also develop after long trips by automobile, bus, or train.8,9 Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips.3
See related patient information material
This article focuses on defining VTE and recognizing its clinical features, as well as providing recommendations and guidelines to prevent, diagnose, and treat this complication in people who travel.
WHAT IS VENOUS THROMBOEMBOLISM?
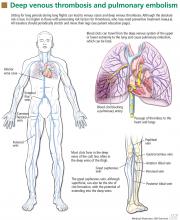
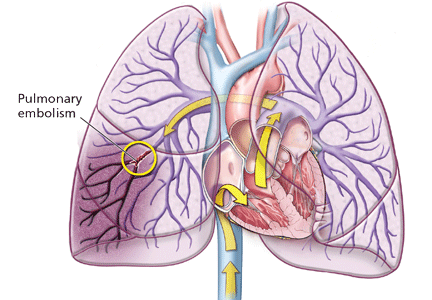
Deep vein thrombosis and pulmonary embolism represent different manifestations of the same clinical entity, ie, VTE. VTE is a common, lethal disease that affects hospitalized and nonhospitalized patients, frequently recurs, is often overlooked, may be asymptomatic, and may result in long-term complications that include pulmonary hypertension and the postthrombotic syndrome.
Deep vein thrombosis of the upper extremities is generally related to an indwelling venous catheter or a central line being used for long-term administration of antibiotics, chemotherapy, or nutrition. A condition known as Paget-Schroetter syndrome or “effort thrombosis” may be seen in younger or athletic people who have a history of strenuous or unusual arm exercise.
RISK FACTORS FOR VTE
Common inherited risk factors include:
- Factor V Leiden mutation
- Prothrombin gene mutation G20210A
- Hyperhomocysteinemia
- Deficiency of the natural anticoagulant proteins C, S, or antithrombin
- Elevated levels of factor VIII (may be inherited or acquired).
Acquired risk factors include:
- Older age
- Immobilization or stasis (such as sitting for long periods of time while traveling)
- Surgery (most notably orthopedic procedures including hip and knee replacement and repair of a hip fracture)
- Trauma
- Stroke
- Acute medical illness (including congestive heart failure, chronic obstructive pulmonary disease, pneumonia)
- The antiphospholipid syndrome (consisting of a lupus anticoagulant, anticardiolipin antibodies, or both)
- Pregnancy and the postpartum state
- Use of oral contraceptives or hormone replacement therapy
- Cancer (including the myeloproliferative disorders) and certain chemotherapeutic agents
- Obesity (a body mass index > 30 kg/m2, see www.nhlbisupport.com/bmi/)
- Inflammatory bowel disease
- Previous VTE
- A central venous catheter or pacemaker
- Nephrotic syndrome.
In addition, emerging risk factors more recently recognized include male sex, persistence of elevated factor VIII levels, and the continued presence of an elevated D-dimer level or deep vein thrombosis on duplex ultrasonography once anticoagulation treatment is completed. There is also evidence of an association between VTE and risk factors for atherosclerotic arterial disease such as smoking, hypertension, hyperlipidemia, and diabetes.
CLINICAL MANIFESTATIONS OF VTE
Patients with deep vein thrombosis may complain of pain, swelling, or both in the leg or arm. Physical examination may reveal increased warmth, tenderness, erythema, edema, or dilated (collateral) veins, most notable on the upper thigh or calf (for deep vein thrombosis in the lower extremity) or the chest wall (for upper-extremity deep vein thrombosis). The examiner may also observe a tender, palpable cord, which represents a superficial vein thrombosis involving the great and small saphenous veins (Figure 1). In extreme situations, the limb may be cyanotic or gangrenous.
DIAGNOSIS OF VTE
Clinical examination alone is generally insufficient to confirm a diagnosis of deep vein thrombosis or pulmonary embolism. Venous duplex ultrasonography is the most dependable investigation for deep vein thrombosis, but other tests include D-dimer and imaging studies such as computed tomographic venography or magnetic resonance venography of the lower extremities. A more invasive approach is venography; formerly considered the gold standard, it is now generally used only when the diagnosis is in doubt after noninvasive testing. The diagnosis of acute pulmonary embolism is best made by spiral computed tomography.
Other studies that may prove helpful include a ventilation-perfusion lung scan for patients who cannot undergo computed tomography due to a contrast allergy or renal insufficiency. Pulmonary angiography, while the gold standard, is less commonly used today, given the specificity and sensitivity of computed tomography.
Echocardiography at the bedside may be useful for patients too sick to move, although the study may not be diagnostic unless thrombi are seen in the heart or pulmonary arteries.
TREATMENT OF VTE
For acute deep venous thrombosis
Acute deep vein thrombosis is now treated on an outpatient basis under most circumstances.
Unfractionated heparin is given intravenously for patients who need to be hospitalized, or subcutaneously in full dose for inpatient or outpatient treatment.
Low-molecular-weight heparins are available in subcutaneous preparations and can be given on an outpatient basis.
Fondaparinux (Arixtra), a factor Xa inhibitor, can also be given subcutaneously on an outpatient basis. Equivalent products are available outside the United States.
Warfarin (Coumadin), an oral vitamin K inhibitor, is the agent of choice for long-term management of deep vein thrombosis.
Other oral agents are available outside the United States.
For pulmonary embolism
Outpatient treatment of pulmonary embolism is not yet advised: an initial hospitalization is necessary. The same anticoagulants used for deep vein thrombosis are also used for acute pulmonary embolism.
Empiric treatment in underdeveloped countries
VTE may be an even greater concern on an outbound trip to a remote area, where medical care capabilities may be less than ideal and diagnostic and treatment options may be limited.
If there is a high pretest probability of acute VTE (Table 2, Table 3) and no diagnostic methods are available, empiric treatment with any of the parenteral anticoagulant agents listed in Table 4 is an option until the diagnosis can be confirmed. Caveats:
- Care must be taken to be certain there is not a strong contraindication to the use of anticoagulation, such as bleeding or a drug allergy.
- Neither unfractionated heparin nor any of the low-molecular-weight heparins should be given to a patient who has a history of heparin-induced thrombocytopenia.
- In patients who have chronic kidney disease (creatinine clearance less than 30 mL/minute), the dosage of low-molecular-weight heparins must be adjusted and factor Xa inhibitors avoided. Both of these types of anticoagulants should be avoided in patients on hemodialysis.
More aggressive therapy
Under select circumstances a more aggressive approach to the treatment of VTE may be necessary. These options are usually indicated for a patient with a massive deep vein thrombosis of a lower extremity and for certain patients with an upper extremity deep vein thrombosis. Treatments include catheter-directed thrombolytic therapy and endovenous or surgical thrombectomy.
Thrombolytic therapy is recommended for a patient with an acute pulmonary embolism who is clinically unstable (systolic blood pressure lower than 90 mm Hg), if there is no contraindication to its use (bleeding risk or recent stroke or surgery). Thrombolytic therapy is also an option for those at low risk of bleeding with an acute pulmonary embolism who have signs and symptoms of right heart failure proven by echocardiography.
Surgical pulmonary embolectomy for acute massive pulmonary embolism and mechanical thrombectomy for extensive deep vein thrombosis are generally available only at highly sophisticated tertiary care centers.
An inferior vena cava filter is advised in patients with acute deep vein thrombosis or pulmonary embolism who cannot be fully anticoagulated, to prevent the clot from migrating from the lower extremities to the lungs. These filters are available as either permanent or temporary implants. Some temporary versions can remain in place for up to 150 days after insertion.
PREVENTION OF VTE
Prevention is the standard of care for all patients admitted to the hospital and in select individuals as outpatients who are at high risk of VTE.
Mechanical compression (graduated compression stockings, intermittent pneumatic compression devices) has proven effective in reducing the incidence of deep vein thrombosis and pulmonary embolism postoperatively in patients who cannot take anticoagulants. One study has demonstrated that compression stockings may also be effective in preventing VTE during travel.12
ABSOLUTE RISK IS LOW
Over the past decade, special attention has been paid to travel as a risk factor for developing VTE.13 Traveler’s thrombosis has become an important public health concern. Numerous publications and epidemiologic studies have targeted air travel in an attempt to determine who is at risk and what precautions are necessary to prevent this complication.1–7,9
The incidence of VTE following air travel is reported to be 3.2 per 1,000 person-years.4 While this incidence is relatively low, it is still 3.2 times higher than in the healthy population that is not flying.
The more serious complication of VTE, ie, acute pulmonary embolism, occurs less often. In three studies, the reported incidence ranged from 1.65 per million patients in flights longer than 8 hours to a high of 4.8 per million patients in flights longer than 12 hours or distances exceeding 10,000 km (6,200 miles).5,14,15 For the 400 passengers on the average long-haul flight of 12 hours, there is at most a 0.2% chance that somebody on the plane will have a symptomatic VTE).
RISK FACTORS IN LONG-DISTANCE TRAVELERS
The risk of traveler’s thrombosis has recently attracted the attention of passengers and the airline industry. Airlines are now openly discussing the risk and providing reminders such as exercises that should be undertaken in-flight (see the patient information page that accompanies this article). Some airlines are recommending that all patients consult their doctor to assess their personal risk of deep vein thrombosis before flying.
The most common risk factors for VTE in travelers are well established and are additive (Table 1). The extent of the additive risk, however, is not entirely clear.
What is clear is that when VTE occurs it is a life-altering and life-threatening event. If it occurs on an outbound trip, the local resources and capabilities available at the destination may not be adequate for optimal treatment. If a traveler experiences a VTE event on an outbound trip, an emergency return trip to the continental United States or a regional center of expertise may be required. There is an additive risk with this subsequent travel event if the patient is not given immediate treatment first (Table 4). Hence, treatment prior to evacuation should be strongly considered.
The traveler must also be aware that VTE can be recognized up to 2 months after a long-haul flight, though it is especially a concern within the first 2 weeks after travel.2,4,16,17
RECOMMENDATIONS FOR LONG-DISTANCE AIR TRAVELERS
Each person should be evaluated on a case-by-case basis for his or her need for VTE prophylaxis. Medical guidelines for airline passengers have been published by the Aerospace Medical Association and the American College of Chest Physicians (ACCP).18,19 In general, travelers should:
- Exercise the legs by flexing and extending the ankles at regular intervals while seated (see the patient information material that accompanies this article) and frequently contracting the calf muscles.
- Walk about the cabin periodically, 5 minutes for every hour on longer-duration flights (over 4 hours) and when flight conditions permit.
- Drink adequate amounts of water and fruit juices to maintain good hydration.17
- Avoid alcohol and caffeinated beverages, which are dehydrating.
- Be careful about eating too much during the flight.
- Request an aisle seat if you are at risk
- Do not place baggage underneath the seat in front of you, because that reduces the ability to move the legs.
- Do not sleep in a cramped position, and avoid the use of any type of sleep aid.
- Avoid wearing constrictive clothing around the lower extremities or waist.
We recommend that all airplane passengers take the steps listed above to reduce venous stasis and avoid dehydration, even though these measures have not been proven effective in clinical trials.19
The ACCP further advises that decisions about pharmacologic prophylaxis of VTE for airplane passengers at high risk should be made on an individual basis, considering that there are potential adverse effects of prophylaxis and that these may outweigh the benefits. For long-distance travelers with additional risk factors for VTE, we suggest the following:
- Use of properly fitted, below-the-knee graduated compression stockings providing 15 to 30 mm Hg of pressure at the ankle (particularly when large varicosities or leg edema is present)
- For people at very high risk, a single prophylactic dose of a low-molecular-weight heparin or a factor Xa inhibitor injected just before departure (Table 5)
- Aspirin is not recommended as it is not effective for the prevention of VTE.20
SUMMARY FOR THE AIR TRAVELER
All travelers on long flights should perform standard VTE prophylaxis exercises (see the patient information pages accompanying this article). Although VTE is uncommon, people with additional risk factors who travel frequently either on multiple flights in a short period of time or on very long flights should be evaluated on a case-by-case basis for a more aggressive approach to prevention (compression support hose or prophylactic administration of a low-molecular-weight heparin or a factor Xa inhibitor).
Should a VTE event occur during travel, the patient should seek medical care immediately. The standard evaluation of a patient with a suspected VTE should include an estimation of the pretest probability of disease (Table 2, Table 3), followed by duplex ultrasonography of the upper or lower extremity to detect a deep vein thrombosis. If symptoms dictate, then spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) should be ordered to diagnose acute pulmonary embolism. A positive D-dimer blood test alone is not diagnostic and may not be available in more remote locations. A negative D-dimer test result is most helpful to exclude VTE.
Standard therapy for VTE is immediate treatment with one of the anticoagulants listed in Table 4, unless the patient has a contraindication to treatment, such as bleeding or allergy. Immediate evacuation is recommended if the patient has a life-threatening pulmonary embolism, defined as hemodynamic instability (hypotension with a blood pressure under 90 mm Hg systolic or signs of right heart failure) that cannot be treated at a local facility. An air ambulance should be used to transport these patients. If the patient has an iliofemoral deep vein thrombosis, it is also advisable that he or she be considered for evacuation if severe symptoms are present, such as pain, swelling, or cyanosis. Unless contraindicated, all patients should be given either full-dose intravenous or full-dose subcutaneous heparin or subcutaneous injection of a readily available low-molecular-weight heparin preparations or factor Xa inhibitor at once.21
- Brenner B. Interventions to prevent venous thrombosis after air travel, are they necessary? Yes. J Thromb Haemost 2006; 4:2302–2305.
- Cannegieter SC, Doggen CJM, van Houwellingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA Study). PLoS Med 2006; 3:1258–1265.
- Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med 2009; 151:180–190.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organizations. PLoS Med 2007; 4:1508–1514.
- Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med 2007; 262:615–634.
- Lehmann R, Suess C, Leus M, et al. Incidence, clinical characteristics, and long-term prognosis of travel-associated pulmonary embolism. Eur Heart J 2009; 30:233–241.
- Philbrick JT, Shumate R, Siadaty MS, et al. Air travel and venous thromboembolism: a systematic review. J Gen Intern Med 2007; 22:107–114.
- Cruickshank JM, Gorlin R, Jennett B. Air travel and thrombotic episodes: the economy class syndrome. Lancet 1988; 2:497–498.
- Bagshaw M. Traveler’s thrombosis: a review of deep vein thrombosis associated with travel. Air Transport Medicine Committee, Aerospace Medical Association. Aviat Space Environ Med 2001; 72:848–851.
- Wells PS, Owens C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Arnason T, Wells PS, Forester AJ. Appropriateness of diagnostic strategies for evaluating suspected venous thromboembolism. Thromb Haemost 2007; 97:195–201.
- Clarke M, Hopewell S, Juszcak E, Eisinga A, Kjeldstrøm M. Compression stockings in preventing deep vein thrombosis in airline passengers. Cochrane Database of Syst Rev 2006; Apr 19( 2):CD004002. DOI: 10.1002/14651858.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. Use of preventive measures for travel-related venous thrombosis in professionals who attend medical conferences. J Thromb Haemost 2006; 4:2373–2376.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism in the Madrid-Barajas Airport. Arch Intern Med 2003; 163:2766–2770.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001; 345:779–783.
- Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ 2003; 327:1072–1076.
- Eklof B, Kistner RL, Masuda EM, et al. Venous thromboembolism in association with prolonged air travel. Dermatol Surg 1996; 22:637–641.
- Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med 2003: 74:1009.
- Geerts WH, Bergqvist B, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133:381S–453S.
- Rosendaal FR. Interventions to prevent venous thrombosis after air travel: are they necessary? No. J Thromb Haemost 2006; 4:2306–2307.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
Editor’s Note: The views expressed in this article are solely those of the authors and do not reflect the official policy or position of the Department of State or the United States Government. This version of the article was peer-reviewed.
Venous thromboembolism (VTE) associated with travel has emerged as an important public health concern over the past decade. Numerous epidemiologic and case control studies have reported air travel as a risk factor for the development of VTE and have attempted to determine who is at risk and which precautions need to be taken to prevent this potentially fatal event.1–7 Often referred to as “traveler’s thrombosis” or “flight-related deep vein thrombosis,” VTE can also develop after long trips by automobile, bus, or train.8,9 Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips.3
See related patient information material
This article focuses on defining VTE and recognizing its clinical features, as well as providing recommendations and guidelines to prevent, diagnose, and treat this complication in people who travel.
WHAT IS VENOUS THROMBOEMBOLISM?
Deep vein thrombosis and pulmonary embolism represent different manifestations of the same clinical entity, ie, VTE. VTE is a common, lethal disease that affects hospitalized and nonhospitalized patients, frequently recurs, is often overlooked, may be asymptomatic, and may result in long-term complications that include pulmonary hypertension and the postthrombotic syndrome.
Deep vein thrombosis of the upper extremities is generally related to an indwelling venous catheter or a central line being used for long-term administration of antibiotics, chemotherapy, or nutrition. A condition known as Paget-Schroetter syndrome or “effort thrombosis” may be seen in younger or athletic people who have a history of strenuous or unusual arm exercise.
RISK FACTORS FOR VTE
Common inherited risk factors include:
- Factor V Leiden mutation
- Prothrombin gene mutation G20210A
- Hyperhomocysteinemia
- Deficiency of the natural anticoagulant proteins C, S, or antithrombin
- Elevated levels of factor VIII (may be inherited or acquired).
Acquired risk factors include:
- Older age
- Immobilization or stasis (such as sitting for long periods of time while traveling)
- Surgery (most notably orthopedic procedures including hip and knee replacement and repair of a hip fracture)
- Trauma
- Stroke
- Acute medical illness (including congestive heart failure, chronic obstructive pulmonary disease, pneumonia)
- The antiphospholipid syndrome (consisting of a lupus anticoagulant, anticardiolipin antibodies, or both)
- Pregnancy and the postpartum state
- Use of oral contraceptives or hormone replacement therapy
- Cancer (including the myeloproliferative disorders) and certain chemotherapeutic agents
- Obesity (a body mass index > 30 kg/m2, see www.nhlbisupport.com/bmi/)
- Inflammatory bowel disease
- Previous VTE
- A central venous catheter or pacemaker
- Nephrotic syndrome.
In addition, emerging risk factors more recently recognized include male sex, persistence of elevated factor VIII levels, and the continued presence of an elevated D-dimer level or deep vein thrombosis on duplex ultrasonography once anticoagulation treatment is completed. There is also evidence of an association between VTE and risk factors for atherosclerotic arterial disease such as smoking, hypertension, hyperlipidemia, and diabetes.
CLINICAL MANIFESTATIONS OF VTE
Patients with deep vein thrombosis may complain of pain, swelling, or both in the leg or arm. Physical examination may reveal increased warmth, tenderness, erythema, edema, or dilated (collateral) veins, most notable on the upper thigh or calf (for deep vein thrombosis in the lower extremity) or the chest wall (for upper-extremity deep vein thrombosis). The examiner may also observe a tender, palpable cord, which represents a superficial vein thrombosis involving the great and small saphenous veins (Figure 1). In extreme situations, the limb may be cyanotic or gangrenous.
DIAGNOSIS OF VTE
Clinical examination alone is generally insufficient to confirm a diagnosis of deep vein thrombosis or pulmonary embolism. Venous duplex ultrasonography is the most dependable investigation for deep vein thrombosis, but other tests include D-dimer and imaging studies such as computed tomographic venography or magnetic resonance venography of the lower extremities. A more invasive approach is venography; formerly considered the gold standard, it is now generally used only when the diagnosis is in doubt after noninvasive testing. The diagnosis of acute pulmonary embolism is best made by spiral computed tomography.
Other studies that may prove helpful include a ventilation-perfusion lung scan for patients who cannot undergo computed tomography due to a contrast allergy or renal insufficiency. Pulmonary angiography, while the gold standard, is less commonly used today, given the specificity and sensitivity of computed tomography.
Echocardiography at the bedside may be useful for patients too sick to move, although the study may not be diagnostic unless thrombi are seen in the heart or pulmonary arteries.
TREATMENT OF VTE
For acute deep venous thrombosis
Acute deep vein thrombosis is now treated on an outpatient basis under most circumstances.
Unfractionated heparin is given intravenously for patients who need to be hospitalized, or subcutaneously in full dose for inpatient or outpatient treatment.
Low-molecular-weight heparins are available in subcutaneous preparations and can be given on an outpatient basis.
Fondaparinux (Arixtra), a factor Xa inhibitor, can also be given subcutaneously on an outpatient basis. Equivalent products are available outside the United States.
Warfarin (Coumadin), an oral vitamin K inhibitor, is the agent of choice for long-term management of deep vein thrombosis.
Other oral agents are available outside the United States.
For pulmonary embolism
Outpatient treatment of pulmonary embolism is not yet advised: an initial hospitalization is necessary. The same anticoagulants used for deep vein thrombosis are also used for acute pulmonary embolism.
Empiric treatment in underdeveloped countries
VTE may be an even greater concern on an outbound trip to a remote area, where medical care capabilities may be less than ideal and diagnostic and treatment options may be limited.
If there is a high pretest probability of acute VTE (Table 2, Table 3) and no diagnostic methods are available, empiric treatment with any of the parenteral anticoagulant agents listed in Table 4 is an option until the diagnosis can be confirmed. Caveats:
- Care must be taken to be certain there is not a strong contraindication to the use of anticoagulation, such as bleeding or a drug allergy.
- Neither unfractionated heparin nor any of the low-molecular-weight heparins should be given to a patient who has a history of heparin-induced thrombocytopenia.
- In patients who have chronic kidney disease (creatinine clearance less than 30 mL/minute), the dosage of low-molecular-weight heparins must be adjusted and factor Xa inhibitors avoided. Both of these types of anticoagulants should be avoided in patients on hemodialysis.
More aggressive therapy
Under select circumstances a more aggressive approach to the treatment of VTE may be necessary. These options are usually indicated for a patient with a massive deep vein thrombosis of a lower extremity and for certain patients with an upper extremity deep vein thrombosis. Treatments include catheter-directed thrombolytic therapy and endovenous or surgical thrombectomy.
Thrombolytic therapy is recommended for a patient with an acute pulmonary embolism who is clinically unstable (systolic blood pressure lower than 90 mm Hg), if there is no contraindication to its use (bleeding risk or recent stroke or surgery). Thrombolytic therapy is also an option for those at low risk of bleeding with an acute pulmonary embolism who have signs and symptoms of right heart failure proven by echocardiography.
Surgical pulmonary embolectomy for acute massive pulmonary embolism and mechanical thrombectomy for extensive deep vein thrombosis are generally available only at highly sophisticated tertiary care centers.
An inferior vena cava filter is advised in patients with acute deep vein thrombosis or pulmonary embolism who cannot be fully anticoagulated, to prevent the clot from migrating from the lower extremities to the lungs. These filters are available as either permanent or temporary implants. Some temporary versions can remain in place for up to 150 days after insertion.
PREVENTION OF VTE
Prevention is the standard of care for all patients admitted to the hospital and in select individuals as outpatients who are at high risk of VTE.
Mechanical compression (graduated compression stockings, intermittent pneumatic compression devices) has proven effective in reducing the incidence of deep vein thrombosis and pulmonary embolism postoperatively in patients who cannot take anticoagulants. One study has demonstrated that compression stockings may also be effective in preventing VTE during travel.12
ABSOLUTE RISK IS LOW
Over the past decade, special attention has been paid to travel as a risk factor for developing VTE.13 Traveler’s thrombosis has become an important public health concern. Numerous publications and epidemiologic studies have targeted air travel in an attempt to determine who is at risk and what precautions are necessary to prevent this complication.1–7,9
The incidence of VTE following air travel is reported to be 3.2 per 1,000 person-years.4 While this incidence is relatively low, it is still 3.2 times higher than in the healthy population that is not flying.
The more serious complication of VTE, ie, acute pulmonary embolism, occurs less often. In three studies, the reported incidence ranged from 1.65 per million patients in flights longer than 8 hours to a high of 4.8 per million patients in flights longer than 12 hours or distances exceeding 10,000 km (6,200 miles).5,14,15 For the 400 passengers on the average long-haul flight of 12 hours, there is at most a 0.2% chance that somebody on the plane will have a symptomatic VTE).
RISK FACTORS IN LONG-DISTANCE TRAVELERS
The risk of traveler’s thrombosis has recently attracted the attention of passengers and the airline industry. Airlines are now openly discussing the risk and providing reminders such as exercises that should be undertaken in-flight (see the patient information page that accompanies this article). Some airlines are recommending that all patients consult their doctor to assess their personal risk of deep vein thrombosis before flying.
The most common risk factors for VTE in travelers are well established and are additive (Table 1). The extent of the additive risk, however, is not entirely clear.
What is clear is that when VTE occurs it is a life-altering and life-threatening event. If it occurs on an outbound trip, the local resources and capabilities available at the destination may not be adequate for optimal treatment. If a traveler experiences a VTE event on an outbound trip, an emergency return trip to the continental United States or a regional center of expertise may be required. There is an additive risk with this subsequent travel event if the patient is not given immediate treatment first (Table 4). Hence, treatment prior to evacuation should be strongly considered.
The traveler must also be aware that VTE can be recognized up to 2 months after a long-haul flight, though it is especially a concern within the first 2 weeks after travel.2,4,16,17
RECOMMENDATIONS FOR LONG-DISTANCE AIR TRAVELERS
Each person should be evaluated on a case-by-case basis for his or her need for VTE prophylaxis. Medical guidelines for airline passengers have been published by the Aerospace Medical Association and the American College of Chest Physicians (ACCP).18,19 In general, travelers should:
- Exercise the legs by flexing and extending the ankles at regular intervals while seated (see the patient information material that accompanies this article) and frequently contracting the calf muscles.
- Walk about the cabin periodically, 5 minutes for every hour on longer-duration flights (over 4 hours) and when flight conditions permit.
- Drink adequate amounts of water and fruit juices to maintain good hydration.17
- Avoid alcohol and caffeinated beverages, which are dehydrating.
- Be careful about eating too much during the flight.
- Request an aisle seat if you are at risk
- Do not place baggage underneath the seat in front of you, because that reduces the ability to move the legs.
- Do not sleep in a cramped position, and avoid the use of any type of sleep aid.
- Avoid wearing constrictive clothing around the lower extremities or waist.
We recommend that all airplane passengers take the steps listed above to reduce venous stasis and avoid dehydration, even though these measures have not been proven effective in clinical trials.19
The ACCP further advises that decisions about pharmacologic prophylaxis of VTE for airplane passengers at high risk should be made on an individual basis, considering that there are potential adverse effects of prophylaxis and that these may outweigh the benefits. For long-distance travelers with additional risk factors for VTE, we suggest the following:
- Use of properly fitted, below-the-knee graduated compression stockings providing 15 to 30 mm Hg of pressure at the ankle (particularly when large varicosities or leg edema is present)
- For people at very high risk, a single prophylactic dose of a low-molecular-weight heparin or a factor Xa inhibitor injected just before departure (Table 5)
- Aspirin is not recommended as it is not effective for the prevention of VTE.20
SUMMARY FOR THE AIR TRAVELER
All travelers on long flights should perform standard VTE prophylaxis exercises (see the patient information pages accompanying this article). Although VTE is uncommon, people with additional risk factors who travel frequently either on multiple flights in a short period of time or on very long flights should be evaluated on a case-by-case basis for a more aggressive approach to prevention (compression support hose or prophylactic administration of a low-molecular-weight heparin or a factor Xa inhibitor).
Should a VTE event occur during travel, the patient should seek medical care immediately. The standard evaluation of a patient with a suspected VTE should include an estimation of the pretest probability of disease (Table 2, Table 3), followed by duplex ultrasonography of the upper or lower extremity to detect a deep vein thrombosis. If symptoms dictate, then spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) should be ordered to diagnose acute pulmonary embolism. A positive D-dimer blood test alone is not diagnostic and may not be available in more remote locations. A negative D-dimer test result is most helpful to exclude VTE.
Standard therapy for VTE is immediate treatment with one of the anticoagulants listed in Table 4, unless the patient has a contraindication to treatment, such as bleeding or allergy. Immediate evacuation is recommended if the patient has a life-threatening pulmonary embolism, defined as hemodynamic instability (hypotension with a blood pressure under 90 mm Hg systolic or signs of right heart failure) that cannot be treated at a local facility. An air ambulance should be used to transport these patients. If the patient has an iliofemoral deep vein thrombosis, it is also advisable that he or she be considered for evacuation if severe symptoms are present, such as pain, swelling, or cyanosis. Unless contraindicated, all patients should be given either full-dose intravenous or full-dose subcutaneous heparin or subcutaneous injection of a readily available low-molecular-weight heparin preparations or factor Xa inhibitor at once.21
Editor’s Note: The views expressed in this article are solely those of the authors and do not reflect the official policy or position of the Department of State or the United States Government. This version of the article was peer-reviewed.
Venous thromboembolism (VTE) associated with travel has emerged as an important public health concern over the past decade. Numerous epidemiologic and case control studies have reported air travel as a risk factor for the development of VTE and have attempted to determine who is at risk and which precautions need to be taken to prevent this potentially fatal event.1–7 Often referred to as “traveler’s thrombosis” or “flight-related deep vein thrombosis,” VTE can also develop after long trips by automobile, bus, or train.8,9 Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips.3
See related patient information material
This article focuses on defining VTE and recognizing its clinical features, as well as providing recommendations and guidelines to prevent, diagnose, and treat this complication in people who travel.
WHAT IS VENOUS THROMBOEMBOLISM?
Deep vein thrombosis and pulmonary embolism represent different manifestations of the same clinical entity, ie, VTE. VTE is a common, lethal disease that affects hospitalized and nonhospitalized patients, frequently recurs, is often overlooked, may be asymptomatic, and may result in long-term complications that include pulmonary hypertension and the postthrombotic syndrome.
Deep vein thrombosis of the upper extremities is generally related to an indwelling venous catheter or a central line being used for long-term administration of antibiotics, chemotherapy, or nutrition. A condition known as Paget-Schroetter syndrome or “effort thrombosis” may be seen in younger or athletic people who have a history of strenuous or unusual arm exercise.
RISK FACTORS FOR VTE
Common inherited risk factors include:
- Factor V Leiden mutation
- Prothrombin gene mutation G20210A
- Hyperhomocysteinemia
- Deficiency of the natural anticoagulant proteins C, S, or antithrombin
- Elevated levels of factor VIII (may be inherited or acquired).
Acquired risk factors include:
- Older age
- Immobilization or stasis (such as sitting for long periods of time while traveling)
- Surgery (most notably orthopedic procedures including hip and knee replacement and repair of a hip fracture)
- Trauma
- Stroke
- Acute medical illness (including congestive heart failure, chronic obstructive pulmonary disease, pneumonia)
- The antiphospholipid syndrome (consisting of a lupus anticoagulant, anticardiolipin antibodies, or both)
- Pregnancy and the postpartum state
- Use of oral contraceptives or hormone replacement therapy
- Cancer (including the myeloproliferative disorders) and certain chemotherapeutic agents
- Obesity (a body mass index > 30 kg/m2, see www.nhlbisupport.com/bmi/)
- Inflammatory bowel disease
- Previous VTE
- A central venous catheter or pacemaker
- Nephrotic syndrome.
In addition, emerging risk factors more recently recognized include male sex, persistence of elevated factor VIII levels, and the continued presence of an elevated D-dimer level or deep vein thrombosis on duplex ultrasonography once anticoagulation treatment is completed. There is also evidence of an association between VTE and risk factors for atherosclerotic arterial disease such as smoking, hypertension, hyperlipidemia, and diabetes.
CLINICAL MANIFESTATIONS OF VTE
Patients with deep vein thrombosis may complain of pain, swelling, or both in the leg or arm. Physical examination may reveal increased warmth, tenderness, erythema, edema, or dilated (collateral) veins, most notable on the upper thigh or calf (for deep vein thrombosis in the lower extremity) or the chest wall (for upper-extremity deep vein thrombosis). The examiner may also observe a tender, palpable cord, which represents a superficial vein thrombosis involving the great and small saphenous veins (Figure 1). In extreme situations, the limb may be cyanotic or gangrenous.
DIAGNOSIS OF VTE
Clinical examination alone is generally insufficient to confirm a diagnosis of deep vein thrombosis or pulmonary embolism. Venous duplex ultrasonography is the most dependable investigation for deep vein thrombosis, but other tests include D-dimer and imaging studies such as computed tomographic venography or magnetic resonance venography of the lower extremities. A more invasive approach is venography; formerly considered the gold standard, it is now generally used only when the diagnosis is in doubt after noninvasive testing. The diagnosis of acute pulmonary embolism is best made by spiral computed tomography.
Other studies that may prove helpful include a ventilation-perfusion lung scan for patients who cannot undergo computed tomography due to a contrast allergy or renal insufficiency. Pulmonary angiography, while the gold standard, is less commonly used today, given the specificity and sensitivity of computed tomography.
Echocardiography at the bedside may be useful for patients too sick to move, although the study may not be diagnostic unless thrombi are seen in the heart or pulmonary arteries.
TREATMENT OF VTE
For acute deep venous thrombosis
Acute deep vein thrombosis is now treated on an outpatient basis under most circumstances.
Unfractionated heparin is given intravenously for patients who need to be hospitalized, or subcutaneously in full dose for inpatient or outpatient treatment.
Low-molecular-weight heparins are available in subcutaneous preparations and can be given on an outpatient basis.
Fondaparinux (Arixtra), a factor Xa inhibitor, can also be given subcutaneously on an outpatient basis. Equivalent products are available outside the United States.
Warfarin (Coumadin), an oral vitamin K inhibitor, is the agent of choice for long-term management of deep vein thrombosis.
Other oral agents are available outside the United States.
For pulmonary embolism
Outpatient treatment of pulmonary embolism is not yet advised: an initial hospitalization is necessary. The same anticoagulants used for deep vein thrombosis are also used for acute pulmonary embolism.
Empiric treatment in underdeveloped countries
VTE may be an even greater concern on an outbound trip to a remote area, where medical care capabilities may be less than ideal and diagnostic and treatment options may be limited.
If there is a high pretest probability of acute VTE (Table 2, Table 3) and no diagnostic methods are available, empiric treatment with any of the parenteral anticoagulant agents listed in Table 4 is an option until the diagnosis can be confirmed. Caveats:
- Care must be taken to be certain there is not a strong contraindication to the use of anticoagulation, such as bleeding or a drug allergy.
- Neither unfractionated heparin nor any of the low-molecular-weight heparins should be given to a patient who has a history of heparin-induced thrombocytopenia.
- In patients who have chronic kidney disease (creatinine clearance less than 30 mL/minute), the dosage of low-molecular-weight heparins must be adjusted and factor Xa inhibitors avoided. Both of these types of anticoagulants should be avoided in patients on hemodialysis.
More aggressive therapy
Under select circumstances a more aggressive approach to the treatment of VTE may be necessary. These options are usually indicated for a patient with a massive deep vein thrombosis of a lower extremity and for certain patients with an upper extremity deep vein thrombosis. Treatments include catheter-directed thrombolytic therapy and endovenous or surgical thrombectomy.
Thrombolytic therapy is recommended for a patient with an acute pulmonary embolism who is clinically unstable (systolic blood pressure lower than 90 mm Hg), if there is no contraindication to its use (bleeding risk or recent stroke or surgery). Thrombolytic therapy is also an option for those at low risk of bleeding with an acute pulmonary embolism who have signs and symptoms of right heart failure proven by echocardiography.
Surgical pulmonary embolectomy for acute massive pulmonary embolism and mechanical thrombectomy for extensive deep vein thrombosis are generally available only at highly sophisticated tertiary care centers.
An inferior vena cava filter is advised in patients with acute deep vein thrombosis or pulmonary embolism who cannot be fully anticoagulated, to prevent the clot from migrating from the lower extremities to the lungs. These filters are available as either permanent or temporary implants. Some temporary versions can remain in place for up to 150 days after insertion.
PREVENTION OF VTE
Prevention is the standard of care for all patients admitted to the hospital and in select individuals as outpatients who are at high risk of VTE.
Mechanical compression (graduated compression stockings, intermittent pneumatic compression devices) has proven effective in reducing the incidence of deep vein thrombosis and pulmonary embolism postoperatively in patients who cannot take anticoagulants. One study has demonstrated that compression stockings may also be effective in preventing VTE during travel.12
ABSOLUTE RISK IS LOW
Over the past decade, special attention has been paid to travel as a risk factor for developing VTE.13 Traveler’s thrombosis has become an important public health concern. Numerous publications and epidemiologic studies have targeted air travel in an attempt to determine who is at risk and what precautions are necessary to prevent this complication.1–7,9
The incidence of VTE following air travel is reported to be 3.2 per 1,000 person-years.4 While this incidence is relatively low, it is still 3.2 times higher than in the healthy population that is not flying.
The more serious complication of VTE, ie, acute pulmonary embolism, occurs less often. In three studies, the reported incidence ranged from 1.65 per million patients in flights longer than 8 hours to a high of 4.8 per million patients in flights longer than 12 hours or distances exceeding 10,000 km (6,200 miles).5,14,15 For the 400 passengers on the average long-haul flight of 12 hours, there is at most a 0.2% chance that somebody on the plane will have a symptomatic VTE).
RISK FACTORS IN LONG-DISTANCE TRAVELERS
The risk of traveler’s thrombosis has recently attracted the attention of passengers and the airline industry. Airlines are now openly discussing the risk and providing reminders such as exercises that should be undertaken in-flight (see the patient information page that accompanies this article). Some airlines are recommending that all patients consult their doctor to assess their personal risk of deep vein thrombosis before flying.
The most common risk factors for VTE in travelers are well established and are additive (Table 1). The extent of the additive risk, however, is not entirely clear.
What is clear is that when VTE occurs it is a life-altering and life-threatening event. If it occurs on an outbound trip, the local resources and capabilities available at the destination may not be adequate for optimal treatment. If a traveler experiences a VTE event on an outbound trip, an emergency return trip to the continental United States or a regional center of expertise may be required. There is an additive risk with this subsequent travel event if the patient is not given immediate treatment first (Table 4). Hence, treatment prior to evacuation should be strongly considered.
The traveler must also be aware that VTE can be recognized up to 2 months after a long-haul flight, though it is especially a concern within the first 2 weeks after travel.2,4,16,17
RECOMMENDATIONS FOR LONG-DISTANCE AIR TRAVELERS
Each person should be evaluated on a case-by-case basis for his or her need for VTE prophylaxis. Medical guidelines for airline passengers have been published by the Aerospace Medical Association and the American College of Chest Physicians (ACCP).18,19 In general, travelers should:
- Exercise the legs by flexing and extending the ankles at regular intervals while seated (see the patient information material that accompanies this article) and frequently contracting the calf muscles.
- Walk about the cabin periodically, 5 minutes for every hour on longer-duration flights (over 4 hours) and when flight conditions permit.
- Drink adequate amounts of water and fruit juices to maintain good hydration.17
- Avoid alcohol and caffeinated beverages, which are dehydrating.
- Be careful about eating too much during the flight.
- Request an aisle seat if you are at risk
- Do not place baggage underneath the seat in front of you, because that reduces the ability to move the legs.
- Do not sleep in a cramped position, and avoid the use of any type of sleep aid.
- Avoid wearing constrictive clothing around the lower extremities or waist.
We recommend that all airplane passengers take the steps listed above to reduce venous stasis and avoid dehydration, even though these measures have not been proven effective in clinical trials.19
The ACCP further advises that decisions about pharmacologic prophylaxis of VTE for airplane passengers at high risk should be made on an individual basis, considering that there are potential adverse effects of prophylaxis and that these may outweigh the benefits. For long-distance travelers with additional risk factors for VTE, we suggest the following:
- Use of properly fitted, below-the-knee graduated compression stockings providing 15 to 30 mm Hg of pressure at the ankle (particularly when large varicosities or leg edema is present)
- For people at very high risk, a single prophylactic dose of a low-molecular-weight heparin or a factor Xa inhibitor injected just before departure (Table 5)
- Aspirin is not recommended as it is not effective for the prevention of VTE.20
SUMMARY FOR THE AIR TRAVELER
All travelers on long flights should perform standard VTE prophylaxis exercises (see the patient information pages accompanying this article). Although VTE is uncommon, people with additional risk factors who travel frequently either on multiple flights in a short period of time or on very long flights should be evaluated on a case-by-case basis for a more aggressive approach to prevention (compression support hose or prophylactic administration of a low-molecular-weight heparin or a factor Xa inhibitor).
Should a VTE event occur during travel, the patient should seek medical care immediately. The standard evaluation of a patient with a suspected VTE should include an estimation of the pretest probability of disease (Table 2, Table 3), followed by duplex ultrasonography of the upper or lower extremity to detect a deep vein thrombosis. If symptoms dictate, then spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) should be ordered to diagnose acute pulmonary embolism. A positive D-dimer blood test alone is not diagnostic and may not be available in more remote locations. A negative D-dimer test result is most helpful to exclude VTE.
Standard therapy for VTE is immediate treatment with one of the anticoagulants listed in Table 4, unless the patient has a contraindication to treatment, such as bleeding or allergy. Immediate evacuation is recommended if the patient has a life-threatening pulmonary embolism, defined as hemodynamic instability (hypotension with a blood pressure under 90 mm Hg systolic or signs of right heart failure) that cannot be treated at a local facility. An air ambulance should be used to transport these patients. If the patient has an iliofemoral deep vein thrombosis, it is also advisable that he or she be considered for evacuation if severe symptoms are present, such as pain, swelling, or cyanosis. Unless contraindicated, all patients should be given either full-dose intravenous or full-dose subcutaneous heparin or subcutaneous injection of a readily available low-molecular-weight heparin preparations or factor Xa inhibitor at once.21
- Brenner B. Interventions to prevent venous thrombosis after air travel, are they necessary? Yes. J Thromb Haemost 2006; 4:2302–2305.
- Cannegieter SC, Doggen CJM, van Houwellingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA Study). PLoS Med 2006; 3:1258–1265.
- Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med 2009; 151:180–190.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organizations. PLoS Med 2007; 4:1508–1514.
- Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med 2007; 262:615–634.
- Lehmann R, Suess C, Leus M, et al. Incidence, clinical characteristics, and long-term prognosis of travel-associated pulmonary embolism. Eur Heart J 2009; 30:233–241.
- Philbrick JT, Shumate R, Siadaty MS, et al. Air travel and venous thromboembolism: a systematic review. J Gen Intern Med 2007; 22:107–114.
- Cruickshank JM, Gorlin R, Jennett B. Air travel and thrombotic episodes: the economy class syndrome. Lancet 1988; 2:497–498.
- Bagshaw M. Traveler’s thrombosis: a review of deep vein thrombosis associated with travel. Air Transport Medicine Committee, Aerospace Medical Association. Aviat Space Environ Med 2001; 72:848–851.
- Wells PS, Owens C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Arnason T, Wells PS, Forester AJ. Appropriateness of diagnostic strategies for evaluating suspected venous thromboembolism. Thromb Haemost 2007; 97:195–201.
- Clarke M, Hopewell S, Juszcak E, Eisinga A, Kjeldstrøm M. Compression stockings in preventing deep vein thrombosis in airline passengers. Cochrane Database of Syst Rev 2006; Apr 19( 2):CD004002. DOI: 10.1002/14651858.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. Use of preventive measures for travel-related venous thrombosis in professionals who attend medical conferences. J Thromb Haemost 2006; 4:2373–2376.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism in the Madrid-Barajas Airport. Arch Intern Med 2003; 163:2766–2770.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001; 345:779–783.
- Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ 2003; 327:1072–1076.
- Eklof B, Kistner RL, Masuda EM, et al. Venous thromboembolism in association with prolonged air travel. Dermatol Surg 1996; 22:637–641.
- Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med 2003: 74:1009.
- Geerts WH, Bergqvist B, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133:381S–453S.
- Rosendaal FR. Interventions to prevent venous thrombosis after air travel: are they necessary? No. J Thromb Haemost 2006; 4:2306–2307.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
- Brenner B. Interventions to prevent venous thrombosis after air travel, are they necessary? Yes. J Thromb Haemost 2006; 4:2302–2305.
- Cannegieter SC, Doggen CJM, van Houwellingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA Study). PLoS Med 2006; 3:1258–1265.
- Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med 2009; 151:180–190.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organizations. PLoS Med 2007; 4:1508–1514.
- Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med 2007; 262:615–634.
- Lehmann R, Suess C, Leus M, et al. Incidence, clinical characteristics, and long-term prognosis of travel-associated pulmonary embolism. Eur Heart J 2009; 30:233–241.
- Philbrick JT, Shumate R, Siadaty MS, et al. Air travel and venous thromboembolism: a systematic review. J Gen Intern Med 2007; 22:107–114.
- Cruickshank JM, Gorlin R, Jennett B. Air travel and thrombotic episodes: the economy class syndrome. Lancet 1988; 2:497–498.
- Bagshaw M. Traveler’s thrombosis: a review of deep vein thrombosis associated with travel. Air Transport Medicine Committee, Aerospace Medical Association. Aviat Space Environ Med 2001; 72:848–851.
- Wells PS, Owens C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Arnason T, Wells PS, Forester AJ. Appropriateness of diagnostic strategies for evaluating suspected venous thromboembolism. Thromb Haemost 2007; 97:195–201.
- Clarke M, Hopewell S, Juszcak E, Eisinga A, Kjeldstrøm M. Compression stockings in preventing deep vein thrombosis in airline passengers. Cochrane Database of Syst Rev 2006; Apr 19( 2):CD004002. DOI: 10.1002/14651858.
- Kuipers S, Cannegieter SC, Middeldorp S, et al. Use of preventive measures for travel-related venous thrombosis in professionals who attend medical conferences. J Thromb Haemost 2006; 4:2373–2376.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism in the Madrid-Barajas Airport. Arch Intern Med 2003; 163:2766–2770.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001; 345:779–783.
- Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ 2003; 327:1072–1076.
- Eklof B, Kistner RL, Masuda EM, et al. Venous thromboembolism in association with prolonged air travel. Dermatol Surg 1996; 22:637–641.
- Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med 2003: 74:1009.
- Geerts WH, Bergqvist B, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133:381S–453S.
- Rosendaal FR. Interventions to prevent venous thrombosis after air travel: are they necessary? No. J Thromb Haemost 2006; 4:2306–2307.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
KEY POINTS
- The risk of VTE is about three times higher in passengers on long-distance flights than in the general population, although the absolute risk is still low.
- All long-distance air passengers should perform stretching exercises once an hour while in flight to prevent VTE. They should also stay hydrated.
- For patients at higher risk due to hypercoagulable conditions, physicians can consider prescribing compression stockings or an anticoagulant drug (a low-molecular-weight heparin or a factor Xa inhibitor) to be taken before the flight, or both.
- The evaluation of a patient with suspected VTE should include an estimation of the pretest probability of disease. If symptoms dictate, duplex ultrasonography of the upper or lower extremity to detect deep vein thrombosis or spiral computed tomography, ventilation-perfusion lung scan, or pulmonary angiography (where available) to diagnose an acute pulmonary embolism should be ordered.