User login
Noninvasive tests for liver disease, fibrosis, and cirrhosis: Is liver biopsy obsolete?
Primary care physicians and specialists alike often encounter patients with chronic liver disease. Fortunately, these days we need to resort to liver biopsy less often than in the past.
The purpose of this review is to provide a critical assessment of the growing number of noninvasive tests available for diagnosing liver disease and assessing hepatic fibrosis, and to discuss the implications of these advances related to the indications for needle liver biopsy.
WHEN IS LIVER BIOPSY USEFUL?
In diagnosis
Needle liver biopsy for diagnosis remains important in cases of:
Diagnostic uncertainty (eg, in patients with atypical features)
Coexisting disorders (eg, human immunodeficiency virus [HIV] and hepatitis C virus infection, or alcoholic liver disease and hepatitis C)
An overlapping syndrome (eg, primary biliary cirrhosis with autoimmune hepatitis).
Fatty liver. Needle liver biopsy can distinguish between benign steatosis and progressive steatohepatitis in a patient with a fatty liver found on imaging, subject to the limitations of sampling error.
Because fatty liver disease is common and proven treatments are few, no consensus has emerged about which patients with suspected fatty liver disease should undergo needle biopsy. Many specialists eschew needle biopsy and treat the underlying risk factors of metabolic syndrome, reserving biopsy for patients with findings that raise the concern of cirrhosis.
Hereditary disorders, eg, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson disease.
In management
Periodic needle biopsy is also valuable in the management of a few diseases.
In autoimmune hepatitis, monitoring the plasma cell score on liver biopsy may help predict relapse when a physician is considering reducing or discontinuing immunosuppressive therapy.1
After liver transplantation, a liver biopsy is highly valuable to assess for rejection and the presence and intensity of disease recurrence.
PROBLEMS WITH LIVER BIOPSY
Liver biopsy is invasive and can cause significant complications. Nearly 30% of patients report having substantial pain after liver biopsy, and some experience serious complications such as pneumothorax, bleeding, or puncture of the biliary tree. In rare cases, patients die of bleeding.2
Furthermore, hepatic pathology, particularly fibrosis, is not always uniformly distributed. Surgical wedge biopsy provides adequate tissue volume to overcome this problem. Needle biopsy, on the other hand, provides a much smaller volume of tissue (1/50,000 of the total mass of the liver).3
As examples of the resulting sampling errors that can occur, consider the two most common chronic liver diseases: hepatitis C and fatty liver disease.
Regev et al4 performed laparoscopically guided biopsy of the right and left hepatic lobes in a series of 124 patients with chronic hepatitis C. Biopsy samples from the right and left lobes differed in the intensity of inflammation in 24.2% of cases, and in the intensity of fibrosis in 33.1%. Differences of more than one grade of inflammation or stage of fibrosis were uncommon. However, in 14.5%, cirrhosis was diagnosed in one lobe but not the other.
In a study in patients with nonalcoholic fatty liver disease, Ratziu et al5 found that none of the features characteristic of nonalcoholic steatohepatitis were highly concordant in paired liver biopsies. Clearly, needle liver biopsy is far from an ideal test.
Increasingly, liver diseases can be diagnosed precisely with laboratory tests, imaging studies, or both. Thus, needle liver biopsy is playing a lesser role in diagnosis.
ADVANCES IN NONINVASIVE DIAGNOSIS OF LIVER DISEASE
Over the past 30 years, substantial strides have been made in our ability to make certain diagnoses through noninvasive means.
Blood tests can be used to diagnose viral hepatitis A, B, and C and many cases of hemochromatosis and primary biliary cirrhosis. For a detailed discussion of how blood tests are used in diagnosing liver diseases, see www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/.
Imaging studies. Primary sclerosing cholangitis can be diagnosed with an imaging study, ie, magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP). The value of needle biopsy in these patients is limited to assessing the degree of fibrosis to help with management of the disease and, less often, to discovering other liver pathologies.6
Most benign space-occupying liver lesions, both cystic and solid, can be fully characterized by imaging, especially in patients who have no underlying chronic liver disease, and no biopsy is needed. Whether biopsy should be performed to investigate liver lesions depends on the clinical scenario; the topic is beyond the scope of this paper but has been reviewed in detail by Rockey et al.2
CAN NONINVASIVE TESTS DETECT HEPATIC FIBROSIS?
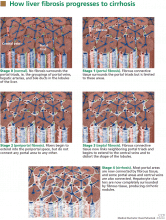
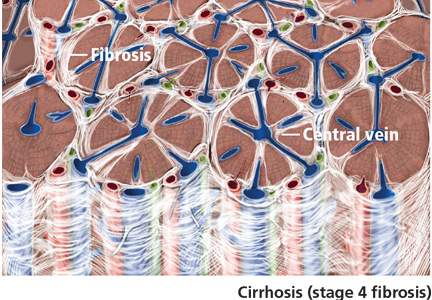
Cirrhosis (stage 4 fibrosis) results in nodular transformation of the liver and impedance of portal blood flow, setting the stage for portal hypertension and its sequelae. Knowing whether cirrhosis is present is important in subsequent management.
In advanced cases, cirrhosis is associated with typical clinical manifestations and laboratory and radiographic findings. In such cases, needle biopsy will add little. However, in most cases, particularly early in the course, clinical, laboratory, and radiologic correlates of cirrhosis are absent. In one study of patients with hepatitis C, 27% had cirrhosis, but in only a small number would cirrhosis have been apparent from clinical signs and laboratory and imaging studies.6
Since a major contemporary role for liver biopsy is in assessing the degree of fibrosis, it is reasonable to ask if newer noninvasive means are available to estimate hepatic fibrosis. The remainder of this review focuses on assessing our increasing ability to stage the degree of fibrosis (including the presence or absence of cirrhosis) by noninvasive means.
Clinical features point to cirrhosis, but not earlier fibrosis
Clinical manifestations help point to the diagnosis of cirrhosis but not to earlier stages of fibrosis.
For example, if a patient is known to have liver disease, the findings of ascites, splenomegaly, or asterixis mean that cirrhosis is highly probable. Similarly, hypersplenism (splenomegaly with a decrease in circulating blood cells but a normal to hyperactive bone marrow) in a patient with liver test abnormalities almost always represents portal hypertension due to cirrhosis, although other, nonhepatic causes are possible, such as congestive heart failure and constrictive pericarditis.
These features generally emerge late in the course of cirrhosis. The absence of such stigmata certainly does not preclude the presence of cirrhosis. Thus, these clinical signs have a high positive predictive value but a low negative predictive value, making them insufficient by themselves to diagnose or stage liver disease.
Laboratory tests are of limited value in assessing the degree of fibrosis
Standard liver tests are of limited value in assessing the degree of fibrosis.
Usual laboratory tests. At one end of the spectrum, anemia, thrombocytopenia, and leukopenia in the presence of liver disease correlate with cirrhosis. At the other end, a serum ferritin concentration of less than 1,000 mg/mL in a patient with hemochromatosis and no confounding features such as hepatitis C, HIV infection, or heavy alcohol use strongly predicts that the patient does not have significant hepatic fibrosis.8
Bilirubin elevation is a late finding in cirrhosis, but in cholestatic diseases bilirubin may be elevated before cirrhosis occurs.
Albumin is made exclusively in the liver, and its concentration falls as liver function worsens with progressive cirrhosis.
The prothrombin time increases as the liver loses its ability to synthesize clotting factors in cirrhosis. Coagulopathy correlates with the degree of liver disease.
Hyponatremia due to impaired ability to excrete free water is seen in patients with cirrhosis and ascites.
In summary, the usual laboratory tests related to liver disease are imprecise and, when abnormal, often indicate not just the presence of cirrhosis, but impending or actual decompensation.
Newer serologic markers, alone or in combination, have been proposed as aids in determining the degree of fibrosis or cirrhosis in the liver. Direct markers of fibrosis measure the turnover or metabolism of extracellular matrix. Indirect markers of fibrosis reflect alterations in hepatic function (see below).
Parkes et al9 reviewed 10 different panels of serum markers of hepatic fibrosis in chronic hepatitis C. Only 35% of patients had fibrosis adequately ruled in or ruled out by these panels, and the stage of fibrosis could not be adequately determined.
These serologic markers have not been validated in other chronic liver diseases or in liver disease due to multiple causes. Thus, although they show promise for use by the general internist, they need to be validated in patients with disease and in normal reference populations before they are ready for “prime time.”
Direct serologic markers of fibrosis
Direct serologic markers of fibrosis include those associated with matrix deposition—eg, procollagen type III amino-terminal peptide (P3NP), type I and IV collagens, laminin, hyaluronic acid, and chondrex.
P3NP is the most widely studied marker of hepatic fibrosis. It is elevated in both acute and chronic liver diseases; serum levels reflect the histologic stage of hepatic fibrosis in various chronic liver diseases, including alcoholic, viral, and primary biliary cirrhosis.10–12 Successful treatment of autoimmune hepatitis has been shown to lead to reductions of P3NP levels.13
Other direct markers of fibrosis are those associated with matrix degradation, ie, matrix metalloproteinases 2 and 3 (MMP-2, MMP-3) and tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1, TIMP-2). Levels of MMP-2 proenzymes and active enzymes are increased in liver disease, but studies are inconsistent in correlating serum levels of MMP-2 to the degree of hepatic fibrosis.14,15 These tests are not commercially available, and the components are not readily available in most clinical laboratories.
Indirect serologic markers of fibrosis
Some indirect markers are readily available:
The AST:ALT ratio. The normal ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is approximately 0.8. A ratio greater than 1.0 provides evidence of cirrhosis. However, findings have been inconsistent.
The AST:platelet ratio index (APRI), a commonly used index, is calculated by the following formula:
In studies of hepatitis C and hepatitis C-HIV, the APRI has shown a sensitivity of 37% to 80% and a specificity of 45% to 98%, depending on the cutoff value and whether a diagnosis of severe fibrosis or cirrhosis was being tested.16–19 These sensitivities and specificities are disappointing and do not provide information equal to that provided by needle liver biopsy in most patients with chronic liver disease.
The combination of prothrombin, gamma glutamyl, and apolipoprotein AI levels (PGA index) has been validated in patients with many types of chronic liver disease, and its accuracy for detecting cirrhosis is highest (66%–72%) in patients with alcoholic liver disease.20,21
FibroIndex uses the platelet count, AST level, and gamma globulin level to detect significant fibrosis in chronic hepatitis C, but its accuracy has yet to be validated.22
The FIB-4 index is based on four independent predictors of fibrosis, ie, age, the platelet count, AST level, and ALT level. It has shown good accuracy for detecting advanced fibrosis in two studies in patients with hepatitis C.23,24
Fibrometer (based on the platelet count; the prothrombin index; the levels of AST, alfa-2 macroglobulin, hyaluronate, and blood urea nitrogen; and age) predicted fibrosis well in chronic viral hepatitis.25,26
Fibrotest and Fibrosure are proprietary commercial tests available in many laboratories. They employ a mathematical formula to predict fibrosis (characterized as mild, significant, or indeterminate) using the levels of alpha-2 macroglobulin, alpha-2 globulin, gamma globulin, apolipoprotein A1, gamma glutamyl transferase, and total bilirubin. For detecting significant fibrosis, these tests are reported to have a sensitivity of about 75% and a specificity of 85%.27–29
ActiTest incorporates the ALT level into the Fibrotest to reflect liver fibrosis and necro-inflammatory activity.
A meta-analysis showed that Fibrotest and ActiTest could be reliable alternatives to liver biopsy in patients with chronic hepatitis C.30 The area under the receiver operator characteristic curve for the diagnosis of significant fibrosis ranged from 0.73 to 0.87; for the diagnosis of significant histologic activity it ranged from 0.75 to 0.86. Fibrotest had a negative predictive value for excluding significant fibrosis of 91% with a cutoff of 0.31. ActiTest’s negative predictive value for excluding significant necrosis was 85% with a cutoff of 0.36. None of these serum tests have become part of standard of practice for diagnosing fibrosis or cirrhosis.
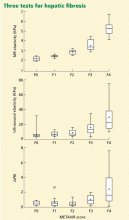
The Sequential Algorithm for Fibrosis Evaluation (SAFE) combines the APRI and Fibrotest-Fibrosure tests in a sequential fashion to test for fibrosis and cirrhosis. In a large multicenter study31 validating this algorithm to detect significant fibrosis (stage F2 or greater by the F0–F4 METAVIR scoring system32), its accuracy was 90.1%, the area under the receiver operating characteristic curve was 0.89 (95% CI 0.87–0.90), and it reduced the number of liver biopsies needed by 46.5%. When the algorithm was used to detect cirrhosis, its accuracy was 92.5%, the area under the curve was 0.92 (95% CI 0.89–0.94), and it reduced the number of liver biopsies needed by 81.5%.
Another algorithm was developed to simultaneously detect significant fibrosis and cirrhosis. It had a 97.4% accuracy, but 64% of patients still required a liver biopsy.31
SAFE algorithms have the potential to reduce the number of needle biopsies needed to assess the degree of hepatic fibrosis.
CONVENTIONAL IMAGING STUDIES ARE NOT SENSITIVE FOR FIBROSIS
Standard imaging studies often show findings of cirrhosis but are not particularly sensitive, with a low negative predictive value.
Ultrasonography can show a small, nodular liver in advanced cirrhosis, but surface nodularity or increased echogenicity can be seen in hepatic steatosis as well as in cirrhosis. In one study,33 ultrasonography identified diffuse parenchymal disease but could not reliably distinguish fat from fibrosis or diagnose cirrhosis.
Often, in cirrhosis, the right lobe of the liver is atrophied and the caudate or left lobes are hypertrophied. Efforts to use the ratio of the widths of the lobes to diagnose cirrhosis have shown varying performance characterstics.34,35
One study of the splenic artery pulsatility index has shown this to be an accurate predictor of cirrhosis.36
Computed tomography provides information similar to that of ultrasonography, and it can identify complications of cirrhosis, including portal hypertension and ascites. On the other hand, it costs more and it exposes the patient to radiation and contrast media.
ELASTOGRAPHY, A PROMISING TEST
Hepatic elastography, a method for estimating liver stiffness, is an exciting recent development in the noninvasive measurement of hepatic fibrosis. Currently, elastography can be accomplished by ultrasound or magnetic resonance.
Ultrasound elastography
The FibroScan device (EchoSens, Paris, France) uses a mild-amplitude, low-frequency (50-Hz) vibration transmitted through the liver.37 It induces an elastic shear wave that is detected by pulse-echo ultrasonography as the wave propagates through the organ.
The velocity of the wave correlates with tissue stiffness: the wave travels faster through denser, fibrotic tissue.38,39
Ultrasound elastography (also called transient elastography) can sample a much larger area than liver biopsy can, providing a better understanding of the entire hepatic parenchyma. 40 Moreover, it can be repeated often without risk. This device is in widespread use in many parts of the world, but it is not yet approved in the United States.
A meta-analysis of 50 studies assessed the overall performance of ultrasound elastography for diagnosing liver fibrosis.41 The areas under the receiver operating characteristic curve were as follows:
- For significant fibrosis: 0.84 (95% CI 0.82–0.86)
- For severe fibrosis: 0.89 (95% CI 0.88–0.91)
- For cirrhosis: 0.94 (95% CI 0.93–0.95).
The type of underlying liver disease influenced the diagnosis of significant fibrosis, which was diagnosed most consistently in patients with hepatitis C. The authors concluded that ultrasound elastography had excellent diagnostic accuracy for diagnosing cirrhosis irrespective of the underlying liver disease, while the diagnosis of significant fibrosis had higher variation, which was dependent on the underlying liver disease.
A meta-analysis of nine studies42 showed ultrasound elastography to have a sensitivity of 87% (95% CI 84%–90%) and a specificity of 91% (95% CI 89%–92%) for the diagnosis of cirrhosis. In seven of the nine studies, it diagnosed stage II to IV fibrosis with 70% sensitivity (95% CI 67%–73%) and 84% specificity (95% CI 80%–88%).
Limitations. Ultrasound elastography is less effective in obese patients, as the adipose tissue attenuates the elastic wave, and it has not been reliable in patients with acute viral hepatitis.43 Male sex, body mass index greater than 30, and metabolic syndrome seem to increase liver stiffness, thus limiting the use of this test.44
Until more data are available, the ultimate value of ultrasound elastography in reducing the number of liver biopsies needed remains unknown. However, this test shows potential as a reliable and noninvasive way to assess the degree of fibrosis in patients with liver disease.
Magnetic resonance elastography
Studies have shown a magnetic resonance scoring system that distinguishes Child-Pugh grade A cirrhosis from other grades to be 93% sensitive and 82% specific.45
Cost may limit the use of magnetic resonance elastography, and some patients may be unable to tolerate the procedure because of claustrophobia. It seems clear, though, that this test currently has the most promise in reducing the need for liver biopsy for grading the severity of hepatic fibrosis.
WHERE ARE WE NOW?
The importance of liver biopsy in arriving at a diagnosis of diffuse parenchymal liver disease is being diminished by accurate blood testing strategies for chronic viral hepatitis, autoimmune hepatitis, and primary biliary cirrhosis. Further, imaging tests are superior to liver biopsy in the diagnosis of primary sclerosing cholangitis.
However, many cases remain in which diagnostic confusion exists even after suitable laboratory testing and imaging studies. Diagnosing infiltrative disease (eg, amyloidosis, sarcoidosis), separating benign fatty liver disease from steatohepatitis, and evaluating liver parenchyma after liver transplantation are best accomplished by liver biopsy.
While needle biopsy is still the mainstay in diagnosing hepatic fibrosis, its days of dominance seem limited as technology improves. When physical examination or standard laboratory tests reveal clear-cut signs of portal hypertension, liver biopsy will seldom add useful information. Similarly, when imaging studies provide compelling evidence of cirrhosis and portal hypertension, needle biopsy is not warranted.
The SAFE algorithms warrant further evaluation in all chronic liver diseases, as they may help decrease the number of liver biopsies required. And we believe elastography will play an ever-increasing role in the assessment of hepatic fibrosis and will significantly reduce the need for biopsy in patients with liver disease.
- Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 2004; 99:1510–1516.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344:495–500.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–2618.
- Ratziu V, Charlotte F, Heurtier A, et al; LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906.
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001; 33:196–200.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19:1409–1417.
- Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med 2003; 138:627–633.
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44:462–474.
- Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med 1996; 25:59–62.
- Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 1993; 342:895–898.
- Trinchet JC, Hartmann DJ, Pateron D, et al. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol 1991; 12:139–144.
- McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med 1987; 109:55–61.
- Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995; 21:787–795.
- Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology 1997; 26:1521–1529.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526.
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43:78–84.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–872.
- Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005; 41:1376–1382.
- Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology 1991; 100:1397–1402.
- Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–1616.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306.
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36.
- Sterling RK, Lissen E, Clumeck N, et al; APRI COT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325.
- Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42:1373–1381.
- Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46:775–782.
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T; MULTIVIRC Group. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci 2003; 48:146–153.
- Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003; 49:450–454.
- Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006; 101:547–555.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3:8.
- Sebastiani G, Halfon P, Castera L, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009; 49:1821–1827.
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretations in patients with chronic hepatitis C. Hepatology 1994; 20:15–20.
- Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985; 89:186–191.
- Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135:273–283.
- Giorgio A, Amoroso P, Lettieri G, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161:443–445.
- Liu CH, Hsu SJ, Lin JW, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007; 5:1199–1206.
- Talawalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008; 135:299–302.
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007; 46:628–634.
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5:1214–1220.
- Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47:380–384.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613.
- Ito K, Mitchell DG, Hann HW, et al. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol 1999; 173:591–596.
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135:32–40.
Primary care physicians and specialists alike often encounter patients with chronic liver disease. Fortunately, these days we need to resort to liver biopsy less often than in the past.
The purpose of this review is to provide a critical assessment of the growing number of noninvasive tests available for diagnosing liver disease and assessing hepatic fibrosis, and to discuss the implications of these advances related to the indications for needle liver biopsy.
WHEN IS LIVER BIOPSY USEFUL?
In diagnosis
Needle liver biopsy for diagnosis remains important in cases of:
Diagnostic uncertainty (eg, in patients with atypical features)
Coexisting disorders (eg, human immunodeficiency virus [HIV] and hepatitis C virus infection, or alcoholic liver disease and hepatitis C)
An overlapping syndrome (eg, primary biliary cirrhosis with autoimmune hepatitis).
Fatty liver. Needle liver biopsy can distinguish between benign steatosis and progressive steatohepatitis in a patient with a fatty liver found on imaging, subject to the limitations of sampling error.
Because fatty liver disease is common and proven treatments are few, no consensus has emerged about which patients with suspected fatty liver disease should undergo needle biopsy. Many specialists eschew needle biopsy and treat the underlying risk factors of metabolic syndrome, reserving biopsy for patients with findings that raise the concern of cirrhosis.
Hereditary disorders, eg, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson disease.
In management
Periodic needle biopsy is also valuable in the management of a few diseases.
In autoimmune hepatitis, monitoring the plasma cell score on liver biopsy may help predict relapse when a physician is considering reducing or discontinuing immunosuppressive therapy.1
After liver transplantation, a liver biopsy is highly valuable to assess for rejection and the presence and intensity of disease recurrence.
PROBLEMS WITH LIVER BIOPSY
Liver biopsy is invasive and can cause significant complications. Nearly 30% of patients report having substantial pain after liver biopsy, and some experience serious complications such as pneumothorax, bleeding, or puncture of the biliary tree. In rare cases, patients die of bleeding.2
Furthermore, hepatic pathology, particularly fibrosis, is not always uniformly distributed. Surgical wedge biopsy provides adequate tissue volume to overcome this problem. Needle biopsy, on the other hand, provides a much smaller volume of tissue (1/50,000 of the total mass of the liver).3
As examples of the resulting sampling errors that can occur, consider the two most common chronic liver diseases: hepatitis C and fatty liver disease.
Regev et al4 performed laparoscopically guided biopsy of the right and left hepatic lobes in a series of 124 patients with chronic hepatitis C. Biopsy samples from the right and left lobes differed in the intensity of inflammation in 24.2% of cases, and in the intensity of fibrosis in 33.1%. Differences of more than one grade of inflammation or stage of fibrosis were uncommon. However, in 14.5%, cirrhosis was diagnosed in one lobe but not the other.
In a study in patients with nonalcoholic fatty liver disease, Ratziu et al5 found that none of the features characteristic of nonalcoholic steatohepatitis were highly concordant in paired liver biopsies. Clearly, needle liver biopsy is far from an ideal test.
Increasingly, liver diseases can be diagnosed precisely with laboratory tests, imaging studies, or both. Thus, needle liver biopsy is playing a lesser role in diagnosis.
ADVANCES IN NONINVASIVE DIAGNOSIS OF LIVER DISEASE
Over the past 30 years, substantial strides have been made in our ability to make certain diagnoses through noninvasive means.
Blood tests can be used to diagnose viral hepatitis A, B, and C and many cases of hemochromatosis and primary biliary cirrhosis. For a detailed discussion of how blood tests are used in diagnosing liver diseases, see www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/.
Imaging studies. Primary sclerosing cholangitis can be diagnosed with an imaging study, ie, magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP). The value of needle biopsy in these patients is limited to assessing the degree of fibrosis to help with management of the disease and, less often, to discovering other liver pathologies.6
Most benign space-occupying liver lesions, both cystic and solid, can be fully characterized by imaging, especially in patients who have no underlying chronic liver disease, and no biopsy is needed. Whether biopsy should be performed to investigate liver lesions depends on the clinical scenario; the topic is beyond the scope of this paper but has been reviewed in detail by Rockey et al.2
CAN NONINVASIVE TESTS DETECT HEPATIC FIBROSIS?
Cirrhosis (stage 4 fibrosis) results in nodular transformation of the liver and impedance of portal blood flow, setting the stage for portal hypertension and its sequelae. Knowing whether cirrhosis is present is important in subsequent management.
In advanced cases, cirrhosis is associated with typical clinical manifestations and laboratory and radiographic findings. In such cases, needle biopsy will add little. However, in most cases, particularly early in the course, clinical, laboratory, and radiologic correlates of cirrhosis are absent. In one study of patients with hepatitis C, 27% had cirrhosis, but in only a small number would cirrhosis have been apparent from clinical signs and laboratory and imaging studies.6
Since a major contemporary role for liver biopsy is in assessing the degree of fibrosis, it is reasonable to ask if newer noninvasive means are available to estimate hepatic fibrosis. The remainder of this review focuses on assessing our increasing ability to stage the degree of fibrosis (including the presence or absence of cirrhosis) by noninvasive means.
Clinical features point to cirrhosis, but not earlier fibrosis
Clinical manifestations help point to the diagnosis of cirrhosis but not to earlier stages of fibrosis.
For example, if a patient is known to have liver disease, the findings of ascites, splenomegaly, or asterixis mean that cirrhosis is highly probable. Similarly, hypersplenism (splenomegaly with a decrease in circulating blood cells but a normal to hyperactive bone marrow) in a patient with liver test abnormalities almost always represents portal hypertension due to cirrhosis, although other, nonhepatic causes are possible, such as congestive heart failure and constrictive pericarditis.
These features generally emerge late in the course of cirrhosis. The absence of such stigmata certainly does not preclude the presence of cirrhosis. Thus, these clinical signs have a high positive predictive value but a low negative predictive value, making them insufficient by themselves to diagnose or stage liver disease.
Laboratory tests are of limited value in assessing the degree of fibrosis
Standard liver tests are of limited value in assessing the degree of fibrosis.
Usual laboratory tests. At one end of the spectrum, anemia, thrombocytopenia, and leukopenia in the presence of liver disease correlate with cirrhosis. At the other end, a serum ferritin concentration of less than 1,000 mg/mL in a patient with hemochromatosis and no confounding features such as hepatitis C, HIV infection, or heavy alcohol use strongly predicts that the patient does not have significant hepatic fibrosis.8
Bilirubin elevation is a late finding in cirrhosis, but in cholestatic diseases bilirubin may be elevated before cirrhosis occurs.
Albumin is made exclusively in the liver, and its concentration falls as liver function worsens with progressive cirrhosis.
The prothrombin time increases as the liver loses its ability to synthesize clotting factors in cirrhosis. Coagulopathy correlates with the degree of liver disease.
Hyponatremia due to impaired ability to excrete free water is seen in patients with cirrhosis and ascites.
In summary, the usual laboratory tests related to liver disease are imprecise and, when abnormal, often indicate not just the presence of cirrhosis, but impending or actual decompensation.
Newer serologic markers, alone or in combination, have been proposed as aids in determining the degree of fibrosis or cirrhosis in the liver. Direct markers of fibrosis measure the turnover or metabolism of extracellular matrix. Indirect markers of fibrosis reflect alterations in hepatic function (see below).
Parkes et al9 reviewed 10 different panels of serum markers of hepatic fibrosis in chronic hepatitis C. Only 35% of patients had fibrosis adequately ruled in or ruled out by these panels, and the stage of fibrosis could not be adequately determined.
These serologic markers have not been validated in other chronic liver diseases or in liver disease due to multiple causes. Thus, although they show promise for use by the general internist, they need to be validated in patients with disease and in normal reference populations before they are ready for “prime time.”
Direct serologic markers of fibrosis
Direct serologic markers of fibrosis include those associated with matrix deposition—eg, procollagen type III amino-terminal peptide (P3NP), type I and IV collagens, laminin, hyaluronic acid, and chondrex.
P3NP is the most widely studied marker of hepatic fibrosis. It is elevated in both acute and chronic liver diseases; serum levels reflect the histologic stage of hepatic fibrosis in various chronic liver diseases, including alcoholic, viral, and primary biliary cirrhosis.10–12 Successful treatment of autoimmune hepatitis has been shown to lead to reductions of P3NP levels.13
Other direct markers of fibrosis are those associated with matrix degradation, ie, matrix metalloproteinases 2 and 3 (MMP-2, MMP-3) and tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1, TIMP-2). Levels of MMP-2 proenzymes and active enzymes are increased in liver disease, but studies are inconsistent in correlating serum levels of MMP-2 to the degree of hepatic fibrosis.14,15 These tests are not commercially available, and the components are not readily available in most clinical laboratories.
Indirect serologic markers of fibrosis
Some indirect markers are readily available:
The AST:ALT ratio. The normal ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is approximately 0.8. A ratio greater than 1.0 provides evidence of cirrhosis. However, findings have been inconsistent.
The AST:platelet ratio index (APRI), a commonly used index, is calculated by the following formula:
In studies of hepatitis C and hepatitis C-HIV, the APRI has shown a sensitivity of 37% to 80% and a specificity of 45% to 98%, depending on the cutoff value and whether a diagnosis of severe fibrosis or cirrhosis was being tested.16–19 These sensitivities and specificities are disappointing and do not provide information equal to that provided by needle liver biopsy in most patients with chronic liver disease.
The combination of prothrombin, gamma glutamyl, and apolipoprotein AI levels (PGA index) has been validated in patients with many types of chronic liver disease, and its accuracy for detecting cirrhosis is highest (66%–72%) in patients with alcoholic liver disease.20,21
FibroIndex uses the platelet count, AST level, and gamma globulin level to detect significant fibrosis in chronic hepatitis C, but its accuracy has yet to be validated.22
The FIB-4 index is based on four independent predictors of fibrosis, ie, age, the platelet count, AST level, and ALT level. It has shown good accuracy for detecting advanced fibrosis in two studies in patients with hepatitis C.23,24
Fibrometer (based on the platelet count; the prothrombin index; the levels of AST, alfa-2 macroglobulin, hyaluronate, and blood urea nitrogen; and age) predicted fibrosis well in chronic viral hepatitis.25,26
Fibrotest and Fibrosure are proprietary commercial tests available in many laboratories. They employ a mathematical formula to predict fibrosis (characterized as mild, significant, or indeterminate) using the levels of alpha-2 macroglobulin, alpha-2 globulin, gamma globulin, apolipoprotein A1, gamma glutamyl transferase, and total bilirubin. For detecting significant fibrosis, these tests are reported to have a sensitivity of about 75% and a specificity of 85%.27–29
ActiTest incorporates the ALT level into the Fibrotest to reflect liver fibrosis and necro-inflammatory activity.
A meta-analysis showed that Fibrotest and ActiTest could be reliable alternatives to liver biopsy in patients with chronic hepatitis C.30 The area under the receiver operator characteristic curve for the diagnosis of significant fibrosis ranged from 0.73 to 0.87; for the diagnosis of significant histologic activity it ranged from 0.75 to 0.86. Fibrotest had a negative predictive value for excluding significant fibrosis of 91% with a cutoff of 0.31. ActiTest’s negative predictive value for excluding significant necrosis was 85% with a cutoff of 0.36. None of these serum tests have become part of standard of practice for diagnosing fibrosis or cirrhosis.
The Sequential Algorithm for Fibrosis Evaluation (SAFE) combines the APRI and Fibrotest-Fibrosure tests in a sequential fashion to test for fibrosis and cirrhosis. In a large multicenter study31 validating this algorithm to detect significant fibrosis (stage F2 or greater by the F0–F4 METAVIR scoring system32), its accuracy was 90.1%, the area under the receiver operating characteristic curve was 0.89 (95% CI 0.87–0.90), and it reduced the number of liver biopsies needed by 46.5%. When the algorithm was used to detect cirrhosis, its accuracy was 92.5%, the area under the curve was 0.92 (95% CI 0.89–0.94), and it reduced the number of liver biopsies needed by 81.5%.
Another algorithm was developed to simultaneously detect significant fibrosis and cirrhosis. It had a 97.4% accuracy, but 64% of patients still required a liver biopsy.31
SAFE algorithms have the potential to reduce the number of needle biopsies needed to assess the degree of hepatic fibrosis.
CONVENTIONAL IMAGING STUDIES ARE NOT SENSITIVE FOR FIBROSIS
Standard imaging studies often show findings of cirrhosis but are not particularly sensitive, with a low negative predictive value.
Ultrasonography can show a small, nodular liver in advanced cirrhosis, but surface nodularity or increased echogenicity can be seen in hepatic steatosis as well as in cirrhosis. In one study,33 ultrasonography identified diffuse parenchymal disease but could not reliably distinguish fat from fibrosis or diagnose cirrhosis.
Often, in cirrhosis, the right lobe of the liver is atrophied and the caudate or left lobes are hypertrophied. Efforts to use the ratio of the widths of the lobes to diagnose cirrhosis have shown varying performance characterstics.34,35
One study of the splenic artery pulsatility index has shown this to be an accurate predictor of cirrhosis.36
Computed tomography provides information similar to that of ultrasonography, and it can identify complications of cirrhosis, including portal hypertension and ascites. On the other hand, it costs more and it exposes the patient to radiation and contrast media.
ELASTOGRAPHY, A PROMISING TEST
Hepatic elastography, a method for estimating liver stiffness, is an exciting recent development in the noninvasive measurement of hepatic fibrosis. Currently, elastography can be accomplished by ultrasound or magnetic resonance.
Ultrasound elastography
The FibroScan device (EchoSens, Paris, France) uses a mild-amplitude, low-frequency (50-Hz) vibration transmitted through the liver.37 It induces an elastic shear wave that is detected by pulse-echo ultrasonography as the wave propagates through the organ.
The velocity of the wave correlates with tissue stiffness: the wave travels faster through denser, fibrotic tissue.38,39
Ultrasound elastography (also called transient elastography) can sample a much larger area than liver biopsy can, providing a better understanding of the entire hepatic parenchyma. 40 Moreover, it can be repeated often without risk. This device is in widespread use in many parts of the world, but it is not yet approved in the United States.
A meta-analysis of 50 studies assessed the overall performance of ultrasound elastography for diagnosing liver fibrosis.41 The areas under the receiver operating characteristic curve were as follows:
- For significant fibrosis: 0.84 (95% CI 0.82–0.86)
- For severe fibrosis: 0.89 (95% CI 0.88–0.91)
- For cirrhosis: 0.94 (95% CI 0.93–0.95).
The type of underlying liver disease influenced the diagnosis of significant fibrosis, which was diagnosed most consistently in patients with hepatitis C. The authors concluded that ultrasound elastography had excellent diagnostic accuracy for diagnosing cirrhosis irrespective of the underlying liver disease, while the diagnosis of significant fibrosis had higher variation, which was dependent on the underlying liver disease.
A meta-analysis of nine studies42 showed ultrasound elastography to have a sensitivity of 87% (95% CI 84%–90%) and a specificity of 91% (95% CI 89%–92%) for the diagnosis of cirrhosis. In seven of the nine studies, it diagnosed stage II to IV fibrosis with 70% sensitivity (95% CI 67%–73%) and 84% specificity (95% CI 80%–88%).
Limitations. Ultrasound elastography is less effective in obese patients, as the adipose tissue attenuates the elastic wave, and it has not been reliable in patients with acute viral hepatitis.43 Male sex, body mass index greater than 30, and metabolic syndrome seem to increase liver stiffness, thus limiting the use of this test.44
Until more data are available, the ultimate value of ultrasound elastography in reducing the number of liver biopsies needed remains unknown. However, this test shows potential as a reliable and noninvasive way to assess the degree of fibrosis in patients with liver disease.
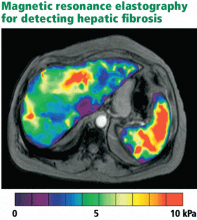
Magnetic resonance elastography
Studies have shown a magnetic resonance scoring system that distinguishes Child-Pugh grade A cirrhosis from other grades to be 93% sensitive and 82% specific.45
Cost may limit the use of magnetic resonance elastography, and some patients may be unable to tolerate the procedure because of claustrophobia. It seems clear, though, that this test currently has the most promise in reducing the need for liver biopsy for grading the severity of hepatic fibrosis.
WHERE ARE WE NOW?
The importance of liver biopsy in arriving at a diagnosis of diffuse parenchymal liver disease is being diminished by accurate blood testing strategies for chronic viral hepatitis, autoimmune hepatitis, and primary biliary cirrhosis. Further, imaging tests are superior to liver biopsy in the diagnosis of primary sclerosing cholangitis.
However, many cases remain in which diagnostic confusion exists even after suitable laboratory testing and imaging studies. Diagnosing infiltrative disease (eg, amyloidosis, sarcoidosis), separating benign fatty liver disease from steatohepatitis, and evaluating liver parenchyma after liver transplantation are best accomplished by liver biopsy.
While needle biopsy is still the mainstay in diagnosing hepatic fibrosis, its days of dominance seem limited as technology improves. When physical examination or standard laboratory tests reveal clear-cut signs of portal hypertension, liver biopsy will seldom add useful information. Similarly, when imaging studies provide compelling evidence of cirrhosis and portal hypertension, needle biopsy is not warranted.
The SAFE algorithms warrant further evaluation in all chronic liver diseases, as they may help decrease the number of liver biopsies required. And we believe elastography will play an ever-increasing role in the assessment of hepatic fibrosis and will significantly reduce the need for biopsy in patients with liver disease.
Primary care physicians and specialists alike often encounter patients with chronic liver disease. Fortunately, these days we need to resort to liver biopsy less often than in the past.
The purpose of this review is to provide a critical assessment of the growing number of noninvasive tests available for diagnosing liver disease and assessing hepatic fibrosis, and to discuss the implications of these advances related to the indications for needle liver biopsy.
WHEN IS LIVER BIOPSY USEFUL?
In diagnosis
Needle liver biopsy for diagnosis remains important in cases of:
Diagnostic uncertainty (eg, in patients with atypical features)
Coexisting disorders (eg, human immunodeficiency virus [HIV] and hepatitis C virus infection, or alcoholic liver disease and hepatitis C)
An overlapping syndrome (eg, primary biliary cirrhosis with autoimmune hepatitis).
Fatty liver. Needle liver biopsy can distinguish between benign steatosis and progressive steatohepatitis in a patient with a fatty liver found on imaging, subject to the limitations of sampling error.
Because fatty liver disease is common and proven treatments are few, no consensus has emerged about which patients with suspected fatty liver disease should undergo needle biopsy. Many specialists eschew needle biopsy and treat the underlying risk factors of metabolic syndrome, reserving biopsy for patients with findings that raise the concern of cirrhosis.
Hereditary disorders, eg, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson disease.
In management
Periodic needle biopsy is also valuable in the management of a few diseases.
In autoimmune hepatitis, monitoring the plasma cell score on liver biopsy may help predict relapse when a physician is considering reducing or discontinuing immunosuppressive therapy.1
After liver transplantation, a liver biopsy is highly valuable to assess for rejection and the presence and intensity of disease recurrence.
PROBLEMS WITH LIVER BIOPSY
Liver biopsy is invasive and can cause significant complications. Nearly 30% of patients report having substantial pain after liver biopsy, and some experience serious complications such as pneumothorax, bleeding, or puncture of the biliary tree. In rare cases, patients die of bleeding.2
Furthermore, hepatic pathology, particularly fibrosis, is not always uniformly distributed. Surgical wedge biopsy provides adequate tissue volume to overcome this problem. Needle biopsy, on the other hand, provides a much smaller volume of tissue (1/50,000 of the total mass of the liver).3
As examples of the resulting sampling errors that can occur, consider the two most common chronic liver diseases: hepatitis C and fatty liver disease.
Regev et al4 performed laparoscopically guided biopsy of the right and left hepatic lobes in a series of 124 patients with chronic hepatitis C. Biopsy samples from the right and left lobes differed in the intensity of inflammation in 24.2% of cases, and in the intensity of fibrosis in 33.1%. Differences of more than one grade of inflammation or stage of fibrosis were uncommon. However, in 14.5%, cirrhosis was diagnosed in one lobe but not the other.
In a study in patients with nonalcoholic fatty liver disease, Ratziu et al5 found that none of the features characteristic of nonalcoholic steatohepatitis were highly concordant in paired liver biopsies. Clearly, needle liver biopsy is far from an ideal test.
Increasingly, liver diseases can be diagnosed precisely with laboratory tests, imaging studies, or both. Thus, needle liver biopsy is playing a lesser role in diagnosis.
ADVANCES IN NONINVASIVE DIAGNOSIS OF LIVER DISEASE
Over the past 30 years, substantial strides have been made in our ability to make certain diagnoses through noninvasive means.
Blood tests can be used to diagnose viral hepatitis A, B, and C and many cases of hemochromatosis and primary biliary cirrhosis. For a detailed discussion of how blood tests are used in diagnosing liver diseases, see www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/.
Imaging studies. Primary sclerosing cholangitis can be diagnosed with an imaging study, ie, magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP). The value of needle biopsy in these patients is limited to assessing the degree of fibrosis to help with management of the disease and, less often, to discovering other liver pathologies.6
Most benign space-occupying liver lesions, both cystic and solid, can be fully characterized by imaging, especially in patients who have no underlying chronic liver disease, and no biopsy is needed. Whether biopsy should be performed to investigate liver lesions depends on the clinical scenario; the topic is beyond the scope of this paper but has been reviewed in detail by Rockey et al.2
CAN NONINVASIVE TESTS DETECT HEPATIC FIBROSIS?
Cirrhosis (stage 4 fibrosis) results in nodular transformation of the liver and impedance of portal blood flow, setting the stage for portal hypertension and its sequelae. Knowing whether cirrhosis is present is important in subsequent management.
In advanced cases, cirrhosis is associated with typical clinical manifestations and laboratory and radiographic findings. In such cases, needle biopsy will add little. However, in most cases, particularly early in the course, clinical, laboratory, and radiologic correlates of cirrhosis are absent. In one study of patients with hepatitis C, 27% had cirrhosis, but in only a small number would cirrhosis have been apparent from clinical signs and laboratory and imaging studies.6
Since a major contemporary role for liver biopsy is in assessing the degree of fibrosis, it is reasonable to ask if newer noninvasive means are available to estimate hepatic fibrosis. The remainder of this review focuses on assessing our increasing ability to stage the degree of fibrosis (including the presence or absence of cirrhosis) by noninvasive means.
Clinical features point to cirrhosis, but not earlier fibrosis
Clinical manifestations help point to the diagnosis of cirrhosis but not to earlier stages of fibrosis.
For example, if a patient is known to have liver disease, the findings of ascites, splenomegaly, or asterixis mean that cirrhosis is highly probable. Similarly, hypersplenism (splenomegaly with a decrease in circulating blood cells but a normal to hyperactive bone marrow) in a patient with liver test abnormalities almost always represents portal hypertension due to cirrhosis, although other, nonhepatic causes are possible, such as congestive heart failure and constrictive pericarditis.
These features generally emerge late in the course of cirrhosis. The absence of such stigmata certainly does not preclude the presence of cirrhosis. Thus, these clinical signs have a high positive predictive value but a low negative predictive value, making them insufficient by themselves to diagnose or stage liver disease.
Laboratory tests are of limited value in assessing the degree of fibrosis
Standard liver tests are of limited value in assessing the degree of fibrosis.
Usual laboratory tests. At one end of the spectrum, anemia, thrombocytopenia, and leukopenia in the presence of liver disease correlate with cirrhosis. At the other end, a serum ferritin concentration of less than 1,000 mg/mL in a patient with hemochromatosis and no confounding features such as hepatitis C, HIV infection, or heavy alcohol use strongly predicts that the patient does not have significant hepatic fibrosis.8
Bilirubin elevation is a late finding in cirrhosis, but in cholestatic diseases bilirubin may be elevated before cirrhosis occurs.
Albumin is made exclusively in the liver, and its concentration falls as liver function worsens with progressive cirrhosis.
The prothrombin time increases as the liver loses its ability to synthesize clotting factors in cirrhosis. Coagulopathy correlates with the degree of liver disease.
Hyponatremia due to impaired ability to excrete free water is seen in patients with cirrhosis and ascites.
In summary, the usual laboratory tests related to liver disease are imprecise and, when abnormal, often indicate not just the presence of cirrhosis, but impending or actual decompensation.
Newer serologic markers, alone or in combination, have been proposed as aids in determining the degree of fibrosis or cirrhosis in the liver. Direct markers of fibrosis measure the turnover or metabolism of extracellular matrix. Indirect markers of fibrosis reflect alterations in hepatic function (see below).
Parkes et al9 reviewed 10 different panels of serum markers of hepatic fibrosis in chronic hepatitis C. Only 35% of patients had fibrosis adequately ruled in or ruled out by these panels, and the stage of fibrosis could not be adequately determined.
These serologic markers have not been validated in other chronic liver diseases or in liver disease due to multiple causes. Thus, although they show promise for use by the general internist, they need to be validated in patients with disease and in normal reference populations before they are ready for “prime time.”
Direct serologic markers of fibrosis
Direct serologic markers of fibrosis include those associated with matrix deposition—eg, procollagen type III amino-terminal peptide (P3NP), type I and IV collagens, laminin, hyaluronic acid, and chondrex.
P3NP is the most widely studied marker of hepatic fibrosis. It is elevated in both acute and chronic liver diseases; serum levels reflect the histologic stage of hepatic fibrosis in various chronic liver diseases, including alcoholic, viral, and primary biliary cirrhosis.10–12 Successful treatment of autoimmune hepatitis has been shown to lead to reductions of P3NP levels.13
Other direct markers of fibrosis are those associated with matrix degradation, ie, matrix metalloproteinases 2 and 3 (MMP-2, MMP-3) and tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1, TIMP-2). Levels of MMP-2 proenzymes and active enzymes are increased in liver disease, but studies are inconsistent in correlating serum levels of MMP-2 to the degree of hepatic fibrosis.14,15 These tests are not commercially available, and the components are not readily available in most clinical laboratories.
Indirect serologic markers of fibrosis
Some indirect markers are readily available:
The AST:ALT ratio. The normal ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is approximately 0.8. A ratio greater than 1.0 provides evidence of cirrhosis. However, findings have been inconsistent.
The AST:platelet ratio index (APRI), a commonly used index, is calculated by the following formula:
In studies of hepatitis C and hepatitis C-HIV, the APRI has shown a sensitivity of 37% to 80% and a specificity of 45% to 98%, depending on the cutoff value and whether a diagnosis of severe fibrosis or cirrhosis was being tested.16–19 These sensitivities and specificities are disappointing and do not provide information equal to that provided by needle liver biopsy in most patients with chronic liver disease.
The combination of prothrombin, gamma glutamyl, and apolipoprotein AI levels (PGA index) has been validated in patients with many types of chronic liver disease, and its accuracy for detecting cirrhosis is highest (66%–72%) in patients with alcoholic liver disease.20,21
FibroIndex uses the platelet count, AST level, and gamma globulin level to detect significant fibrosis in chronic hepatitis C, but its accuracy has yet to be validated.22
The FIB-4 index is based on four independent predictors of fibrosis, ie, age, the platelet count, AST level, and ALT level. It has shown good accuracy for detecting advanced fibrosis in two studies in patients with hepatitis C.23,24
Fibrometer (based on the platelet count; the prothrombin index; the levels of AST, alfa-2 macroglobulin, hyaluronate, and blood urea nitrogen; and age) predicted fibrosis well in chronic viral hepatitis.25,26
Fibrotest and Fibrosure are proprietary commercial tests available in many laboratories. They employ a mathematical formula to predict fibrosis (characterized as mild, significant, or indeterminate) using the levels of alpha-2 macroglobulin, alpha-2 globulin, gamma globulin, apolipoprotein A1, gamma glutamyl transferase, and total bilirubin. For detecting significant fibrosis, these tests are reported to have a sensitivity of about 75% and a specificity of 85%.27–29
ActiTest incorporates the ALT level into the Fibrotest to reflect liver fibrosis and necro-inflammatory activity.
A meta-analysis showed that Fibrotest and ActiTest could be reliable alternatives to liver biopsy in patients with chronic hepatitis C.30 The area under the receiver operator characteristic curve for the diagnosis of significant fibrosis ranged from 0.73 to 0.87; for the diagnosis of significant histologic activity it ranged from 0.75 to 0.86. Fibrotest had a negative predictive value for excluding significant fibrosis of 91% with a cutoff of 0.31. ActiTest’s negative predictive value for excluding significant necrosis was 85% with a cutoff of 0.36. None of these serum tests have become part of standard of practice for diagnosing fibrosis or cirrhosis.
The Sequential Algorithm for Fibrosis Evaluation (SAFE) combines the APRI and Fibrotest-Fibrosure tests in a sequential fashion to test for fibrosis and cirrhosis. In a large multicenter study31 validating this algorithm to detect significant fibrosis (stage F2 or greater by the F0–F4 METAVIR scoring system32), its accuracy was 90.1%, the area under the receiver operating characteristic curve was 0.89 (95% CI 0.87–0.90), and it reduced the number of liver biopsies needed by 46.5%. When the algorithm was used to detect cirrhosis, its accuracy was 92.5%, the area under the curve was 0.92 (95% CI 0.89–0.94), and it reduced the number of liver biopsies needed by 81.5%.
Another algorithm was developed to simultaneously detect significant fibrosis and cirrhosis. It had a 97.4% accuracy, but 64% of patients still required a liver biopsy.31
SAFE algorithms have the potential to reduce the number of needle biopsies needed to assess the degree of hepatic fibrosis.
CONVENTIONAL IMAGING STUDIES ARE NOT SENSITIVE FOR FIBROSIS
Standard imaging studies often show findings of cirrhosis but are not particularly sensitive, with a low negative predictive value.
Ultrasonography can show a small, nodular liver in advanced cirrhosis, but surface nodularity or increased echogenicity can be seen in hepatic steatosis as well as in cirrhosis. In one study,33 ultrasonography identified diffuse parenchymal disease but could not reliably distinguish fat from fibrosis or diagnose cirrhosis.
Often, in cirrhosis, the right lobe of the liver is atrophied and the caudate or left lobes are hypertrophied. Efforts to use the ratio of the widths of the lobes to diagnose cirrhosis have shown varying performance characterstics.34,35
One study of the splenic artery pulsatility index has shown this to be an accurate predictor of cirrhosis.36
Computed tomography provides information similar to that of ultrasonography, and it can identify complications of cirrhosis, including portal hypertension and ascites. On the other hand, it costs more and it exposes the patient to radiation and contrast media.
ELASTOGRAPHY, A PROMISING TEST
Hepatic elastography, a method for estimating liver stiffness, is an exciting recent development in the noninvasive measurement of hepatic fibrosis. Currently, elastography can be accomplished by ultrasound or magnetic resonance.
Ultrasound elastography
The FibroScan device (EchoSens, Paris, France) uses a mild-amplitude, low-frequency (50-Hz) vibration transmitted through the liver.37 It induces an elastic shear wave that is detected by pulse-echo ultrasonography as the wave propagates through the organ.
The velocity of the wave correlates with tissue stiffness: the wave travels faster through denser, fibrotic tissue.38,39
Ultrasound elastography (also called transient elastography) can sample a much larger area than liver biopsy can, providing a better understanding of the entire hepatic parenchyma. 40 Moreover, it can be repeated often without risk. This device is in widespread use in many parts of the world, but it is not yet approved in the United States.
A meta-analysis of 50 studies assessed the overall performance of ultrasound elastography for diagnosing liver fibrosis.41 The areas under the receiver operating characteristic curve were as follows:
- For significant fibrosis: 0.84 (95% CI 0.82–0.86)
- For severe fibrosis: 0.89 (95% CI 0.88–0.91)
- For cirrhosis: 0.94 (95% CI 0.93–0.95).
The type of underlying liver disease influenced the diagnosis of significant fibrosis, which was diagnosed most consistently in patients with hepatitis C. The authors concluded that ultrasound elastography had excellent diagnostic accuracy for diagnosing cirrhosis irrespective of the underlying liver disease, while the diagnosis of significant fibrosis had higher variation, which was dependent on the underlying liver disease.
A meta-analysis of nine studies42 showed ultrasound elastography to have a sensitivity of 87% (95% CI 84%–90%) and a specificity of 91% (95% CI 89%–92%) for the diagnosis of cirrhosis. In seven of the nine studies, it diagnosed stage II to IV fibrosis with 70% sensitivity (95% CI 67%–73%) and 84% specificity (95% CI 80%–88%).
Limitations. Ultrasound elastography is less effective in obese patients, as the adipose tissue attenuates the elastic wave, and it has not been reliable in patients with acute viral hepatitis.43 Male sex, body mass index greater than 30, and metabolic syndrome seem to increase liver stiffness, thus limiting the use of this test.44
Until more data are available, the ultimate value of ultrasound elastography in reducing the number of liver biopsies needed remains unknown. However, this test shows potential as a reliable and noninvasive way to assess the degree of fibrosis in patients with liver disease.
Magnetic resonance elastography
Studies have shown a magnetic resonance scoring system that distinguishes Child-Pugh grade A cirrhosis from other grades to be 93% sensitive and 82% specific.45
Cost may limit the use of magnetic resonance elastography, and some patients may be unable to tolerate the procedure because of claustrophobia. It seems clear, though, that this test currently has the most promise in reducing the need for liver biopsy for grading the severity of hepatic fibrosis.
WHERE ARE WE NOW?
The importance of liver biopsy in arriving at a diagnosis of diffuse parenchymal liver disease is being diminished by accurate blood testing strategies for chronic viral hepatitis, autoimmune hepatitis, and primary biliary cirrhosis. Further, imaging tests are superior to liver biopsy in the diagnosis of primary sclerosing cholangitis.
However, many cases remain in which diagnostic confusion exists even after suitable laboratory testing and imaging studies. Diagnosing infiltrative disease (eg, amyloidosis, sarcoidosis), separating benign fatty liver disease from steatohepatitis, and evaluating liver parenchyma after liver transplantation are best accomplished by liver biopsy.
While needle biopsy is still the mainstay in diagnosing hepatic fibrosis, its days of dominance seem limited as technology improves. When physical examination or standard laboratory tests reveal clear-cut signs of portal hypertension, liver biopsy will seldom add useful information. Similarly, when imaging studies provide compelling evidence of cirrhosis and portal hypertension, needle biopsy is not warranted.
The SAFE algorithms warrant further evaluation in all chronic liver diseases, as they may help decrease the number of liver biopsies required. And we believe elastography will play an ever-increasing role in the assessment of hepatic fibrosis and will significantly reduce the need for biopsy in patients with liver disease.
- Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 2004; 99:1510–1516.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344:495–500.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–2618.
- Ratziu V, Charlotte F, Heurtier A, et al; LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906.
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001; 33:196–200.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19:1409–1417.
- Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med 2003; 138:627–633.
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44:462–474.
- Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med 1996; 25:59–62.
- Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 1993; 342:895–898.
- Trinchet JC, Hartmann DJ, Pateron D, et al. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol 1991; 12:139–144.
- McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med 1987; 109:55–61.
- Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995; 21:787–795.
- Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology 1997; 26:1521–1529.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526.
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43:78–84.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–872.
- Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005; 41:1376–1382.
- Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology 1991; 100:1397–1402.
- Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–1616.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306.
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36.
- Sterling RK, Lissen E, Clumeck N, et al; APRI COT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325.
- Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42:1373–1381.
- Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46:775–782.
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T; MULTIVIRC Group. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci 2003; 48:146–153.
- Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003; 49:450–454.
- Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006; 101:547–555.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3:8.
- Sebastiani G, Halfon P, Castera L, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009; 49:1821–1827.
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretations in patients with chronic hepatitis C. Hepatology 1994; 20:15–20.
- Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985; 89:186–191.
- Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135:273–283.
- Giorgio A, Amoroso P, Lettieri G, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161:443–445.
- Liu CH, Hsu SJ, Lin JW, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007; 5:1199–1206.
- Talawalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008; 135:299–302.
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007; 46:628–634.
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5:1214–1220.
- Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47:380–384.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613.
- Ito K, Mitchell DG, Hann HW, et al. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol 1999; 173:591–596.
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135:32–40.
- Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 2004; 99:1510–1516.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344:495–500.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–2618.
- Ratziu V, Charlotte F, Heurtier A, et al; LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906.
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001; 33:196–200.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19:1409–1417.
- Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med 2003; 138:627–633.
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44:462–474.
- Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med 1996; 25:59–62.
- Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 1993; 342:895–898.
- Trinchet JC, Hartmann DJ, Pateron D, et al. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol 1991; 12:139–144.
- McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med 1987; 109:55–61.
- Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995; 21:787–795.
- Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology 1997; 26:1521–1529.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526.
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43:78–84.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–872.
- Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005; 41:1376–1382.
- Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology 1991; 100:1397–1402.
- Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–1616.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306.
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36.
- Sterling RK, Lissen E, Clumeck N, et al; APRI COT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325.
- Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42:1373–1381.
- Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46:775–782.
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T; MULTIVIRC Group. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci 2003; 48:146–153.
- Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003; 49:450–454.
- Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006; 101:547–555.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3:8.
- Sebastiani G, Halfon P, Castera L, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009; 49:1821–1827.
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretations in patients with chronic hepatitis C. Hepatology 1994; 20:15–20.
- Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985; 89:186–191.
- Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135:273–283.
- Giorgio A, Amoroso P, Lettieri G, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161:443–445.
- Liu CH, Hsu SJ, Lin JW, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007; 5:1199–1206.
- Talawalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008; 135:299–302.
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007; 46:628–634.
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5:1214–1220.
- Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47:380–384.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613.
- Ito K, Mitchell DG, Hann HW, et al. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol 1999; 173:591–596.
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135:32–40.
KEY POINTS
- Liver biopsy remains an important tool in the evaluation and management of liver disease.
- The role of liver biopsy for diagnosis of chronic liver disease has diminished, owing to accurate blood tests and imaging studies.
- Noninvasive tests for assessing the degree of hepatic fibrosis are showing more promise and may further reduce the need for liver biopsy. Elastography, in particular, shows promise in measuring hepatic fibrosis.
- Liver biopsy is still needed if laboratory testing and imaging studies are inconclusive.