User login
Pseudomembranous colitis: Not always Clostridium difficile
Pseudomembranous colitis is most often due to Clostridium difficile infection, but it has a variety of other causes, including other infections, ischemia, medications, and inflammatory mucosal diseases (Table 1). When pseudomembranes are found, one should consider these other causes if tests for C difficile are negative or if anti-C difficile therapy does not produce a response.
These less common causes are important to consider to avoid needlessly escalating anti-C difficile antibiotic therapy and to provide appropriate treatment. Pseudomembranous colitis is a nonspecific finding that suggests a larger disease process. Associated signs and symptoms, including fever, abdominal pain, leukocytosis, diarrhea, toxic megacolon, and electrolyte imbalances, may portend a life-threatening condition.1 Awareness of causes of pseudomembranous colitis other than C difficile infection, the focus of this review, is key to prompt diagnosis and potentially life-saving patient care.
PSEUDOMEMBRANES ARE NONSPECIFIC
A pseudomembrane is a layer of fibropurulent exudate composed of acute inflammatory cells and mucus originating from inflamed and erupting crypts.2 Although most often seen in C difficile infection, pseudomembranous colitis is a nonspecific pattern of injury resulting from decreased oxygenation, endothelial damage, and impaired blood flow to the mucosa that can be triggered by a number of disease states.2
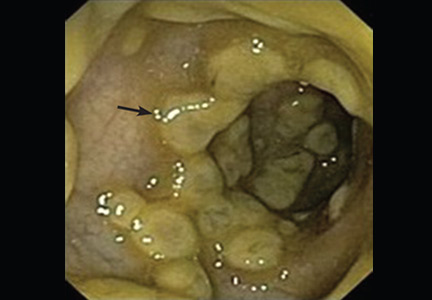
On endoscopy, pseudomembranes appear as raised whitish or yellowish plaques that may be scattered or confluent in distribution (Figure 1).2 They are usually found in the rectosigmoid colon but may be isolated to more proximal segments.3 Lower endoscopy is often performed in the diagnostic evaluation of patients with unexplained diarrhea, hematochezia, and abnormal abdominal computed tomographic findings (eg, colonic thickening).
CAUSES OF NON-C DIFFICLE PSEUDOMEMBRANOUS COLITIS
When pseudomembranous colitis is confirmed endoscopically, C difficile infection naturally comes to mind, but the two terms are not interchangeable. A wide differential diagnosis should be maintained, especially when there are clues that C difficile infection may not be the correct diagnosis.
Chemicals and medications
Several chemicals and medications can injure the bowel and predispose to pseudomembrane formation.
Glutaraldehyde has long been used to sanitize endoscopes because of its broad antimicrobial activity. Nevertheless, if the disinfecting solution is not adequately rinsed off the endoscope, direct contact with colonic mucosa can produce an allergic and a chemical reaction, resulting in an acute self-limited colitis with pseudomembrane formation.4
Chemotherapeutic and antiproliferative agents can be toxic to the bowel, generally through production of free radicals and up-regulation of inflammatory cytokines. The colonic epithelium is then more susceptible to ulceration and mucosal necrosis with pseudomembrane development. Cisplatin, cyclosporine A, docetaxel, and 5-fluorouracil are prominent examples.5–8
Nonsteroidal anti-inflammatory drugs can damage the mucosa at all levels of the gastrointestinal tract. Although gastric ulcerations are more typical (from nonselective cyclooxygenase inhibition), colonic ulcerations and colitis can occur.9 These drugs, particularly diclofenac and indomethacin, have been associated with non-C difficile pseudomembranous colitis when used by themselves or in conjunction with other agents such as cyclosporine A.8,10,11
Infections
C difficile is the organism most commonly linked to pseudomembranous colitis, but other bacterial, viral, and parasitic pathogens have also been implicated.
Staphylococcus aureus was believed to be responsible for enterocolitis in a series of 155 surgical patients between 1958 and 1962 receiving antibiotic therapy. All had a positive stool culture for S aureus, and nine were found to have pseudomembranes at autopsy.12
Although this finding has been disputed as a misdiagnosis, since C difficile infection was not widely recognized until the 1970s, there is evidence that S aureus may indeed be a cause of non-C difficile pseudomembranous colitis. In a review of 36 cases of methicillin-resistant S aureus bacteremia in Japan, four patients were documented to have intestinal pseudomembranes either by endoscopy or autopsy. In two of these patients, biopsies of the pseudomembranes were positive for methicillin-resistant S aureus.13
Escherichia coli O157:H7. Pseudomembranes have been seen endoscopically in several adults and children with enterohemorrhagic E coli O157:H7 infection.14,15 This invasive gram-negative rod normally resides in the gastrointestinal tract of cattle, sheep, and other animals and can be pathogenic to people who eat undercooked beef. The organism attaches to and effaces intestinal epithelial cells, and bacterial proteins and the Shiga toxin then damage the vasculature, precipitating bloody diarrhea. Colonic damage can range from mild hemorrhagic colitis to severe colitis with ischemic changes. In patients with enterohemorrhagic E coli O157:H7 infection, pseudomembrane formation results from colon ischemia due to microvascular thrombosis or from destructive effects of bacterial enterotoxin.1,15
Cytomegalovirus is a ubiquitous human herpes virus that can affect nearly all organ systems. Infection is often reported in immunocompromised patients, eg, those with acquired immunodeficiency syndrome, chronic corticosteroid use, inflammatory bowel disease, malignancy, or solid-organ transplants. Gastrointestinal manifestations can be nonspecific and range from abdominal discomfort to diarrhea to tenesmus. Pseudomembranous colitis can be a presenting feature of cytomegalovirus involvement.16,17 Ulcerative lesions in the colonic mucosa are a frequent accompanying finding on endoscopy.18,19
Other rarer infectious causes of pseudomembranous colitis identified in case reports include Clostridium ramosum, Entamoeba histolytica, Klebsiella oxytoca, Plesiomonas shigelloides, Schistosomiasis mansoni, and Salmonella and Shigella species.20–26 Additionally, there have been two reports of Strongyloides stercoralis hyperinfection manifesting as pseudomembranous colitis.27,28
Ischemia
Colon ischemia usually affects elderly or debilitated patients who have multiple comorbidities. Known risk factors include aortoiliac surgery, cardiovascular disease, diabetes mellitus, hemodialysis, and pulmonary disease. The ischemia can be related to an occlusive arterial or venous thromboembolism, but hypoperfusion without occlusion of the mesenteric or internal iliac arteries is the primary mechanism. Low blood flow states such as atherosclerosis and septic shock can affect the watershed areas, typically the splenic flexure and rectosigmoid junction.
We reported a case of a patient with vascular disease who was incorrectly diagnosed and treated as having refractory C difficile infection when pseudomembranes were seen on flexible sigmoidoscopy. Further investigation revealed ischemic colitis secondary to a high-grade inferior mesenteric artery stenosis as the true cause.29
Microvascular thrombosis is the likely mechanism in a number of non-C difficile causes of pseudomembranous colitis. For example, in most patients with enterohemorrhagic E coli O157:H7 infection, histologic review of colonic mucosal biopsies has revealed fibrin and platelet thrombi in the capillaries, suggesting microvascular thrombosis.15,30
Cocaine has been associated with pseudomembranes in the setting of ischemia in the cecum and ascending colon. Cocaine can cause vasoconstriction after stimulation of alpha-adrenergic receptors and hence intestinal ischemia, thrombosis of vessels in the large and small intestines, and direct toxic effects.31
Inflammatory conditions
Collagenous colitis is an inflammatory disease that often affects middle-aged women and presents with copious watery diarrhea. It is a type of microscopic colitis—the endoscopic appearance is often normal, while the histologic appearance is abnormal and characterized by collagen deposition in the lamina propria. Medications that have been implicated in microscopic colitis include acid-suppressive agents (eg, histamine receptor antagonists, proton pump inhibitors) and nonsteroidal anti-inflammatory drugs.
An increasing number of cases of pseudomembranous changes are being reported in patients diagnosed with collagenous colitis.32–36 Although the pathophysiologic mechanism is unknown, some authors have suggested that pseudomembrane formation is actually part of the presenting spectrum of collagenous colitis.36
Inflammatory bowel disease. Crohn disease and ulcerative colitis have been associated with pseudomembranous colitis. Pseudomembranes can be found on endoscopy in patients with inflammatory bowel disease during a disease exacerbation with or without C difficile.37,38 In patients with inflammatory bowel disease and C difficile infection, pseudomembranes can be found endoscopically in up to 13% of cases.39 Pseudomembranous colitis has been reported in a patient with ulcerative colitis exacerbation in association with cytomegalovirus colitis.40
Behçet disease is a rare, immune-mediated small-vessel systemic vasculitis. It usually presents with mucous membrane ulcerations and ocular disease but can affect any organ.41 Pseudomembranous colitis can occur in Behçet disease in the absence of C difficile infection or any infectious colitis. Treatment includes corticosteroids and immunosuppressants such as azathioprine and anti-tumor necrosis factor agents.41
INITIAL EVALUATION
The initial evaluation of a patient with suspected or confirmed pseudomembranous colitis should include a comprehensive medical history with information on recent hospitalizations or procedures, antibiotic use, infections, exposure to sick contacts, recent travel, and medications taken.
Testing for C difficile
As most patients with pseudomembranous colitis have C difficile infection, it should be excluded first. Empiric anti-C difficile treatment is recommended in seriously ill-appearing patients, ideally starting after a stool sample is obtained.
Diagnosis of C difficile infection requires laboratory demonstration of the toxin or detection of toxigenic organisms. The gold standard test is the cell culture cytotoxicity assay, but it is labor- and time-intensive.42 More widely available tests are polymerase chain reaction for the toxin gene or genes, enzyme immunoassay, and stool evaluation for glutamate dehydrogenase, which can yield results readily within hours.
Polymerase chain reaction has a sensitivity of 97% and a specificity of 93%. Results can be falsely positive if empiric treatment is started before specimen collection, in which case C difficile DNA may still be present and detectable, but not the organism itself.43
Enzyme immunoassay for toxins A and B carries a sensitivity of 75% and a specificity of 99%, but 100 to 1,000 pg of toxin must be present for a positive result.44,45
If the initial enzyme immunoassay or polymerase chain reaction result is negative, current guidelines do not recommend repeat testing, which has limited value.44,46 Repeat testing after a negative result is positive in less than 5% of samples and greatly increases the chances of false-positive results.44,46 Nevertheless, if a laboratory’s enzyme immunoassay test has a low sensitivity, repeating negative tests may improve its sensitivity.
Glutamate dehydrogenase is an enzyme produced by both toxigenic and nontoxigenic strains of C difficile. As a result, stool testing for glutamate dehydrogenase is sensitive but not specific for C difficile infection, although multistep testing sequences (glutamate dehydrogenase followed by polymerase chain reaction) have proven to be useful screening tools.44
Treatment for C difficile infection
If testing for C difficile is positive, treatment is generally based on the severity and the complications of the illness46:
- Mild or moderate C difficile infection should be treated with oral metronidazole 500 mg three times per day for 10 to 14 days.
- Severe infection, which is defined as a white blood cell count of 15.0 × 109/L or higher or a serum creatinine level greater than or equal to 1.5 times the premorbid level, should be treated with oral vancomycin 125 mg four times per day for 10 to 14 days.
- Severe C difficile infection complicated by hypotension, shock, ileus, or megacolon should be treated with a combination of high-dose oral vancomycin (and possibly rectal vancomycin as well) at 500 mg four times per day plus intravenous metronidazole.
Additional treatment recommendations for individualized situations, recurrent C difficile infection, and comorbid conditions are discussed elsewhere.46
ENDOSCOPIC EVALUATION
Colonoscopy or flexible sigmoidoscopy is the primary means by which pseudomembranous colitis is diagnosed. Lower endoscopy should be pursued as an adjunctive tool when C difficile infection remains strongly suspected despite negative testing, when presumed C difficile infection does not respond to medical therapy, and when non-C difficile diagnoses are considered. If pseudomembranes are demonstrated on lower endoscopy, obtaining biopsy samples of normal- and abnormal-appearing mucosa is recommended. Pseudomembranes are suggestive but not diagnostic of C difficile infection, and microscopic evaluation of the mucosa is warranted to explore causes of pseudomembranous colitis not related to C difficile.
The pattern and distribution of pseudomembranes may provide clues to the etiology and the degree of mucosal injury. In intestinal ischemia, for example, a localized segment of the bowel is typically involved, and mucosal changes are often well delineated from normal mucosa. On endoscopic examination, mild ischemia is characterized by granular mucosa with decreased vascularity, whereas friable, edematous, ulcerated, and at times necrotic mucosa is evident in severe cases. Punctate pseudomembrane formation is seen in early ischemia, but as injury progresses, the pseudomembranes may grow and merge. In fact, diffuse involvement of the mucosal surface of the biopsy specimen by pseudomembranes has been shown to be more closely associated with ischemic colitis than C difficile infection.47
MICROSCOPIC EVALUATION
Histologic study can differentiate the various causes of pseudomembranous colitis.
In C difficile infection and drug reaction, there is acute crypt injury and dilation. The upper lamina propria is usually involved, and affected crypts are filled with an exudate similar to that found in pseudomembranes.1 However, in drug reaction, there is also prominent apoptosis and increased intraepithelial lymphocytosis.9
In colon ischemia, hyalinization of the lamina propria is a sensitive and specific marker.47 This has been shown in a study comparing histologic characteristics of colonic biopsies in patients with pseudomembranous colitis due to either known colon ischemia or C difficile infection.47 Crypt atrophy, lamina propria hemorrhage, full-thickness mucosal necrosis, and layering of pseudomembranes would further favor the diagnosis.
In collagenous colitis, a thickened subepithelial collagen band and intraepithelial lymphocytosis are often seen.
In inflammatory bowel disease, even with secondary pseudomembranes, ulcerative colitis and Crohn disease retain the characteristics of inflammatory bowel disease with crypt architectural distortion and focal or diffuse basal lymphoplasmacytosis on microscopy.48
- Gebhard RL, Gerding DN, Olson MM, et al. Clinical and endoscopic findings in patients early in the course of clostridium difficile-associated pseudomembranous colitis. Am J Med 1985; 78:45–48.
- Carpenter HA, Talley NJ. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: histological patterns with clinical implications. Am J Gastroenterol 2000; 95:878–896.
- Seppälä K. Colonoscopy in the diagnosis of pseudomembranous colitis. Br Med J 1978; 2:435.
- Stein BL, Lamoureux E, Miller M, Vasilevsky CA, Julien L, Gordon PH. Glutaraldehyde-induced colitis. Can J Surg 2001; 44:113–116.
- Trevisani F, Simoncini M, Alampi G, Bernardi M. Colitis associated to chemotherapy with 5-fluorouracil. Hepatogastroenterology 1997; 44:710–712.
- Takao T, Nishida M, Maeda Y, Takao K, Oka M. The study of continuous infusion chemotherapy with low-dose cisplatin and 5-fluorouracil for patients with primary liver cancer. Gan To Kagaku Ryoho 1997; 24:1724–1727. Japanese.
- Carrion AF, Hosein PJ, Cooper EM, Lopes G, Pelaez L, Rocha-Lima CM. Severe colitis associated with docetaxel use: a report of four cases. World J Gastrointest Oncol 2010; 2:390-394.
- Constantopoulos A. Colitis induced by interaction of cyclosporine A and non-steroidal anti-inflammatory drugs. Pediatr Int 1999; 41:184–186.
- Price AB. Pathology of drug-associated gastrointestinal disease. Br J Clin Pharmacol 2003; 56:477–482.
- Gentric A, Pennec YL. Diclofenac-induced pseudomembranous colitis. Lancet 1992; 340:126–127.
- Romero-Gómez M, Suárez García E, Castro Fernández M. Pseudomembranous colitis induced by diclofenac. J Clin Gastroenterol 1998; 26:228.
- Altemeier WA, Hummel RP, Hill EO. Staphylococcal enterocolitis following antibiotic therapy. Ann Surg 1963; 157:847–858.
- Ogawa Y, Saraya T, Koide T, et al. Methicillin-resistant Staphylococcus aureus enterocolitis sequentially complicated with septic arthritis: a case report and review of the literature. BMC Res Notes 2014; 7:21.
- Griffin PM, Olmstead LC, Petras RE. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 1990; 99:142–149.
- Uc A, Mitros FA, Kao SC, Sanders KD. Pseudomembranous colitis with Escherichia coli O157:H7. J Pediatr Gastroenterol Nutr 1997; 24:590–593.
- Battaglino MP, Rockey DC. Cytomegalovirus colitis presenting with the endoscopic appearance of pseudomembranous colitis. Gastrointest Endosc 1999; 50:697–700.
- Olofinlade O, Chiang C. Cytomegalovirus infection as a cause of pseudomembrane colitis: a report of four cases. J Clin Gastroenterol 2001; 32:82–84.
- Wilcox CM, Chalasani N, Lazenby A, Schwartz DA. Cytomegalovirus colitis in acquired immunodeficiency syndrome: a clinical and endoscopic study. Gastrointest Endosc 1998; 48:39–43.
- Seo TH, Kim JH, Ko SY, et al. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology 2012; 59:2137–2141.
- Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 2006; 355:2418–2426.
- Hovius SE, Rietra PJ. Salmonella colitis clinically presenting as a pseudomembranous colitis. Neth J Surg 1982; 34:81–82.
- Kelber M, Ament ME. Shigella dysenteriae I: a forgotten cause of pseudomembranous colitis. J Pediatr 1976; 89:595–596.
- Neves J, Raso P, Pinto Dde M, da Silva SP, Alvarenga RJ. Ischaemic colitis (necrotizing colitis, pseudomembranous colitis) in acute schistosomiasis mansoni: report of two cases. Trans R Soc Trop Med Hyg 1993; 87:449–452.
- Alcalde-Vargas A, Trigo-Salado C, Leo Carnerero E, De-la-Cruz-Ramírez D, Herrera-Justiniano JM. Pseudomembranous colitis and bacteremia in an immunocompetent patient associated with a rare specie of Clostridium (C. ramosum). Rev Esp Enferm Dig 2012; 104:498–499.
- Koo JS, Choi WS, Park DW. Fulminant amebic colitis mimicking pseudomembranous colitis. Gastrointest Endosc 2010; 71:400–401.
- van Loon FP, Rahim Z, Chowdhury KA, Kay BA, Rahman SA. Case report of Plesiomonas shigelloides-associated persistent dysentery and pseudomembranous colitis. J Clin Microbiol 1989; 27:1913–1915.
- Janvier J, Kuhn S, Church D. Not all pseudomembranous colitis is caused by Clostridium difficile. Can J Infect Dis Med Microbiol 2008; 19:256–257.
- Jain AK, Agarwal SK, el-Sadr W. Streptococcus bovis bacteremia and meningitis associated with Strongyloides stercoralis colitis in a patient infected with human immunodeficiency virus. Clin Infect Dis 1994; 18:253–254.
- Tang DM, Urrunaga NH, De Groot H, von Rosenvinge EC, Xie G, Ghazi LJ. Pseudomembranous colitis: not always caused by Clostridium difficile. Case Rep Med 2014; 2014:812704.
- Kendrick JB, Risbano M, Groshong SD, Frankel SK. A rare presentation of ischemic pseudomembranous colitis due to Escherichia coli O157:H7. Clin Infect Dis 2007; 45:217–219.
- Fishel R, Hamamoto G, Barbul A, Jiji V, Efron G. Cocaine colitis. Is this a new syndrome? Dis Colon Rectum 1985; 28:264–266.
- Khan-Kheil AM, Disney B, Ruban E, Wood G. Pseudomembranous collagenous colitis: an unusual cause of chronic diarrhoea. BMJ Case Rep 2014; 2014.
- Villanacci V, Cristina S, Muscarà M, et al. Pseudomembranous collagenous colitis with superimposed drug damage. Pathol Res Pract 2013; 209:735–739.
- Denız K, Coban G, Ozbakir O, Denız E. Pseudomembranous collagenous colitis. Turk J Gastroenterol 2012; 23:93–95.
- Vesoulis Z, Lozanski G, Loiudice T. Synchronous occurrence of collagenous colitis and pseudomembranous colitis. Can J Gastroenterol 2000; 14:353–358.
- Yuan S, Reyes V, Bronner MP. Pseudomembranous collagenous colitis. Am J Surg Pathol 2003; 27:1375–1379.
- Berdichevski T, Barshack I, Bar-Meir S, Ben-Horin S. Pseudomembranes in a patient with flare-up of inflammatory bowel disease (IBD): is it only Clostridium difficile or is it still an IBD exacerbation? Endoscopy 2010; 42(suppl 2):E131.
- Kilinçalp S, Altinbas A, Basar O, Deveci M, Yüksel O. A case of ulcerative colitis co-existing with pseudo-membranous enterocolitis. J Crohns Colitis 2011; 5:506–507.
- Ben-Horin S, Margalit M, Bossuyt P, et al; European Crohn’s and Colitis Organization (ECCO). Prevalence and clinical impact of endoscopic pseudomembranes in patients with inflammatory bowel disease and Clostridium difficile infection. J Crohns Colitis 2010; 4:194–198.
- Chiba M, Abe T, Tsuda S, Ono I. Cytomegalovirus infection associated with onset of ulcerative colitis. BMC Res Notes 2013; 6:40.
- Shukla A, Tolan RW. Behcet disease presenting with pseudomembranous colitis and progression to neurological involvement: case report and review of the literature. Clin Pediatr (Phila) 2012; 51:1197–1201.
- Shanholtzer CJ, Willard KE, Holter JJ, Olson MM, Gerding DN, Peterson LR. Comparison of the VIDAS Clostridium difficile toxin A immunoassay with C. difficile culture and cytotoxin and latex tests. J Clin Microbiol 1992; 30:1837–1840.
- Huang H, Weintraub A, Fang H, Nord CE. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of Clostridium difficile infections. J Clin Microbiol 2009; 47:3729–3731.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society For Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002; 346:334–339.
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–498.
- Dignan CR, Greenson JK. Can ischemic colitis be differentiated from C difficile colitis in biopsy specimens? Am J Surg Pathol 1997; 21:706–710.
- Jenkins D, Balsitis M, Gallivan S, et al. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol 1997; 50:93–105.
Pseudomembranous colitis is most often due to Clostridium difficile infection, but it has a variety of other causes, including other infections, ischemia, medications, and inflammatory mucosal diseases (Table 1). When pseudomembranes are found, one should consider these other causes if tests for C difficile are negative or if anti-C difficile therapy does not produce a response.
These less common causes are important to consider to avoid needlessly escalating anti-C difficile antibiotic therapy and to provide appropriate treatment. Pseudomembranous colitis is a nonspecific finding that suggests a larger disease process. Associated signs and symptoms, including fever, abdominal pain, leukocytosis, diarrhea, toxic megacolon, and electrolyte imbalances, may portend a life-threatening condition.1 Awareness of causes of pseudomembranous colitis other than C difficile infection, the focus of this review, is key to prompt diagnosis and potentially life-saving patient care.
PSEUDOMEMBRANES ARE NONSPECIFIC
A pseudomembrane is a layer of fibropurulent exudate composed of acute inflammatory cells and mucus originating from inflamed and erupting crypts.2 Although most often seen in C difficile infection, pseudomembranous colitis is a nonspecific pattern of injury resulting from decreased oxygenation, endothelial damage, and impaired blood flow to the mucosa that can be triggered by a number of disease states.2
On endoscopy, pseudomembranes appear as raised whitish or yellowish plaques that may be scattered or confluent in distribution (Figure 1).2 They are usually found in the rectosigmoid colon but may be isolated to more proximal segments.3 Lower endoscopy is often performed in the diagnostic evaluation of patients with unexplained diarrhea, hematochezia, and abnormal abdominal computed tomographic findings (eg, colonic thickening).
CAUSES OF NON-C DIFFICLE PSEUDOMEMBRANOUS COLITIS
When pseudomembranous colitis is confirmed endoscopically, C difficile infection naturally comes to mind, but the two terms are not interchangeable. A wide differential diagnosis should be maintained, especially when there are clues that C difficile infection may not be the correct diagnosis.
Chemicals and medications
Several chemicals and medications can injure the bowel and predispose to pseudomembrane formation.
Glutaraldehyde has long been used to sanitize endoscopes because of its broad antimicrobial activity. Nevertheless, if the disinfecting solution is not adequately rinsed off the endoscope, direct contact with colonic mucosa can produce an allergic and a chemical reaction, resulting in an acute self-limited colitis with pseudomembrane formation.4
Chemotherapeutic and antiproliferative agents can be toxic to the bowel, generally through production of free radicals and up-regulation of inflammatory cytokines. The colonic epithelium is then more susceptible to ulceration and mucosal necrosis with pseudomembrane development. Cisplatin, cyclosporine A, docetaxel, and 5-fluorouracil are prominent examples.5–8
Nonsteroidal anti-inflammatory drugs can damage the mucosa at all levels of the gastrointestinal tract. Although gastric ulcerations are more typical (from nonselective cyclooxygenase inhibition), colonic ulcerations and colitis can occur.9 These drugs, particularly diclofenac and indomethacin, have been associated with non-C difficile pseudomembranous colitis when used by themselves or in conjunction with other agents such as cyclosporine A.8,10,11
Infections
C difficile is the organism most commonly linked to pseudomembranous colitis, but other bacterial, viral, and parasitic pathogens have also been implicated.
Staphylococcus aureus was believed to be responsible for enterocolitis in a series of 155 surgical patients between 1958 and 1962 receiving antibiotic therapy. All had a positive stool culture for S aureus, and nine were found to have pseudomembranes at autopsy.12
Although this finding has been disputed as a misdiagnosis, since C difficile infection was not widely recognized until the 1970s, there is evidence that S aureus may indeed be a cause of non-C difficile pseudomembranous colitis. In a review of 36 cases of methicillin-resistant S aureus bacteremia in Japan, four patients were documented to have intestinal pseudomembranes either by endoscopy or autopsy. In two of these patients, biopsies of the pseudomembranes were positive for methicillin-resistant S aureus.13
Escherichia coli O157:H7. Pseudomembranes have been seen endoscopically in several adults and children with enterohemorrhagic E coli O157:H7 infection.14,15 This invasive gram-negative rod normally resides in the gastrointestinal tract of cattle, sheep, and other animals and can be pathogenic to people who eat undercooked beef. The organism attaches to and effaces intestinal epithelial cells, and bacterial proteins and the Shiga toxin then damage the vasculature, precipitating bloody diarrhea. Colonic damage can range from mild hemorrhagic colitis to severe colitis with ischemic changes. In patients with enterohemorrhagic E coli O157:H7 infection, pseudomembrane formation results from colon ischemia due to microvascular thrombosis or from destructive effects of bacterial enterotoxin.1,15
Cytomegalovirus is a ubiquitous human herpes virus that can affect nearly all organ systems. Infection is often reported in immunocompromised patients, eg, those with acquired immunodeficiency syndrome, chronic corticosteroid use, inflammatory bowel disease, malignancy, or solid-organ transplants. Gastrointestinal manifestations can be nonspecific and range from abdominal discomfort to diarrhea to tenesmus. Pseudomembranous colitis can be a presenting feature of cytomegalovirus involvement.16,17 Ulcerative lesions in the colonic mucosa are a frequent accompanying finding on endoscopy.18,19
Other rarer infectious causes of pseudomembranous colitis identified in case reports include Clostridium ramosum, Entamoeba histolytica, Klebsiella oxytoca, Plesiomonas shigelloides, Schistosomiasis mansoni, and Salmonella and Shigella species.20–26 Additionally, there have been two reports of Strongyloides stercoralis hyperinfection manifesting as pseudomembranous colitis.27,28
Ischemia
Colon ischemia usually affects elderly or debilitated patients who have multiple comorbidities. Known risk factors include aortoiliac surgery, cardiovascular disease, diabetes mellitus, hemodialysis, and pulmonary disease. The ischemia can be related to an occlusive arterial or venous thromboembolism, but hypoperfusion without occlusion of the mesenteric or internal iliac arteries is the primary mechanism. Low blood flow states such as atherosclerosis and septic shock can affect the watershed areas, typically the splenic flexure and rectosigmoid junction.
We reported a case of a patient with vascular disease who was incorrectly diagnosed and treated as having refractory C difficile infection when pseudomembranes were seen on flexible sigmoidoscopy. Further investigation revealed ischemic colitis secondary to a high-grade inferior mesenteric artery stenosis as the true cause.29
Microvascular thrombosis is the likely mechanism in a number of non-C difficile causes of pseudomembranous colitis. For example, in most patients with enterohemorrhagic E coli O157:H7 infection, histologic review of colonic mucosal biopsies has revealed fibrin and platelet thrombi in the capillaries, suggesting microvascular thrombosis.15,30
Cocaine has been associated with pseudomembranes in the setting of ischemia in the cecum and ascending colon. Cocaine can cause vasoconstriction after stimulation of alpha-adrenergic receptors and hence intestinal ischemia, thrombosis of vessels in the large and small intestines, and direct toxic effects.31
Inflammatory conditions
Collagenous colitis is an inflammatory disease that often affects middle-aged women and presents with copious watery diarrhea. It is a type of microscopic colitis—the endoscopic appearance is often normal, while the histologic appearance is abnormal and characterized by collagen deposition in the lamina propria. Medications that have been implicated in microscopic colitis include acid-suppressive agents (eg, histamine receptor antagonists, proton pump inhibitors) and nonsteroidal anti-inflammatory drugs.
An increasing number of cases of pseudomembranous changes are being reported in patients diagnosed with collagenous colitis.32–36 Although the pathophysiologic mechanism is unknown, some authors have suggested that pseudomembrane formation is actually part of the presenting spectrum of collagenous colitis.36
Inflammatory bowel disease. Crohn disease and ulcerative colitis have been associated with pseudomembranous colitis. Pseudomembranes can be found on endoscopy in patients with inflammatory bowel disease during a disease exacerbation with or without C difficile.37,38 In patients with inflammatory bowel disease and C difficile infection, pseudomembranes can be found endoscopically in up to 13% of cases.39 Pseudomembranous colitis has been reported in a patient with ulcerative colitis exacerbation in association with cytomegalovirus colitis.40
Behçet disease is a rare, immune-mediated small-vessel systemic vasculitis. It usually presents with mucous membrane ulcerations and ocular disease but can affect any organ.41 Pseudomembranous colitis can occur in Behçet disease in the absence of C difficile infection or any infectious colitis. Treatment includes corticosteroids and immunosuppressants such as azathioprine and anti-tumor necrosis factor agents.41
INITIAL EVALUATION
The initial evaluation of a patient with suspected or confirmed pseudomembranous colitis should include a comprehensive medical history with information on recent hospitalizations or procedures, antibiotic use, infections, exposure to sick contacts, recent travel, and medications taken.
Testing for C difficile
As most patients with pseudomembranous colitis have C difficile infection, it should be excluded first. Empiric anti-C difficile treatment is recommended in seriously ill-appearing patients, ideally starting after a stool sample is obtained.
Diagnosis of C difficile infection requires laboratory demonstration of the toxin or detection of toxigenic organisms. The gold standard test is the cell culture cytotoxicity assay, but it is labor- and time-intensive.42 More widely available tests are polymerase chain reaction for the toxin gene or genes, enzyme immunoassay, and stool evaluation for glutamate dehydrogenase, which can yield results readily within hours.
Polymerase chain reaction has a sensitivity of 97% and a specificity of 93%. Results can be falsely positive if empiric treatment is started before specimen collection, in which case C difficile DNA may still be present and detectable, but not the organism itself.43
Enzyme immunoassay for toxins A and B carries a sensitivity of 75% and a specificity of 99%, but 100 to 1,000 pg of toxin must be present for a positive result.44,45
If the initial enzyme immunoassay or polymerase chain reaction result is negative, current guidelines do not recommend repeat testing, which has limited value.44,46 Repeat testing after a negative result is positive in less than 5% of samples and greatly increases the chances of false-positive results.44,46 Nevertheless, if a laboratory’s enzyme immunoassay test has a low sensitivity, repeating negative tests may improve its sensitivity.
Glutamate dehydrogenase is an enzyme produced by both toxigenic and nontoxigenic strains of C difficile. As a result, stool testing for glutamate dehydrogenase is sensitive but not specific for C difficile infection, although multistep testing sequences (glutamate dehydrogenase followed by polymerase chain reaction) have proven to be useful screening tools.44
Treatment for C difficile infection
If testing for C difficile is positive, treatment is generally based on the severity and the complications of the illness46:
- Mild or moderate C difficile infection should be treated with oral metronidazole 500 mg three times per day for 10 to 14 days.
- Severe infection, which is defined as a white blood cell count of 15.0 × 109/L or higher or a serum creatinine level greater than or equal to 1.5 times the premorbid level, should be treated with oral vancomycin 125 mg four times per day for 10 to 14 days.
- Severe C difficile infection complicated by hypotension, shock, ileus, or megacolon should be treated with a combination of high-dose oral vancomycin (and possibly rectal vancomycin as well) at 500 mg four times per day plus intravenous metronidazole.
Additional treatment recommendations for individualized situations, recurrent C difficile infection, and comorbid conditions are discussed elsewhere.46
ENDOSCOPIC EVALUATION
Colonoscopy or flexible sigmoidoscopy is the primary means by which pseudomembranous colitis is diagnosed. Lower endoscopy should be pursued as an adjunctive tool when C difficile infection remains strongly suspected despite negative testing, when presumed C difficile infection does not respond to medical therapy, and when non-C difficile diagnoses are considered. If pseudomembranes are demonstrated on lower endoscopy, obtaining biopsy samples of normal- and abnormal-appearing mucosa is recommended. Pseudomembranes are suggestive but not diagnostic of C difficile infection, and microscopic evaluation of the mucosa is warranted to explore causes of pseudomembranous colitis not related to C difficile.
The pattern and distribution of pseudomembranes may provide clues to the etiology and the degree of mucosal injury. In intestinal ischemia, for example, a localized segment of the bowel is typically involved, and mucosal changes are often well delineated from normal mucosa. On endoscopic examination, mild ischemia is characterized by granular mucosa with decreased vascularity, whereas friable, edematous, ulcerated, and at times necrotic mucosa is evident in severe cases. Punctate pseudomembrane formation is seen in early ischemia, but as injury progresses, the pseudomembranes may grow and merge. In fact, diffuse involvement of the mucosal surface of the biopsy specimen by pseudomembranes has been shown to be more closely associated with ischemic colitis than C difficile infection.47
MICROSCOPIC EVALUATION
Histologic study can differentiate the various causes of pseudomembranous colitis.
In C difficile infection and drug reaction, there is acute crypt injury and dilation. The upper lamina propria is usually involved, and affected crypts are filled with an exudate similar to that found in pseudomembranes.1 However, in drug reaction, there is also prominent apoptosis and increased intraepithelial lymphocytosis.9
In colon ischemia, hyalinization of the lamina propria is a sensitive and specific marker.47 This has been shown in a study comparing histologic characteristics of colonic biopsies in patients with pseudomembranous colitis due to either known colon ischemia or C difficile infection.47 Crypt atrophy, lamina propria hemorrhage, full-thickness mucosal necrosis, and layering of pseudomembranes would further favor the diagnosis.
In collagenous colitis, a thickened subepithelial collagen band and intraepithelial lymphocytosis are often seen.
In inflammatory bowel disease, even with secondary pseudomembranes, ulcerative colitis and Crohn disease retain the characteristics of inflammatory bowel disease with crypt architectural distortion and focal or diffuse basal lymphoplasmacytosis on microscopy.48
Pseudomembranous colitis is most often due to Clostridium difficile infection, but it has a variety of other causes, including other infections, ischemia, medications, and inflammatory mucosal diseases (Table 1). When pseudomembranes are found, one should consider these other causes if tests for C difficile are negative or if anti-C difficile therapy does not produce a response.
These less common causes are important to consider to avoid needlessly escalating anti-C difficile antibiotic therapy and to provide appropriate treatment. Pseudomembranous colitis is a nonspecific finding that suggests a larger disease process. Associated signs and symptoms, including fever, abdominal pain, leukocytosis, diarrhea, toxic megacolon, and electrolyte imbalances, may portend a life-threatening condition.1 Awareness of causes of pseudomembranous colitis other than C difficile infection, the focus of this review, is key to prompt diagnosis and potentially life-saving patient care.
PSEUDOMEMBRANES ARE NONSPECIFIC
A pseudomembrane is a layer of fibropurulent exudate composed of acute inflammatory cells and mucus originating from inflamed and erupting crypts.2 Although most often seen in C difficile infection, pseudomembranous colitis is a nonspecific pattern of injury resulting from decreased oxygenation, endothelial damage, and impaired blood flow to the mucosa that can be triggered by a number of disease states.2
On endoscopy, pseudomembranes appear as raised whitish or yellowish plaques that may be scattered or confluent in distribution (Figure 1).2 They are usually found in the rectosigmoid colon but may be isolated to more proximal segments.3 Lower endoscopy is often performed in the diagnostic evaluation of patients with unexplained diarrhea, hematochezia, and abnormal abdominal computed tomographic findings (eg, colonic thickening).
CAUSES OF NON-C DIFFICLE PSEUDOMEMBRANOUS COLITIS
When pseudomembranous colitis is confirmed endoscopically, C difficile infection naturally comes to mind, but the two terms are not interchangeable. A wide differential diagnosis should be maintained, especially when there are clues that C difficile infection may not be the correct diagnosis.
Chemicals and medications
Several chemicals and medications can injure the bowel and predispose to pseudomembrane formation.
Glutaraldehyde has long been used to sanitize endoscopes because of its broad antimicrobial activity. Nevertheless, if the disinfecting solution is not adequately rinsed off the endoscope, direct contact with colonic mucosa can produce an allergic and a chemical reaction, resulting in an acute self-limited colitis with pseudomembrane formation.4
Chemotherapeutic and antiproliferative agents can be toxic to the bowel, generally through production of free radicals and up-regulation of inflammatory cytokines. The colonic epithelium is then more susceptible to ulceration and mucosal necrosis with pseudomembrane development. Cisplatin, cyclosporine A, docetaxel, and 5-fluorouracil are prominent examples.5–8
Nonsteroidal anti-inflammatory drugs can damage the mucosa at all levels of the gastrointestinal tract. Although gastric ulcerations are more typical (from nonselective cyclooxygenase inhibition), colonic ulcerations and colitis can occur.9 These drugs, particularly diclofenac and indomethacin, have been associated with non-C difficile pseudomembranous colitis when used by themselves or in conjunction with other agents such as cyclosporine A.8,10,11
Infections
C difficile is the organism most commonly linked to pseudomembranous colitis, but other bacterial, viral, and parasitic pathogens have also been implicated.
Staphylococcus aureus was believed to be responsible for enterocolitis in a series of 155 surgical patients between 1958 and 1962 receiving antibiotic therapy. All had a positive stool culture for S aureus, and nine were found to have pseudomembranes at autopsy.12
Although this finding has been disputed as a misdiagnosis, since C difficile infection was not widely recognized until the 1970s, there is evidence that S aureus may indeed be a cause of non-C difficile pseudomembranous colitis. In a review of 36 cases of methicillin-resistant S aureus bacteremia in Japan, four patients were documented to have intestinal pseudomembranes either by endoscopy or autopsy. In two of these patients, biopsies of the pseudomembranes were positive for methicillin-resistant S aureus.13
Escherichia coli O157:H7. Pseudomembranes have been seen endoscopically in several adults and children with enterohemorrhagic E coli O157:H7 infection.14,15 This invasive gram-negative rod normally resides in the gastrointestinal tract of cattle, sheep, and other animals and can be pathogenic to people who eat undercooked beef. The organism attaches to and effaces intestinal epithelial cells, and bacterial proteins and the Shiga toxin then damage the vasculature, precipitating bloody diarrhea. Colonic damage can range from mild hemorrhagic colitis to severe colitis with ischemic changes. In patients with enterohemorrhagic E coli O157:H7 infection, pseudomembrane formation results from colon ischemia due to microvascular thrombosis or from destructive effects of bacterial enterotoxin.1,15
Cytomegalovirus is a ubiquitous human herpes virus that can affect nearly all organ systems. Infection is often reported in immunocompromised patients, eg, those with acquired immunodeficiency syndrome, chronic corticosteroid use, inflammatory bowel disease, malignancy, or solid-organ transplants. Gastrointestinal manifestations can be nonspecific and range from abdominal discomfort to diarrhea to tenesmus. Pseudomembranous colitis can be a presenting feature of cytomegalovirus involvement.16,17 Ulcerative lesions in the colonic mucosa are a frequent accompanying finding on endoscopy.18,19
Other rarer infectious causes of pseudomembranous colitis identified in case reports include Clostridium ramosum, Entamoeba histolytica, Klebsiella oxytoca, Plesiomonas shigelloides, Schistosomiasis mansoni, and Salmonella and Shigella species.20–26 Additionally, there have been two reports of Strongyloides stercoralis hyperinfection manifesting as pseudomembranous colitis.27,28
Ischemia
Colon ischemia usually affects elderly or debilitated patients who have multiple comorbidities. Known risk factors include aortoiliac surgery, cardiovascular disease, diabetes mellitus, hemodialysis, and pulmonary disease. The ischemia can be related to an occlusive arterial or venous thromboembolism, but hypoperfusion without occlusion of the mesenteric or internal iliac arteries is the primary mechanism. Low blood flow states such as atherosclerosis and septic shock can affect the watershed areas, typically the splenic flexure and rectosigmoid junction.
We reported a case of a patient with vascular disease who was incorrectly diagnosed and treated as having refractory C difficile infection when pseudomembranes were seen on flexible sigmoidoscopy. Further investigation revealed ischemic colitis secondary to a high-grade inferior mesenteric artery stenosis as the true cause.29
Microvascular thrombosis is the likely mechanism in a number of non-C difficile causes of pseudomembranous colitis. For example, in most patients with enterohemorrhagic E coli O157:H7 infection, histologic review of colonic mucosal biopsies has revealed fibrin and platelet thrombi in the capillaries, suggesting microvascular thrombosis.15,30
Cocaine has been associated with pseudomembranes in the setting of ischemia in the cecum and ascending colon. Cocaine can cause vasoconstriction after stimulation of alpha-adrenergic receptors and hence intestinal ischemia, thrombosis of vessels in the large and small intestines, and direct toxic effects.31
Inflammatory conditions
Collagenous colitis is an inflammatory disease that often affects middle-aged women and presents with copious watery diarrhea. It is a type of microscopic colitis—the endoscopic appearance is often normal, while the histologic appearance is abnormal and characterized by collagen deposition in the lamina propria. Medications that have been implicated in microscopic colitis include acid-suppressive agents (eg, histamine receptor antagonists, proton pump inhibitors) and nonsteroidal anti-inflammatory drugs.
An increasing number of cases of pseudomembranous changes are being reported in patients diagnosed with collagenous colitis.32–36 Although the pathophysiologic mechanism is unknown, some authors have suggested that pseudomembrane formation is actually part of the presenting spectrum of collagenous colitis.36
Inflammatory bowel disease. Crohn disease and ulcerative colitis have been associated with pseudomembranous colitis. Pseudomembranes can be found on endoscopy in patients with inflammatory bowel disease during a disease exacerbation with or without C difficile.37,38 In patients with inflammatory bowel disease and C difficile infection, pseudomembranes can be found endoscopically in up to 13% of cases.39 Pseudomembranous colitis has been reported in a patient with ulcerative colitis exacerbation in association with cytomegalovirus colitis.40
Behçet disease is a rare, immune-mediated small-vessel systemic vasculitis. It usually presents with mucous membrane ulcerations and ocular disease but can affect any organ.41 Pseudomembranous colitis can occur in Behçet disease in the absence of C difficile infection or any infectious colitis. Treatment includes corticosteroids and immunosuppressants such as azathioprine and anti-tumor necrosis factor agents.41
INITIAL EVALUATION
The initial evaluation of a patient with suspected or confirmed pseudomembranous colitis should include a comprehensive medical history with information on recent hospitalizations or procedures, antibiotic use, infections, exposure to sick contacts, recent travel, and medications taken.
Testing for C difficile
As most patients with pseudomembranous colitis have C difficile infection, it should be excluded first. Empiric anti-C difficile treatment is recommended in seriously ill-appearing patients, ideally starting after a stool sample is obtained.
Diagnosis of C difficile infection requires laboratory demonstration of the toxin or detection of toxigenic organisms. The gold standard test is the cell culture cytotoxicity assay, but it is labor- and time-intensive.42 More widely available tests are polymerase chain reaction for the toxin gene or genes, enzyme immunoassay, and stool evaluation for glutamate dehydrogenase, which can yield results readily within hours.
Polymerase chain reaction has a sensitivity of 97% and a specificity of 93%. Results can be falsely positive if empiric treatment is started before specimen collection, in which case C difficile DNA may still be present and detectable, but not the organism itself.43
Enzyme immunoassay for toxins A and B carries a sensitivity of 75% and a specificity of 99%, but 100 to 1,000 pg of toxin must be present for a positive result.44,45
If the initial enzyme immunoassay or polymerase chain reaction result is negative, current guidelines do not recommend repeat testing, which has limited value.44,46 Repeat testing after a negative result is positive in less than 5% of samples and greatly increases the chances of false-positive results.44,46 Nevertheless, if a laboratory’s enzyme immunoassay test has a low sensitivity, repeating negative tests may improve its sensitivity.
Glutamate dehydrogenase is an enzyme produced by both toxigenic and nontoxigenic strains of C difficile. As a result, stool testing for glutamate dehydrogenase is sensitive but not specific for C difficile infection, although multistep testing sequences (glutamate dehydrogenase followed by polymerase chain reaction) have proven to be useful screening tools.44
Treatment for C difficile infection
If testing for C difficile is positive, treatment is generally based on the severity and the complications of the illness46:
- Mild or moderate C difficile infection should be treated with oral metronidazole 500 mg three times per day for 10 to 14 days.
- Severe infection, which is defined as a white blood cell count of 15.0 × 109/L or higher or a serum creatinine level greater than or equal to 1.5 times the premorbid level, should be treated with oral vancomycin 125 mg four times per day for 10 to 14 days.
- Severe C difficile infection complicated by hypotension, shock, ileus, or megacolon should be treated with a combination of high-dose oral vancomycin (and possibly rectal vancomycin as well) at 500 mg four times per day plus intravenous metronidazole.
Additional treatment recommendations for individualized situations, recurrent C difficile infection, and comorbid conditions are discussed elsewhere.46
ENDOSCOPIC EVALUATION
Colonoscopy or flexible sigmoidoscopy is the primary means by which pseudomembranous colitis is diagnosed. Lower endoscopy should be pursued as an adjunctive tool when C difficile infection remains strongly suspected despite negative testing, when presumed C difficile infection does not respond to medical therapy, and when non-C difficile diagnoses are considered. If pseudomembranes are demonstrated on lower endoscopy, obtaining biopsy samples of normal- and abnormal-appearing mucosa is recommended. Pseudomembranes are suggestive but not diagnostic of C difficile infection, and microscopic evaluation of the mucosa is warranted to explore causes of pseudomembranous colitis not related to C difficile.
The pattern and distribution of pseudomembranes may provide clues to the etiology and the degree of mucosal injury. In intestinal ischemia, for example, a localized segment of the bowel is typically involved, and mucosal changes are often well delineated from normal mucosa. On endoscopic examination, mild ischemia is characterized by granular mucosa with decreased vascularity, whereas friable, edematous, ulcerated, and at times necrotic mucosa is evident in severe cases. Punctate pseudomembrane formation is seen in early ischemia, but as injury progresses, the pseudomembranes may grow and merge. In fact, diffuse involvement of the mucosal surface of the biopsy specimen by pseudomembranes has been shown to be more closely associated with ischemic colitis than C difficile infection.47
MICROSCOPIC EVALUATION
Histologic study can differentiate the various causes of pseudomembranous colitis.
In C difficile infection and drug reaction, there is acute crypt injury and dilation. The upper lamina propria is usually involved, and affected crypts are filled with an exudate similar to that found in pseudomembranes.1 However, in drug reaction, there is also prominent apoptosis and increased intraepithelial lymphocytosis.9
In colon ischemia, hyalinization of the lamina propria is a sensitive and specific marker.47 This has been shown in a study comparing histologic characteristics of colonic biopsies in patients with pseudomembranous colitis due to either known colon ischemia or C difficile infection.47 Crypt atrophy, lamina propria hemorrhage, full-thickness mucosal necrosis, and layering of pseudomembranes would further favor the diagnosis.
In collagenous colitis, a thickened subepithelial collagen band and intraepithelial lymphocytosis are often seen.
In inflammatory bowel disease, even with secondary pseudomembranes, ulcerative colitis and Crohn disease retain the characteristics of inflammatory bowel disease with crypt architectural distortion and focal or diffuse basal lymphoplasmacytosis on microscopy.48
- Gebhard RL, Gerding DN, Olson MM, et al. Clinical and endoscopic findings in patients early in the course of clostridium difficile-associated pseudomembranous colitis. Am J Med 1985; 78:45–48.
- Carpenter HA, Talley NJ. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: histological patterns with clinical implications. Am J Gastroenterol 2000; 95:878–896.
- Seppälä K. Colonoscopy in the diagnosis of pseudomembranous colitis. Br Med J 1978; 2:435.
- Stein BL, Lamoureux E, Miller M, Vasilevsky CA, Julien L, Gordon PH. Glutaraldehyde-induced colitis. Can J Surg 2001; 44:113–116.
- Trevisani F, Simoncini M, Alampi G, Bernardi M. Colitis associated to chemotherapy with 5-fluorouracil. Hepatogastroenterology 1997; 44:710–712.
- Takao T, Nishida M, Maeda Y, Takao K, Oka M. The study of continuous infusion chemotherapy with low-dose cisplatin and 5-fluorouracil for patients with primary liver cancer. Gan To Kagaku Ryoho 1997; 24:1724–1727. Japanese.
- Carrion AF, Hosein PJ, Cooper EM, Lopes G, Pelaez L, Rocha-Lima CM. Severe colitis associated with docetaxel use: a report of four cases. World J Gastrointest Oncol 2010; 2:390-394.
- Constantopoulos A. Colitis induced by interaction of cyclosporine A and non-steroidal anti-inflammatory drugs. Pediatr Int 1999; 41:184–186.
- Price AB. Pathology of drug-associated gastrointestinal disease. Br J Clin Pharmacol 2003; 56:477–482.
- Gentric A, Pennec YL. Diclofenac-induced pseudomembranous colitis. Lancet 1992; 340:126–127.
- Romero-Gómez M, Suárez García E, Castro Fernández M. Pseudomembranous colitis induced by diclofenac. J Clin Gastroenterol 1998; 26:228.
- Altemeier WA, Hummel RP, Hill EO. Staphylococcal enterocolitis following antibiotic therapy. Ann Surg 1963; 157:847–858.
- Ogawa Y, Saraya T, Koide T, et al. Methicillin-resistant Staphylococcus aureus enterocolitis sequentially complicated with septic arthritis: a case report and review of the literature. BMC Res Notes 2014; 7:21.
- Griffin PM, Olmstead LC, Petras RE. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 1990; 99:142–149.
- Uc A, Mitros FA, Kao SC, Sanders KD. Pseudomembranous colitis with Escherichia coli O157:H7. J Pediatr Gastroenterol Nutr 1997; 24:590–593.
- Battaglino MP, Rockey DC. Cytomegalovirus colitis presenting with the endoscopic appearance of pseudomembranous colitis. Gastrointest Endosc 1999; 50:697–700.
- Olofinlade O, Chiang C. Cytomegalovirus infection as a cause of pseudomembrane colitis: a report of four cases. J Clin Gastroenterol 2001; 32:82–84.
- Wilcox CM, Chalasani N, Lazenby A, Schwartz DA. Cytomegalovirus colitis in acquired immunodeficiency syndrome: a clinical and endoscopic study. Gastrointest Endosc 1998; 48:39–43.
- Seo TH, Kim JH, Ko SY, et al. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology 2012; 59:2137–2141.
- Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 2006; 355:2418–2426.
- Hovius SE, Rietra PJ. Salmonella colitis clinically presenting as a pseudomembranous colitis. Neth J Surg 1982; 34:81–82.
- Kelber M, Ament ME. Shigella dysenteriae I: a forgotten cause of pseudomembranous colitis. J Pediatr 1976; 89:595–596.
- Neves J, Raso P, Pinto Dde M, da Silva SP, Alvarenga RJ. Ischaemic colitis (necrotizing colitis, pseudomembranous colitis) in acute schistosomiasis mansoni: report of two cases. Trans R Soc Trop Med Hyg 1993; 87:449–452.
- Alcalde-Vargas A, Trigo-Salado C, Leo Carnerero E, De-la-Cruz-Ramírez D, Herrera-Justiniano JM. Pseudomembranous colitis and bacteremia in an immunocompetent patient associated with a rare specie of Clostridium (C. ramosum). Rev Esp Enferm Dig 2012; 104:498–499.
- Koo JS, Choi WS, Park DW. Fulminant amebic colitis mimicking pseudomembranous colitis. Gastrointest Endosc 2010; 71:400–401.
- van Loon FP, Rahim Z, Chowdhury KA, Kay BA, Rahman SA. Case report of Plesiomonas shigelloides-associated persistent dysentery and pseudomembranous colitis. J Clin Microbiol 1989; 27:1913–1915.
- Janvier J, Kuhn S, Church D. Not all pseudomembranous colitis is caused by Clostridium difficile. Can J Infect Dis Med Microbiol 2008; 19:256–257.
- Jain AK, Agarwal SK, el-Sadr W. Streptococcus bovis bacteremia and meningitis associated with Strongyloides stercoralis colitis in a patient infected with human immunodeficiency virus. Clin Infect Dis 1994; 18:253–254.
- Tang DM, Urrunaga NH, De Groot H, von Rosenvinge EC, Xie G, Ghazi LJ. Pseudomembranous colitis: not always caused by Clostridium difficile. Case Rep Med 2014; 2014:812704.
- Kendrick JB, Risbano M, Groshong SD, Frankel SK. A rare presentation of ischemic pseudomembranous colitis due to Escherichia coli O157:H7. Clin Infect Dis 2007; 45:217–219.
- Fishel R, Hamamoto G, Barbul A, Jiji V, Efron G. Cocaine colitis. Is this a new syndrome? Dis Colon Rectum 1985; 28:264–266.
- Khan-Kheil AM, Disney B, Ruban E, Wood G. Pseudomembranous collagenous colitis: an unusual cause of chronic diarrhoea. BMJ Case Rep 2014; 2014.
- Villanacci V, Cristina S, Muscarà M, et al. Pseudomembranous collagenous colitis with superimposed drug damage. Pathol Res Pract 2013; 209:735–739.
- Denız K, Coban G, Ozbakir O, Denız E. Pseudomembranous collagenous colitis. Turk J Gastroenterol 2012; 23:93–95.
- Vesoulis Z, Lozanski G, Loiudice T. Synchronous occurrence of collagenous colitis and pseudomembranous colitis. Can J Gastroenterol 2000; 14:353–358.
- Yuan S, Reyes V, Bronner MP. Pseudomembranous collagenous colitis. Am J Surg Pathol 2003; 27:1375–1379.
- Berdichevski T, Barshack I, Bar-Meir S, Ben-Horin S. Pseudomembranes in a patient with flare-up of inflammatory bowel disease (IBD): is it only Clostridium difficile or is it still an IBD exacerbation? Endoscopy 2010; 42(suppl 2):E131.
- Kilinçalp S, Altinbas A, Basar O, Deveci M, Yüksel O. A case of ulcerative colitis co-existing with pseudo-membranous enterocolitis. J Crohns Colitis 2011; 5:506–507.
- Ben-Horin S, Margalit M, Bossuyt P, et al; European Crohn’s and Colitis Organization (ECCO). Prevalence and clinical impact of endoscopic pseudomembranes in patients with inflammatory bowel disease and Clostridium difficile infection. J Crohns Colitis 2010; 4:194–198.
- Chiba M, Abe T, Tsuda S, Ono I. Cytomegalovirus infection associated with onset of ulcerative colitis. BMC Res Notes 2013; 6:40.
- Shukla A, Tolan RW. Behcet disease presenting with pseudomembranous colitis and progression to neurological involvement: case report and review of the literature. Clin Pediatr (Phila) 2012; 51:1197–1201.
- Shanholtzer CJ, Willard KE, Holter JJ, Olson MM, Gerding DN, Peterson LR. Comparison of the VIDAS Clostridium difficile toxin A immunoassay with C. difficile culture and cytotoxin and latex tests. J Clin Microbiol 1992; 30:1837–1840.
- Huang H, Weintraub A, Fang H, Nord CE. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of Clostridium difficile infections. J Clin Microbiol 2009; 47:3729–3731.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society For Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002; 346:334–339.
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–498.
- Dignan CR, Greenson JK. Can ischemic colitis be differentiated from C difficile colitis in biopsy specimens? Am J Surg Pathol 1997; 21:706–710.
- Jenkins D, Balsitis M, Gallivan S, et al. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol 1997; 50:93–105.
- Gebhard RL, Gerding DN, Olson MM, et al. Clinical and endoscopic findings in patients early in the course of clostridium difficile-associated pseudomembranous colitis. Am J Med 1985; 78:45–48.
- Carpenter HA, Talley NJ. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: histological patterns with clinical implications. Am J Gastroenterol 2000; 95:878–896.
- Seppälä K. Colonoscopy in the diagnosis of pseudomembranous colitis. Br Med J 1978; 2:435.
- Stein BL, Lamoureux E, Miller M, Vasilevsky CA, Julien L, Gordon PH. Glutaraldehyde-induced colitis. Can J Surg 2001; 44:113–116.
- Trevisani F, Simoncini M, Alampi G, Bernardi M. Colitis associated to chemotherapy with 5-fluorouracil. Hepatogastroenterology 1997; 44:710–712.
- Takao T, Nishida M, Maeda Y, Takao K, Oka M. The study of continuous infusion chemotherapy with low-dose cisplatin and 5-fluorouracil for patients with primary liver cancer. Gan To Kagaku Ryoho 1997; 24:1724–1727. Japanese.
- Carrion AF, Hosein PJ, Cooper EM, Lopes G, Pelaez L, Rocha-Lima CM. Severe colitis associated with docetaxel use: a report of four cases. World J Gastrointest Oncol 2010; 2:390-394.
- Constantopoulos A. Colitis induced by interaction of cyclosporine A and non-steroidal anti-inflammatory drugs. Pediatr Int 1999; 41:184–186.
- Price AB. Pathology of drug-associated gastrointestinal disease. Br J Clin Pharmacol 2003; 56:477–482.
- Gentric A, Pennec YL. Diclofenac-induced pseudomembranous colitis. Lancet 1992; 340:126–127.
- Romero-Gómez M, Suárez García E, Castro Fernández M. Pseudomembranous colitis induced by diclofenac. J Clin Gastroenterol 1998; 26:228.
- Altemeier WA, Hummel RP, Hill EO. Staphylococcal enterocolitis following antibiotic therapy. Ann Surg 1963; 157:847–858.
- Ogawa Y, Saraya T, Koide T, et al. Methicillin-resistant Staphylococcus aureus enterocolitis sequentially complicated with septic arthritis: a case report and review of the literature. BMC Res Notes 2014; 7:21.
- Griffin PM, Olmstead LC, Petras RE. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 1990; 99:142–149.
- Uc A, Mitros FA, Kao SC, Sanders KD. Pseudomembranous colitis with Escherichia coli O157:H7. J Pediatr Gastroenterol Nutr 1997; 24:590–593.
- Battaglino MP, Rockey DC. Cytomegalovirus colitis presenting with the endoscopic appearance of pseudomembranous colitis. Gastrointest Endosc 1999; 50:697–700.
- Olofinlade O, Chiang C. Cytomegalovirus infection as a cause of pseudomembrane colitis: a report of four cases. J Clin Gastroenterol 2001; 32:82–84.
- Wilcox CM, Chalasani N, Lazenby A, Schwartz DA. Cytomegalovirus colitis in acquired immunodeficiency syndrome: a clinical and endoscopic study. Gastrointest Endosc 1998; 48:39–43.
- Seo TH, Kim JH, Ko SY, et al. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology 2012; 59:2137–2141.
- Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 2006; 355:2418–2426.
- Hovius SE, Rietra PJ. Salmonella colitis clinically presenting as a pseudomembranous colitis. Neth J Surg 1982; 34:81–82.
- Kelber M, Ament ME. Shigella dysenteriae I: a forgotten cause of pseudomembranous colitis. J Pediatr 1976; 89:595–596.
- Neves J, Raso P, Pinto Dde M, da Silva SP, Alvarenga RJ. Ischaemic colitis (necrotizing colitis, pseudomembranous colitis) in acute schistosomiasis mansoni: report of two cases. Trans R Soc Trop Med Hyg 1993; 87:449–452.
- Alcalde-Vargas A, Trigo-Salado C, Leo Carnerero E, De-la-Cruz-Ramírez D, Herrera-Justiniano JM. Pseudomembranous colitis and bacteremia in an immunocompetent patient associated with a rare specie of Clostridium (C. ramosum). Rev Esp Enferm Dig 2012; 104:498–499.
- Koo JS, Choi WS, Park DW. Fulminant amebic colitis mimicking pseudomembranous colitis. Gastrointest Endosc 2010; 71:400–401.
- van Loon FP, Rahim Z, Chowdhury KA, Kay BA, Rahman SA. Case report of Plesiomonas shigelloides-associated persistent dysentery and pseudomembranous colitis. J Clin Microbiol 1989; 27:1913–1915.
- Janvier J, Kuhn S, Church D. Not all pseudomembranous colitis is caused by Clostridium difficile. Can J Infect Dis Med Microbiol 2008; 19:256–257.
- Jain AK, Agarwal SK, el-Sadr W. Streptococcus bovis bacteremia and meningitis associated with Strongyloides stercoralis colitis in a patient infected with human immunodeficiency virus. Clin Infect Dis 1994; 18:253–254.
- Tang DM, Urrunaga NH, De Groot H, von Rosenvinge EC, Xie G, Ghazi LJ. Pseudomembranous colitis: not always caused by Clostridium difficile. Case Rep Med 2014; 2014:812704.
- Kendrick JB, Risbano M, Groshong SD, Frankel SK. A rare presentation of ischemic pseudomembranous colitis due to Escherichia coli O157:H7. Clin Infect Dis 2007; 45:217–219.
- Fishel R, Hamamoto G, Barbul A, Jiji V, Efron G. Cocaine colitis. Is this a new syndrome? Dis Colon Rectum 1985; 28:264–266.
- Khan-Kheil AM, Disney B, Ruban E, Wood G. Pseudomembranous collagenous colitis: an unusual cause of chronic diarrhoea. BMJ Case Rep 2014; 2014.
- Villanacci V, Cristina S, Muscarà M, et al. Pseudomembranous collagenous colitis with superimposed drug damage. Pathol Res Pract 2013; 209:735–739.
- Denız K, Coban G, Ozbakir O, Denız E. Pseudomembranous collagenous colitis. Turk J Gastroenterol 2012; 23:93–95.
- Vesoulis Z, Lozanski G, Loiudice T. Synchronous occurrence of collagenous colitis and pseudomembranous colitis. Can J Gastroenterol 2000; 14:353–358.
- Yuan S, Reyes V, Bronner MP. Pseudomembranous collagenous colitis. Am J Surg Pathol 2003; 27:1375–1379.
- Berdichevski T, Barshack I, Bar-Meir S, Ben-Horin S. Pseudomembranes in a patient with flare-up of inflammatory bowel disease (IBD): is it only Clostridium difficile or is it still an IBD exacerbation? Endoscopy 2010; 42(suppl 2):E131.
- Kilinçalp S, Altinbas A, Basar O, Deveci M, Yüksel O. A case of ulcerative colitis co-existing with pseudo-membranous enterocolitis. J Crohns Colitis 2011; 5:506–507.
- Ben-Horin S, Margalit M, Bossuyt P, et al; European Crohn’s and Colitis Organization (ECCO). Prevalence and clinical impact of endoscopic pseudomembranes in patients with inflammatory bowel disease and Clostridium difficile infection. J Crohns Colitis 2010; 4:194–198.
- Chiba M, Abe T, Tsuda S, Ono I. Cytomegalovirus infection associated with onset of ulcerative colitis. BMC Res Notes 2013; 6:40.
- Shukla A, Tolan RW. Behcet disease presenting with pseudomembranous colitis and progression to neurological involvement: case report and review of the literature. Clin Pediatr (Phila) 2012; 51:1197–1201.
- Shanholtzer CJ, Willard KE, Holter JJ, Olson MM, Gerding DN, Peterson LR. Comparison of the VIDAS Clostridium difficile toxin A immunoassay with C. difficile culture and cytotoxin and latex tests. J Clin Microbiol 1992; 30:1837–1840.
- Huang H, Weintraub A, Fang H, Nord CE. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of Clostridium difficile infections. J Clin Microbiol 2009; 47:3729–3731.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society For Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002; 346:334–339.
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–498.
- Dignan CR, Greenson JK. Can ischemic colitis be differentiated from C difficile colitis in biopsy specimens? Am J Surg Pathol 1997; 21:706–710.
- Jenkins D, Balsitis M, Gallivan S, et al. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol 1997; 50:93–105.
KEY POINTS
- Pseudomembranous colitis is a nonspecific pattern of injury resulting from decreased oxygenation, endothelial damage, and impaired blood flow to the mucosa that can be triggered by a number of disease states.
- Chemicals, medications, ischemia, microscopic colitis, other infectious organisms, and inflammatory conditions can all predispose to pseudomembrane formation and should be included in the differential diagnosis.
- As most patients with pseudomembranous colitis have C difficile infection, it should be excluded first. Empiric treatment for C difficile should be started if the patient is seriously ill.
- Testing for C difficile is with polymerase chain reaction, enzyme immunoassay for toxins A and B, and glutamate dehydrogenase measurement.
Rash from hepatitis C treatment
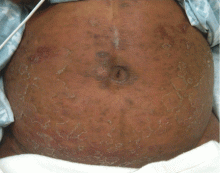
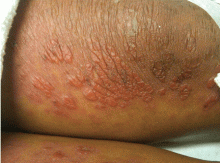
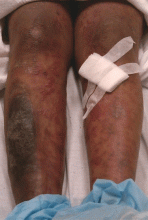
A 54-year-old woman with hepatitis C virus infection presents with generalized rash, pruritus, and fever over the past week. The rash appeared on her left arm after she received her fifth weekly injection of pegylated interferon alfa 2b, in combination with daily oral ribavirin (Copegus, Rebetol). Over the course of 3 days, it spread to her face and the rest of her body.
Q: What is the most likely clinical diagnosis?
- Stevens-Johnson syndrome
- Mixed cryoglobulinemia
- Acute eczematous drug eruption
- Lichen planus
A: Acute eczematous drug eruption is the most likely diagnosis.
The clinical presentation and laboratory findings suggest (the latter by exclusion) that our patient had an allergic drug reaction to the interferon or to the ribavirin therapy, or to both. Although this combination is a standard treatment for chronic hepatitis C, some patients experience adverse reactions that lead to its discontinuation. Local injection-site reactions are the most prevalent, affecting up to 12% of patients, whereas eczematous dermatoses manifest less commonly, occurring in up to 5% of patients.1
While awaiting the results of skin biopsy, a careful evaluation of the clinical features of the physical examination and an appropriate laboratory evaluation can rule out other important conditions in the differential diagnosis.
The absence of mucous membrane involvement steers the diagnosis away from Stevens-Johnson syndrome, a life-threatening hypersensitivity condition often triggered by drugs, malignant tumors, and viral infections, which may also affect internal organs. In this condition, skin biopsy specimens would be distinguished by subepidermal bullae and epidermal cell necrosis—neither of which was seen in our patient.
Mixed cryoglobulinemia should always be considered in hepatitis C patients because of the strong association between this infection and the development of cryoglobulins. The rash usually is purpuric, but it may be pleomorphic.2,3 This vasculitis often manifests with excess cryoglobulins, elevated rheumatoid factor, and low titers of complement in the blood due to consumption by immune complexes. Tissue biopsy would usually show typical vascular changes if performed on fresh lesions.4,5 The normal levels of these components in our patient coupled with the appearance of her skin makes cryoglobulinemia a less likely cause.
Furthermore, hepatitis C infection, whether or not treated with interferon and ribavirin, can cause an onset or recurrence of other dermatologic conditions, notably lichen planus, psoriasis, vitiligo, and systemic lupus erythematosus.1–4
In lichen planus, the rash is often described as flat-topped, pruritic, and violaceous. It may involve the extremities, the genitalia, and the oral cavity.4,5 The difference in quality of the rash compared with the rash in our patient makes lichen planus less likely.
Exclusion of the other conditions in the differential diagnosis, in addition to results from a definitive punch biopsy, solidified the diagnosis in our patient. Skin biopsy of the patient’s lower-extremity lesions revealed spongiotic dermatitis with lymphocytes, neutrophils, and few eosinophils—a finding characteristic of an acute eczematous drug eruption. Improvement of her rash after discontinuation of interferon and ribavirin further supported this conclusion, although it was unclear whether one or both agents were responsible.
OUTCOME
Management of acute eczematous drug eruption entails stopping the offending drug and alleviating the symptoms. Our patient’s non-life-threatening rash improved dramatically with cessation of interferon and ribavirin. She received a single dose of a systemic corticosteroid initially, out of concern for a severe medication-induced reaction (ie, Stevens-Johnson syndrome), but she was otherwise maintained with diphenhydramine (Benadryl) and a multivitamin ointment for the rash throughout her 9-day hospital stay. Her pruritus was well controlled with hydroxyzine (Atarax, Vistaril). At discharge, she was referred back to her hepatologist for further treatment of her hepatitis C, possibly with interferon and ribavirin again.
TAKE-HOME MESSAGE
Adverse reactions to interferon and ribavirin treatment in hepatitis C patients can manifest dermatologically, and the combination therapy should be discontinued to prevent further insult. A broad variety of conditions in the differential diagnosis should be taken into account, but dermatologic conditions that occur or recur specifically in hepatitis C patients should be considered as well.
- Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 2002; 147:1142–1146.
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13.
- Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010; 22:967–972.
- Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009; 145:1040–1047.
- Aamir S, Ullah Z, Iqbal Z, Khan AA, Yaqub F, Malik K. Cutaneous manifestations of interferon alfa and ribavirin for hepatitis C. J Pak Assoc Dermatol 2008; 18:14–20.
A 54-year-old woman with hepatitis C virus infection presents with generalized rash, pruritus, and fever over the past week. The rash appeared on her left arm after she received her fifth weekly injection of pegylated interferon alfa 2b, in combination with daily oral ribavirin (Copegus, Rebetol). Over the course of 3 days, it spread to her face and the rest of her body.
Q: What is the most likely clinical diagnosis?
- Stevens-Johnson syndrome
- Mixed cryoglobulinemia
- Acute eczematous drug eruption
- Lichen planus
A: Acute eczematous drug eruption is the most likely diagnosis.
The clinical presentation and laboratory findings suggest (the latter by exclusion) that our patient had an allergic drug reaction to the interferon or to the ribavirin therapy, or to both. Although this combination is a standard treatment for chronic hepatitis C, some patients experience adverse reactions that lead to its discontinuation. Local injection-site reactions are the most prevalent, affecting up to 12% of patients, whereas eczematous dermatoses manifest less commonly, occurring in up to 5% of patients.1
While awaiting the results of skin biopsy, a careful evaluation of the clinical features of the physical examination and an appropriate laboratory evaluation can rule out other important conditions in the differential diagnosis.
The absence of mucous membrane involvement steers the diagnosis away from Stevens-Johnson syndrome, a life-threatening hypersensitivity condition often triggered by drugs, malignant tumors, and viral infections, which may also affect internal organs. In this condition, skin biopsy specimens would be distinguished by subepidermal bullae and epidermal cell necrosis—neither of which was seen in our patient.
Mixed cryoglobulinemia should always be considered in hepatitis C patients because of the strong association between this infection and the development of cryoglobulins. The rash usually is purpuric, but it may be pleomorphic.2,3 This vasculitis often manifests with excess cryoglobulins, elevated rheumatoid factor, and low titers of complement in the blood due to consumption by immune complexes. Tissue biopsy would usually show typical vascular changes if performed on fresh lesions.4,5 The normal levels of these components in our patient coupled with the appearance of her skin makes cryoglobulinemia a less likely cause.
Furthermore, hepatitis C infection, whether or not treated with interferon and ribavirin, can cause an onset or recurrence of other dermatologic conditions, notably lichen planus, psoriasis, vitiligo, and systemic lupus erythematosus.1–4
In lichen planus, the rash is often described as flat-topped, pruritic, and violaceous. It may involve the extremities, the genitalia, and the oral cavity.4,5 The difference in quality of the rash compared with the rash in our patient makes lichen planus less likely.
Exclusion of the other conditions in the differential diagnosis, in addition to results from a definitive punch biopsy, solidified the diagnosis in our patient. Skin biopsy of the patient’s lower-extremity lesions revealed spongiotic dermatitis with lymphocytes, neutrophils, and few eosinophils—a finding characteristic of an acute eczematous drug eruption. Improvement of her rash after discontinuation of interferon and ribavirin further supported this conclusion, although it was unclear whether one or both agents were responsible.
OUTCOME
Management of acute eczematous drug eruption entails stopping the offending drug and alleviating the symptoms. Our patient’s non-life-threatening rash improved dramatically with cessation of interferon and ribavirin. She received a single dose of a systemic corticosteroid initially, out of concern for a severe medication-induced reaction (ie, Stevens-Johnson syndrome), but she was otherwise maintained with diphenhydramine (Benadryl) and a multivitamin ointment for the rash throughout her 9-day hospital stay. Her pruritus was well controlled with hydroxyzine (Atarax, Vistaril). At discharge, she was referred back to her hepatologist for further treatment of her hepatitis C, possibly with interferon and ribavirin again.
TAKE-HOME MESSAGE
Adverse reactions to interferon and ribavirin treatment in hepatitis C patients can manifest dermatologically, and the combination therapy should be discontinued to prevent further insult. A broad variety of conditions in the differential diagnosis should be taken into account, but dermatologic conditions that occur or recur specifically in hepatitis C patients should be considered as well.
A 54-year-old woman with hepatitis C virus infection presents with generalized rash, pruritus, and fever over the past week. The rash appeared on her left arm after she received her fifth weekly injection of pegylated interferon alfa 2b, in combination with daily oral ribavirin (Copegus, Rebetol). Over the course of 3 days, it spread to her face and the rest of her body.
Q: What is the most likely clinical diagnosis?
- Stevens-Johnson syndrome
- Mixed cryoglobulinemia
- Acute eczematous drug eruption
- Lichen planus
A: Acute eczematous drug eruption is the most likely diagnosis.
The clinical presentation and laboratory findings suggest (the latter by exclusion) that our patient had an allergic drug reaction to the interferon or to the ribavirin therapy, or to both. Although this combination is a standard treatment for chronic hepatitis C, some patients experience adverse reactions that lead to its discontinuation. Local injection-site reactions are the most prevalent, affecting up to 12% of patients, whereas eczematous dermatoses manifest less commonly, occurring in up to 5% of patients.1
While awaiting the results of skin biopsy, a careful evaluation of the clinical features of the physical examination and an appropriate laboratory evaluation can rule out other important conditions in the differential diagnosis.
The absence of mucous membrane involvement steers the diagnosis away from Stevens-Johnson syndrome, a life-threatening hypersensitivity condition often triggered by drugs, malignant tumors, and viral infections, which may also affect internal organs. In this condition, skin biopsy specimens would be distinguished by subepidermal bullae and epidermal cell necrosis—neither of which was seen in our patient.
Mixed cryoglobulinemia should always be considered in hepatitis C patients because of the strong association between this infection and the development of cryoglobulins. The rash usually is purpuric, but it may be pleomorphic.2,3 This vasculitis often manifests with excess cryoglobulins, elevated rheumatoid factor, and low titers of complement in the blood due to consumption by immune complexes. Tissue biopsy would usually show typical vascular changes if performed on fresh lesions.4,5 The normal levels of these components in our patient coupled with the appearance of her skin makes cryoglobulinemia a less likely cause.
Furthermore, hepatitis C infection, whether or not treated with interferon and ribavirin, can cause an onset or recurrence of other dermatologic conditions, notably lichen planus, psoriasis, vitiligo, and systemic lupus erythematosus.1–4
In lichen planus, the rash is often described as flat-topped, pruritic, and violaceous. It may involve the extremities, the genitalia, and the oral cavity.4,5 The difference in quality of the rash compared with the rash in our patient makes lichen planus less likely.
Exclusion of the other conditions in the differential diagnosis, in addition to results from a definitive punch biopsy, solidified the diagnosis in our patient. Skin biopsy of the patient’s lower-extremity lesions revealed spongiotic dermatitis with lymphocytes, neutrophils, and few eosinophils—a finding characteristic of an acute eczematous drug eruption. Improvement of her rash after discontinuation of interferon and ribavirin further supported this conclusion, although it was unclear whether one or both agents were responsible.
OUTCOME
Management of acute eczematous drug eruption entails stopping the offending drug and alleviating the symptoms. Our patient’s non-life-threatening rash improved dramatically with cessation of interferon and ribavirin. She received a single dose of a systemic corticosteroid initially, out of concern for a severe medication-induced reaction (ie, Stevens-Johnson syndrome), but she was otherwise maintained with diphenhydramine (Benadryl) and a multivitamin ointment for the rash throughout her 9-day hospital stay. Her pruritus was well controlled with hydroxyzine (Atarax, Vistaril). At discharge, she was referred back to her hepatologist for further treatment of her hepatitis C, possibly with interferon and ribavirin again.
TAKE-HOME MESSAGE
Adverse reactions to interferon and ribavirin treatment in hepatitis C patients can manifest dermatologically, and the combination therapy should be discontinued to prevent further insult. A broad variety of conditions in the differential diagnosis should be taken into account, but dermatologic conditions that occur or recur specifically in hepatitis C patients should be considered as well.
- Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 2002; 147:1142–1146.
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13.
- Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010; 22:967–972.
- Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009; 145:1040–1047.
- Aamir S, Ullah Z, Iqbal Z, Khan AA, Yaqub F, Malik K. Cutaneous manifestations of interferon alfa and ribavirin for hepatitis C. J Pak Assoc Dermatol 2008; 18:14–20.
- Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 2002; 147:1142–1146.
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13.
- Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010; 22:967–972.
- Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009; 145:1040–1047.
- Aamir S, Ullah Z, Iqbal Z, Khan AA, Yaqub F, Malik K. Cutaneous manifestations of interferon alfa and ribavirin for hepatitis C. J Pak Assoc Dermatol 2008; 18:14–20.