User login
UPDATE ON FERTILITY
Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care. Dr. Abusief reports no financial relationships relevant to this article.
The field of reproductive endocrinology has advanced at warp speed over the past few decades—and shows no sign of stopping any time soon. In this article, we outline noteworthy developments of the past year:
- publication of two important Committee Opinions from the American Society for Reproductive Medicine (ASRM)—one of them on the need to reduce the rate of multiple gestation among women undergoing treatment for infertility and the other focusing on a method of achieving this goal: elective single embryo transfer
- two studies of vitrification for cryopreservation of embryos and oocytes
- a trio of investigations into the utility of anti-Müllerian hormone as a means of assessing ovarian reserve and reproductive potential.
Goal of non-ART infertility therapy should be to produce a single child
Practice Committee of the American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion [published online ahead of print December 20, 2011]. Fertil Steril. doi:10.1016/j.fertnstert.2011.11.048.
The goal of infertility treatment is for each patient to have one healthy child at a time, according to a new Practice Committee Opinion from the American Society for Reproductive Medicine (ASRM).
In women who experience oligo-ovulation or anovulation, ovulation induction is typically offered. For ovulatory women who have unexplained or age-related infertility, the treatment often is controlled ovarian stimulation. Either intervention can lead to ovulation from multiple follicles and, ultimately, increase the risk of multiple gestation.
Multiple gestation increases maternal morbidity and both fetal and neonatal morbidity and mortality. Most of the poor perinatal outcomes relate directly to preterm birth. Treatment of women who have infertility, therefore, requires achieving a balance between two competing needs:
- maximizing the probability of pregnancy
- minimizing the risk of multiple (two fetuses or more) or high-order multiple (more than two fetuses) gestation.
Many multiple births are iatrogenic
Approximately 60% of twin births result from natural conception, 30% from ovulation induction and controlled ovarian stimulation, and 10% from assisted reproductive technologies (ART). For high-order multiple gestation, the figures are 20% for natural conception, 50% for ovulation induction and controlled ovarian stimulation, and 30% for ART. These statistics reveal that a very large percentage of multiple births are iatrogenic, with fertility treatment increasing the risk of twins by a factor of approximately 20 and the risk of high-order multiples by a factor of more than 100. The risk of monozygotic twinning also increases by a factor of 2 or 3 after ovulation induction, compared with natural conception.
Triplets should be a rarity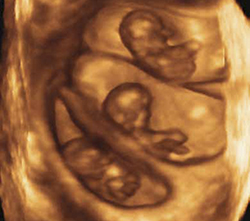
Three-dimensional sonogram of triplets.
Multiple gestation is expensive
The economic costs associated with excess perinatal and maternal morbidity are substantial. They include the immediate costs associated with maternal hospitalization and neonatal intensive care and lifetime costs associated with care for chronic illness, rehabilitation, and special education. Although these costs might be offset by the productivity of individuals, the overall benefit to society is clearly greater when a singleton is born. Personal and familial nonfinancial costs of morbidity and mortality can also be significant.
A sense of urgency on the part of the patient may contribute to an increased risk of multiple gestation by prompting more aggressive treatment. Other contributors include limited health coverage, which creates a personal financial burden, and inadequate patient education about the risks of multiple gestation.
Strategies for limiting the risk of multiple gestation
Appropriate treatment goals are the foundation of risk-reducing strategies. For example, ovulation induction in women who have oligo-ovulation or anovulation should aim toward producing a single oocyte. These women tend to respond to lower dosages of ovarian-stimulation drugs than are typically given. Therefore, women undergoing ovulation induction should receive a lower dosage of gonadotropins and be monitored very carefully for the number of developing follicles and ovarian hyperstimulation syndrome.
In contrast, the goal of controlled ovarian stimulation in ovulatory women who have unexplained or age-related subfertility is to stimulate the development and ovulation of more than one mature follicle to increase cycle fecundity.
Regrettably, efforts have failed to identify estradiol levels and the specific size and number of follicles that prevent multiple gestation. The most likely reason is that follicular size cannot accurately predict the maturity of the oocyte within—follicles as small as 10 mm sometimes yield mature and fertilizable oocytes. Moreover, the population that undergoes ovulation induction or controlled ovarian stimulation is very heterogenous. Therefore, it is not possible to propose valid guidelines to reduce the rate of multiple gestation.
Nevertheless, multiple gestation is sufficiently problematic that we recommend some strategies to reduce its incidence:
- Use low-dosage gonadotropin stimulation with careful monitoring, and limit the number of follicles that are roughly 15 mm or larger to two in patients 37 years of age or younger; three in patient 38 to 40 years old; and more in patients older than 40
- Develop specific cancellation criteria, which should be explained to and accepted by patients undergoing controlled ovarian stimulation. Gonadotropin-releasing hormone (GnRH) antagonists may be of benefit.1
- When clomiphene citrate stimulates the development of two or more mature follicles, outcomes do not differ from those obtained with controlled ovarian stimulation using gonadotropins and intrauterine insemination (IUI).2 Therefore, a reasonable strategy in many patients is to consider initiating treatment with clomiphene citrate and IUI and to proceed directly to in vitro fertilization (IVF) when treatment fails, thereby avoiding controlled ovarian stimulation altogether.3
- Pre-ovulatory ultrasonography-guided aspiration of excess follicles to reduce the risk of multiple gestation has potential benefit but needs further study.
Overall, regardless of the medication or regimen employed, it may not be possible to entirely eliminate the risk of multiple gestation associated with ovulation induction or controlled ovarian stimulation.
When to consider gestation reduction
High-order multifetal gestation reduction has been utilized as a strategy to reduce complications associated with ovulation induction and controlled ovarian stimulation, but use of this technology must be regarded as an adverse outcome of infertility treatment. Overall, data suggest that multifetal gestation reduction is associated with a reduced risk of prematurity, although its true benefit is difficult to elucidate due to potential bias in the interpretation of data. A small percentage of patients lose the entire pregnancy, and the procedure can present patients with a profound ethical dilemma and psychological trauma. Thorough counseling is imperative.
Despite feelings of loss and guilt that persist for a year or longer, most patients report that they would make the decision to undergo gestation reduction again if a similar situation arose in the future.4
The procedure should be performed only in a specialized center by an experienced practitioner.
When performing ovulation induction and controlled ovarian stimulation, use the lowest dose of drug necessary to obtain a single mature follicle in anovulatory women, two follicles in young ovulatory women, and three follicles in women 38 to 40 years old. Because of the high risk of multiple gestation associated with controlled ovarian stimulation followed by IUI, consider moving directly to IVF after use of clomiphene citrate and IUI.
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Elective single-embryo transfer [published online ahead of print December 22, 2011]. Fertil Steril. doi:10.1016/j.fertnstert.2011.11.050.
As IVF implantation rates have improved, the practice of transferring multiple embryos has resulted in a much-increased pregnancy rate but also a high percentage of multiple gestations. Elective single embryo transfer (eSET) has been advocated as the only effective means to avoid multiple pregnancy in IVF cycles, but there is significant concern that it might ultimately reduce the pregnancy rate.
ASRM recently published a Practice Committee Opinion that offers guidance for patient selection and describes barriers to eSET. Patient selection is critical.
Utilization of eSET in the United States has increased over the past decade but still lags behind other countries. Use of double embryo transfer (DET) has increased, significantly reducing the likelihood of high-order multiple pregnancies associated with ART but producing no change in the twin pregnancy rate (FIGURE). Randomized, controlled trials and other studies have demonstrated that the cumulative pregnancy rate per retrieval is no different for eSET followed by frozen embryo transfer than it is for DET in properly selected patients.
Most transfers involve two embryos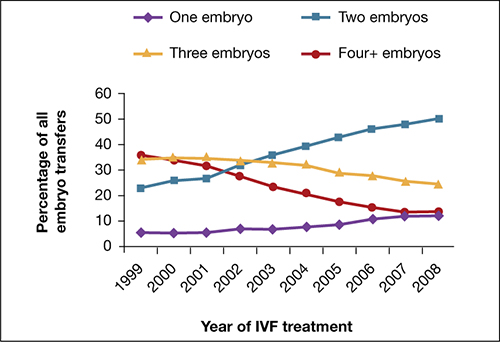
Percentage of transfer of one, two, three, or four or more embryos among all in vitro fertilization cycles performed in the United States, 1999–2008.
SOURCE: ASRM. Reproduced with permission.eSET is most appropriate for women who have a good prognosis:
- age younger than 35 years
- >1 top-quality embryo available for transfer
- first or second treatment cycle
- prior successful IVF
- recipients of embryos from donated eggs.
Women 35 to 40 years old can be considered for eSET if they have top-quality, blastocyst-stage embryos available for transfer.
Barriers to eSET include a lack of provider and patient education about it, financial considerations, embryo selection, and successful cryopreservation. When insurance coverage or refund guarantees are available, patient acceptance of eSET increases.
Elective single embryo transfer is the only ART embryo transfer strategy that will reduce the twin pregnancy rate. However, it is not a good approach for all patients and must be carefully utilized in selected patients who have a good prognosis.
Vitrification for cryopreservation of embryos appears to be superior to slow freezing
Leibo S, Pool T. The principal variables of cryopreservation: solutions, temperatures, and rate changes. Fertil Steril. 2011;96(2):269–276.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–285.
Cryopreservation is a method by which cells are suspended in a solution of salts and low-molecular-weight organic compound, cooled to subzero temperatures (approximately –196°C) in liquid nitrogen, stored, and then rewarmed. Cryopreservation has become a major component of the practice of assisted reproduction, with more than 37,000 pregnancies produced from cryopreserved embryos from 2005 through 2009 in the United States alone.5,6
Standard (slow) freezing methods for embryo cryopreservation involve suspension of the embryos in a 10% solution of propylene glycol supplemented with 3.4% sucrose, cooling them to –35°C at a rate of 0.3°C/min, submerging them in liquid nitrogen for storage, and rewarming the frozen embryos at a rate of approximately 300°C/min to thaw them.5
A major advance in the science of cryopreservation is the use of vitrification, a method of freezing in which the embryos are equilibrated with a 10% or 15% solution of cryoprotectant and then exposed briefly (30–60 seconds) to a 20% to 40% solution of cryoprotectant to achieve relative cellular dehydration. The embryos are then placed in a storage container and submerged in liquid nitrogen. During vitrification, embryos can be cooled at a rate exceeding 1,000°C/min. Vitrified embryos are stored at approximately –196°C and thawed in ultra-rapid fashion.
The development of vitrification methods has significantly advanced the technology of oocyte cryopreservation, which has been utilized for:
- preservation of fertility in cancer patients
- social reasons (e.g., lack of a partner)
- egg-donation programs
- minimization of the risk of ovarian hyperstimulation syndrome
- storage of surplus eggs when embryo cryopreservation is not feasible.
Cobo and Diaz recently conducted a systematic review and meta-analysis of randomized, controlled trials of oocyte vitrification. They found that the potential for fertilization, embryogenesis, and pregnancy from oocytes that had undergone vitrification and warming was not significantly different from the potential for fresh oocytes and was better than the potential for oocytes that had undergone freezing and thawing from standard freezing cycles.
Although the findings of the meta-analysis were limited by the small number of studies and possible selection bias, an increasing body of evidence supports the use of vitrification for cryopreservation of oocytes. Large-scale controlled trials are needed. Until they are performed, the findings of the meta-analysis should be interpreted with caution.
Newer ultra-rapid freezing of oocytes and embryos using vitrification appears to produce results that are superior to those obtained with traditional slow freezing. Large randomized, controlled trials are needed to confirm the improved efficacy of vitrification.
Anti-Müllerian hormone is an informative test of ovarian reserve—but lacks a nod from the FDA
Buyuk E, Seifer D, Younger J, Grazi R, Lieman H. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369–2372.
Li H, Yeung PW, Lau E, Ho PC, Ng EH. Evaluating the performance of serum anti-Müllerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94(6):2177–2181.
Lee J, Kim S, Jee B, Suh C, Kim KC, Moon SY. Anti-Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95(8):2602–2604.
Although it is well understood that both the quantity and quality of oocytes decline with age, the assessment of ovarian reserve continues to be a clinical challenge. Accurate evaluation can predict a woman’s response to infertility treatment, including IVF, and estimate her chance of conception. Noninvasive tests of ovarian reserve are a critical component of any evaluation of fertility. Although a woman’s age is the single most important historical factor in the assessment of reproductive capacity, there is significant variation in ovarian aging among women.
Historically, age, antral follicle count (AFC), and measurement of cycle day 3 follicle-stimulating hormone (FSH) and estradiol (E2) levels have been the most widely used measures of ovarian reserve, but mounting evidence suggests that assessment of the anti-Müllerian hormone (AMH) level may be even more informative.
AMH, also known as Müllerian-inhibiting substance, is a dimeric glycoprotein. A member of the transforming growth factor–ß family, AMH is closely related to inhibin and activin and is secreted by granulosa cells of preantral and small antral follicles in post-pubertal females.7 AMH aids in the coordination of ovarian follicular development by inhibiting recruitment of additional primordial follicles and decreasing the sensitivity of preantral and small antral follicles to FSH.8,9
AMH levels, measurable in serum, decline with age and are undetectable after menopause.10 Unlike FSH, which fluctuates during the menstrual cycle, AMH exhibits minimal intercycle and intracycle variation. The AMH level remains stable in women taking oral contraceptives and even in women who are pregnant.11
AMH is independently and significantly correlated with the ovarian response to gonadotropin therapy, with decreased levels of AMH associated with a poor response, and increased levels associated with a strong response.12 In the first cycle of IVF, an elevated AMH level has been associated with excessive response to gonadotropins and an increased risk of ovarian hyperstimulation syndrome (OHSS), independent of age and the presence of polycystic ovary syndrome.12
In a recent study of women who had an elevated FSH level and were undergoing IVF, the AMH level was strongly associated with the number of oocytes retrieved.13 Women who had an elevated FSH level but a serum AMH level of 0.6 ng/mL or above had a greater number of oocytes and day-3 embryos retrieved; they also had a lower cancellation rate than women who had a lower AMH level.13
Although no single test can predict the outcome of treatment for infertility, AMH concentrations are significantly higher in women who have a live birth (from the first cycle of stimulated IUI or after three cycles) than in women who do not.14
Two ELISA kits, one value?
Two types of enzyme-linked immunosorbent assay (ELISA) kits are commercially available for measurement of the AMH level: one from Immunotech Beckman Coulter and the other from Diagnostic Systems Laboratories. Neither kit has been approved for clinical use by the US Food and Drug Administration.
Studies comparing the values obtained using each kit have been inconsistent, generating controversy about the measurement of AMH. A recent study of women who were undergoing controlled ovarian stimulation found that the AMH levels obtained by the two kits were similar and significantly correlated with each other.15 In that study, the AMH level was measured on the day before gonadotropin administration or on the day of oocyte retrieval.15 In addition, the AMH concentrations measured by both kits were significantly associated with age, basal FSH levels, AFC, and the outcome of controlled ovarian stimulation.15 The authors concluded:
- The two commercially available kits provide reliable and similar results.
- The AMH level measured by either kit can predict the outcome of controlled ovarian stimulation, with similar reference values.15
Measurement of the AMH level can be an informative aspect of the evaluation of a patient’s fertility, as well as a valuable tool in the assessment of ovarian reserve. The AMH level can also help clinicians identify the appropriate dose of gonadotropins and predict which patients might be likely to over- or under-respond to stimulation—ultimately reducing the length and cost of treatment. Knowledge of the patient’s AMH level might inform pretreatment counseling and help women achieve reasonable expectations.
AMH is a useful test to help predict a patient’s response to ovarian stimulation and her chances of achieving pregnancy. However, AMH is only one measure of ovarian reserve and should not be used alone as a reason to exclude patients from treatment. In our practice, we use the AMH level along with cycle day 3 antral follicle count and FSH and estradiol levels.
We want to hear from you! Tell us what you think.
1. Ragni G, Caliari I, Nicolosi AE, Arnoldi M, Somigliana E, Crosignani PG. Preventing high-order multiple pregnancies during controlled ovarian hyperstimulation and intrauterine insemination: 3 years’ experience using low-dose recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2006;85(3):619-624.
2. Ghesquiere SL, Castelain EG, Spiessens C, et al. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauterine insemination. Am J Obstet Gynecol. 2007;197(6):589.e1-5.
3. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94(3):888-899.
4. Schreiner-Engel P, Walther VN, Mindes J, et al. First-trimester multifetal pregnancy reduction: acute and persistent psychologic reactions. Am J Obstet Gynecol. 1995;172(2 Pt 1):541.-
5. Leibo S, Pool T. The principal variables of cryopreservation: solutions temperatures, and rate changes. Fertil Steril. 2011;96(2):269-276.
6. Society for Assisted Reproductive Technology; American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2001 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology registry. Fertil Steril. 2007;87(6):1253-1266.
7. Vigier JA, Picard JY, Tran D, Legeai L, Josso N. Production of anti-Müllerian hormone: another homology between Sertoli and granulosa cells. Endocrinology. 1984;114(4):1315-1320.
8. Durlinger All, Gruijters MJG, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891-4899.
9. Salmon NA, Handyside AH, Joyce IM. Oocyte regulation and anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201-208.
10. Shin SY, Lee JR, Noh GW, et al. Analysis of serum levels of anti-Müllerian hormone, inhibin B, insulin-like growth factor-I, insulin-like growth factor binding protein-3, and follicle-stimulating hormone with respect to age and menopausal status. J Korean Med Sci. 2008;23(1):104-110.
11. Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, de Ziegler D. Clinical uses of anti-Müllerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91(1):226-230.
12. Nardo L, Gelbaya T, Wilkinson H, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586-1593.
13. Buyuk E, Seifer D, Younger J, Grazi R, Lieman H. Random anti-Müllerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369-2372.
14. Li HW, Yeung PW, Lau E&, Ho PC, Ng EH. Evaluating the performance of serum anti-Müllerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94(6):2177-2181.
15. Lee J, Kim S, Jee B, Suh C, Kim KC, Moon SY. Anti- Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95(8):2602-2604.
Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care. Dr. Abusief reports no financial relationships relevant to this article.
The field of reproductive endocrinology has advanced at warp speed over the past few decades—and shows no sign of stopping any time soon. In this article, we outline noteworthy developments of the past year:
- publication of two important Committee Opinions from the American Society for Reproductive Medicine (ASRM)—one of them on the need to reduce the rate of multiple gestation among women undergoing treatment for infertility and the other focusing on a method of achieving this goal: elective single embryo transfer
- two studies of vitrification for cryopreservation of embryos and oocytes
- a trio of investigations into the utility of anti-Müllerian hormone as a means of assessing ovarian reserve and reproductive potential.
Goal of non-ART infertility therapy should be to produce a single child
Practice Committee of the American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion [published online ahead of print December 20, 2011]. Fertil Steril. doi:10.1016/j.fertnstert.2011.11.048.
The goal of infertility treatment is for each patient to have one healthy child at a time, according to a new Practice Committee Opinion from the American Society for Reproductive Medicine (ASRM).
In women who experience oligo-ovulation or anovulation, ovulation induction is typically offered. For ovulatory women who have unexplained or age-related infertility, the treatment often is controlled ovarian stimulation. Either intervention can lead to ovulation from multiple follicles and, ultimately, increase the risk of multiple gestation.
Multiple gestation increases maternal morbidity and both fetal and neonatal morbidity and mortality. Most of the poor perinatal outcomes relate directly to preterm birth. Treatment of women who have infertility, therefore, requires achieving a balance between two competing needs:
- maximizing the probability of pregnancy
- minimizing the risk of multiple (two fetuses or more) or high-order multiple (more than two fetuses) gestation.
Many multiple births are iatrogenic
Approximately 60% of twin births result from natural conception, 30% from ovulation induction and controlled ovarian stimulation, and 10% from assisted reproductive technologies (ART). For high-order multiple gestation, the figures are 20% for natural conception, 50% for ovulation induction and controlled ovarian stimulation, and 30% for ART. These statistics reveal that a very large percentage of multiple births are iatrogenic, with fertility treatment increasing the risk of twins by a factor of approximately 20 and the risk of high-order multiples by a factor of more than 100. The risk of monozygotic twinning also increases by a factor of 2 or 3 after ovulation induction, compared with natural conception.
Triplets should be a rarity
Three-dimensional sonogram of triplets.
Multiple gestation is expensive
The economic costs associated with excess perinatal and maternal morbidity are substantial. They include the immediate costs associated with maternal hospitalization and neonatal intensive care and lifetime costs associated with care for chronic illness, rehabilitation, and special education. Although these costs might be offset by the productivity of individuals, the overall benefit to society is clearly greater when a singleton is born. Personal and familial nonfinancial costs of morbidity and mortality can also be significant.
A sense of urgency on the part of the patient may contribute to an increased risk of multiple gestation by prompting more aggressive treatment. Other contributors include limited health coverage, which creates a personal financial burden, and inadequate patient education about the risks of multiple gestation.
Strategies for limiting the risk of multiple gestation
Appropriate treatment goals are the foundation of risk-reducing strategies. For example, ovulation induction in women who have oligo-ovulation or anovulation should aim toward producing a single oocyte. These women tend to respond to lower dosages of ovarian-stimulation drugs than are typically given. Therefore, women undergoing ovulation induction should receive a lower dosage of gonadotropins and be monitored very carefully for the number of developing follicles and ovarian hyperstimulation syndrome.
In contrast, the goal of controlled ovarian stimulation in ovulatory women who have unexplained or age-related subfertility is to stimulate the development and ovulation of more than one mature follicle to increase cycle fecundity.
Regrettably, efforts have failed to identify estradiol levels and the specific size and number of follicles that prevent multiple gestation. The most likely reason is that follicular size cannot accurately predict the maturity of the oocyte within—follicles as small as 10 mm sometimes yield mature and fertilizable oocytes. Moreover, the population that undergoes ovulation induction or controlled ovarian stimulation is very heterogenous. Therefore, it is not possible to propose valid guidelines to reduce the rate of multiple gestation.
Nevertheless, multiple gestation is sufficiently problematic that we recommend some strategies to reduce its incidence:
- Use low-dosage gonadotropin stimulation with careful monitoring, and limit the number of follicles that are roughly 15 mm or larger to two in patients 37 years of age or younger; three in patient 38 to 40 years old; and more in patients older than 40
- Develop specific cancellation criteria, which should be explained to and accepted by patients undergoing controlled ovarian stimulation. Gonadotropin-releasing hormone (GnRH) antagonists may be of benefit.1
- When clomiphene citrate stimulates the development of two or more mature follicles, outcomes do not differ from those obtained with controlled ovarian stimulation using gonadotropins and intrauterine insemination (IUI).2 Therefore, a reasonable strategy in many patients is to consider initiating treatment with clomiphene citrate and IUI and to proceed directly to in vitro fertilization (IVF) when treatment fails, thereby avoiding controlled ovarian stimulation altogether.3
- Pre-ovulatory ultrasonography-guided aspiration of excess follicles to reduce the risk of multiple gestation has potential benefit but needs further study.
Overall, regardless of the medication or regimen employed, it may not be possible to entirely eliminate the risk of multiple gestation associated with ovulation induction or controlled ovarian stimulation.
When to consider gestation reduction
High-order multifetal gestation reduction has been utilized as a strategy to reduce complications associated with ovulation induction and controlled ovarian stimulation, but use of this technology must be regarded as an adverse outcome of infertility treatment. Overall, data suggest that multifetal gestation reduction is associated with a reduced risk of prematurity, although its true benefit is difficult to elucidate due to potential bias in the interpretation of data. A small percentage of patients lose the entire pregnancy, and the procedure can present patients with a profound ethical dilemma and psychological trauma. Thorough counseling is imperative.
Despite feelings of loss and guilt that persist for a year or longer, most patients report that they would make the decision to undergo gestation reduction again if a similar situation arose in the future.4
The procedure should be performed only in a specialized center by an experienced practitioner.
When performing ovulation induction and controlled ovarian stimulation, use the lowest dose of drug necessary to obtain a single mature follicle in anovulatory women, two follicles in young ovulatory women, and three follicles in women 38 to 40 years old. Because of the high risk of multiple gestation associated with controlled ovarian stimulation followed by IUI, consider moving directly to IVF after use of clomiphene citrate and IUI.
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Elective single-embryo transfer [published online ahead of print December 22, 2011]. Fertil Steril. doi:10.1016/j.fertnstert.2011.11.050.
As IVF implantation rates have improved, the practice of transferring multiple embryos has resulted in a much-increased pregnancy rate but also a high percentage of multiple gestations. Elective single embryo transfer (eSET) has been advocated as the only effective means to avoid multiple pregnancy in IVF cycles, but there is significant concern that it might ultimately reduce the pregnancy rate.
ASRM recently published a Practice Committee Opinion that offers guidance for patient selection and describes barriers to eSET. Patient selection is critical.
Utilization of eSET in the United States has increased over the past decade but still lags behind other countries. Use of double embryo transfer (DET) has increased, significantly reducing the likelihood of high-order multiple pregnancies associated with ART but producing no change in the twin pregnancy rate (FIGURE). Randomized, controlled trials and other studies have demonstrated that the cumulative pregnancy rate per retrieval is no different for eSET followed by frozen embryo transfer than it is for DET in properly selected patients.
Most transfers involve two embryos
Percentage of transfer of one, two, three, or four or more embryos among all in vitro fertilization cycles performed in the United States, 1999–2008.
SOURCE: ASRM. Reproduced with permission.eSET is most appropriate for women who have a good prognosis:
- age younger than 35 years
- >1 top-quality embryo available for transfer
- first or second treatment cycle
- prior successful IVF
- recipients of embryos from donated eggs.
Women 35 to 40 years old can be considered for eSET if they have top-quality, blastocyst-stage embryos available for transfer.
Barriers to eSET include a lack of provider and patient education about it, financial considerations, embryo selection, and successful cryopreservation. When insurance coverage or refund guarantees are available, patient acceptance of eSET increases.
Elective single embryo transfer is the only ART embryo transfer strategy that will reduce the twin pregnancy rate. However, it is not a good approach for all patients and must be carefully utilized in selected patients who have a good prognosis.
Vitrification for cryopreservation of embryos appears to be superior to slow freezing
Leibo S, Pool T. The principal variables of cryopreservation: solutions, temperatures, and rate changes. Fertil Steril. 2011;96(2):269–276.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–285.
Cryopreservation is a method by which cells are suspended in a solution of salts and low-molecular-weight organic compound, cooled to subzero temperatures (approximately –196°C) in liquid nitrogen, stored, and then rewarmed. Cryopreservation has become a major component of the practice of assisted reproduction, with more than 37,000 pregnancies produced from cryopreserved embryos from 2005 through 2009 in the United States alone.5,6
Standard (slow) freezing methods for embryo cryopreservation involve suspension of the embryos in a 10% solution of propylene glycol supplemented with 3.4% sucrose, cooling them to –35°C at a rate of 0.3°C/min, submerging them in liquid nitrogen for storage, and rewarming the frozen embryos at a rate of approximately 300°C/min to thaw them.5
A major advance in the science of cryopreservation is the use of vitrification, a method of freezing in which the embryos are equilibrated with a 10% or 15% solution of cryoprotectant and then exposed briefly (30–60 seconds) to a 20% to 40% solution of cryoprotectant to achieve relative cellular dehydration. The embryos are then placed in a storage container and submerged in liquid nitrogen. During vitrification, embryos can be cooled at a rate exceeding 1,000°C/min. Vitrified embryos are stored at approximately –196°C and thawed in ultra-rapid fashion.
The development of vitrification methods has significantly advanced the technology of oocyte cryopreservation, which has been utilized for:
- preservation of fertility in cancer patients
- social reasons (e.g., lack of a partner)
- egg-donation programs
- minimization of the risk of ovarian hyperstimulation syndrome
- storage of surplus eggs when embryo cryopreservation is not feasible.
Cobo and Diaz recently conducted a systematic review and meta-analysis of randomized, controlled trials of oocyte vitrification. They found that the potential for fertilization, embryogenesis, and pregnancy from oocytes that had undergone vitrification and warming was not significantly different from the potential for fresh oocytes and was better than the potential for oocytes that had undergone freezing and thawing from standard freezing cycles.
Although the findings of the meta-analysis were limited by the small number of studies and possible selection bias, an increasing body of evidence supports the use of vitrification for cryopreservation of oocytes. Large-scale controlled trials are needed. Until they are performed, the findings of the meta-analysis should be interpreted with caution.
Newer ultra-rapid freezing of oocytes and embryos using vitrification appears to produce results that are superior to those obtained with traditional slow freezing. Large randomized, controlled trials are needed to confirm the improved efficacy of vitrification.
Anti-Müllerian hormone is an informative test of ovarian reserve—but lacks a nod from the FDA
Buyuk E, Seifer D, Younger J, Grazi R, Lieman H. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369–2372.
Li H, Yeung PW, Lau E, Ho PC, Ng EH. Evaluating the performance of serum anti-Müllerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94(6):2177–2181.
Lee J, Kim S, Jee B, Suh C, Kim KC, Moon SY. Anti-Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95(8):2602–2604.
Although it is well understood that both the quantity and quality of oocytes decline with age, the assessment of ovarian reserve continues to be a clinical challenge. Accurate evaluation can predict a woman’s response to infertility treatment, including IVF, and estimate her chance of conception. Noninvasive tests of ovarian reserve are a critical component of any evaluation of fertility. Although a woman’s age is the single most important historical factor in the assessment of reproductive capacity, there is significant variation in ovarian aging among women.
Historically, age, antral follicle count (AFC), and measurement of cycle day 3 follicle-stimulating hormone (FSH) and estradiol (E2) levels have been the most widely used measures of ovarian reserve, but mounting evidence suggests that assessment of the anti-Müllerian hormone (AMH) level may be even more informative.
AMH, also known as Müllerian-inhibiting substance, is a dimeric glycoprotein. A member of the transforming growth factor–ß family, AMH is closely related to inhibin and activin and is secreted by granulosa cells of preantral and small antral follicles in post-pubertal females.7 AMH aids in the coordination of ovarian follicular development by inhibiting recruitment of additional primordial follicles and decreasing the sensitivity of preantral and small antral follicles to FSH.8,9
AMH levels, measurable in serum, decline with age and are undetectable after menopause.10 Unlike FSH, which fluctuates during the menstrual cycle, AMH exhibits minimal intercycle and intracycle variation. The AMH level remains stable in women taking oral contraceptives and even in women who are pregnant.11
AMH is independently and significantly correlated with the ovarian response to gonadotropin therapy, with decreased levels of AMH associated with a poor response, and increased levels associated with a strong response.12 In the first cycle of IVF, an elevated AMH level has been associated with excessive response to gonadotropins and an increased risk of ovarian hyperstimulation syndrome (OHSS), independent of age and the presence of polycystic ovary syndrome.12
In a recent study of women who had an elevated FSH level and were undergoing IVF, the AMH level was strongly associated with the number of oocytes retrieved.13 Women who had an elevated FSH level but a serum AMH level of 0.6 ng/mL or above had a greater number of oocytes and day-3 embryos retrieved; they also had a lower cancellation rate than women who had a lower AMH level.13
Although no single test can predict the outcome of treatment for infertility, AMH concentrations are significantly higher in women who have a live birth (from the first cycle of stimulated IUI or after three cycles) than in women who do not.14
Two ELISA kits, one value?
Two types of enzyme-linked immunosorbent assay (ELISA) kits are commercially available for measurement of the AMH level: one from Immunotech Beckman Coulter and the other from Diagnostic Systems Laboratories. Neither kit has been approved for clinical use by the US Food and Drug Administration.
Studies comparing the values obtained using each kit have been inconsistent, generating controversy about the measurement of AMH. A recent study of women who were undergoing controlled ovarian stimulation found that the AMH levels obtained by the two kits were similar and significantly correlated with each other.15 In that study, the AMH level was measured on the day before gonadotropin administration or on the day of oocyte retrieval.15 In addition, the AMH concentrations measured by both kits were significantly associated with age, basal FSH levels, AFC, and the outcome of controlled ovarian stimulation.15 The authors concluded:
- The two commercially available kits provide reliable and similar results.
- The AMH level measured by either kit can predict the outcome of controlled ovarian stimulation, with similar reference values.15
Measurement of the AMH level can be an informative aspect of the evaluation of a patient’s fertility, as well as a valuable tool in the assessment of ovarian reserve. The AMH level can also help clinicians identify the appropriate dose of gonadotropins and predict which patients might be likely to over- or under-respond to stimulation—ultimately reducing the length and cost of treatment. Knowledge of the patient’s AMH level might inform pretreatment counseling and help women achieve reasonable expectations.
AMH is a useful test to help predict a patient’s response to ovarian stimulation and her chances of achieving pregnancy. However, AMH is only one measure of ovarian reserve and should not be used alone as a reason to exclude patients from treatment. In our practice, we use the AMH level along with cycle day 3 antral follicle count and FSH and estradiol levels.
We want to hear from you! Tell us what you think.
Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care. Dr. Abusief reports no financial relationships relevant to this article.
The field of reproductive endocrinology has advanced at warp speed over the past few decades—and shows no sign of stopping any time soon. In this article, we outline noteworthy developments of the past year:
- publication of two important Committee Opinions from the American Society for Reproductive Medicine (ASRM)—one of them on the need to reduce the rate of multiple gestation among women undergoing treatment for infertility and the other focusing on a method of achieving this goal: elective single embryo transfer
- two studies of vitrification for cryopreservation of embryos and oocytes
- a trio of investigations into the utility of anti-Müllerian hormone as a means of assessing ovarian reserve and reproductive potential.
Goal of non-ART infertility therapy should be to produce a single child
Practice Committee of the American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion [published online ahead of print December 20, 2011]. Fertil Steril. doi:10.1016/j.fertnstert.2011.11.048.
The goal of infertility treatment is for each patient to have one healthy child at a time, according to a new Practice Committee Opinion from the American Society for Reproductive Medicine (ASRM).
In women who experience oligo-ovulation or anovulation, ovulation induction is typically offered. For ovulatory women who have unexplained or age-related infertility, the treatment often is controlled ovarian stimulation. Either intervention can lead to ovulation from multiple follicles and, ultimately, increase the risk of multiple gestation.
Multiple gestation increases maternal morbidity and both fetal and neonatal morbidity and mortality. Most of the poor perinatal outcomes relate directly to preterm birth. Treatment of women who have infertility, therefore, requires achieving a balance between two competing needs:
- maximizing the probability of pregnancy
- minimizing the risk of multiple (two fetuses or more) or high-order multiple (more than two fetuses) gestation.
Many multiple births are iatrogenic
Approximately 60% of twin births result from natural conception, 30% from ovulation induction and controlled ovarian stimulation, and 10% from assisted reproductive technologies (ART). For high-order multiple gestation, the figures are 20% for natural conception, 50% for ovulation induction and controlled ovarian stimulation, and 30% for ART. These statistics reveal that a very large percentage of multiple births are iatrogenic, with fertility treatment increasing the risk of twins by a factor of approximately 20 and the risk of high-order multiples by a factor of more than 100. The risk of monozygotic twinning also increases by a factor of 2 or 3 after ovulation induction, compared with natural conception.
Triplets should be a rarity
Three-dimensional sonogram of triplets.
Multiple gestation is expensive
The economic costs associated with excess perinatal and maternal morbidity are substantial. They include the immediate costs associated with maternal hospitalization and neonatal intensive care and lifetime costs associated with care for chronic illness, rehabilitation, and special education. Although these costs might be offset by the productivity of individuals, the overall benefit to society is clearly greater when a singleton is born. Personal and familial nonfinancial costs of morbidity and mortality can also be significant.
A sense of urgency on the part of the patient may contribute to an increased risk of multiple gestation by prompting more aggressive treatment. Other contributors include limited health coverage, which creates a personal financial burden, and inadequate patient education about the risks of multiple gestation.
Strategies for limiting the risk of multiple gestation
Appropriate treatment goals are the foundation of risk-reducing strategies. For example, ovulation induction in women who have oligo-ovulation or anovulation should aim toward producing a single oocyte. These women tend to respond to lower dosages of ovarian-stimulation drugs than are typically given. Therefore, women undergoing ovulation induction should receive a lower dosage of gonadotropins and be monitored very carefully for the number of developing follicles and ovarian hyperstimulation syndrome.
In contrast, the goal of controlled ovarian stimulation in ovulatory women who have unexplained or age-related subfertility is to stimulate the development and ovulation of more than one mature follicle to increase cycle fecundity.
Regrettably, efforts have failed to identify estradiol levels and the specific size and number of follicles that prevent multiple gestation. The most likely reason is that follicular size cannot accurately predict the maturity of the oocyte within—follicles as small as 10 mm sometimes yield mature and fertilizable oocytes. Moreover, the population that undergoes ovulation induction or controlled ovarian stimulation is very heterogenous. Therefore, it is not possible to propose valid guidelines to reduce the rate of multiple gestation.
Nevertheless, multiple gestation is sufficiently problematic that we recommend some strategies to reduce its incidence:
- Use low-dosage gonadotropin stimulation with careful monitoring, and limit the number of follicles that are roughly 15 mm or larger to two in patients 37 years of age or younger; three in patient 38 to 40 years old; and more in patients older than 40
- Develop specific cancellation criteria, which should be explained to and accepted by patients undergoing controlled ovarian stimulation. Gonadotropin-releasing hormone (GnRH) antagonists may be of benefit.1
- When clomiphene citrate stimulates the development of two or more mature follicles, outcomes do not differ from those obtained with controlled ovarian stimulation using gonadotropins and intrauterine insemination (IUI).2 Therefore, a reasonable strategy in many patients is to consider initiating treatment with clomiphene citrate and IUI and to proceed directly to in vitro fertilization (IVF) when treatment fails, thereby avoiding controlled ovarian stimulation altogether.3
- Pre-ovulatory ultrasonography-guided aspiration of excess follicles to reduce the risk of multiple gestation has potential benefit but needs further study.
Overall, regardless of the medication or regimen employed, it may not be possible to entirely eliminate the risk of multiple gestation associated with ovulation induction or controlled ovarian stimulation.
When to consider gestation reduction
High-order multifetal gestation reduction has been utilized as a strategy to reduce complications associated with ovulation induction and controlled ovarian stimulation, but use of this technology must be regarded as an adverse outcome of infertility treatment. Overall, data suggest that multifetal gestation reduction is associated with a reduced risk of prematurity, although its true benefit is difficult to elucidate due to potential bias in the interpretation of data. A small percentage of patients lose the entire pregnancy, and the procedure can present patients with a profound ethical dilemma and psychological trauma. Thorough counseling is imperative.
Despite feelings of loss and guilt that persist for a year or longer, most patients report that they would make the decision to undergo gestation reduction again if a similar situation arose in the future.4
The procedure should be performed only in a specialized center by an experienced practitioner.
When performing ovulation induction and controlled ovarian stimulation, use the lowest dose of drug necessary to obtain a single mature follicle in anovulatory women, two follicles in young ovulatory women, and three follicles in women 38 to 40 years old. Because of the high risk of multiple gestation associated with controlled ovarian stimulation followed by IUI, consider moving directly to IVF after use of clomiphene citrate and IUI.
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Elective single-embryo transfer [published online ahead of print December 22, 2011]. Fertil Steril. doi:10.1016/j.fertnstert.2011.11.050.
As IVF implantation rates have improved, the practice of transferring multiple embryos has resulted in a much-increased pregnancy rate but also a high percentage of multiple gestations. Elective single embryo transfer (eSET) has been advocated as the only effective means to avoid multiple pregnancy in IVF cycles, but there is significant concern that it might ultimately reduce the pregnancy rate.
ASRM recently published a Practice Committee Opinion that offers guidance for patient selection and describes barriers to eSET. Patient selection is critical.
Utilization of eSET in the United States has increased over the past decade but still lags behind other countries. Use of double embryo transfer (DET) has increased, significantly reducing the likelihood of high-order multiple pregnancies associated with ART but producing no change in the twin pregnancy rate (FIGURE). Randomized, controlled trials and other studies have demonstrated that the cumulative pregnancy rate per retrieval is no different for eSET followed by frozen embryo transfer than it is for DET in properly selected patients.
Most transfers involve two embryos
Percentage of transfer of one, two, three, or four or more embryos among all in vitro fertilization cycles performed in the United States, 1999–2008.
SOURCE: ASRM. Reproduced with permission.eSET is most appropriate for women who have a good prognosis:
- age younger than 35 years
- >1 top-quality embryo available for transfer
- first or second treatment cycle
- prior successful IVF
- recipients of embryos from donated eggs.
Women 35 to 40 years old can be considered for eSET if they have top-quality, blastocyst-stage embryos available for transfer.
Barriers to eSET include a lack of provider and patient education about it, financial considerations, embryo selection, and successful cryopreservation. When insurance coverage or refund guarantees are available, patient acceptance of eSET increases.
Elective single embryo transfer is the only ART embryo transfer strategy that will reduce the twin pregnancy rate. However, it is not a good approach for all patients and must be carefully utilized in selected patients who have a good prognosis.
Vitrification for cryopreservation of embryos appears to be superior to slow freezing
Leibo S, Pool T. The principal variables of cryopreservation: solutions, temperatures, and rate changes. Fertil Steril. 2011;96(2):269–276.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–285.
Cryopreservation is a method by which cells are suspended in a solution of salts and low-molecular-weight organic compound, cooled to subzero temperatures (approximately –196°C) in liquid nitrogen, stored, and then rewarmed. Cryopreservation has become a major component of the practice of assisted reproduction, with more than 37,000 pregnancies produced from cryopreserved embryos from 2005 through 2009 in the United States alone.5,6
Standard (slow) freezing methods for embryo cryopreservation involve suspension of the embryos in a 10% solution of propylene glycol supplemented with 3.4% sucrose, cooling them to –35°C at a rate of 0.3°C/min, submerging them in liquid nitrogen for storage, and rewarming the frozen embryos at a rate of approximately 300°C/min to thaw them.5
A major advance in the science of cryopreservation is the use of vitrification, a method of freezing in which the embryos are equilibrated with a 10% or 15% solution of cryoprotectant and then exposed briefly (30–60 seconds) to a 20% to 40% solution of cryoprotectant to achieve relative cellular dehydration. The embryos are then placed in a storage container and submerged in liquid nitrogen. During vitrification, embryos can be cooled at a rate exceeding 1,000°C/min. Vitrified embryos are stored at approximately –196°C and thawed in ultra-rapid fashion.
The development of vitrification methods has significantly advanced the technology of oocyte cryopreservation, which has been utilized for:
- preservation of fertility in cancer patients
- social reasons (e.g., lack of a partner)
- egg-donation programs
- minimization of the risk of ovarian hyperstimulation syndrome
- storage of surplus eggs when embryo cryopreservation is not feasible.
Cobo and Diaz recently conducted a systematic review and meta-analysis of randomized, controlled trials of oocyte vitrification. They found that the potential for fertilization, embryogenesis, and pregnancy from oocytes that had undergone vitrification and warming was not significantly different from the potential for fresh oocytes and was better than the potential for oocytes that had undergone freezing and thawing from standard freezing cycles.
Although the findings of the meta-analysis were limited by the small number of studies and possible selection bias, an increasing body of evidence supports the use of vitrification for cryopreservation of oocytes. Large-scale controlled trials are needed. Until they are performed, the findings of the meta-analysis should be interpreted with caution.
Newer ultra-rapid freezing of oocytes and embryos using vitrification appears to produce results that are superior to those obtained with traditional slow freezing. Large randomized, controlled trials are needed to confirm the improved efficacy of vitrification.
Anti-Müllerian hormone is an informative test of ovarian reserve—but lacks a nod from the FDA
Buyuk E, Seifer D, Younger J, Grazi R, Lieman H. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369–2372.
Li H, Yeung PW, Lau E, Ho PC, Ng EH. Evaluating the performance of serum anti-Müllerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94(6):2177–2181.
Lee J, Kim S, Jee B, Suh C, Kim KC, Moon SY. Anti-Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95(8):2602–2604.
Although it is well understood that both the quantity and quality of oocytes decline with age, the assessment of ovarian reserve continues to be a clinical challenge. Accurate evaluation can predict a woman’s response to infertility treatment, including IVF, and estimate her chance of conception. Noninvasive tests of ovarian reserve are a critical component of any evaluation of fertility. Although a woman’s age is the single most important historical factor in the assessment of reproductive capacity, there is significant variation in ovarian aging among women.
Historically, age, antral follicle count (AFC), and measurement of cycle day 3 follicle-stimulating hormone (FSH) and estradiol (E2) levels have been the most widely used measures of ovarian reserve, but mounting evidence suggests that assessment of the anti-Müllerian hormone (AMH) level may be even more informative.
AMH, also known as Müllerian-inhibiting substance, is a dimeric glycoprotein. A member of the transforming growth factor–ß family, AMH is closely related to inhibin and activin and is secreted by granulosa cells of preantral and small antral follicles in post-pubertal females.7 AMH aids in the coordination of ovarian follicular development by inhibiting recruitment of additional primordial follicles and decreasing the sensitivity of preantral and small antral follicles to FSH.8,9
AMH levels, measurable in serum, decline with age and are undetectable after menopause.10 Unlike FSH, which fluctuates during the menstrual cycle, AMH exhibits minimal intercycle and intracycle variation. The AMH level remains stable in women taking oral contraceptives and even in women who are pregnant.11
AMH is independently and significantly correlated with the ovarian response to gonadotropin therapy, with decreased levels of AMH associated with a poor response, and increased levels associated with a strong response.12 In the first cycle of IVF, an elevated AMH level has been associated with excessive response to gonadotropins and an increased risk of ovarian hyperstimulation syndrome (OHSS), independent of age and the presence of polycystic ovary syndrome.12
In a recent study of women who had an elevated FSH level and were undergoing IVF, the AMH level was strongly associated with the number of oocytes retrieved.13 Women who had an elevated FSH level but a serum AMH level of 0.6 ng/mL or above had a greater number of oocytes and day-3 embryos retrieved; they also had a lower cancellation rate than women who had a lower AMH level.13
Although no single test can predict the outcome of treatment for infertility, AMH concentrations are significantly higher in women who have a live birth (from the first cycle of stimulated IUI or after three cycles) than in women who do not.14
Two ELISA kits, one value?
Two types of enzyme-linked immunosorbent assay (ELISA) kits are commercially available for measurement of the AMH level: one from Immunotech Beckman Coulter and the other from Diagnostic Systems Laboratories. Neither kit has been approved for clinical use by the US Food and Drug Administration.
Studies comparing the values obtained using each kit have been inconsistent, generating controversy about the measurement of AMH. A recent study of women who were undergoing controlled ovarian stimulation found that the AMH levels obtained by the two kits were similar and significantly correlated with each other.15 In that study, the AMH level was measured on the day before gonadotropin administration or on the day of oocyte retrieval.15 In addition, the AMH concentrations measured by both kits were significantly associated with age, basal FSH levels, AFC, and the outcome of controlled ovarian stimulation.15 The authors concluded:
- The two commercially available kits provide reliable and similar results.
- The AMH level measured by either kit can predict the outcome of controlled ovarian stimulation, with similar reference values.15
Measurement of the AMH level can be an informative aspect of the evaluation of a patient’s fertility, as well as a valuable tool in the assessment of ovarian reserve. The AMH level can also help clinicians identify the appropriate dose of gonadotropins and predict which patients might be likely to over- or under-respond to stimulation—ultimately reducing the length and cost of treatment. Knowledge of the patient’s AMH level might inform pretreatment counseling and help women achieve reasonable expectations.
AMH is a useful test to help predict a patient’s response to ovarian stimulation and her chances of achieving pregnancy. However, AMH is only one measure of ovarian reserve and should not be used alone as a reason to exclude patients from treatment. In our practice, we use the AMH level along with cycle day 3 antral follicle count and FSH and estradiol levels.
We want to hear from you! Tell us what you think.
1. Ragni G, Caliari I, Nicolosi AE, Arnoldi M, Somigliana E, Crosignani PG. Preventing high-order multiple pregnancies during controlled ovarian hyperstimulation and intrauterine insemination: 3 years’ experience using low-dose recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2006;85(3):619-624.
2. Ghesquiere SL, Castelain EG, Spiessens C, et al. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauterine insemination. Am J Obstet Gynecol. 2007;197(6):589.e1-5.
3. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94(3):888-899.
4. Schreiner-Engel P, Walther VN, Mindes J, et al. First-trimester multifetal pregnancy reduction: acute and persistent psychologic reactions. Am J Obstet Gynecol. 1995;172(2 Pt 1):541.-
5. Leibo S, Pool T. The principal variables of cryopreservation: solutions temperatures, and rate changes. Fertil Steril. 2011;96(2):269-276.
6. Society for Assisted Reproductive Technology; American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2001 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology registry. Fertil Steril. 2007;87(6):1253-1266.
7. Vigier JA, Picard JY, Tran D, Legeai L, Josso N. Production of anti-Müllerian hormone: another homology between Sertoli and granulosa cells. Endocrinology. 1984;114(4):1315-1320.
8. Durlinger All, Gruijters MJG, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891-4899.
9. Salmon NA, Handyside AH, Joyce IM. Oocyte regulation and anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201-208.
10. Shin SY, Lee JR, Noh GW, et al. Analysis of serum levels of anti-Müllerian hormone, inhibin B, insulin-like growth factor-I, insulin-like growth factor binding protein-3, and follicle-stimulating hormone with respect to age and menopausal status. J Korean Med Sci. 2008;23(1):104-110.
11. Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, de Ziegler D. Clinical uses of anti-Müllerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91(1):226-230.
12. Nardo L, Gelbaya T, Wilkinson H, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586-1593.
13. Buyuk E, Seifer D, Younger J, Grazi R, Lieman H. Random anti-Müllerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369-2372.
14. Li HW, Yeung PW, Lau E&, Ho PC, Ng EH. Evaluating the performance of serum anti-Müllerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94(6):2177-2181.
15. Lee J, Kim S, Jee B, Suh C, Kim KC, Moon SY. Anti- Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95(8):2602-2604.
1. Ragni G, Caliari I, Nicolosi AE, Arnoldi M, Somigliana E, Crosignani PG. Preventing high-order multiple pregnancies during controlled ovarian hyperstimulation and intrauterine insemination: 3 years’ experience using low-dose recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2006;85(3):619-624.
2. Ghesquiere SL, Castelain EG, Spiessens C, et al. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauterine insemination. Am J Obstet Gynecol. 2007;197(6):589.e1-5.
3. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94(3):888-899.
4. Schreiner-Engel P, Walther VN, Mindes J, et al. First-trimester multifetal pregnancy reduction: acute and persistent psychologic reactions. Am J Obstet Gynecol. 1995;172(2 Pt 1):541.-
5. Leibo S, Pool T. The principal variables of cryopreservation: solutions temperatures, and rate changes. Fertil Steril. 2011;96(2):269-276.
6. Society for Assisted Reproductive Technology; American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2001 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology registry. Fertil Steril. 2007;87(6):1253-1266.
7. Vigier JA, Picard JY, Tran D, Legeai L, Josso N. Production of anti-Müllerian hormone: another homology between Sertoli and granulosa cells. Endocrinology. 1984;114(4):1315-1320.
8. Durlinger All, Gruijters MJG, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891-4899.
9. Salmon NA, Handyside AH, Joyce IM. Oocyte regulation and anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201-208.
10. Shin SY, Lee JR, Noh GW, et al. Analysis of serum levels of anti-Müllerian hormone, inhibin B, insulin-like growth factor-I, insulin-like growth factor binding protein-3, and follicle-stimulating hormone with respect to age and menopausal status. J Korean Med Sci. 2008;23(1):104-110.
11. Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, de Ziegler D. Clinical uses of anti-Müllerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91(1):226-230.
12. Nardo L, Gelbaya T, Wilkinson H, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586-1593.
13. Buyuk E, Seifer D, Younger J, Grazi R, Lieman H. Random anti-Müllerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369-2372.
14. Li HW, Yeung PW, Lau E&, Ho PC, Ng EH. Evaluating the performance of serum anti-Müllerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94(6):2177-2181.
15. Lee J, Kim S, Jee B, Suh C, Kim KC, Moon SY. Anti- Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95(8):2602-2604.
UPDATE: FERTILITY
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.
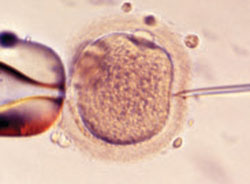
FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).
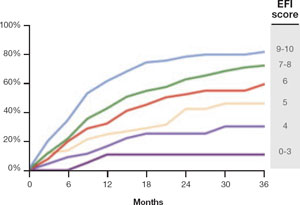
FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.

FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).

FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.

FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).

FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
FERTILITY
The diagnosis and treatment of fertility are evolving rapidly as a result of clinical studies, scientific research, and changing socioeconomic and ethical perspectives. These developments benefit health-care consumers, but they also pose new challenges to general ObGyns and other practitioners committed to the best possible care for their patients.
In this Update, I focus on a number of these areas of change:
- care of women who have polycystic ovary syndrome (PCOS)
- the impact of myomas on fertility
- treatment of infertility in women who have endometriosis
- when tubal reconstruction is appropriate
- the impact of a woman’s age on fertility
- patient-friendly strategies to enhance fertility
- cross-border reproductive travel.
Use clomiphene citrate to stimulate ovulation in women who have PCOS
Practice Committee of the American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90(5 Suppl):S69–S73.
A new Committee Opinion from the American Society for Reproductive Medicine (ASRM) Practice Committee tackles the challenge of treating women with PCOS for infertility.
PCOS is associated with an increased risk of insulin resistance and diabetes mellitus. The first line of treatment for all women who have PCOS, especially those with an elevated body mass index, is lifestyle modification through diet and exercise, with the goal of losing weight.
Clomiphene is first-line therapy when ovulation is the aim
Metformin and other insulin-sensitizing agents may enhance ovulation and increase the response to clomiphene citrate in women who have PCOS and insulin resistance, but their use solely to enhance ovulation is unwarranted, and they do not reduce the rate of miscarriage. Clomiphene citrate should be the first-line treatment because it is much more effective. Long-term use of metformin to prevent disease is not advised.
Screen for insulin resistance at the time of diagnosis
Women who have PCOS should be given a 2-hour oral glucose tolerance test and have their lipid profile measured at the time of diagnosis and then at an interval of every 2 years. Insulin-sensitizing agents should be used for long-term health issues only after impaired glucose tolerance has been measured, if diet and exercise alone prove to be ineffective.
My strategy for stimulating ovulation in this population involves the following:
- Perform vaginal ultrasonography (US) on cycle day 3 for an antral follicle count and to rule out ovarian cysts >1 cm.
- Give clomiphene citrate, 50 mg, on cycle days 3 through 7 (or 5 through 9).
- Repeat vaginal US on cycle day 11 (or 13) to evaluate ovarian response. The optimal response is 1 to 2, and not more than 3, follicles ≥15 mm in size.
- Recommend timed intercourse, starting on cycle day 10 and then every 2±0.5 days until 1 to 2 days after ovulation.
- Measure urinary luteinizing hormone (uLH) daily, to detect uLH surge, starting on cycle day 11. A positive surge indicates that ovulation is likely within the next 12–48 hours. Absence of a surge indicates the likely absence of ovulation, which can be treated by giving 10,000 IU of human chorionic gonadotropin (hCG) subcutaneously or intramuscularly when the largest follicle is 18 to 25 mm in size.—G. DAVID ADAMSON, MD
When choosing a treatment for myoma, consider impacts on fertility
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproduction Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 Suppl): S125–S130.
A recent educational bulletin from the ASRM Practice Committee examined the relationship between myomas and reproductive function and reviewed management of this pathology.
The effects of myomas on reproductive outcome are ill-defined, but fibroids that distort the uterine cavity, as well as larger intramural myomas, may have adverse effects on fertility.
Select interventions carefully
Among women who have infertility and those who have recurrent pregnancy loss, myomectomy should be considered only after thorough evaluation. The reason? Postoperative adhesions as a result of abdominal myomectomy are common and may reduce subsequent fertility.
As for uterine artery embolization, myolysis, and MRI-guided ultrasonic treatment, these are not recommended for women who have myomas and who are seeking to maintain or improve fertility. The safety and efficacy of these procedures in this population have not been established.
Is a GnRH agonist useful?
Treatment of myomas with a gonadotropin-releasing hormone (GnRH) agonist does not improve fertility but may be helpful before surgery in anemic women and in those who might be able to undergo a less invasive procedure if the myoma volume were moderately smaller.
Sequence of infertility treatments is critical in endometriosis patients
Adamson G. Management of endometriosis and infertility following surgery. In: Sutton C, Jones K, Adamson GD, eds. Modern Management of Endometriosis. London: Taylor & Francis; 2006:273–287.
New data make it easier to treat infertility in women thought to have endometriosis, although further randomized trials are needed. If other fertility variables are normal, and minimal to mild endometriosis is suspected but not confirmed, clomiphene citrate, 100 mg on cycle days 3 through 7, followed by intrauterine insemination (IUI) for 3 to 6 cycles, is a reasonable initial treatment, with the higher number of cycles being reserved for younger patients and those who have a better prognosis.
When is surgery helpful?
Diagnostic or operative laparoscopy, or both, is often indicated when one or more of the following are present:
- The patient experiences pain
- She fails to conceive after clomiphene citrate is administered and IUI is attempted for 3 to 6 cycles
- She has other factors associated with infertility.
Generally, if pregnancy does not occur within 9 to 15 months after surgery, repeat surgery is of limited benefit for infertility, but may have some benefit for pain. In women who do not conceive after surgery, ovarian suppression for 2 months is of possible benefit before assisted reproductive technology (ART) and should be considered in patients who are also suffering from pain. Pre-ART surgery for large endometriomas is frequently indicated, and excision of the cyst capsule produces results superior to those of drainage, coagulation, or both.
Postoperative management
After complete destruction of endometriosis in women who have infertility, ovarian suppression is not indicated. Rather, the patient should usually attempt to conceive for 9 to 15 months, with an outside range of 3 to 24 months for much older women who have an unfavorable prognosis, and for much younger women who have a good prognosis, respectively. If pregnancy does not occur, clomiphene citrate and IUI for 3 to 6 months are then indicated.
If this last strategy is unsuccessful, the options include:
- gonadotropins and IUI for 3 months to a maximum of 6 months in the young patient who has a good prognosis
- repeat laparoscopy (although this option is rare), possibly in conjunction with gamete intrafallopian transfer (GIFT), or, alternatively, in vitro fertilization (IVF). If the patient had a technically inadequate operation the first time, it sometimes is appropriate to repeat the surgery or go directly to IVF.
Consider tubal reconstruction in carefully selected patients
Practice Committee of the American Society for Reproductive Medicine. The role of tubal reconstructive surgery in the era of assisted reproductive technologies. Fertil Steril. 2008;90(5 Suppl):S250–S253.
In the era of ART, tubal reconstruction has fewer indications but is still appropriate and effective in properly selected individuals.
Determine the extent of tubal disease before reconstructive surgery
Hysterosalpingography is a useful initial test for the evaluation of tubal patency, but laparoscopy often is necessary to identify the nature and extent of pelvic disease. Selective salpingography or hysteroscopic tubal recanalization can help confirm the diagnosis of true proximal tubal occlusion.
Advise the patient of risks of surgery
Generally, the risk of ectopic pregnancy after tubal reconstruction is comparable to the risk of ectopic pregnancy associated with IVF, but the extent of tubal disease and pelvic pathology are important variables in predicting intrauterine and ectopic pregnancy rates.
The pregnancy rate after reversal of tubal sterilization depends on 1) the type of sterilization procedure that was performed, 2) site of anastomosis, and 3) postoperative tubal length, as well as 4) sperm quality and 5) the age of the female patient.
Maternal age, number of children desired, coexisting infertility variables, risk of ectopic and multiple pregnancy, and treatment cost are important considerations when counseling patients about the relative advantages and disadvantages of tubal surgery and IVF.
IVF is the best treatment for older women of reproductive age who have significant tubal pathology, and for women who have both proximal and distal occlusion.
Age, and duration of infertility, are key determinants of treatment
Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists, and Practice Committee of the American Society for Reproductive Medicine. Age-related fertility decline: a Committee Opinion. Fertil Steril. 2008;90(5 Suppl):S154–S155.
Women older than 35 years should receive expedited evaluation and treatment for infertility if they have not conceived after 6 months, or earlier if clinically indicated. That’s one of the conclusions from a recent ACOG–ASRM joint Committee Opinion on age-related fertility decline.
Age remains a major variable influencing a woman’s fertility and risk of pregnancy loss, and is increasingly important because of the social trend toward deferred child-bearing. The fertility rate peaks in a woman’s mid-20s and decreases by approximately 25% by age 35 and 50% by age 40, with a concomitant (and significant) increase in rates of aneuploidy and miscarriage.
The duration of infertility also is key. Of any given 100 women attempting to conceive:
- 78 will succeed within 1 year
- 88 will conceive within 2 years
- only an additional two or three women will conceive in the third year
- one more will conceive in each of the fourth and fifth years
- only three more will conceive over the rest of their reproductive life.
Recurrent pregnancy loss and infertility are separate entities
By definition, recurrent pregnancy loss entails the loss of two or more pregnancies. When the cause is unknown, each loss merits careful review to determine whether specific evaluation may be appropriate. After three losses, thorough evaluation is warranted.1,2
To distinguish infertility from recurrent pregnancy loss, define clinical pregnancy as one documented by US or histopathology.
New technologies remain unproven
Although ovarian tissue and oocyte cryopreservation offer the promise of female fertility preservation, these technologies remain investigational to date.
The greatest benefit to patients who wish to preserve their fertility is appropriate counseling about their reproductive health.3, 4
Fertility can be enhanced with a few patient-friendly strategies
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(5 Suppl):S1–S6.
Another Committee Opinion from ASRM, in collaboration with the Society for Reproductive Endocrinology and Infertility, offers simple but effective steps for patients to take to optimize fertility. ObGyns should recommend these strategies to any woman planning to conceive in the near future.
Frequent intercourse is best
Intercourse every day or every other day yields the highest pregnancy rate, but intercourse two to three times a week is nearly equivalent. There is a “fertile window” that spans the 6-day interval ending on the day of ovulation, and it correlates with the volume and character of cervical mucus.
Among women who have regular menstrual cycles, frequent intercourse that begins soon after the cessation of menses can help maximize fecundity.
Devices that determine or predict the time of ovulation may be useful for couples who have infrequent intercourse.
Neither specific coital timing, nor position during coitus, nor rest in a supine position after intercourse has a significant impact on fertility.
Caffeine, alcohol OK—in moderation
Moderate caffeine or alcohol consumption (1 or 2 drinks daily) has no demonstrable adverse effect on fertility. Smoking, a higher level of alcohol consumption (≥2 drinks daily), use of recreational drugs, and most commercially available vaginal lubricants should be discouraged among patients who are trying to conceive.
Fertility rates are lower in women who are very thin or obese, but there is little evidence that dietary variations improve fertility or affect the gender of the infant.
Elevated blood mercury levels from heavy seafood consumption have been associated with infertility.
Saunas do not reduce fertility in women. In normal men, attempts to protect the testicles from excessive heat are unjustified.
Avoid solvents and pesticides
- Fecundity may be diminished in women who are exposed to certain toxins and solvents, such as those used in the dry-cleaning and printing industries.
- Men who are exposed to heavy metals may be more likely to have abnormal semen parameters.
- Pesticide exposure may be a problem for both male and female agricultural workers.
- Despite limited data on exposure to lead and use of industrial microwaves, they are probably best avoided or minimized.
- Prescription drug use should be carefully controlled and managed on an individual basis.
Recommend 400 µg of folic acid daily
Any woman hoping to conceive should be advised to initiate this regimen to reduce the risk of neural tube defects.
The Centers for Disease Control and Prevention (CDC) held its first Public Health Symposium on Infertility in September 2008. Consensus is growing that infertility is a common disease or disability that has serious consequences for the well-being of families—making it a public health concern.
Because only approximately 50% of patients who have infertility ever seek treatment, it is hoped that new programs will improve access to fertility treatment for many more women.
For more information on the CDC’s initiatives in reproductive health, visit: http://www.cdc.gov/reproductivehealth/
WHO focuses on international inequities
The World Health Organization (WHO) held a meeting in Geneva in December 2008 to modify its glossary of ART definitions and develop new terminology to allow the collection of better data on the use of IVF internationally.5, 6
The prevalence of infertility is about the same in all countries of the world, affecting, on average, about 9% of people of reproductive age. However, there is a greater degree of secondary infertility—mostly as a result of infectious disease and obstetric complications—in low-resource (developing) countries.
Infertility is a major burden with serious medical and psychological consequences in American society, but its impact on women in other cultures is often more profound, with loss of personal status, divorce, and social ostracism adding to the burden.
More and more women seek care in countries other than their own
“Medical tourism” is an interesting phenomenon that has received widespread media attention. When it applies to infertility, a more appropriate term may be “cross-border reproductive care.”
This is an international phenomenon that is, so far, poorly documented. Common reasons to travel for medical care include cost and availability of specialized services. Women grappling with infertility may also seek to bypass regulations or ethical issues that limit availability of treatment in their home country. Among the issues that prompt travel are:
- gamete and embryo donation
- payment of donors and surrogates
- nontraditional relationships.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60.-
2. Adamson GD. Update in fertility. OBG Management. 2007;19(2):37-38, 41-44,-76.
3. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241-S246.
4. Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134-S135.
5. Zegers-Hochschild F, Nygren K-G, Adamson GD, et al. International Committee Monitoring Assisted Reproductive Technologies. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16-19.
6. International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization for year 2000. Fertil Steril. 2006;85:1586-1622.
The diagnosis and treatment of fertility are evolving rapidly as a result of clinical studies, scientific research, and changing socioeconomic and ethical perspectives. These developments benefit health-care consumers, but they also pose new challenges to general ObGyns and other practitioners committed to the best possible care for their patients.
In this Update, I focus on a number of these areas of change:
- care of women who have polycystic ovary syndrome (PCOS)
- the impact of myomas on fertility
- treatment of infertility in women who have endometriosis
- when tubal reconstruction is appropriate
- the impact of a woman’s age on fertility
- patient-friendly strategies to enhance fertility
- cross-border reproductive travel.
Use clomiphene citrate to stimulate ovulation in women who have PCOS
Practice Committee of the American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90(5 Suppl):S69–S73.
A new Committee Opinion from the American Society for Reproductive Medicine (ASRM) Practice Committee tackles the challenge of treating women with PCOS for infertility.
PCOS is associated with an increased risk of insulin resistance and diabetes mellitus. The first line of treatment for all women who have PCOS, especially those with an elevated body mass index, is lifestyle modification through diet and exercise, with the goal of losing weight.
Clomiphene is first-line therapy when ovulation is the aim
Metformin and other insulin-sensitizing agents may enhance ovulation and increase the response to clomiphene citrate in women who have PCOS and insulin resistance, but their use solely to enhance ovulation is unwarranted, and they do not reduce the rate of miscarriage. Clomiphene citrate should be the first-line treatment because it is much more effective. Long-term use of metformin to prevent disease is not advised.
Screen for insulin resistance at the time of diagnosis
Women who have PCOS should be given a 2-hour oral glucose tolerance test and have their lipid profile measured at the time of diagnosis and then at an interval of every 2 years. Insulin-sensitizing agents should be used for long-term health issues only after impaired glucose tolerance has been measured, if diet and exercise alone prove to be ineffective.
My strategy for stimulating ovulation in this population involves the following:
- Perform vaginal ultrasonography (US) on cycle day 3 for an antral follicle count and to rule out ovarian cysts >1 cm.
- Give clomiphene citrate, 50 mg, on cycle days 3 through 7 (or 5 through 9).
- Repeat vaginal US on cycle day 11 (or 13) to evaluate ovarian response. The optimal response is 1 to 2, and not more than 3, follicles ≥15 mm in size.
- Recommend timed intercourse, starting on cycle day 10 and then every 2±0.5 days until 1 to 2 days after ovulation.
- Measure urinary luteinizing hormone (uLH) daily, to detect uLH surge, starting on cycle day 11. A positive surge indicates that ovulation is likely within the next 12–48 hours. Absence of a surge indicates the likely absence of ovulation, which can be treated by giving 10,000 IU of human chorionic gonadotropin (hCG) subcutaneously or intramuscularly when the largest follicle is 18 to 25 mm in size.—G. DAVID ADAMSON, MD
When choosing a treatment for myoma, consider impacts on fertility
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproduction Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 Suppl): S125–S130.
A recent educational bulletin from the ASRM Practice Committee examined the relationship between myomas and reproductive function and reviewed management of this pathology.
The effects of myomas on reproductive outcome are ill-defined, but fibroids that distort the uterine cavity, as well as larger intramural myomas, may have adverse effects on fertility.
Select interventions carefully
Among women who have infertility and those who have recurrent pregnancy loss, myomectomy should be considered only after thorough evaluation. The reason? Postoperative adhesions as a result of abdominal myomectomy are common and may reduce subsequent fertility.
As for uterine artery embolization, myolysis, and MRI-guided ultrasonic treatment, these are not recommended for women who have myomas and who are seeking to maintain or improve fertility. The safety and efficacy of these procedures in this population have not been established.
Is a GnRH agonist useful?
Treatment of myomas with a gonadotropin-releasing hormone (GnRH) agonist does not improve fertility but may be helpful before surgery in anemic women and in those who might be able to undergo a less invasive procedure if the myoma volume were moderately smaller.
Sequence of infertility treatments is critical in endometriosis patients
Adamson G. Management of endometriosis and infertility following surgery. In: Sutton C, Jones K, Adamson GD, eds. Modern Management of Endometriosis. London: Taylor & Francis; 2006:273–287.
New data make it easier to treat infertility in women thought to have endometriosis, although further randomized trials are needed. If other fertility variables are normal, and minimal to mild endometriosis is suspected but not confirmed, clomiphene citrate, 100 mg on cycle days 3 through 7, followed by intrauterine insemination (IUI) for 3 to 6 cycles, is a reasonable initial treatment, with the higher number of cycles being reserved for younger patients and those who have a better prognosis.
When is surgery helpful?
Diagnostic or operative laparoscopy, or both, is often indicated when one or more of the following are present:
- The patient experiences pain
- She fails to conceive after clomiphene citrate is administered and IUI is attempted for 3 to 6 cycles
- She has other factors associated with infertility.
Generally, if pregnancy does not occur within 9 to 15 months after surgery, repeat surgery is of limited benefit for infertility, but may have some benefit for pain. In women who do not conceive after surgery, ovarian suppression for 2 months is of possible benefit before assisted reproductive technology (ART) and should be considered in patients who are also suffering from pain. Pre-ART surgery for large endometriomas is frequently indicated, and excision of the cyst capsule produces results superior to those of drainage, coagulation, or both.
Postoperative management
After complete destruction of endometriosis in women who have infertility, ovarian suppression is not indicated. Rather, the patient should usually attempt to conceive for 9 to 15 months, with an outside range of 3 to 24 months for much older women who have an unfavorable prognosis, and for much younger women who have a good prognosis, respectively. If pregnancy does not occur, clomiphene citrate and IUI for 3 to 6 months are then indicated.
If this last strategy is unsuccessful, the options include:
- gonadotropins and IUI for 3 months to a maximum of 6 months in the young patient who has a good prognosis
- repeat laparoscopy (although this option is rare), possibly in conjunction with gamete intrafallopian transfer (GIFT), or, alternatively, in vitro fertilization (IVF). If the patient had a technically inadequate operation the first time, it sometimes is appropriate to repeat the surgery or go directly to IVF.
Consider tubal reconstruction in carefully selected patients
Practice Committee of the American Society for Reproductive Medicine. The role of tubal reconstructive surgery in the era of assisted reproductive technologies. Fertil Steril. 2008;90(5 Suppl):S250–S253.
In the era of ART, tubal reconstruction has fewer indications but is still appropriate and effective in properly selected individuals.
Determine the extent of tubal disease before reconstructive surgery
Hysterosalpingography is a useful initial test for the evaluation of tubal patency, but laparoscopy often is necessary to identify the nature and extent of pelvic disease. Selective salpingography or hysteroscopic tubal recanalization can help confirm the diagnosis of true proximal tubal occlusion.
Advise the patient of risks of surgery
Generally, the risk of ectopic pregnancy after tubal reconstruction is comparable to the risk of ectopic pregnancy associated with IVF, but the extent of tubal disease and pelvic pathology are important variables in predicting intrauterine and ectopic pregnancy rates.
The pregnancy rate after reversal of tubal sterilization depends on 1) the type of sterilization procedure that was performed, 2) site of anastomosis, and 3) postoperative tubal length, as well as 4) sperm quality and 5) the age of the female patient.
Maternal age, number of children desired, coexisting infertility variables, risk of ectopic and multiple pregnancy, and treatment cost are important considerations when counseling patients about the relative advantages and disadvantages of tubal surgery and IVF.
IVF is the best treatment for older women of reproductive age who have significant tubal pathology, and for women who have both proximal and distal occlusion.
Age, and duration of infertility, are key determinants of treatment
Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists, and Practice Committee of the American Society for Reproductive Medicine. Age-related fertility decline: a Committee Opinion. Fertil Steril. 2008;90(5 Suppl):S154–S155.
Women older than 35 years should receive expedited evaluation and treatment for infertility if they have not conceived after 6 months, or earlier if clinically indicated. That’s one of the conclusions from a recent ACOG–ASRM joint Committee Opinion on age-related fertility decline.
Age remains a major variable influencing a woman’s fertility and risk of pregnancy loss, and is increasingly important because of the social trend toward deferred child-bearing. The fertility rate peaks in a woman’s mid-20s and decreases by approximately 25% by age 35 and 50% by age 40, with a concomitant (and significant) increase in rates of aneuploidy and miscarriage.
The duration of infertility also is key. Of any given 100 women attempting to conceive:
- 78 will succeed within 1 year
- 88 will conceive within 2 years
- only an additional two or three women will conceive in the third year
- one more will conceive in each of the fourth and fifth years
- only three more will conceive over the rest of their reproductive life.
Recurrent pregnancy loss and infertility are separate entities
By definition, recurrent pregnancy loss entails the loss of two or more pregnancies. When the cause is unknown, each loss merits careful review to determine whether specific evaluation may be appropriate. After three losses, thorough evaluation is warranted.1,2
To distinguish infertility from recurrent pregnancy loss, define clinical pregnancy as one documented by US or histopathology.
New technologies remain unproven
Although ovarian tissue and oocyte cryopreservation offer the promise of female fertility preservation, these technologies remain investigational to date.
The greatest benefit to patients who wish to preserve their fertility is appropriate counseling about their reproductive health.3, 4
Fertility can be enhanced with a few patient-friendly strategies
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(5 Suppl):S1–S6.
Another Committee Opinion from ASRM, in collaboration with the Society for Reproductive Endocrinology and Infertility, offers simple but effective steps for patients to take to optimize fertility. ObGyns should recommend these strategies to any woman planning to conceive in the near future.
Frequent intercourse is best
Intercourse every day or every other day yields the highest pregnancy rate, but intercourse two to three times a week is nearly equivalent. There is a “fertile window” that spans the 6-day interval ending on the day of ovulation, and it correlates with the volume and character of cervical mucus.
Among women who have regular menstrual cycles, frequent intercourse that begins soon after the cessation of menses can help maximize fecundity.
Devices that determine or predict the time of ovulation may be useful for couples who have infrequent intercourse.
Neither specific coital timing, nor position during coitus, nor rest in a supine position after intercourse has a significant impact on fertility.
Caffeine, alcohol OK—in moderation
Moderate caffeine or alcohol consumption (1 or 2 drinks daily) has no demonstrable adverse effect on fertility. Smoking, a higher level of alcohol consumption (≥2 drinks daily), use of recreational drugs, and most commercially available vaginal lubricants should be discouraged among patients who are trying to conceive.
Fertility rates are lower in women who are very thin or obese, but there is little evidence that dietary variations improve fertility or affect the gender of the infant.
Elevated blood mercury levels from heavy seafood consumption have been associated with infertility.
Saunas do not reduce fertility in women. In normal men, attempts to protect the testicles from excessive heat are unjustified.
Avoid solvents and pesticides
- Fecundity may be diminished in women who are exposed to certain toxins and solvents, such as those used in the dry-cleaning and printing industries.
- Men who are exposed to heavy metals may be more likely to have abnormal semen parameters.
- Pesticide exposure may be a problem for both male and female agricultural workers.
- Despite limited data on exposure to lead and use of industrial microwaves, they are probably best avoided or minimized.
- Prescription drug use should be carefully controlled and managed on an individual basis.
Recommend 400 µg of folic acid daily
Any woman hoping to conceive should be advised to initiate this regimen to reduce the risk of neural tube defects.
The Centers for Disease Control and Prevention (CDC) held its first Public Health Symposium on Infertility in September 2008. Consensus is growing that infertility is a common disease or disability that has serious consequences for the well-being of families—making it a public health concern.
Because only approximately 50% of patients who have infertility ever seek treatment, it is hoped that new programs will improve access to fertility treatment for many more women.
For more information on the CDC’s initiatives in reproductive health, visit: http://www.cdc.gov/reproductivehealth/
WHO focuses on international inequities
The World Health Organization (WHO) held a meeting in Geneva in December 2008 to modify its glossary of ART definitions and develop new terminology to allow the collection of better data on the use of IVF internationally.5, 6
The prevalence of infertility is about the same in all countries of the world, affecting, on average, about 9% of people of reproductive age. However, there is a greater degree of secondary infertility—mostly as a result of infectious disease and obstetric complications—in low-resource (developing) countries.
Infertility is a major burden with serious medical and psychological consequences in American society, but its impact on women in other cultures is often more profound, with loss of personal status, divorce, and social ostracism adding to the burden.
More and more women seek care in countries other than their own
“Medical tourism” is an interesting phenomenon that has received widespread media attention. When it applies to infertility, a more appropriate term may be “cross-border reproductive care.”
This is an international phenomenon that is, so far, poorly documented. Common reasons to travel for medical care include cost and availability of specialized services. Women grappling with infertility may also seek to bypass regulations or ethical issues that limit availability of treatment in their home country. Among the issues that prompt travel are:
- gamete and embryo donation
- payment of donors and surrogates
- nontraditional relationships.
The diagnosis and treatment of fertility are evolving rapidly as a result of clinical studies, scientific research, and changing socioeconomic and ethical perspectives. These developments benefit health-care consumers, but they also pose new challenges to general ObGyns and other practitioners committed to the best possible care for their patients.
In this Update, I focus on a number of these areas of change:
- care of women who have polycystic ovary syndrome (PCOS)
- the impact of myomas on fertility
- treatment of infertility in women who have endometriosis
- when tubal reconstruction is appropriate
- the impact of a woman’s age on fertility
- patient-friendly strategies to enhance fertility
- cross-border reproductive travel.
Use clomiphene citrate to stimulate ovulation in women who have PCOS
Practice Committee of the American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90(5 Suppl):S69–S73.
A new Committee Opinion from the American Society for Reproductive Medicine (ASRM) Practice Committee tackles the challenge of treating women with PCOS for infertility.
PCOS is associated with an increased risk of insulin resistance and diabetes mellitus. The first line of treatment for all women who have PCOS, especially those with an elevated body mass index, is lifestyle modification through diet and exercise, with the goal of losing weight.
Clomiphene is first-line therapy when ovulation is the aim
Metformin and other insulin-sensitizing agents may enhance ovulation and increase the response to clomiphene citrate in women who have PCOS and insulin resistance, but their use solely to enhance ovulation is unwarranted, and they do not reduce the rate of miscarriage. Clomiphene citrate should be the first-line treatment because it is much more effective. Long-term use of metformin to prevent disease is not advised.
Screen for insulin resistance at the time of diagnosis
Women who have PCOS should be given a 2-hour oral glucose tolerance test and have their lipid profile measured at the time of diagnosis and then at an interval of every 2 years. Insulin-sensitizing agents should be used for long-term health issues only after impaired glucose tolerance has been measured, if diet and exercise alone prove to be ineffective.
My strategy for stimulating ovulation in this population involves the following:
- Perform vaginal ultrasonography (US) on cycle day 3 for an antral follicle count and to rule out ovarian cysts >1 cm.
- Give clomiphene citrate, 50 mg, on cycle days 3 through 7 (or 5 through 9).
- Repeat vaginal US on cycle day 11 (or 13) to evaluate ovarian response. The optimal response is 1 to 2, and not more than 3, follicles ≥15 mm in size.
- Recommend timed intercourse, starting on cycle day 10 and then every 2±0.5 days until 1 to 2 days after ovulation.
- Measure urinary luteinizing hormone (uLH) daily, to detect uLH surge, starting on cycle day 11. A positive surge indicates that ovulation is likely within the next 12–48 hours. Absence of a surge indicates the likely absence of ovulation, which can be treated by giving 10,000 IU of human chorionic gonadotropin (hCG) subcutaneously or intramuscularly when the largest follicle is 18 to 25 mm in size.—G. DAVID ADAMSON, MD
When choosing a treatment for myoma, consider impacts on fertility
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproduction Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 Suppl): S125–S130.
A recent educational bulletin from the ASRM Practice Committee examined the relationship between myomas and reproductive function and reviewed management of this pathology.
The effects of myomas on reproductive outcome are ill-defined, but fibroids that distort the uterine cavity, as well as larger intramural myomas, may have adverse effects on fertility.
Select interventions carefully
Among women who have infertility and those who have recurrent pregnancy loss, myomectomy should be considered only after thorough evaluation. The reason? Postoperative adhesions as a result of abdominal myomectomy are common and may reduce subsequent fertility.
As for uterine artery embolization, myolysis, and MRI-guided ultrasonic treatment, these are not recommended for women who have myomas and who are seeking to maintain or improve fertility. The safety and efficacy of these procedures in this population have not been established.
Is a GnRH agonist useful?
Treatment of myomas with a gonadotropin-releasing hormone (GnRH) agonist does not improve fertility but may be helpful before surgery in anemic women and in those who might be able to undergo a less invasive procedure if the myoma volume were moderately smaller.
Sequence of infertility treatments is critical in endometriosis patients
Adamson G. Management of endometriosis and infertility following surgery. In: Sutton C, Jones K, Adamson GD, eds. Modern Management of Endometriosis. London: Taylor & Francis; 2006:273–287.
New data make it easier to treat infertility in women thought to have endometriosis, although further randomized trials are needed. If other fertility variables are normal, and minimal to mild endometriosis is suspected but not confirmed, clomiphene citrate, 100 mg on cycle days 3 through 7, followed by intrauterine insemination (IUI) for 3 to 6 cycles, is a reasonable initial treatment, with the higher number of cycles being reserved for younger patients and those who have a better prognosis.
When is surgery helpful?
Diagnostic or operative laparoscopy, or both, is often indicated when one or more of the following are present:
- The patient experiences pain
- She fails to conceive after clomiphene citrate is administered and IUI is attempted for 3 to 6 cycles
- She has other factors associated with infertility.
Generally, if pregnancy does not occur within 9 to 15 months after surgery, repeat surgery is of limited benefit for infertility, but may have some benefit for pain. In women who do not conceive after surgery, ovarian suppression for 2 months is of possible benefit before assisted reproductive technology (ART) and should be considered in patients who are also suffering from pain. Pre-ART surgery for large endometriomas is frequently indicated, and excision of the cyst capsule produces results superior to those of drainage, coagulation, or both.
Postoperative management
After complete destruction of endometriosis in women who have infertility, ovarian suppression is not indicated. Rather, the patient should usually attempt to conceive for 9 to 15 months, with an outside range of 3 to 24 months for much older women who have an unfavorable prognosis, and for much younger women who have a good prognosis, respectively. If pregnancy does not occur, clomiphene citrate and IUI for 3 to 6 months are then indicated.
If this last strategy is unsuccessful, the options include:
- gonadotropins and IUI for 3 months to a maximum of 6 months in the young patient who has a good prognosis
- repeat laparoscopy (although this option is rare), possibly in conjunction with gamete intrafallopian transfer (GIFT), or, alternatively, in vitro fertilization (IVF). If the patient had a technically inadequate operation the first time, it sometimes is appropriate to repeat the surgery or go directly to IVF.
Consider tubal reconstruction in carefully selected patients
Practice Committee of the American Society for Reproductive Medicine. The role of tubal reconstructive surgery in the era of assisted reproductive technologies. Fertil Steril. 2008;90(5 Suppl):S250–S253.
In the era of ART, tubal reconstruction has fewer indications but is still appropriate and effective in properly selected individuals.
Determine the extent of tubal disease before reconstructive surgery
Hysterosalpingography is a useful initial test for the evaluation of tubal patency, but laparoscopy often is necessary to identify the nature and extent of pelvic disease. Selective salpingography or hysteroscopic tubal recanalization can help confirm the diagnosis of true proximal tubal occlusion.
Advise the patient of risks of surgery
Generally, the risk of ectopic pregnancy after tubal reconstruction is comparable to the risk of ectopic pregnancy associated with IVF, but the extent of tubal disease and pelvic pathology are important variables in predicting intrauterine and ectopic pregnancy rates.
The pregnancy rate after reversal of tubal sterilization depends on 1) the type of sterilization procedure that was performed, 2) site of anastomosis, and 3) postoperative tubal length, as well as 4) sperm quality and 5) the age of the female patient.
Maternal age, number of children desired, coexisting infertility variables, risk of ectopic and multiple pregnancy, and treatment cost are important considerations when counseling patients about the relative advantages and disadvantages of tubal surgery and IVF.
IVF is the best treatment for older women of reproductive age who have significant tubal pathology, and for women who have both proximal and distal occlusion.
Age, and duration of infertility, are key determinants of treatment
Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists, and Practice Committee of the American Society for Reproductive Medicine. Age-related fertility decline: a Committee Opinion. Fertil Steril. 2008;90(5 Suppl):S154–S155.
Women older than 35 years should receive expedited evaluation and treatment for infertility if they have not conceived after 6 months, or earlier if clinically indicated. That’s one of the conclusions from a recent ACOG–ASRM joint Committee Opinion on age-related fertility decline.
Age remains a major variable influencing a woman’s fertility and risk of pregnancy loss, and is increasingly important because of the social trend toward deferred child-bearing. The fertility rate peaks in a woman’s mid-20s and decreases by approximately 25% by age 35 and 50% by age 40, with a concomitant (and significant) increase in rates of aneuploidy and miscarriage.
The duration of infertility also is key. Of any given 100 women attempting to conceive:
- 78 will succeed within 1 year
- 88 will conceive within 2 years
- only an additional two or three women will conceive in the third year
- one more will conceive in each of the fourth and fifth years
- only three more will conceive over the rest of their reproductive life.
Recurrent pregnancy loss and infertility are separate entities
By definition, recurrent pregnancy loss entails the loss of two or more pregnancies. When the cause is unknown, each loss merits careful review to determine whether specific evaluation may be appropriate. After three losses, thorough evaluation is warranted.1,2
To distinguish infertility from recurrent pregnancy loss, define clinical pregnancy as one documented by US or histopathology.
New technologies remain unproven
Although ovarian tissue and oocyte cryopreservation offer the promise of female fertility preservation, these technologies remain investigational to date.
The greatest benefit to patients who wish to preserve their fertility is appropriate counseling about their reproductive health.3, 4
Fertility can be enhanced with a few patient-friendly strategies
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(5 Suppl):S1–S6.
Another Committee Opinion from ASRM, in collaboration with the Society for Reproductive Endocrinology and Infertility, offers simple but effective steps for patients to take to optimize fertility. ObGyns should recommend these strategies to any woman planning to conceive in the near future.
Frequent intercourse is best
Intercourse every day or every other day yields the highest pregnancy rate, but intercourse two to three times a week is nearly equivalent. There is a “fertile window” that spans the 6-day interval ending on the day of ovulation, and it correlates with the volume and character of cervical mucus.
Among women who have regular menstrual cycles, frequent intercourse that begins soon after the cessation of menses can help maximize fecundity.
Devices that determine or predict the time of ovulation may be useful for couples who have infrequent intercourse.
Neither specific coital timing, nor position during coitus, nor rest in a supine position after intercourse has a significant impact on fertility.
Caffeine, alcohol OK—in moderation
Moderate caffeine or alcohol consumption (1 or 2 drinks daily) has no demonstrable adverse effect on fertility. Smoking, a higher level of alcohol consumption (≥2 drinks daily), use of recreational drugs, and most commercially available vaginal lubricants should be discouraged among patients who are trying to conceive.
Fertility rates are lower in women who are very thin or obese, but there is little evidence that dietary variations improve fertility or affect the gender of the infant.
Elevated blood mercury levels from heavy seafood consumption have been associated with infertility.
Saunas do not reduce fertility in women. In normal men, attempts to protect the testicles from excessive heat are unjustified.
Avoid solvents and pesticides
- Fecundity may be diminished in women who are exposed to certain toxins and solvents, such as those used in the dry-cleaning and printing industries.
- Men who are exposed to heavy metals may be more likely to have abnormal semen parameters.
- Pesticide exposure may be a problem for both male and female agricultural workers.
- Despite limited data on exposure to lead and use of industrial microwaves, they are probably best avoided or minimized.
- Prescription drug use should be carefully controlled and managed on an individual basis.
Recommend 400 µg of folic acid daily
Any woman hoping to conceive should be advised to initiate this regimen to reduce the risk of neural tube defects.
The Centers for Disease Control and Prevention (CDC) held its first Public Health Symposium on Infertility in September 2008. Consensus is growing that infertility is a common disease or disability that has serious consequences for the well-being of families—making it a public health concern.
Because only approximately 50% of patients who have infertility ever seek treatment, it is hoped that new programs will improve access to fertility treatment for many more women.
For more information on the CDC’s initiatives in reproductive health, visit: http://www.cdc.gov/reproductivehealth/
WHO focuses on international inequities
The World Health Organization (WHO) held a meeting in Geneva in December 2008 to modify its glossary of ART definitions and develop new terminology to allow the collection of better data on the use of IVF internationally.5, 6
The prevalence of infertility is about the same in all countries of the world, affecting, on average, about 9% of people of reproductive age. However, there is a greater degree of secondary infertility—mostly as a result of infectious disease and obstetric complications—in low-resource (developing) countries.
Infertility is a major burden with serious medical and psychological consequences in American society, but its impact on women in other cultures is often more profound, with loss of personal status, divorce, and social ostracism adding to the burden.
More and more women seek care in countries other than their own
“Medical tourism” is an interesting phenomenon that has received widespread media attention. When it applies to infertility, a more appropriate term may be “cross-border reproductive care.”
This is an international phenomenon that is, so far, poorly documented. Common reasons to travel for medical care include cost and availability of specialized services. Women grappling with infertility may also seek to bypass regulations or ethical issues that limit availability of treatment in their home country. Among the issues that prompt travel are:
- gamete and embryo donation
- payment of donors and surrogates
- nontraditional relationships.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60.-
2. Adamson GD. Update in fertility. OBG Management. 2007;19(2):37-38, 41-44,-76.
3. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241-S246.
4. Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134-S135.
5. Zegers-Hochschild F, Nygren K-G, Adamson GD, et al. International Committee Monitoring Assisted Reproductive Technologies. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16-19.
6. International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization for year 2000. Fertil Steril. 2006;85:1586-1622.
1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90(5 Suppl):S60.-
2. Adamson GD. Update in fertility. OBG Management. 2007;19(2):37-38, 41-44,-76.
3. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241-S246.
4. Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134-S135.
5. Zegers-Hochschild F, Nygren K-G, Adamson GD, et al. International Committee Monitoring Assisted Reproductive Technologies. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16-19.
6. International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F. World collaborative report on in vitro fertilization for year 2000. Fertil Steril. 2006;85:1586-1622.
FERTILITY
The field of reproductive endocrinology and infertility is anything but stagnant. New technologies continue to enter the market at a brisk pace, and a greater emphasis on evidence has produced better-designed randomized controlled trials, meta-analyses, and practice guidelines. This means greater availability of standardized protocols that reflect best practice and can be tailored to a patient’s condition and needs.
Highlighted here are notable studies and guidelines from the past year, including advice on:
- preventing peritoneal adhesions
- expediting in vitro fertilization (IVF) for unexplained infertility
- counseling the patient about the real limitations of preimplantation genetic screening for aneuploidy
- informing patients that oocyte cryopreservation is unlikely to lead to live birth.
Guideline urges good surgical technique in battle against adhesions
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Re-productive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery. Fertil Steril. 2007;88:21–26.
This newly released practice guideline from the American Society of Reproductive Medicine (ASRM) focuses on adhesions and their impact on fertility. The guideline reiterates that peritoneal adhesions are a common and serious complication of gynecologic surgery and emphasizes key principles to reduce their likelihood and extent. These principles include the need to:
- Perform surgery only when the benefits of doing so clearly outweigh the risks
- Handle tissue gently (this is the most important preventive technique)
- Don’t assume laparoscopy is superior to laparotomy—it will be only if less tissue injury occurs
- Be especially careful when operating on or near ovaries, which form adhesions easily.
Ovarian surgery often necessitates additional operations
Studies have demonstrated that approximately 33% of patients who undergo open abdominal or pelvic surgery are readmitted, on average, two times over the subsequent 10 years for conditions directly or possibly related to adhesions or for further surgery that could be complicated by adhesions. The highest readmission rate directly related to adhesions—7.5 for every 100 initial operations—was associated with ovarian surgery performed via laparotomy.
Adhesion-related complications of gynecologic surgery include small-bowel obstruction, which occurs in approximately 1.5% of women who have undergone abdominal hysterectomy.
The relationship between adhesions and pelvic pain is unclear, although severe bowel adhesions can cause visceral pain. The ASRM guideline notes that “the impact that lysis of bowel or adnexal adhesions may have on abdominal and pelvic pain cannot be predicted confidently.” Postoperative adhesions increase subsequent operating times and risk of bowel injury.
How adhesions affect fertility
Adhesions may impair fertility by distorting adnexal anatomy and interfering with gamete and embryo transport. Among infertile women who have adnexal adhesions, adhesiolysis is associated with pregnancy rates of 32% at 12 months and 45% at 24 months, compared with 11% and 16%, respectively, for untreated women.1 Pregnancy rates are inversely correlated with adhesion scores on the ASRM classification system for adnexal adhesions.2
Some, but not all, adhesion-reducing measures work
According to the ASRM guideline, adhesions may be prevented, at least theoretically, by:
- minimizing peritoneal injury during surgery
- avoiding the introduction of reactive foreign bodies
- reducing the local inflammatory response
- inhibiting the coagulation cascade and promoting fibrinolysis
- placing barriers between damaged tissues.
Pharmacotherapeutic and fluid agents. ASRM found no evidence of improved pregnancy outcomes for pharmacologic and fluid agents used as an adjunct during pelvic surgery. For example, anti-inflammatory agents that have been evaluated, both locally and systemically, including dexamethasone and promethazine, have not reduced postoperative adhesions. Antibiotic solutions, 32% Dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids have been used to separate adjacent peritoneal surfaces via “hydroflotation,” but none have reduced adhesion formation.
Surgical barriers may help decrease postoperative adhesion formation but cannot compensate for poor surgical technique. I rarely use adhesion barriers because I feel that careful tissue handling, excellent hemostasis, avoiding trauma to healthy tissue, and removal of all diseased tissue are the key ways to obtain good postsurgical results and reduce adhesions.
Hyaluronic acid agents may decrease the prevalence of adhesions and prevent the deterioration of preexisting adhesions, but because of the limited number of studies available, these data should be interpreted with caution.3 However, ASRM found no substantial evidence that they improve fertility, decrease pain, or reduce the incidence of postoperative bowel obstruction.
Averting adhesions: Surgical techniques and tools
By Togas Tulandi, MD, MHCM, and Mohammed Al-Sunaidi, MD It’s available in our archive at www.obgmanagement.com
A move from clomiphene directly to IVF may cut time to pregnancy
Reindollar RH, Regan MM, Neumann PJ, Thornton KL, Alper MM, Goldman MB. A randomized controlled trial of 503 couples assigned to conventional infertility treatment or an accelerated track to IVF: Preliminary results of the fast track and standard treatment (FASTT) trial. Fertil Steril. 2007;88(Suppl 1):S41.
This very important abstract, presented at the annual meeting of ASRM, has the potential to dramatically change fertility treatment. The multicenter randomized controlled clinical trial measured the efficacy and time to pregnancy of an accelerated treatment strategy for women 21 to 39 years old who had unexplained infertility. A similar percentage of patients—approximately 75%—became pregnant in each arm (traditional versus accelerated), with a shorter time to pregnancy in the accelerated arm.
The new paradigm for management of unexplained infertility includes:
- comprehensive fertility history and physical examination
- targeted laboratory testing and other investigation, as needed
- counseling and psychological support for the patient once the diagnosis is made
- empiric treatment with clomiphene citrate plus intrauterine insemination (IUI) for as many as three cycles
- immediate IVF for as many as six cycles.
Details of the trial
Women in the trial had attempted to conceive for 12 months and had normal ovarian reserve (and semen analysis) and no pelvic pathology. Couples already treated for infertility were excluded.
Participants were randomized to:
- a conventional treatment regimen of three cycles of clomiphene citrate with IUI, three cycles of folliclestimulating hormone (FSH) and IUI, and as many as six cycles of IVF or
- three cycles of clomiphene citrate with IUI and then as many as six cycles of IVF.
Regimen likely reduces cost, stress
Major issues affecting the eventual success rate for infertile couples are cost and psychological stress, which can cause even patients who have a good prognosis to drop out of treatment. The major complication of fertility treatment is multiple pregnancy. By avoiding the use of gonadotropins in couples with unexplained infertility and accelerating the transition to IVF, physicians can lower the cost and psychological stress of treatment. They can also reduce the likelihood of multiple pregnancy because it is easier to control the number of embryos transferred in IVF than the number of follicles that develop with gonadotropins.
In women younger than 35 years on the first IVF cycle who have a good prognosis, ASRM now recommends that only one or two day-3 embryos be transferred, and not more than one day-5 blastocyst.4 The multiple-birth rate has declined in recent years, as more and more IVF clinics place fewer embryos; the rate should continue to fall with wider application of elective single-embryo transfer.5,6
Because this accelerated protocol produces a similar number of births over a shorter period and has the potential to lower cost, psychological stress, and the multiple-birth rate, it deserves implementation for many patients and warrants further evaluation for potential benefits in other populations.
It’s no help, after all: Preimplantation genetic screening for aneuploidy
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: A Practice Committee report. Fertil Steril. 2007;88:1497–1504.
Mastenbroek S, Twisk M, van Echten-Arends J, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17.
Preimplantation genetic diagnosis of known single-gene defects, structural chromosomal rearrangements, X-linked disorders, and human leukocyte antigen typing is a major benefit to couples known to be at risk of passing on a heritable and debilitating genetic disease. Aneuploidy is the most common cause of early pregnancy loss, and its prevalence increases with maternal age and may increase in chromosomally normal couples who experience recurrent early pregnancy loss or repeated failure of IVF cycles. Preimplantation genetic screening (PGS) has been advocated to identify and transfer only euploid embryos and increase the chance of successful pregnancy.
New data from Mastenbroek and colleagues indicate that PGS for aneuploidy does not increase the rate of pregnancy or live birth. After several years of increasing utilization and studies suggesting that PGS has benefit, the first multicenter, randomized, doubleblind, controlled study that compared three cycles of IVF with and without PGS in women 35 to 41 years old concluded that PGS does not increase but, in fact, significantly reduces the rate of pregnancy and live birth in this group.
Findings sparked controversy
This trial generated controversy within the genetics and reproductive endocrinology specialties because it challenged the intuitive view that screening of embryos before transfer into the uterus should be beneficial—or, at least, harmless. Some now argue that the benefits of PGS, if any, cannot be intuitively assumed and assert that the burden of proof of those benefits rests with proponents of PGS.
The practice committees of the Society for Assisted Reproductive Technology (SART) and ASRM found insufficient evidence to support the use of PGS to improve the live birth rate in women of advanced age or in those who have had implantation failure or recurrent pregnancy loss (TABLE). Many physicians believe, however, that technologies under development will soon bring verifiable benefits of PGS to patients.
SART and ASRM weigh in on use of preimplantation genetic testing
| TEST | RECOMMENDATION |
|---|---|
| Pre-implantation genetic diagnosis | |
| Pre- implantation genetic screening | |
| SOURCE: Society for Assisted Reproductive Technology and American Society for Reproductive Medicine | |
Advise your patients that oocyte cryopreservation is “a long shot”
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a practice committee opinion. Fertil Steril. 2007;88:1495–1496.
Oocyte cryopreservation is an experimental procedure that should not be offered or marketed as a means to defer reproductive aging, primarily because data on clinical outcomes are limited. That is the conclusion of this guideline from SART and ASRM. Consequently, women who may be considering the procedure should be fully informed about the process and likely outcomes and counseled by a qualified mental health professional.
Counseling is crucial
According to the SART and ASRM guideline, pretreatment counseling should include comprehensive information on a range of topics (see the box below). In addition, women considering oocyte cryopreservation should be counseled thoroughly about reproductive aging and life planning.7,8
Few alternatives for some women
Women who have cancer should receive the same counseling. Unlike healthy women, however, they may have no other options, and cryopreservation may be more appropriate for them despite experimental status.
Patients considering this procedure need comprehensive information about:
- Ovarian stimulation and oocyte retrieval
- Methods of oocyte cryopreservation
- Storage fees
- The expected thaw survival rate
- The requirement for intracytoplasmic sperm injection
- Clinic-specific data and outcomes or, in their absence, literature estimates of a 2% overall live birth rate per oocyte thawed using slow-freeze methods and 4% for vitrification, compared with age-related probabilities of success per IVF cycle using fresh nondonor oocytes
- The relatively low likelihood that a woman who cryopreserves her eggs before age 35 will ever need or use them
- State and federal screening laws for potential donation of cryopreserved oocytes
- Potential risks of basing important life decisions and expectations on a limited number of cryopreserved oocytes
- The possibility that the facility may cease operation, necessitating transfer of cryopreserved oocytes to another facility
- The possibility that cryopreserved oocytes might be lost or damaged as a result of laboratory error or other events beyond control.
1. Tulandi T, Collins JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
2. Marana R, Rizzi M, Muzii L, Catalano GF, Caruana P, Mancuso S. Correlation between the American Fertility Society classification of adnexal adhesions and distal tubal occlusion, salpingoscopy, and reproductive outcome in tubal surgery. Fertil Steril. 1995;64:924-929.
3. Metwally M, Gorvy D, Watson A, Li TC. Hyaluronic acid fluid agents for the prevention of adhesions after fertility-preserving gynecological surgery: a metaanalysis of randomized controlled trials. Fertil Steril. 2007;87:1139-1146.
4. Practice Committee of the Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-S52.
5. Adamson GD, Baker VL. Multiple births from assisted reproductive technologies: a challenge that must be met. Fertil Steril. 2004;81:517-522.
6. Stern JE, Cedars MI, Jain T, et al. for the Society for Assisted Reproductive Technology Writing Group Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275-282.
7. Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389-1394.
8. Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548-1553.
The field of reproductive endocrinology and infertility is anything but stagnant. New technologies continue to enter the market at a brisk pace, and a greater emphasis on evidence has produced better-designed randomized controlled trials, meta-analyses, and practice guidelines. This means greater availability of standardized protocols that reflect best practice and can be tailored to a patient’s condition and needs.
Highlighted here are notable studies and guidelines from the past year, including advice on:
- preventing peritoneal adhesions
- expediting in vitro fertilization (IVF) for unexplained infertility
- counseling the patient about the real limitations of preimplantation genetic screening for aneuploidy
- informing patients that oocyte cryopreservation is unlikely to lead to live birth.
Guideline urges good surgical technique in battle against adhesions
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Re-productive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery. Fertil Steril. 2007;88:21–26.
This newly released practice guideline from the American Society of Reproductive Medicine (ASRM) focuses on adhesions and their impact on fertility. The guideline reiterates that peritoneal adhesions are a common and serious complication of gynecologic surgery and emphasizes key principles to reduce their likelihood and extent. These principles include the need to:
- Perform surgery only when the benefits of doing so clearly outweigh the risks
- Handle tissue gently (this is the most important preventive technique)
- Don’t assume laparoscopy is superior to laparotomy—it will be only if less tissue injury occurs
- Be especially careful when operating on or near ovaries, which form adhesions easily.
Ovarian surgery often necessitates additional operations
Studies have demonstrated that approximately 33% of patients who undergo open abdominal or pelvic surgery are readmitted, on average, two times over the subsequent 10 years for conditions directly or possibly related to adhesions or for further surgery that could be complicated by adhesions. The highest readmission rate directly related to adhesions—7.5 for every 100 initial operations—was associated with ovarian surgery performed via laparotomy.
Adhesion-related complications of gynecologic surgery include small-bowel obstruction, which occurs in approximately 1.5% of women who have undergone abdominal hysterectomy.
The relationship between adhesions and pelvic pain is unclear, although severe bowel adhesions can cause visceral pain. The ASRM guideline notes that “the impact that lysis of bowel or adnexal adhesions may have on abdominal and pelvic pain cannot be predicted confidently.” Postoperative adhesions increase subsequent operating times and risk of bowel injury.
How adhesions affect fertility
Adhesions may impair fertility by distorting adnexal anatomy and interfering with gamete and embryo transport. Among infertile women who have adnexal adhesions, adhesiolysis is associated with pregnancy rates of 32% at 12 months and 45% at 24 months, compared with 11% and 16%, respectively, for untreated women.1 Pregnancy rates are inversely correlated with adhesion scores on the ASRM classification system for adnexal adhesions.2
Some, but not all, adhesion-reducing measures work
According to the ASRM guideline, adhesions may be prevented, at least theoretically, by:
- minimizing peritoneal injury during surgery
- avoiding the introduction of reactive foreign bodies
- reducing the local inflammatory response
- inhibiting the coagulation cascade and promoting fibrinolysis
- placing barriers between damaged tissues.
Pharmacotherapeutic and fluid agents. ASRM found no evidence of improved pregnancy outcomes for pharmacologic and fluid agents used as an adjunct during pelvic surgery. For example, anti-inflammatory agents that have been evaluated, both locally and systemically, including dexamethasone and promethazine, have not reduced postoperative adhesions. Antibiotic solutions, 32% Dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids have been used to separate adjacent peritoneal surfaces via “hydroflotation,” but none have reduced adhesion formation.
Surgical barriers may help decrease postoperative adhesion formation but cannot compensate for poor surgical technique. I rarely use adhesion barriers because I feel that careful tissue handling, excellent hemostasis, avoiding trauma to healthy tissue, and removal of all diseased tissue are the key ways to obtain good postsurgical results and reduce adhesions.
Hyaluronic acid agents may decrease the prevalence of adhesions and prevent the deterioration of preexisting adhesions, but because of the limited number of studies available, these data should be interpreted with caution.3 However, ASRM found no substantial evidence that they improve fertility, decrease pain, or reduce the incidence of postoperative bowel obstruction.
Averting adhesions: Surgical techniques and tools
By Togas Tulandi, MD, MHCM, and Mohammed Al-Sunaidi, MD It’s available in our archive at www.obgmanagement.com
A move from clomiphene directly to IVF may cut time to pregnancy
Reindollar RH, Regan MM, Neumann PJ, Thornton KL, Alper MM, Goldman MB. A randomized controlled trial of 503 couples assigned to conventional infertility treatment or an accelerated track to IVF: Preliminary results of the fast track and standard treatment (FASTT) trial. Fertil Steril. 2007;88(Suppl 1):S41.
This very important abstract, presented at the annual meeting of ASRM, has the potential to dramatically change fertility treatment. The multicenter randomized controlled clinical trial measured the efficacy and time to pregnancy of an accelerated treatment strategy for women 21 to 39 years old who had unexplained infertility. A similar percentage of patients—approximately 75%—became pregnant in each arm (traditional versus accelerated), with a shorter time to pregnancy in the accelerated arm.
The new paradigm for management of unexplained infertility includes:
- comprehensive fertility history and physical examination
- targeted laboratory testing and other investigation, as needed
- counseling and psychological support for the patient once the diagnosis is made
- empiric treatment with clomiphene citrate plus intrauterine insemination (IUI) for as many as three cycles
- immediate IVF for as many as six cycles.
Details of the trial
Women in the trial had attempted to conceive for 12 months and had normal ovarian reserve (and semen analysis) and no pelvic pathology. Couples already treated for infertility were excluded.
Participants were randomized to:
- a conventional treatment regimen of three cycles of clomiphene citrate with IUI, three cycles of folliclestimulating hormone (FSH) and IUI, and as many as six cycles of IVF or
- three cycles of clomiphene citrate with IUI and then as many as six cycles of IVF.
Regimen likely reduces cost, stress
Major issues affecting the eventual success rate for infertile couples are cost and psychological stress, which can cause even patients who have a good prognosis to drop out of treatment. The major complication of fertility treatment is multiple pregnancy. By avoiding the use of gonadotropins in couples with unexplained infertility and accelerating the transition to IVF, physicians can lower the cost and psychological stress of treatment. They can also reduce the likelihood of multiple pregnancy because it is easier to control the number of embryos transferred in IVF than the number of follicles that develop with gonadotropins.
In women younger than 35 years on the first IVF cycle who have a good prognosis, ASRM now recommends that only one or two day-3 embryos be transferred, and not more than one day-5 blastocyst.4 The multiple-birth rate has declined in recent years, as more and more IVF clinics place fewer embryos; the rate should continue to fall with wider application of elective single-embryo transfer.5,6
Because this accelerated protocol produces a similar number of births over a shorter period and has the potential to lower cost, psychological stress, and the multiple-birth rate, it deserves implementation for many patients and warrants further evaluation for potential benefits in other populations.
It’s no help, after all: Preimplantation genetic screening for aneuploidy
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: A Practice Committee report. Fertil Steril. 2007;88:1497–1504.
Mastenbroek S, Twisk M, van Echten-Arends J, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17.
Preimplantation genetic diagnosis of known single-gene defects, structural chromosomal rearrangements, X-linked disorders, and human leukocyte antigen typing is a major benefit to couples known to be at risk of passing on a heritable and debilitating genetic disease. Aneuploidy is the most common cause of early pregnancy loss, and its prevalence increases with maternal age and may increase in chromosomally normal couples who experience recurrent early pregnancy loss or repeated failure of IVF cycles. Preimplantation genetic screening (PGS) has been advocated to identify and transfer only euploid embryos and increase the chance of successful pregnancy.
New data from Mastenbroek and colleagues indicate that PGS for aneuploidy does not increase the rate of pregnancy or live birth. After several years of increasing utilization and studies suggesting that PGS has benefit, the first multicenter, randomized, doubleblind, controlled study that compared three cycles of IVF with and without PGS in women 35 to 41 years old concluded that PGS does not increase but, in fact, significantly reduces the rate of pregnancy and live birth in this group.
Findings sparked controversy
This trial generated controversy within the genetics and reproductive endocrinology specialties because it challenged the intuitive view that screening of embryos before transfer into the uterus should be beneficial—or, at least, harmless. Some now argue that the benefits of PGS, if any, cannot be intuitively assumed and assert that the burden of proof of those benefits rests with proponents of PGS.
The practice committees of the Society for Assisted Reproductive Technology (SART) and ASRM found insufficient evidence to support the use of PGS to improve the live birth rate in women of advanced age or in those who have had implantation failure or recurrent pregnancy loss (TABLE). Many physicians believe, however, that technologies under development will soon bring verifiable benefits of PGS to patients.
SART and ASRM weigh in on use of preimplantation genetic testing
| TEST | RECOMMENDATION |
|---|---|
| Pre-implantation genetic diagnosis | |
| Pre- implantation genetic screening | |
| SOURCE: Society for Assisted Reproductive Technology and American Society for Reproductive Medicine | |
Advise your patients that oocyte cryopreservation is “a long shot”
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a practice committee opinion. Fertil Steril. 2007;88:1495–1496.
Oocyte cryopreservation is an experimental procedure that should not be offered or marketed as a means to defer reproductive aging, primarily because data on clinical outcomes are limited. That is the conclusion of this guideline from SART and ASRM. Consequently, women who may be considering the procedure should be fully informed about the process and likely outcomes and counseled by a qualified mental health professional.
Counseling is crucial
According to the SART and ASRM guideline, pretreatment counseling should include comprehensive information on a range of topics (see the box below). In addition, women considering oocyte cryopreservation should be counseled thoroughly about reproductive aging and life planning.7,8
Few alternatives for some women
Women who have cancer should receive the same counseling. Unlike healthy women, however, they may have no other options, and cryopreservation may be more appropriate for them despite experimental status.
Patients considering this procedure need comprehensive information about:
- Ovarian stimulation and oocyte retrieval
- Methods of oocyte cryopreservation
- Storage fees
- The expected thaw survival rate
- The requirement for intracytoplasmic sperm injection
- Clinic-specific data and outcomes or, in their absence, literature estimates of a 2% overall live birth rate per oocyte thawed using slow-freeze methods and 4% for vitrification, compared with age-related probabilities of success per IVF cycle using fresh nondonor oocytes
- The relatively low likelihood that a woman who cryopreserves her eggs before age 35 will ever need or use them
- State and federal screening laws for potential donation of cryopreserved oocytes
- Potential risks of basing important life decisions and expectations on a limited number of cryopreserved oocytes
- The possibility that the facility may cease operation, necessitating transfer of cryopreserved oocytes to another facility
- The possibility that cryopreserved oocytes might be lost or damaged as a result of laboratory error or other events beyond control.
The field of reproductive endocrinology and infertility is anything but stagnant. New technologies continue to enter the market at a brisk pace, and a greater emphasis on evidence has produced better-designed randomized controlled trials, meta-analyses, and practice guidelines. This means greater availability of standardized protocols that reflect best practice and can be tailored to a patient’s condition and needs.
Highlighted here are notable studies and guidelines from the past year, including advice on:
- preventing peritoneal adhesions
- expediting in vitro fertilization (IVF) for unexplained infertility
- counseling the patient about the real limitations of preimplantation genetic screening for aneuploidy
- informing patients that oocyte cryopreservation is unlikely to lead to live birth.
Guideline urges good surgical technique in battle against adhesions
Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Re-productive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery. Fertil Steril. 2007;88:21–26.
This newly released practice guideline from the American Society of Reproductive Medicine (ASRM) focuses on adhesions and their impact on fertility. The guideline reiterates that peritoneal adhesions are a common and serious complication of gynecologic surgery and emphasizes key principles to reduce their likelihood and extent. These principles include the need to:
- Perform surgery only when the benefits of doing so clearly outweigh the risks
- Handle tissue gently (this is the most important preventive technique)
- Don’t assume laparoscopy is superior to laparotomy—it will be only if less tissue injury occurs
- Be especially careful when operating on or near ovaries, which form adhesions easily.
Ovarian surgery often necessitates additional operations
Studies have demonstrated that approximately 33% of patients who undergo open abdominal or pelvic surgery are readmitted, on average, two times over the subsequent 10 years for conditions directly or possibly related to adhesions or for further surgery that could be complicated by adhesions. The highest readmission rate directly related to adhesions—7.5 for every 100 initial operations—was associated with ovarian surgery performed via laparotomy.
Adhesion-related complications of gynecologic surgery include small-bowel obstruction, which occurs in approximately 1.5% of women who have undergone abdominal hysterectomy.
The relationship between adhesions and pelvic pain is unclear, although severe bowel adhesions can cause visceral pain. The ASRM guideline notes that “the impact that lysis of bowel or adnexal adhesions may have on abdominal and pelvic pain cannot be predicted confidently.” Postoperative adhesions increase subsequent operating times and risk of bowel injury.
How adhesions affect fertility
Adhesions may impair fertility by distorting adnexal anatomy and interfering with gamete and embryo transport. Among infertile women who have adnexal adhesions, adhesiolysis is associated with pregnancy rates of 32% at 12 months and 45% at 24 months, compared with 11% and 16%, respectively, for untreated women.1 Pregnancy rates are inversely correlated with adhesion scores on the ASRM classification system for adnexal adhesions.2
Some, but not all, adhesion-reducing measures work
According to the ASRM guideline, adhesions may be prevented, at least theoretically, by:
- minimizing peritoneal injury during surgery
- avoiding the introduction of reactive foreign bodies
- reducing the local inflammatory response
- inhibiting the coagulation cascade and promoting fibrinolysis
- placing barriers between damaged tissues.
Pharmacotherapeutic and fluid agents. ASRM found no evidence of improved pregnancy outcomes for pharmacologic and fluid agents used as an adjunct during pelvic surgery. For example, anti-inflammatory agents that have been evaluated, both locally and systemically, including dexamethasone and promethazine, have not reduced postoperative adhesions. Antibiotic solutions, 32% Dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids have been used to separate adjacent peritoneal surfaces via “hydroflotation,” but none have reduced adhesion formation.
Surgical barriers may help decrease postoperative adhesion formation but cannot compensate for poor surgical technique. I rarely use adhesion barriers because I feel that careful tissue handling, excellent hemostasis, avoiding trauma to healthy tissue, and removal of all diseased tissue are the key ways to obtain good postsurgical results and reduce adhesions.
Hyaluronic acid agents may decrease the prevalence of adhesions and prevent the deterioration of preexisting adhesions, but because of the limited number of studies available, these data should be interpreted with caution.3 However, ASRM found no substantial evidence that they improve fertility, decrease pain, or reduce the incidence of postoperative bowel obstruction.
Averting adhesions: Surgical techniques and tools
By Togas Tulandi, MD, MHCM, and Mohammed Al-Sunaidi, MD It’s available in our archive at www.obgmanagement.com
A move from clomiphene directly to IVF may cut time to pregnancy
Reindollar RH, Regan MM, Neumann PJ, Thornton KL, Alper MM, Goldman MB. A randomized controlled trial of 503 couples assigned to conventional infertility treatment or an accelerated track to IVF: Preliminary results of the fast track and standard treatment (FASTT) trial. Fertil Steril. 2007;88(Suppl 1):S41.
This very important abstract, presented at the annual meeting of ASRM, has the potential to dramatically change fertility treatment. The multicenter randomized controlled clinical trial measured the efficacy and time to pregnancy of an accelerated treatment strategy for women 21 to 39 years old who had unexplained infertility. A similar percentage of patients—approximately 75%—became pregnant in each arm (traditional versus accelerated), with a shorter time to pregnancy in the accelerated arm.
The new paradigm for management of unexplained infertility includes:
- comprehensive fertility history and physical examination
- targeted laboratory testing and other investigation, as needed
- counseling and psychological support for the patient once the diagnosis is made
- empiric treatment with clomiphene citrate plus intrauterine insemination (IUI) for as many as three cycles
- immediate IVF for as many as six cycles.
Details of the trial
Women in the trial had attempted to conceive for 12 months and had normal ovarian reserve (and semen analysis) and no pelvic pathology. Couples already treated for infertility were excluded.
Participants were randomized to:
- a conventional treatment regimen of three cycles of clomiphene citrate with IUI, three cycles of folliclestimulating hormone (FSH) and IUI, and as many as six cycles of IVF or
- three cycles of clomiphene citrate with IUI and then as many as six cycles of IVF.
Regimen likely reduces cost, stress
Major issues affecting the eventual success rate for infertile couples are cost and psychological stress, which can cause even patients who have a good prognosis to drop out of treatment. The major complication of fertility treatment is multiple pregnancy. By avoiding the use of gonadotropins in couples with unexplained infertility and accelerating the transition to IVF, physicians can lower the cost and psychological stress of treatment. They can also reduce the likelihood of multiple pregnancy because it is easier to control the number of embryos transferred in IVF than the number of follicles that develop with gonadotropins.
In women younger than 35 years on the first IVF cycle who have a good prognosis, ASRM now recommends that only one or two day-3 embryos be transferred, and not more than one day-5 blastocyst.4 The multiple-birth rate has declined in recent years, as more and more IVF clinics place fewer embryos; the rate should continue to fall with wider application of elective single-embryo transfer.5,6
Because this accelerated protocol produces a similar number of births over a shorter period and has the potential to lower cost, psychological stress, and the multiple-birth rate, it deserves implementation for many patients and warrants further evaluation for potential benefits in other populations.
It’s no help, after all: Preimplantation genetic screening for aneuploidy
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: A Practice Committee report. Fertil Steril. 2007;88:1497–1504.
Mastenbroek S, Twisk M, van Echten-Arends J, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17.
Preimplantation genetic diagnosis of known single-gene defects, structural chromosomal rearrangements, X-linked disorders, and human leukocyte antigen typing is a major benefit to couples known to be at risk of passing on a heritable and debilitating genetic disease. Aneuploidy is the most common cause of early pregnancy loss, and its prevalence increases with maternal age and may increase in chromosomally normal couples who experience recurrent early pregnancy loss or repeated failure of IVF cycles. Preimplantation genetic screening (PGS) has been advocated to identify and transfer only euploid embryos and increase the chance of successful pregnancy.
New data from Mastenbroek and colleagues indicate that PGS for aneuploidy does not increase the rate of pregnancy or live birth. After several years of increasing utilization and studies suggesting that PGS has benefit, the first multicenter, randomized, doubleblind, controlled study that compared three cycles of IVF with and without PGS in women 35 to 41 years old concluded that PGS does not increase but, in fact, significantly reduces the rate of pregnancy and live birth in this group.
Findings sparked controversy
This trial generated controversy within the genetics and reproductive endocrinology specialties because it challenged the intuitive view that screening of embryos before transfer into the uterus should be beneficial—or, at least, harmless. Some now argue that the benefits of PGS, if any, cannot be intuitively assumed and assert that the burden of proof of those benefits rests with proponents of PGS.
The practice committees of the Society for Assisted Reproductive Technology (SART) and ASRM found insufficient evidence to support the use of PGS to improve the live birth rate in women of advanced age or in those who have had implantation failure or recurrent pregnancy loss (TABLE). Many physicians believe, however, that technologies under development will soon bring verifiable benefits of PGS to patients.
SART and ASRM weigh in on use of preimplantation genetic testing
| TEST | RECOMMENDATION |
|---|---|
| Pre-implantation genetic diagnosis | |
| Pre- implantation genetic screening | |
| SOURCE: Society for Assisted Reproductive Technology and American Society for Reproductive Medicine | |
Advise your patients that oocyte cryopreservation is “a long shot”
Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a practice committee opinion. Fertil Steril. 2007;88:1495–1496.
Oocyte cryopreservation is an experimental procedure that should not be offered or marketed as a means to defer reproductive aging, primarily because data on clinical outcomes are limited. That is the conclusion of this guideline from SART and ASRM. Consequently, women who may be considering the procedure should be fully informed about the process and likely outcomes and counseled by a qualified mental health professional.
Counseling is crucial
According to the SART and ASRM guideline, pretreatment counseling should include comprehensive information on a range of topics (see the box below). In addition, women considering oocyte cryopreservation should be counseled thoroughly about reproductive aging and life planning.7,8
Few alternatives for some women
Women who have cancer should receive the same counseling. Unlike healthy women, however, they may have no other options, and cryopreservation may be more appropriate for them despite experimental status.
Patients considering this procedure need comprehensive information about:
- Ovarian stimulation and oocyte retrieval
- Methods of oocyte cryopreservation
- Storage fees
- The expected thaw survival rate
- The requirement for intracytoplasmic sperm injection
- Clinic-specific data and outcomes or, in their absence, literature estimates of a 2% overall live birth rate per oocyte thawed using slow-freeze methods and 4% for vitrification, compared with age-related probabilities of success per IVF cycle using fresh nondonor oocytes
- The relatively low likelihood that a woman who cryopreserves her eggs before age 35 will ever need or use them
- State and federal screening laws for potential donation of cryopreserved oocytes
- Potential risks of basing important life decisions and expectations on a limited number of cryopreserved oocytes
- The possibility that the facility may cease operation, necessitating transfer of cryopreserved oocytes to another facility
- The possibility that cryopreserved oocytes might be lost or damaged as a result of laboratory error or other events beyond control.
1. Tulandi T, Collins JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
2. Marana R, Rizzi M, Muzii L, Catalano GF, Caruana P, Mancuso S. Correlation between the American Fertility Society classification of adnexal adhesions and distal tubal occlusion, salpingoscopy, and reproductive outcome in tubal surgery. Fertil Steril. 1995;64:924-929.
3. Metwally M, Gorvy D, Watson A, Li TC. Hyaluronic acid fluid agents for the prevention of adhesions after fertility-preserving gynecological surgery: a metaanalysis of randomized controlled trials. Fertil Steril. 2007;87:1139-1146.
4. Practice Committee of the Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-S52.
5. Adamson GD, Baker VL. Multiple births from assisted reproductive technologies: a challenge that must be met. Fertil Steril. 2004;81:517-522.
6. Stern JE, Cedars MI, Jain T, et al. for the Society for Assisted Reproductive Technology Writing Group Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275-282.
7. Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389-1394.
8. Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548-1553.
1. Tulandi T, Collins JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
2. Marana R, Rizzi M, Muzii L, Catalano GF, Caruana P, Mancuso S. Correlation between the American Fertility Society classification of adnexal adhesions and distal tubal occlusion, salpingoscopy, and reproductive outcome in tubal surgery. Fertil Steril. 1995;64:924-929.
3. Metwally M, Gorvy D, Watson A, Li TC. Hyaluronic acid fluid agents for the prevention of adhesions after fertility-preserving gynecological surgery: a metaanalysis of randomized controlled trials. Fertil Steril. 2007;87:1139-1146.
4. Practice Committee of the Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-S52.
5. Adamson GD, Baker VL. Multiple births from assisted reproductive technologies: a challenge that must be met. Fertil Steril. 2004;81:517-522.
6. Stern JE, Cedars MI, Jain T, et al. for the Society for Assisted Reproductive Technology Writing Group Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275-282.
7. Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389-1394.
8. Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548-1553.
FERTILITY
Changes are occurring so quickly it is often difficult for the general ObGyn to know the most advanced and appropriate treatment for a given patient. The American Society for Reproductive Medicine (ASRM) Practice Committee establishes guidelines based upon well-designed studies to help physicians keep abreast of the best clinical practices. In this article, I focus on recent ASRM guidelines in 5 topical areas associated with substantial misinformation in both the professional and public sectors:
- when and how gynecologists should initiate infertility testing and treatment
- how to evaluate and manage recurrent pregnancy loss
- the need to reduce the rate of multiple gestation from IVF and ART
- the expanded applications for preimplantation genetic diagnosis
- the truth about fertility-sparing efforts in young women planning to undergo cancer therapy and other treatments.
When and how to evaluate patients complaining of infertility
Infertility is a disease, but there are different opinions about when a woman reporting this condition should be assessed (TABLE 1). According to the ASRM, a couple should not be considered infertile until they have tried to conceive spontaneously for at least 12 months, unless the medical history and physical findings dictate earlier evaluation and treatment.1
For example, approximately 25% of couples experience infertility when the woman is age 35, and about 50% experience it when the woman is age 40. Therefore, it is reasonable to investigate infertility after 6 months of attempted conception when the woman is over 35 and after 3 months if she is over age 40.2 The primary reason for this age-related reduction in fertility is the diminishing number and quality of oocytes over time.
Other risk factors for infertility include smoking, family history of premature ovarian failure, significant ovarian pathology, previous ovarian surgery, history of oligomenorrhea or amenorrhea, known or suspected disease of the uterus or fallopian tubes, endometriosis, or a partner known to be subfertile.3,4
TABLE 1
When to investigate infertility, treat, and refer
| INVESTIGATE |
| After 12 months of unprotected intercourse if age |
| After 6 months of unprotected intercourse if age 35–39 |
| After 3 months of unprotected intercourse if age ≥40 |
| After 0–6 months if patient has history of or risk factors for infertility |
| TREAT |
| Treat identifiable causes of infertility |
| Optimize factors influencing fertility: |
|
| Treat empirically (eg, clomiphene, insemination) for 3–6 months in patients |
| REFER |
| History of infertility or significant risk factors |
| Significant fertility problems identified during investigation |
| Age ≥40 |
| After 3–6 months of failed treatment for identifiable causes |
| After 3–6 months of failed empiric treatment |
How to evaluate ovarian function
A careful history and physical examination are key components of systematic, expeditious, and cost-effective identification of the cause of infertility (TABLE 2). A menstrual history and basal body temperature recordings are useful in the diagnosis of ovulatory dysfunction and are easy to obtain. Measurements of urinary luteinizing hormone (LH) using ovulation-prediction kits and mid-luteal-phase serum progesterone are also helpful.
Endometrial biopsy is rarely indicated because of its lack of clinical relevance.
Serial vaginal ultrasonography of the size and number of ovarian follicles may be indicated when simpler methods are inconclusive.
Other tests to evaluate ovarian function may include thyroid-stimulating hormone (TSH), serum prolactin, cycle day 3 follicle-stimulating hormone (FSH) and estradiol, and the clomiphene citrate challenge test in selected patients at higher risk of ovarian dysfunction.
TABLE 2
Current status of tests and treatments
| OLD, NOW RARELY INDICATED |
| Postcoital test |
| Endometrial biopsy |
| Antisperm antibodies testing |
| Intracervical insemination |
| Clomiphene for more than 3–6 cycles |
| Routine hCG injection to stimulate ovulation in clomiphene cycles |
| NEW AND HELPFUL |
| Clomiphene citrate challenge test in selected patients |
| Serial vaginal ultrasounds to evaluate response to ovarian stimulation |
| Saline sonohysterography |
| Preimplantation genetic diagnosis for single-gene defects |
| Embryo cryopreservation |
| Single-embryo transfer to reduce multiple pregnancy rates |
| NEW BUT STILL EXPERIMENTAL* |
| Preimplantation genetic screening for aneuploidy in older patients |
| Human lymphocyte antigen typing for recurrent pregnancy loss |
| Intravenous immunoglobulin for recurrent pregnancy loss |
| Ovarian tissue or oocyte cryopreservation for fertility preservation |
| * Should be performed only in clinical trials |
Clomiphene citrate is preferred
Ovarian dysfunction can be treated with clomiphene for 3 to 6 cycles5 starting at 50 mg per day from cycle day 5 to 9 and increasing to 100 and then 150 mg per day if ovulation does not occur. The drug may also be effective empiric treatment for unexplained infertility using 100 mg per day from cycle days 3 through 7 for a maximum of 3 to 4 cycles.
Only gynecologists experienced with ovarian stimulation drugs and with access to daily ultrasonographic monitoring and estradiol levels should use them, because of the risk of multiple pregnancy and ovarian hyperstimulation.
For women with polycystic ovary syndrome (PCOS), clomiphene alone is more effective than metformin alone. Ovarian drilling may be an effective surgical treatment for PCOS if clomiphene fails, but the cost and risk of adhesions must be considered.
Human chorionic gonadotropin (hCG) injections during clomiphene treatment to stimulate ovulation should be given only if the patient’s own urinary LH surge cannot be detected.
A single intrauterine insemination (IUI) improves the pregnancy rate slightly in conjunction with clomiphene, and by an odds ratio of approximately 2 in conjunction with gonadotropins. The gonadotropin dosage ranges from about 75 to 600 IU per day for 8 to 12 days, based on patient need and careful monitoring.
When to give up on ovarian stimulation. Failure to achieve pregnancy after 3 to 6 cycles signals the need to expand diagnostic evaluation or change treatment strategies.
Evaluate the uterus and tubes
Uterine factors rarely cause infertility but warrant thorough investigation all the same, including assessment of uterine cavity size and shape. A number of methods are available:
- hysterosalpingography (HSG)
- ultrasonography
- saline sonohysterography
Peritoneal factors such as endometriosis or pelvic or adnexal adhesions may occasionally be identified by ultrasonography if there is a mass, but are more likely to require laparoscopy.
When laparoscopy is indicated
If there is evidence or a strong suspicion of endometriosis, pelvic or adnexal adhesions, or significant tubal disease, laparoscopy is warranted. It also may be helpful in younger patients (eg,
Because they reduce pregnancy rates by 50%, hydrosalpinges should be removed or the fallopian tube should be ligated proximally before IVF. It also is important to consider the number of patients needed to treat by laparoscopy to obtain 1 additional pregnancy.
Only gynecologists with expertise should perform laparoscopy, because it is important to make the correct diagnosis and be capable of surgically treating conditions found during the surgery.
Skip the postcoital test, but keep the semen analysis
Abnormalities of the cervical mucus or sperm–mucus interaction rarely cause infertility. Therefore, the postcoital test has questionable predictive value and is probably only useful to confirm that the couple can have properly timed intercourse during the cycle.3
A male factor is solely responsible in about 20% of infertile couples and contributory in another 30% to 40%. For this reason, semen analysis is always warranted when the female is being evaluated for infertility.
Examination of the male partner should be performed by the gynecologist, or the male should be referred to a urologist interested in infertility.6
For recurrent pregnancy loss, best treatment is TLC
Recurrent pregnancy loss is challenging because it is so emotionally charged for the patient, the cause is often unclear, and we lack specific treatments. A methodical and empathetic approach is therefore recommended.
What the history can reveal
Many women with recurrent pregnancy loss will eventually have a live birth, but increasing numbers of miscarriages do predict a poorer overall chance of success, as does increasing age.
Lifestyle factors rarely, if ever, cause recurrent pregnancy loss, but the following factors may increase the risk of miscarriage: obesity, high daily caffeine intake, alcohol consumption, use of nonsteroidal anti-inflammatory drugs, and social class and occupation. A previous diagnosis of or treatment for infertility also increases the risk of recurrent loss.
Smoking should be discouraged and healthy lifestyles should be promoted.7
Causes of recurrent pregnancy loss
Definite causal factors include chromosomal abnormalities, such as translocations, in approximately 5% of couples with 2 or more losses.
Probable factors include uterine abnormalities (both congenital abnormalities such as septate, and acquired defects such as adhesions and intrauterine or submucous myomas), uncontrolled thyroid disease or diabetes, PCOS, and antiphospholipid antibody syndrome.
Other thrombophilias, such as those associated with factor V Leiden mutation, activated protein C resistance, and possibly prothrombin G20210A and protein S deficiency, have been found by some investigators to be associated with recurrent pregnancy loss. It is doubtful that antithyroid antibodies and sharing of parental human lymphocyte antigen (HLA) cause recurrent miscarriage.7
Genetic component likely. The risk of recurrent pregnancy loss in first-degree relatives of women with unexplained repeated pregnancy loss who have normal chromosomes is approximately 6 times higher than the risk in the background population, suggesting a polygenic mode of inheritance.7,8
Other possible causes include low plasma folate levels, which have been associated with an increased risk of first-trimester pregnancy loss. Environmental toxins such as ionizing radiation, organic solvents, alcohol, mercury, and lead are confirmed causes of recurrent pregnancy loss; hyperthermia is a suspected cause.8
Recommended evaluation
Investigations that have been proven in many studies include:
- HSG, hysteroscopy, and sonohysterography
- karyotyping of the couple
- measurement of thyroid hormone
- hemoglobin A1C and serum glucose assessment
- activated partial thromboplastin time, dilute Russell viper venom time, and lupus anticoagulant assessment
- measurement of immunoglobulin G and immunoglobulin M anticardiolipin antibodies
- test for factor V Leiden mutation
Examine products of conception?
Although it is routine practice to send products of conception for histologic examination, mainly to exclude a gestational trophoblastic disorder, the usefulness of this practice is unclear.8 In couples with recurrent pregnancy loss, chromosomal analysis of the products of conception indicates that a normal conceptus karyotype in a previous pregnancy is a predictor of a higher rate of miscarriage in a subsequent pregnancy.8 When stratified by maternal age, there is no difference in the distribution of cytogenetically abnormal miscarriages in couples with recurrent pregnancy loss, compared with controls.8 The cost-effectiveness of karyotyping is therefore unclear.
High levels of homocysteine (ie, hyperhomocysteinemia) can be associated with recurrent pregnancy loss. Among genetic causes is polymorphism at position 677 in the methylene tetrahydrofolate reductase (MTHFR) gene, which is often evaluated to rule out this condition.
Infections with bacteria, viruses, or parasites can all interfere with early pregnancy development, but none seem to be a significant cause of recurrent pregnancy loss.8 Testing is most useful in the context of an acute infectious episode.
Can recurrent loss be treated?
The hallmark of treatment is empathetic care, along with counseling emphasizing the complexity of this condition. Any endocrinologic, anatomic, or other abnormality that is identified during evaluation should be treated, if possible.
Progesterone supplementation is not proven treatment. This therapy is commonly prescribed but has not been proved to improve live birth rates.
Prednisone, aspirin, and NSAIDs have no benefits but potential risks and should not be used.
Current immunologic therapies for recurrent pregnancy loss have no sound scientific basis, except for the use of heparin and aspirin in patients with well-documented antiphospholipid antibodies.7 Specifically, intravenous immunoglobulin remains unproven, is experimental, and should be provided only in approved research settings.9 Paternal leukocyte immunization does not work, has been proscribed by the US Food and Drug Administration, and should be avoided.
Careful counseling and education of the patient about the history, pathophysiology, testing, test results, and treatment of recurrent pregnancy loss are necessary. Women with subfertility who have taken a long time to conceive should be treated empirically with ovarian stimulation in an attempt to shorten the time to conception.
Singleton births can be encouraged without jeopardizing IVF, ART
Multiple gestations have increased over the past 15 years, largely because of:
- ovulation induction for management of oligo-ovulation
- superovulation to produce more than 1 ovulated egg for fertility treatments
- assisted reproductive technologies (ART), in which more than 1 embryo is replaced to increase the pregnancy rate
Multiple gestations are a bad idea
Risks include a higher complication rate for gravidas and fetuses, as well as higher short- and long-term costs to patients and society. It is therefore important to reduce the incidence of multiple gestation associated with fertility treatments.10
How to reduce the likelihood of multiple fetuses
- Closely monitor cycles involving ovulation induction and superovulation for efficacy and safety, to avoid ovarian hyperstimulation and reduce the risk of multiple gestation. Although attempts to limit multiple gestation during ovulation induction or superovulation using ultrasonographic criteria and serum estradiol limits have been ineffective,10 it is my opinion that we should err on the side of conservatism, even though the optimal parameters for doing so have not been determined by high-quality trials. I recommend that hCG or IUI be avoided if more than 4 mature follicles (>15 mm) or 6 large follicles (>12 mm) are present on a sonogram, and the couple should be instructed to refrain from intercourse.
- Focus on the objective of a single healthy baby as the optimal outcome. Data published by the Society for Assisted Reproductive Technology (SART) clearly demonstrate the clinical impact of a reduction in the number of embryos transferred, which reduced triplet pregnancy rates in 2005 to less than half the rate in the late 1990s. Fewer embryos are transferred today than just a few years ago, and the trend is continuing. This will help reduce the triplet rate further and also reduce twin pregnancies. In the past 6 months, guidelines have recommended replacement of only 1 blastocyst at day 5 or 1 to 2 embryos at day 3 in women under age 35 with a favorable prognosis.
What the future holds
We can expect more elective single-embryo or single-blastocyst transfers as we gain further expertise in this area. However, this practice should be implemented carefully in selected patients to maintain adequate pregnancy rates while reducing multiple gestations.
The United States has the highest ART success rates in the world (approximately 40% higher than in Europe) despite a reduction in the number of triplet or higher-order pregnancies resulting in live births after ART—from 7.0% in 1996 to 2.4% in 2004. The twin rate has remained stable at approximately 30%, but should decrease as 1- and 2-embryo transfers become more common.11
Preimplantation genetic diagnosis now has multiple applications
Preimplantation genetic diagnosis (PGD) is over 15 years old, and at least 1,000 babies worldwide have been born after its use, with no reports of increased fetal malformation or other problems.12
Two basic techniques are employed to analyze the genomic status of the 1 or 2 blastomeres usually removed from the 8-cell embryo on day 3 after fertilization:
- Polymerase chain reaction (PCR) is used to amplify a specific DNA sequence harboring a mutation. A mismatch (eg, due to a genetic deletion) leads to differential migration on the gel, thus permitting diagnosis. The error rate, primarily due to allelic dropout, in which 1 of the 2 alleles selectively amplifies and thus contributes to diagnostic errors, is approximately 0.3% to 5.6%.
- Fluorescent in situ hybridization (FISH) allows determination of the ploidy of a blastomere. Labeled probes bind to chromosomes and are viewed under a fluorescent microscope. The error rate is 1% to 10% for a variety of technical reasons.12 Testing takes about 1 day while the embryos are developing to blastocysts, at which time those that are viable and tested to be normal are transferred back into the uterus.
Not just for gender determination
PGD initially was used to determine gender (by FISH) as an indirect method of avoiding X-linked genetic diseases such as hemophilia. The error rate for gender determination is less than 1%. Since then, single-gene-defect disorders have been diagnosed using PCR and heteroduplex formation or restriction endonuclease digestion, both of which distinguish normal from mutant alleles. PGD has been performed broadly to diagnose Tay-Sachs, Huntington’s disease, and hundreds of other diseases.
Testing for translocation by PGD has been especially useful and may reduce the risk of spontaneous abortion from as much as 95% to 13% if one of the parents is a known translocation carrier.
Still under investigation is the routine use of FISH to detect aneuploidy in cases of recurrent pregnancy loss. The use of FISH for gender selection for family balancing is not recommended by the ASRM.
More young women seek to preserve their fertility
Fertility preservation through ovarian tissue or oocyte cryopreservation or vitrification has recently been popularized by cancer survival consumer groups, the media, and other interests. In addition to cancer patients planning to undergo chemotherapy or radiotherapy, candidates for fertility preservation include women undergoing bone marrow or stem cell transplantation or oophorectomy (for a benign tumor, endometriosis, or prophylaxis) and patients with severe autoimmune disease needing chemotherapy.
In cancer patients, fertility is preserved using one of several methods:
- shielding or moving the ovaries to a different anatomic site during radiation
- use of gonadotropin-releasing hormone analogs or oral contraceptives during chemotherapy (unproven)
- changes to chemotherapy regimen
- IVF cycle followed by cryopreservation of embryos if the patient has a male partner or is prepared to use donor sperm (provided the oncologist confirms that ovarian stimulation and high estradiol levels are acceptable and there is time to undergo an IVF cycle before cancer treatment begins).
Unresolved issues
Concerns about cryopreservation of ovarian tissue in cancer patients13,14 include the possibility of reseeding tumor cells after ovarian transplantation, malignant transformation of transplanted ovarian tissue, and the possibility of congenital abnormalities as a result of cryopreservation—although no increase has been found in the patients studied so far.
The pregnancy rate is low
For cancer patients, the preservation of ovarian tissue or oocytes yields pregnancy rates significantly lower than those observed with standard IVF procedures. For cancer patients facing chemotherapy, however, oocyte cryopreservation may be one of the few options available and is acceptable in experimental protocols approved by the institutional review board.
Physicians should inform cancer patients about the options for fertility preservation prior to treatment.14 We lack data to recommend ovarian tissue or oocyte cryopreservation for the sole purpose of circumventing reproductive aging in healthy women.13
1. Practice Committee. American Society for Reproductive Medicine. Definition of “infertility.” Fertil Steril. 2006;86(Suppl 4):S228.-
2. Practice Committee, American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86(Suppl 4):S248-252.
3. Practice Committee, American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(Suppl 4):S264-267.
4. Practice Committee, American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2006;86(Suppl 4):S172-177.
5. Practice committee, American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86(Suppl 4):S187-193.
6. Male Infertility Best Practice Committee, American Urological Association, and Practice Committee, American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(Suppl 4):S202-209.
7. Christiansen OB, Andersen A-MN, Bosch E, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83:821-839.
8. Jauniauz E, Farquharson RG, Christiansen OB, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21:2216-2222.
9. Practice Committee, American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril. 2006;86(Suppl 4):S226-227.
10. Practice committee, American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(Suppl 4):S106-110.
11. Practice Committee, Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-52.
12. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Preimplantation genetic diagnosis. Fertil Steril. 2006;86(Suppl 4):S257-258.
13. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86(Suppl 4):S142-147.
14. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622-1628.
Dr. Adamson reports no financial relationships with any company whose products are mentioned in this article. He receives grant/research support from IBSA, Serono, and ViaCell and is a consultant to ViaCell.
Changes are occurring so quickly it is often difficult for the general ObGyn to know the most advanced and appropriate treatment for a given patient. The American Society for Reproductive Medicine (ASRM) Practice Committee establishes guidelines based upon well-designed studies to help physicians keep abreast of the best clinical practices. In this article, I focus on recent ASRM guidelines in 5 topical areas associated with substantial misinformation in both the professional and public sectors:
- when and how gynecologists should initiate infertility testing and treatment
- how to evaluate and manage recurrent pregnancy loss
- the need to reduce the rate of multiple gestation from IVF and ART
- the expanded applications for preimplantation genetic diagnosis
- the truth about fertility-sparing efforts in young women planning to undergo cancer therapy and other treatments.
When and how to evaluate patients complaining of infertility
Infertility is a disease, but there are different opinions about when a woman reporting this condition should be assessed (TABLE 1). According to the ASRM, a couple should not be considered infertile until they have tried to conceive spontaneously for at least 12 months, unless the medical history and physical findings dictate earlier evaluation and treatment.1
For example, approximately 25% of couples experience infertility when the woman is age 35, and about 50% experience it when the woman is age 40. Therefore, it is reasonable to investigate infertility after 6 months of attempted conception when the woman is over 35 and after 3 months if she is over age 40.2 The primary reason for this age-related reduction in fertility is the diminishing number and quality of oocytes over time.
Other risk factors for infertility include smoking, family history of premature ovarian failure, significant ovarian pathology, previous ovarian surgery, history of oligomenorrhea or amenorrhea, known or suspected disease of the uterus or fallopian tubes, endometriosis, or a partner known to be subfertile.3,4
TABLE 1
When to investigate infertility, treat, and refer
| INVESTIGATE |
| After 12 months of unprotected intercourse if age |
| After 6 months of unprotected intercourse if age 35–39 |
| After 3 months of unprotected intercourse if age ≥40 |
| After 0–6 months if patient has history of or risk factors for infertility |
| TREAT |
| Treat identifiable causes of infertility |
| Optimize factors influencing fertility: |
|
| Treat empirically (eg, clomiphene, insemination) for 3–6 months in patients |
| REFER |
| History of infertility or significant risk factors |
| Significant fertility problems identified during investigation |
| Age ≥40 |
| After 3–6 months of failed treatment for identifiable causes |
| After 3–6 months of failed empiric treatment |
How to evaluate ovarian function
A careful history and physical examination are key components of systematic, expeditious, and cost-effective identification of the cause of infertility (TABLE 2). A menstrual history and basal body temperature recordings are useful in the diagnosis of ovulatory dysfunction and are easy to obtain. Measurements of urinary luteinizing hormone (LH) using ovulation-prediction kits and mid-luteal-phase serum progesterone are also helpful.
Endometrial biopsy is rarely indicated because of its lack of clinical relevance.
Serial vaginal ultrasonography of the size and number of ovarian follicles may be indicated when simpler methods are inconclusive.
Other tests to evaluate ovarian function may include thyroid-stimulating hormone (TSH), serum prolactin, cycle day 3 follicle-stimulating hormone (FSH) and estradiol, and the clomiphene citrate challenge test in selected patients at higher risk of ovarian dysfunction.
TABLE 2
Current status of tests and treatments
| OLD, NOW RARELY INDICATED |
| Postcoital test |
| Endometrial biopsy |
| Antisperm antibodies testing |
| Intracervical insemination |
| Clomiphene for more than 3–6 cycles |
| Routine hCG injection to stimulate ovulation in clomiphene cycles |
| NEW AND HELPFUL |
| Clomiphene citrate challenge test in selected patients |
| Serial vaginal ultrasounds to evaluate response to ovarian stimulation |
| Saline sonohysterography |
| Preimplantation genetic diagnosis for single-gene defects |
| Embryo cryopreservation |
| Single-embryo transfer to reduce multiple pregnancy rates |
| NEW BUT STILL EXPERIMENTAL* |
| Preimplantation genetic screening for aneuploidy in older patients |
| Human lymphocyte antigen typing for recurrent pregnancy loss |
| Intravenous immunoglobulin for recurrent pregnancy loss |
| Ovarian tissue or oocyte cryopreservation for fertility preservation |
| * Should be performed only in clinical trials |
Clomiphene citrate is preferred
Ovarian dysfunction can be treated with clomiphene for 3 to 6 cycles5 starting at 50 mg per day from cycle day 5 to 9 and increasing to 100 and then 150 mg per day if ovulation does not occur. The drug may also be effective empiric treatment for unexplained infertility using 100 mg per day from cycle days 3 through 7 for a maximum of 3 to 4 cycles.
Only gynecologists experienced with ovarian stimulation drugs and with access to daily ultrasonographic monitoring and estradiol levels should use them, because of the risk of multiple pregnancy and ovarian hyperstimulation.
For women with polycystic ovary syndrome (PCOS), clomiphene alone is more effective than metformin alone. Ovarian drilling may be an effective surgical treatment for PCOS if clomiphene fails, but the cost and risk of adhesions must be considered.
Human chorionic gonadotropin (hCG) injections during clomiphene treatment to stimulate ovulation should be given only if the patient’s own urinary LH surge cannot be detected.
A single intrauterine insemination (IUI) improves the pregnancy rate slightly in conjunction with clomiphene, and by an odds ratio of approximately 2 in conjunction with gonadotropins. The gonadotropin dosage ranges from about 75 to 600 IU per day for 8 to 12 days, based on patient need and careful monitoring.
When to give up on ovarian stimulation. Failure to achieve pregnancy after 3 to 6 cycles signals the need to expand diagnostic evaluation or change treatment strategies.
Evaluate the uterus and tubes
Uterine factors rarely cause infertility but warrant thorough investigation all the same, including assessment of uterine cavity size and shape. A number of methods are available:
- hysterosalpingography (HSG)
- ultrasonography
- saline sonohysterography
Peritoneal factors such as endometriosis or pelvic or adnexal adhesions may occasionally be identified by ultrasonography if there is a mass, but are more likely to require laparoscopy.
When laparoscopy is indicated
If there is evidence or a strong suspicion of endometriosis, pelvic or adnexal adhesions, or significant tubal disease, laparoscopy is warranted. It also may be helpful in younger patients (eg,
Because they reduce pregnancy rates by 50%, hydrosalpinges should be removed or the fallopian tube should be ligated proximally before IVF. It also is important to consider the number of patients needed to treat by laparoscopy to obtain 1 additional pregnancy.
Only gynecologists with expertise should perform laparoscopy, because it is important to make the correct diagnosis and be capable of surgically treating conditions found during the surgery.
Skip the postcoital test, but keep the semen analysis
Abnormalities of the cervical mucus or sperm–mucus interaction rarely cause infertility. Therefore, the postcoital test has questionable predictive value and is probably only useful to confirm that the couple can have properly timed intercourse during the cycle.3
A male factor is solely responsible in about 20% of infertile couples and contributory in another 30% to 40%. For this reason, semen analysis is always warranted when the female is being evaluated for infertility.
Examination of the male partner should be performed by the gynecologist, or the male should be referred to a urologist interested in infertility.6
For recurrent pregnancy loss, best treatment is TLC
Recurrent pregnancy loss is challenging because it is so emotionally charged for the patient, the cause is often unclear, and we lack specific treatments. A methodical and empathetic approach is therefore recommended.
What the history can reveal
Many women with recurrent pregnancy loss will eventually have a live birth, but increasing numbers of miscarriages do predict a poorer overall chance of success, as does increasing age.
Lifestyle factors rarely, if ever, cause recurrent pregnancy loss, but the following factors may increase the risk of miscarriage: obesity, high daily caffeine intake, alcohol consumption, use of nonsteroidal anti-inflammatory drugs, and social class and occupation. A previous diagnosis of or treatment for infertility also increases the risk of recurrent loss.
Smoking should be discouraged and healthy lifestyles should be promoted.7
Causes of recurrent pregnancy loss
Definite causal factors include chromosomal abnormalities, such as translocations, in approximately 5% of couples with 2 or more losses.
Probable factors include uterine abnormalities (both congenital abnormalities such as septate, and acquired defects such as adhesions and intrauterine or submucous myomas), uncontrolled thyroid disease or diabetes, PCOS, and antiphospholipid antibody syndrome.
Other thrombophilias, such as those associated with factor V Leiden mutation, activated protein C resistance, and possibly prothrombin G20210A and protein S deficiency, have been found by some investigators to be associated with recurrent pregnancy loss. It is doubtful that antithyroid antibodies and sharing of parental human lymphocyte antigen (HLA) cause recurrent miscarriage.7
Genetic component likely. The risk of recurrent pregnancy loss in first-degree relatives of women with unexplained repeated pregnancy loss who have normal chromosomes is approximately 6 times higher than the risk in the background population, suggesting a polygenic mode of inheritance.7,8
Other possible causes include low plasma folate levels, which have been associated with an increased risk of first-trimester pregnancy loss. Environmental toxins such as ionizing radiation, organic solvents, alcohol, mercury, and lead are confirmed causes of recurrent pregnancy loss; hyperthermia is a suspected cause.8
Recommended evaluation
Investigations that have been proven in many studies include:
- HSG, hysteroscopy, and sonohysterography
- karyotyping of the couple
- measurement of thyroid hormone
- hemoglobin A1C and serum glucose assessment
- activated partial thromboplastin time, dilute Russell viper venom time, and lupus anticoagulant assessment
- measurement of immunoglobulin G and immunoglobulin M anticardiolipin antibodies
- test for factor V Leiden mutation
Examine products of conception?
Although it is routine practice to send products of conception for histologic examination, mainly to exclude a gestational trophoblastic disorder, the usefulness of this practice is unclear.8 In couples with recurrent pregnancy loss, chromosomal analysis of the products of conception indicates that a normal conceptus karyotype in a previous pregnancy is a predictor of a higher rate of miscarriage in a subsequent pregnancy.8 When stratified by maternal age, there is no difference in the distribution of cytogenetically abnormal miscarriages in couples with recurrent pregnancy loss, compared with controls.8 The cost-effectiveness of karyotyping is therefore unclear.
High levels of homocysteine (ie, hyperhomocysteinemia) can be associated with recurrent pregnancy loss. Among genetic causes is polymorphism at position 677 in the methylene tetrahydrofolate reductase (MTHFR) gene, which is often evaluated to rule out this condition.
Infections with bacteria, viruses, or parasites can all interfere with early pregnancy development, but none seem to be a significant cause of recurrent pregnancy loss.8 Testing is most useful in the context of an acute infectious episode.
Can recurrent loss be treated?
The hallmark of treatment is empathetic care, along with counseling emphasizing the complexity of this condition. Any endocrinologic, anatomic, or other abnormality that is identified during evaluation should be treated, if possible.
Progesterone supplementation is not proven treatment. This therapy is commonly prescribed but has not been proved to improve live birth rates.
Prednisone, aspirin, and NSAIDs have no benefits but potential risks and should not be used.
Current immunologic therapies for recurrent pregnancy loss have no sound scientific basis, except for the use of heparin and aspirin in patients with well-documented antiphospholipid antibodies.7 Specifically, intravenous immunoglobulin remains unproven, is experimental, and should be provided only in approved research settings.9 Paternal leukocyte immunization does not work, has been proscribed by the US Food and Drug Administration, and should be avoided.
Careful counseling and education of the patient about the history, pathophysiology, testing, test results, and treatment of recurrent pregnancy loss are necessary. Women with subfertility who have taken a long time to conceive should be treated empirically with ovarian stimulation in an attempt to shorten the time to conception.
Singleton births can be encouraged without jeopardizing IVF, ART
Multiple gestations have increased over the past 15 years, largely because of:
- ovulation induction for management of oligo-ovulation
- superovulation to produce more than 1 ovulated egg for fertility treatments
- assisted reproductive technologies (ART), in which more than 1 embryo is replaced to increase the pregnancy rate
Multiple gestations are a bad idea
Risks include a higher complication rate for gravidas and fetuses, as well as higher short- and long-term costs to patients and society. It is therefore important to reduce the incidence of multiple gestation associated with fertility treatments.10
How to reduce the likelihood of multiple fetuses
- Closely monitor cycles involving ovulation induction and superovulation for efficacy and safety, to avoid ovarian hyperstimulation and reduce the risk of multiple gestation. Although attempts to limit multiple gestation during ovulation induction or superovulation using ultrasonographic criteria and serum estradiol limits have been ineffective,10 it is my opinion that we should err on the side of conservatism, even though the optimal parameters for doing so have not been determined by high-quality trials. I recommend that hCG or IUI be avoided if more than 4 mature follicles (>15 mm) or 6 large follicles (>12 mm) are present on a sonogram, and the couple should be instructed to refrain from intercourse.
- Focus on the objective of a single healthy baby as the optimal outcome. Data published by the Society for Assisted Reproductive Technology (SART) clearly demonstrate the clinical impact of a reduction in the number of embryos transferred, which reduced triplet pregnancy rates in 2005 to less than half the rate in the late 1990s. Fewer embryos are transferred today than just a few years ago, and the trend is continuing. This will help reduce the triplet rate further and also reduce twin pregnancies. In the past 6 months, guidelines have recommended replacement of only 1 blastocyst at day 5 or 1 to 2 embryos at day 3 in women under age 35 with a favorable prognosis.
What the future holds
We can expect more elective single-embryo or single-blastocyst transfers as we gain further expertise in this area. However, this practice should be implemented carefully in selected patients to maintain adequate pregnancy rates while reducing multiple gestations.
The United States has the highest ART success rates in the world (approximately 40% higher than in Europe) despite a reduction in the number of triplet or higher-order pregnancies resulting in live births after ART—from 7.0% in 1996 to 2.4% in 2004. The twin rate has remained stable at approximately 30%, but should decrease as 1- and 2-embryo transfers become more common.11
Preimplantation genetic diagnosis now has multiple applications
Preimplantation genetic diagnosis (PGD) is over 15 years old, and at least 1,000 babies worldwide have been born after its use, with no reports of increased fetal malformation or other problems.12
Two basic techniques are employed to analyze the genomic status of the 1 or 2 blastomeres usually removed from the 8-cell embryo on day 3 after fertilization:
- Polymerase chain reaction (PCR) is used to amplify a specific DNA sequence harboring a mutation. A mismatch (eg, due to a genetic deletion) leads to differential migration on the gel, thus permitting diagnosis. The error rate, primarily due to allelic dropout, in which 1 of the 2 alleles selectively amplifies and thus contributes to diagnostic errors, is approximately 0.3% to 5.6%.
- Fluorescent in situ hybridization (FISH) allows determination of the ploidy of a blastomere. Labeled probes bind to chromosomes and are viewed under a fluorescent microscope. The error rate is 1% to 10% for a variety of technical reasons.12 Testing takes about 1 day while the embryos are developing to blastocysts, at which time those that are viable and tested to be normal are transferred back into the uterus.
Not just for gender determination
PGD initially was used to determine gender (by FISH) as an indirect method of avoiding X-linked genetic diseases such as hemophilia. The error rate for gender determination is less than 1%. Since then, single-gene-defect disorders have been diagnosed using PCR and heteroduplex formation or restriction endonuclease digestion, both of which distinguish normal from mutant alleles. PGD has been performed broadly to diagnose Tay-Sachs, Huntington’s disease, and hundreds of other diseases.
Testing for translocation by PGD has been especially useful and may reduce the risk of spontaneous abortion from as much as 95% to 13% if one of the parents is a known translocation carrier.
Still under investigation is the routine use of FISH to detect aneuploidy in cases of recurrent pregnancy loss. The use of FISH for gender selection for family balancing is not recommended by the ASRM.
More young women seek to preserve their fertility
Fertility preservation through ovarian tissue or oocyte cryopreservation or vitrification has recently been popularized by cancer survival consumer groups, the media, and other interests. In addition to cancer patients planning to undergo chemotherapy or radiotherapy, candidates for fertility preservation include women undergoing bone marrow or stem cell transplantation or oophorectomy (for a benign tumor, endometriosis, or prophylaxis) and patients with severe autoimmune disease needing chemotherapy.
In cancer patients, fertility is preserved using one of several methods:
- shielding or moving the ovaries to a different anatomic site during radiation
- use of gonadotropin-releasing hormone analogs or oral contraceptives during chemotherapy (unproven)
- changes to chemotherapy regimen
- IVF cycle followed by cryopreservation of embryos if the patient has a male partner or is prepared to use donor sperm (provided the oncologist confirms that ovarian stimulation and high estradiol levels are acceptable and there is time to undergo an IVF cycle before cancer treatment begins).
Unresolved issues
Concerns about cryopreservation of ovarian tissue in cancer patients13,14 include the possibility of reseeding tumor cells after ovarian transplantation, malignant transformation of transplanted ovarian tissue, and the possibility of congenital abnormalities as a result of cryopreservation—although no increase has been found in the patients studied so far.
The pregnancy rate is low
For cancer patients, the preservation of ovarian tissue or oocytes yields pregnancy rates significantly lower than those observed with standard IVF procedures. For cancer patients facing chemotherapy, however, oocyte cryopreservation may be one of the few options available and is acceptable in experimental protocols approved by the institutional review board.
Physicians should inform cancer patients about the options for fertility preservation prior to treatment.14 We lack data to recommend ovarian tissue or oocyte cryopreservation for the sole purpose of circumventing reproductive aging in healthy women.13
Changes are occurring so quickly it is often difficult for the general ObGyn to know the most advanced and appropriate treatment for a given patient. The American Society for Reproductive Medicine (ASRM) Practice Committee establishes guidelines based upon well-designed studies to help physicians keep abreast of the best clinical practices. In this article, I focus on recent ASRM guidelines in 5 topical areas associated with substantial misinformation in both the professional and public sectors:
- when and how gynecologists should initiate infertility testing and treatment
- how to evaluate and manage recurrent pregnancy loss
- the need to reduce the rate of multiple gestation from IVF and ART
- the expanded applications for preimplantation genetic diagnosis
- the truth about fertility-sparing efforts in young women planning to undergo cancer therapy and other treatments.
When and how to evaluate patients complaining of infertility
Infertility is a disease, but there are different opinions about when a woman reporting this condition should be assessed (TABLE 1). According to the ASRM, a couple should not be considered infertile until they have tried to conceive spontaneously for at least 12 months, unless the medical history and physical findings dictate earlier evaluation and treatment.1
For example, approximately 25% of couples experience infertility when the woman is age 35, and about 50% experience it when the woman is age 40. Therefore, it is reasonable to investigate infertility after 6 months of attempted conception when the woman is over 35 and after 3 months if she is over age 40.2 The primary reason for this age-related reduction in fertility is the diminishing number and quality of oocytes over time.
Other risk factors for infertility include smoking, family history of premature ovarian failure, significant ovarian pathology, previous ovarian surgery, history of oligomenorrhea or amenorrhea, known or suspected disease of the uterus or fallopian tubes, endometriosis, or a partner known to be subfertile.3,4
TABLE 1
When to investigate infertility, treat, and refer
| INVESTIGATE |
| After 12 months of unprotected intercourse if age |
| After 6 months of unprotected intercourse if age 35–39 |
| After 3 months of unprotected intercourse if age ≥40 |
| After 0–6 months if patient has history of or risk factors for infertility |
| TREAT |
| Treat identifiable causes of infertility |
| Optimize factors influencing fertility: |
|
| Treat empirically (eg, clomiphene, insemination) for 3–6 months in patients |
| REFER |
| History of infertility or significant risk factors |
| Significant fertility problems identified during investigation |
| Age ≥40 |
| After 3–6 months of failed treatment for identifiable causes |
| After 3–6 months of failed empiric treatment |
How to evaluate ovarian function
A careful history and physical examination are key components of systematic, expeditious, and cost-effective identification of the cause of infertility (TABLE 2). A menstrual history and basal body temperature recordings are useful in the diagnosis of ovulatory dysfunction and are easy to obtain. Measurements of urinary luteinizing hormone (LH) using ovulation-prediction kits and mid-luteal-phase serum progesterone are also helpful.
Endometrial biopsy is rarely indicated because of its lack of clinical relevance.
Serial vaginal ultrasonography of the size and number of ovarian follicles may be indicated when simpler methods are inconclusive.
Other tests to evaluate ovarian function may include thyroid-stimulating hormone (TSH), serum prolactin, cycle day 3 follicle-stimulating hormone (FSH) and estradiol, and the clomiphene citrate challenge test in selected patients at higher risk of ovarian dysfunction.
TABLE 2
Current status of tests and treatments
| OLD, NOW RARELY INDICATED |
| Postcoital test |
| Endometrial biopsy |
| Antisperm antibodies testing |
| Intracervical insemination |
| Clomiphene for more than 3–6 cycles |
| Routine hCG injection to stimulate ovulation in clomiphene cycles |
| NEW AND HELPFUL |
| Clomiphene citrate challenge test in selected patients |
| Serial vaginal ultrasounds to evaluate response to ovarian stimulation |
| Saline sonohysterography |
| Preimplantation genetic diagnosis for single-gene defects |
| Embryo cryopreservation |
| Single-embryo transfer to reduce multiple pregnancy rates |
| NEW BUT STILL EXPERIMENTAL* |
| Preimplantation genetic screening for aneuploidy in older patients |
| Human lymphocyte antigen typing for recurrent pregnancy loss |
| Intravenous immunoglobulin for recurrent pregnancy loss |
| Ovarian tissue or oocyte cryopreservation for fertility preservation |
| * Should be performed only in clinical trials |
Clomiphene citrate is preferred
Ovarian dysfunction can be treated with clomiphene for 3 to 6 cycles5 starting at 50 mg per day from cycle day 5 to 9 and increasing to 100 and then 150 mg per day if ovulation does not occur. The drug may also be effective empiric treatment for unexplained infertility using 100 mg per day from cycle days 3 through 7 for a maximum of 3 to 4 cycles.
Only gynecologists experienced with ovarian stimulation drugs and with access to daily ultrasonographic monitoring and estradiol levels should use them, because of the risk of multiple pregnancy and ovarian hyperstimulation.
For women with polycystic ovary syndrome (PCOS), clomiphene alone is more effective than metformin alone. Ovarian drilling may be an effective surgical treatment for PCOS if clomiphene fails, but the cost and risk of adhesions must be considered.
Human chorionic gonadotropin (hCG) injections during clomiphene treatment to stimulate ovulation should be given only if the patient’s own urinary LH surge cannot be detected.
A single intrauterine insemination (IUI) improves the pregnancy rate slightly in conjunction with clomiphene, and by an odds ratio of approximately 2 in conjunction with gonadotropins. The gonadotropin dosage ranges from about 75 to 600 IU per day for 8 to 12 days, based on patient need and careful monitoring.
When to give up on ovarian stimulation. Failure to achieve pregnancy after 3 to 6 cycles signals the need to expand diagnostic evaluation or change treatment strategies.
Evaluate the uterus and tubes
Uterine factors rarely cause infertility but warrant thorough investigation all the same, including assessment of uterine cavity size and shape. A number of methods are available:
- hysterosalpingography (HSG)
- ultrasonography
- saline sonohysterography
Peritoneal factors such as endometriosis or pelvic or adnexal adhesions may occasionally be identified by ultrasonography if there is a mass, but are more likely to require laparoscopy.
When laparoscopy is indicated
If there is evidence or a strong suspicion of endometriosis, pelvic or adnexal adhesions, or significant tubal disease, laparoscopy is warranted. It also may be helpful in younger patients (eg,
Because they reduce pregnancy rates by 50%, hydrosalpinges should be removed or the fallopian tube should be ligated proximally before IVF. It also is important to consider the number of patients needed to treat by laparoscopy to obtain 1 additional pregnancy.
Only gynecologists with expertise should perform laparoscopy, because it is important to make the correct diagnosis and be capable of surgically treating conditions found during the surgery.
Skip the postcoital test, but keep the semen analysis
Abnormalities of the cervical mucus or sperm–mucus interaction rarely cause infertility. Therefore, the postcoital test has questionable predictive value and is probably only useful to confirm that the couple can have properly timed intercourse during the cycle.3
A male factor is solely responsible in about 20% of infertile couples and contributory in another 30% to 40%. For this reason, semen analysis is always warranted when the female is being evaluated for infertility.
Examination of the male partner should be performed by the gynecologist, or the male should be referred to a urologist interested in infertility.6
For recurrent pregnancy loss, best treatment is TLC
Recurrent pregnancy loss is challenging because it is so emotionally charged for the patient, the cause is often unclear, and we lack specific treatments. A methodical and empathetic approach is therefore recommended.
What the history can reveal
Many women with recurrent pregnancy loss will eventually have a live birth, but increasing numbers of miscarriages do predict a poorer overall chance of success, as does increasing age.
Lifestyle factors rarely, if ever, cause recurrent pregnancy loss, but the following factors may increase the risk of miscarriage: obesity, high daily caffeine intake, alcohol consumption, use of nonsteroidal anti-inflammatory drugs, and social class and occupation. A previous diagnosis of or treatment for infertility also increases the risk of recurrent loss.
Smoking should be discouraged and healthy lifestyles should be promoted.7
Causes of recurrent pregnancy loss
Definite causal factors include chromosomal abnormalities, such as translocations, in approximately 5% of couples with 2 or more losses.
Probable factors include uterine abnormalities (both congenital abnormalities such as septate, and acquired defects such as adhesions and intrauterine or submucous myomas), uncontrolled thyroid disease or diabetes, PCOS, and antiphospholipid antibody syndrome.
Other thrombophilias, such as those associated with factor V Leiden mutation, activated protein C resistance, and possibly prothrombin G20210A and protein S deficiency, have been found by some investigators to be associated with recurrent pregnancy loss. It is doubtful that antithyroid antibodies and sharing of parental human lymphocyte antigen (HLA) cause recurrent miscarriage.7
Genetic component likely. The risk of recurrent pregnancy loss in first-degree relatives of women with unexplained repeated pregnancy loss who have normal chromosomes is approximately 6 times higher than the risk in the background population, suggesting a polygenic mode of inheritance.7,8
Other possible causes include low plasma folate levels, which have been associated with an increased risk of first-trimester pregnancy loss. Environmental toxins such as ionizing radiation, organic solvents, alcohol, mercury, and lead are confirmed causes of recurrent pregnancy loss; hyperthermia is a suspected cause.8
Recommended evaluation
Investigations that have been proven in many studies include:
- HSG, hysteroscopy, and sonohysterography
- karyotyping of the couple
- measurement of thyroid hormone
- hemoglobin A1C and serum glucose assessment
- activated partial thromboplastin time, dilute Russell viper venom time, and lupus anticoagulant assessment
- measurement of immunoglobulin G and immunoglobulin M anticardiolipin antibodies
- test for factor V Leiden mutation
Examine products of conception?
Although it is routine practice to send products of conception for histologic examination, mainly to exclude a gestational trophoblastic disorder, the usefulness of this practice is unclear.8 In couples with recurrent pregnancy loss, chromosomal analysis of the products of conception indicates that a normal conceptus karyotype in a previous pregnancy is a predictor of a higher rate of miscarriage in a subsequent pregnancy.8 When stratified by maternal age, there is no difference in the distribution of cytogenetically abnormal miscarriages in couples with recurrent pregnancy loss, compared with controls.8 The cost-effectiveness of karyotyping is therefore unclear.
High levels of homocysteine (ie, hyperhomocysteinemia) can be associated with recurrent pregnancy loss. Among genetic causes is polymorphism at position 677 in the methylene tetrahydrofolate reductase (MTHFR) gene, which is often evaluated to rule out this condition.
Infections with bacteria, viruses, or parasites can all interfere with early pregnancy development, but none seem to be a significant cause of recurrent pregnancy loss.8 Testing is most useful in the context of an acute infectious episode.
Can recurrent loss be treated?
The hallmark of treatment is empathetic care, along with counseling emphasizing the complexity of this condition. Any endocrinologic, anatomic, or other abnormality that is identified during evaluation should be treated, if possible.
Progesterone supplementation is not proven treatment. This therapy is commonly prescribed but has not been proved to improve live birth rates.
Prednisone, aspirin, and NSAIDs have no benefits but potential risks and should not be used.
Current immunologic therapies for recurrent pregnancy loss have no sound scientific basis, except for the use of heparin and aspirin in patients with well-documented antiphospholipid antibodies.7 Specifically, intravenous immunoglobulin remains unproven, is experimental, and should be provided only in approved research settings.9 Paternal leukocyte immunization does not work, has been proscribed by the US Food and Drug Administration, and should be avoided.
Careful counseling and education of the patient about the history, pathophysiology, testing, test results, and treatment of recurrent pregnancy loss are necessary. Women with subfertility who have taken a long time to conceive should be treated empirically with ovarian stimulation in an attempt to shorten the time to conception.
Singleton births can be encouraged without jeopardizing IVF, ART
Multiple gestations have increased over the past 15 years, largely because of:
- ovulation induction for management of oligo-ovulation
- superovulation to produce more than 1 ovulated egg for fertility treatments
- assisted reproductive technologies (ART), in which more than 1 embryo is replaced to increase the pregnancy rate
Multiple gestations are a bad idea
Risks include a higher complication rate for gravidas and fetuses, as well as higher short- and long-term costs to patients and society. It is therefore important to reduce the incidence of multiple gestation associated with fertility treatments.10
How to reduce the likelihood of multiple fetuses
- Closely monitor cycles involving ovulation induction and superovulation for efficacy and safety, to avoid ovarian hyperstimulation and reduce the risk of multiple gestation. Although attempts to limit multiple gestation during ovulation induction or superovulation using ultrasonographic criteria and serum estradiol limits have been ineffective,10 it is my opinion that we should err on the side of conservatism, even though the optimal parameters for doing so have not been determined by high-quality trials. I recommend that hCG or IUI be avoided if more than 4 mature follicles (>15 mm) or 6 large follicles (>12 mm) are present on a sonogram, and the couple should be instructed to refrain from intercourse.
- Focus on the objective of a single healthy baby as the optimal outcome. Data published by the Society for Assisted Reproductive Technology (SART) clearly demonstrate the clinical impact of a reduction in the number of embryos transferred, which reduced triplet pregnancy rates in 2005 to less than half the rate in the late 1990s. Fewer embryos are transferred today than just a few years ago, and the trend is continuing. This will help reduce the triplet rate further and also reduce twin pregnancies. In the past 6 months, guidelines have recommended replacement of only 1 blastocyst at day 5 or 1 to 2 embryos at day 3 in women under age 35 with a favorable prognosis.
What the future holds
We can expect more elective single-embryo or single-blastocyst transfers as we gain further expertise in this area. However, this practice should be implemented carefully in selected patients to maintain adequate pregnancy rates while reducing multiple gestations.
The United States has the highest ART success rates in the world (approximately 40% higher than in Europe) despite a reduction in the number of triplet or higher-order pregnancies resulting in live births after ART—from 7.0% in 1996 to 2.4% in 2004. The twin rate has remained stable at approximately 30%, but should decrease as 1- and 2-embryo transfers become more common.11
Preimplantation genetic diagnosis now has multiple applications
Preimplantation genetic diagnosis (PGD) is over 15 years old, and at least 1,000 babies worldwide have been born after its use, with no reports of increased fetal malformation or other problems.12
Two basic techniques are employed to analyze the genomic status of the 1 or 2 blastomeres usually removed from the 8-cell embryo on day 3 after fertilization:
- Polymerase chain reaction (PCR) is used to amplify a specific DNA sequence harboring a mutation. A mismatch (eg, due to a genetic deletion) leads to differential migration on the gel, thus permitting diagnosis. The error rate, primarily due to allelic dropout, in which 1 of the 2 alleles selectively amplifies and thus contributes to diagnostic errors, is approximately 0.3% to 5.6%.
- Fluorescent in situ hybridization (FISH) allows determination of the ploidy of a blastomere. Labeled probes bind to chromosomes and are viewed under a fluorescent microscope. The error rate is 1% to 10% for a variety of technical reasons.12 Testing takes about 1 day while the embryos are developing to blastocysts, at which time those that are viable and tested to be normal are transferred back into the uterus.
Not just for gender determination
PGD initially was used to determine gender (by FISH) as an indirect method of avoiding X-linked genetic diseases such as hemophilia. The error rate for gender determination is less than 1%. Since then, single-gene-defect disorders have been diagnosed using PCR and heteroduplex formation or restriction endonuclease digestion, both of which distinguish normal from mutant alleles. PGD has been performed broadly to diagnose Tay-Sachs, Huntington’s disease, and hundreds of other diseases.
Testing for translocation by PGD has been especially useful and may reduce the risk of spontaneous abortion from as much as 95% to 13% if one of the parents is a known translocation carrier.
Still under investigation is the routine use of FISH to detect aneuploidy in cases of recurrent pregnancy loss. The use of FISH for gender selection for family balancing is not recommended by the ASRM.
More young women seek to preserve their fertility
Fertility preservation through ovarian tissue or oocyte cryopreservation or vitrification has recently been popularized by cancer survival consumer groups, the media, and other interests. In addition to cancer patients planning to undergo chemotherapy or radiotherapy, candidates for fertility preservation include women undergoing bone marrow or stem cell transplantation or oophorectomy (for a benign tumor, endometriosis, or prophylaxis) and patients with severe autoimmune disease needing chemotherapy.
In cancer patients, fertility is preserved using one of several methods:
- shielding or moving the ovaries to a different anatomic site during radiation
- use of gonadotropin-releasing hormone analogs or oral contraceptives during chemotherapy (unproven)
- changes to chemotherapy regimen
- IVF cycle followed by cryopreservation of embryos if the patient has a male partner or is prepared to use donor sperm (provided the oncologist confirms that ovarian stimulation and high estradiol levels are acceptable and there is time to undergo an IVF cycle before cancer treatment begins).
Unresolved issues
Concerns about cryopreservation of ovarian tissue in cancer patients13,14 include the possibility of reseeding tumor cells after ovarian transplantation, malignant transformation of transplanted ovarian tissue, and the possibility of congenital abnormalities as a result of cryopreservation—although no increase has been found in the patients studied so far.
The pregnancy rate is low
For cancer patients, the preservation of ovarian tissue or oocytes yields pregnancy rates significantly lower than those observed with standard IVF procedures. For cancer patients facing chemotherapy, however, oocyte cryopreservation may be one of the few options available and is acceptable in experimental protocols approved by the institutional review board.
Physicians should inform cancer patients about the options for fertility preservation prior to treatment.14 We lack data to recommend ovarian tissue or oocyte cryopreservation for the sole purpose of circumventing reproductive aging in healthy women.13
1. Practice Committee. American Society for Reproductive Medicine. Definition of “infertility.” Fertil Steril. 2006;86(Suppl 4):S228.-
2. Practice Committee, American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86(Suppl 4):S248-252.
3. Practice Committee, American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(Suppl 4):S264-267.
4. Practice Committee, American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2006;86(Suppl 4):S172-177.
5. Practice committee, American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86(Suppl 4):S187-193.
6. Male Infertility Best Practice Committee, American Urological Association, and Practice Committee, American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(Suppl 4):S202-209.
7. Christiansen OB, Andersen A-MN, Bosch E, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83:821-839.
8. Jauniauz E, Farquharson RG, Christiansen OB, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21:2216-2222.
9. Practice Committee, American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril. 2006;86(Suppl 4):S226-227.
10. Practice committee, American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(Suppl 4):S106-110.
11. Practice Committee, Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-52.
12. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Preimplantation genetic diagnosis. Fertil Steril. 2006;86(Suppl 4):S257-258.
13. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86(Suppl 4):S142-147.
14. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622-1628.
Dr. Adamson reports no financial relationships with any company whose products are mentioned in this article. He receives grant/research support from IBSA, Serono, and ViaCell and is a consultant to ViaCell.
1. Practice Committee. American Society for Reproductive Medicine. Definition of “infertility.” Fertil Steril. 2006;86(Suppl 4):S228.-
2. Practice Committee, American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86(Suppl 4):S248-252.
3. Practice Committee, American Society for Reproductive Medicine. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(Suppl 4):S264-267.
4. Practice Committee, American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2006;86(Suppl 4):S172-177.
5. Practice committee, American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86(Suppl 4):S187-193.
6. Male Infertility Best Practice Committee, American Urological Association, and Practice Committee, American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(Suppl 4):S202-209.
7. Christiansen OB, Andersen A-MN, Bosch E, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83:821-839.
8. Jauniauz E, Farquharson RG, Christiansen OB, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21:2216-2222.
9. Practice Committee, American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril. 2006;86(Suppl 4):S226-227.
10. Practice committee, American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(Suppl 4):S106-110.
11. Practice Committee, Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86(Suppl 4):S51-52.
12. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Preimplantation genetic diagnosis. Fertil Steril. 2006;86(Suppl 4):S257-258.
13. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86(Suppl 4):S142-147.
14. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622-1628.
Dr. Adamson reports no financial relationships with any company whose products are mentioned in this article. He receives grant/research support from IBSA, Serono, and ViaCell and is a consultant to ViaCell.

