User login
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.
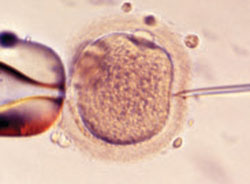
FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).
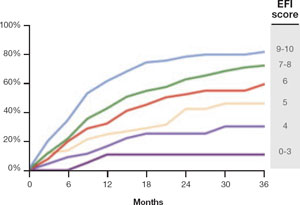
FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.

FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).

FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.

FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).

FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.