User login
Prediction of Disposition Within 48 Hours of Hospital Admission Using Patient Mobility Scores
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
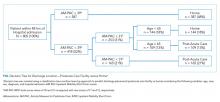
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
© 2019 Society of Hospital Medicine
Inpatient Mobility Technicians: One Step Forward?
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
©2019 Society of Hospital Medicine