User login
Discoid Lupus Erythematosus Following Herpes Zoster
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
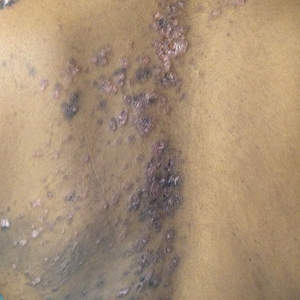
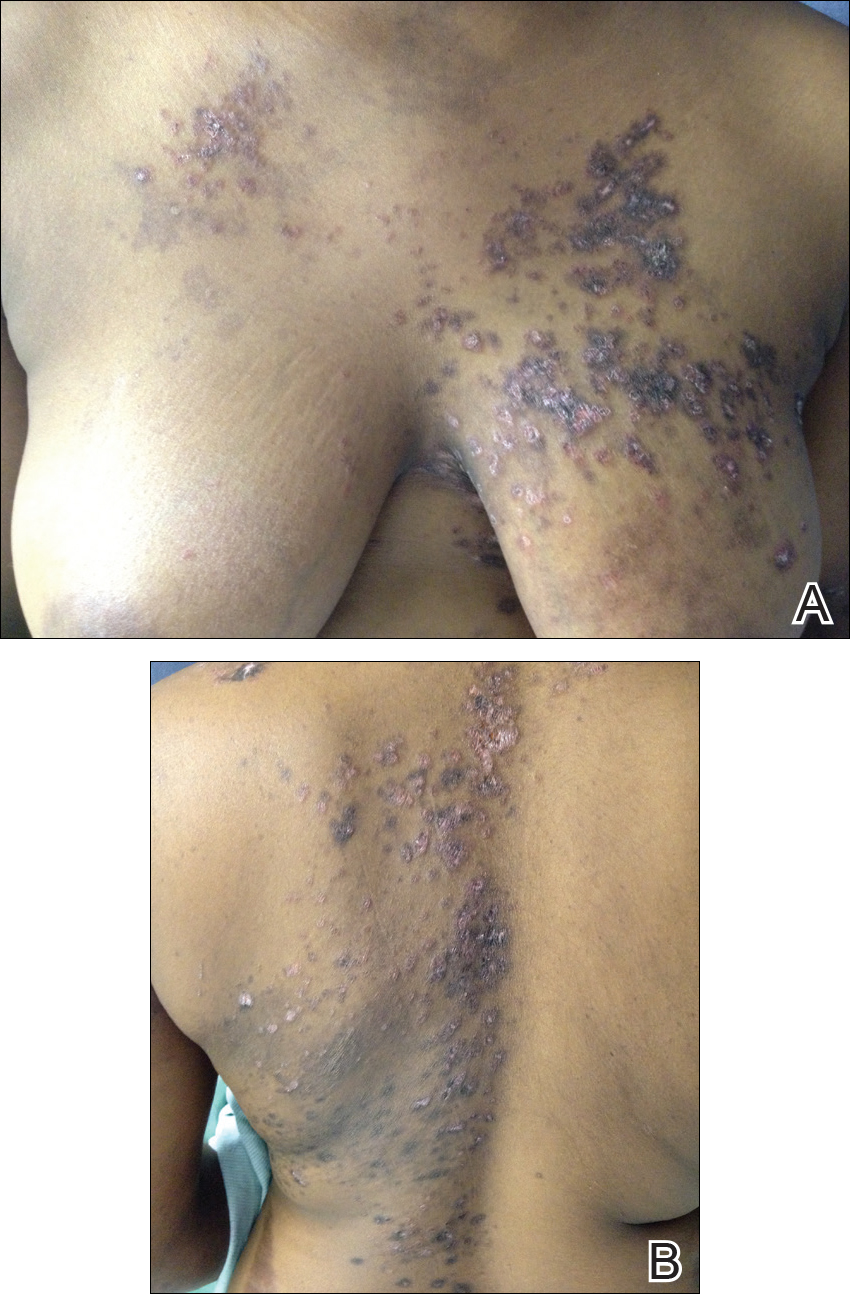
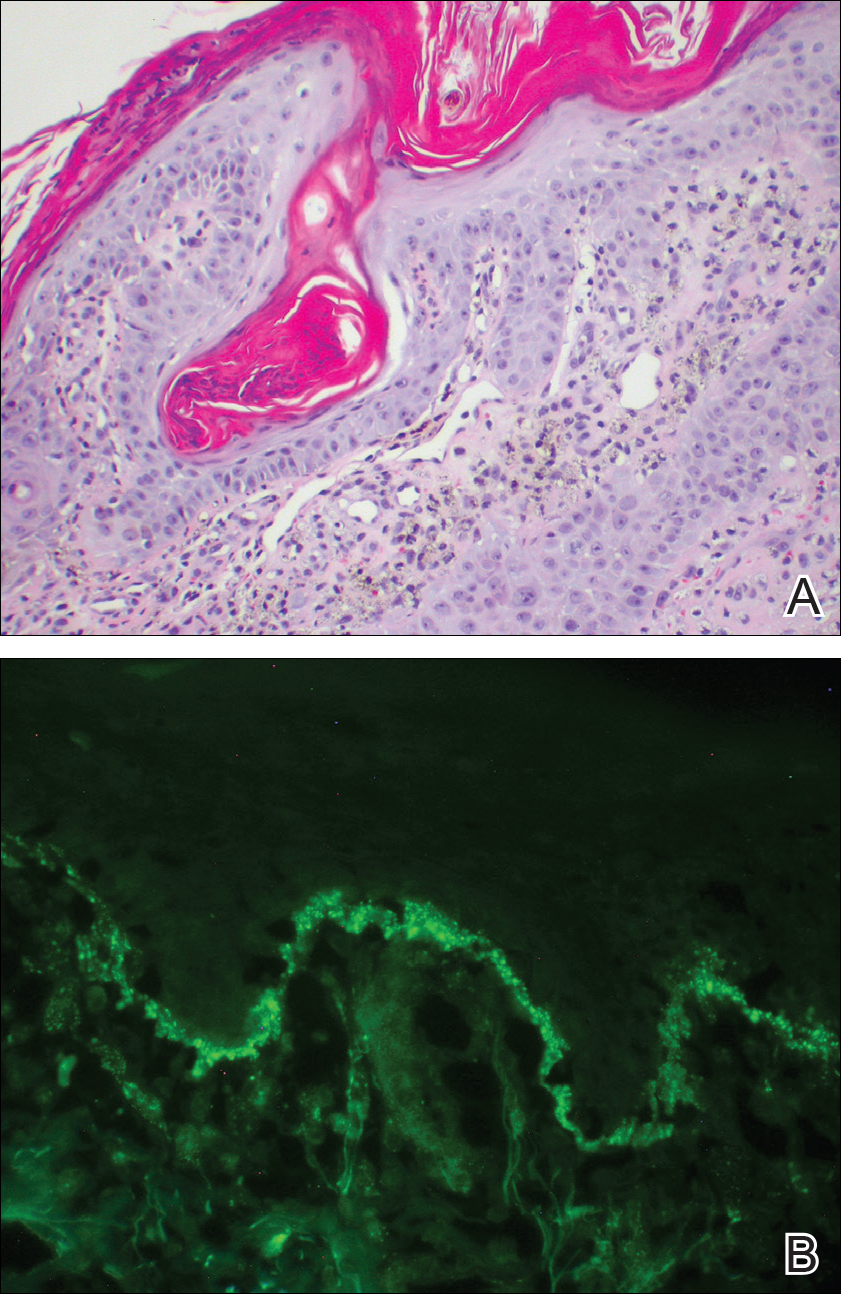
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.
Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.


Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.


Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
Practice Points
- Discoid lupus erythematosus (DLE) most commonly presents as scaling and crusted plaques in sun-exposed areas of the face and arms. It also may present in skin traumatized by tattoos, scratches, scars, prolonged heat exposure, andherpes zoster (HZ).
- Patients with a history of DLE who subsequently develop HZ should be followed closely for the development of DLE in HZ-affected dermatomes.
- Following resolution of HZ, topical corticosteroids may have a role in prevention of DLE in HZ-affected dermatomes.