User login
You Need a Plan: A Stepwise Protocol for Operating Room Preparedness During an Infectious Pandemic
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
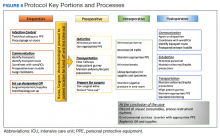
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.