User login
The surgical approach to the obliterated anterior cul-de-sac

Additional videos from SGS are available here, including these recent offerings:
• A minimally invasive modification for fascia lata mid-urethral sling
• Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
• Maintaining and reclaiming hemostasis in laparoscopic surgery

Additional videos from SGS are available here, including these recent offerings:
• A minimally invasive modification for fascia lata mid-urethral sling
• Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
• Maintaining and reclaiming hemostasis in laparoscopic surgery

Additional videos from SGS are available here, including these recent offerings:
• A minimally invasive modification for fascia lata mid-urethral sling
• Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
• Maintaining and reclaiming hemostasis in laparoscopic surgery
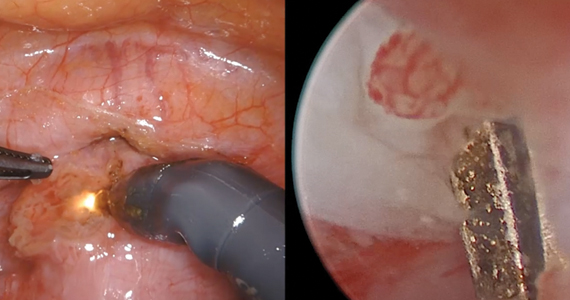
Isthmocele repair: Simultaneous hysteroscopy and robotic-assisted laparoscopy

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues
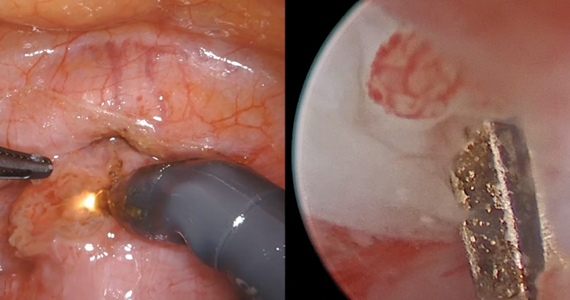
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues


An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
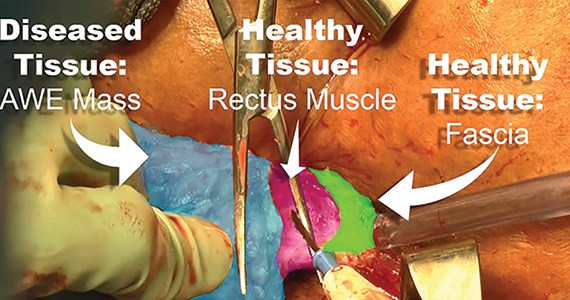
Excision of abdominal wall endometriosis
Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
2018 Update on minimally invasive gynecologic surgery
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel
Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.
Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

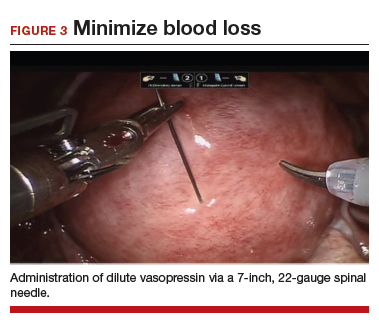
To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28
Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
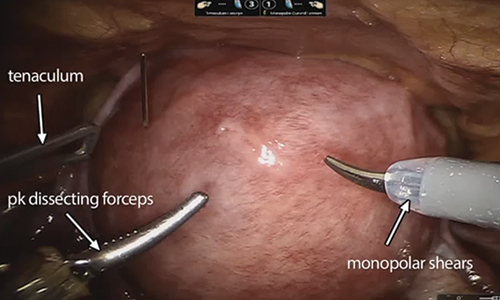
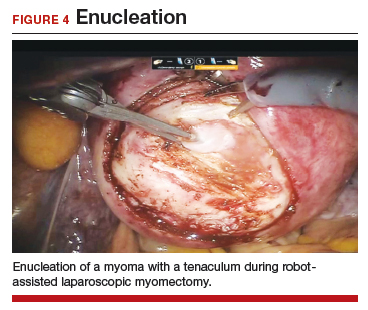
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.
As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
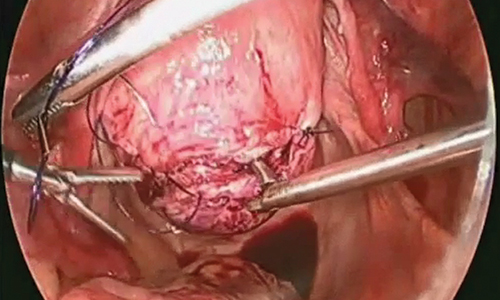
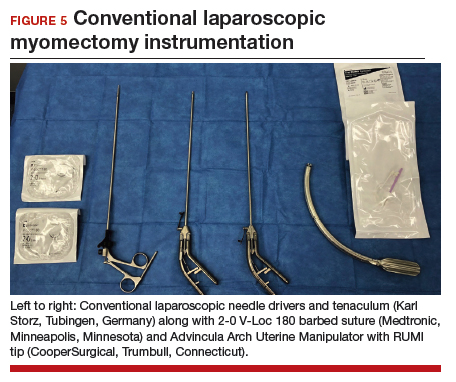
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.
Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
2017 Update on minimally invasive gynecologic surgery
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.
The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11
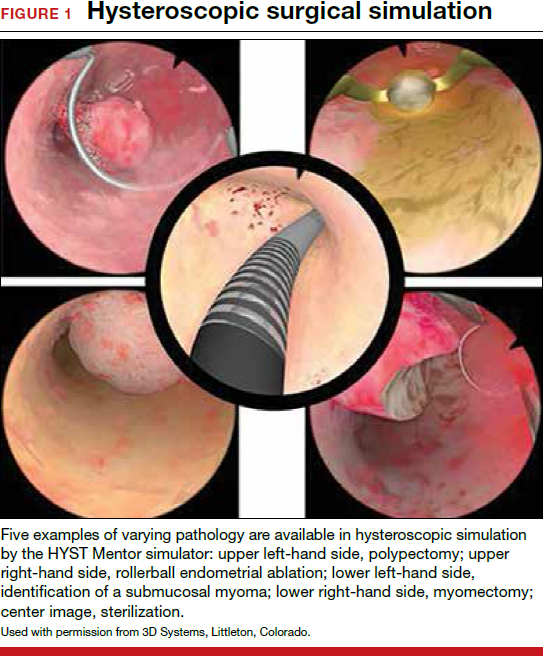
Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18
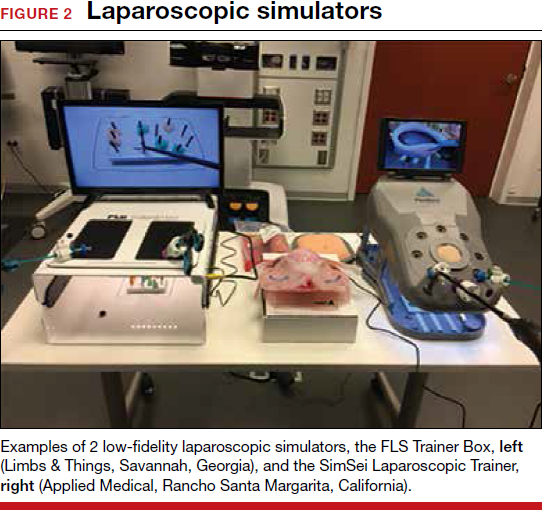
The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).
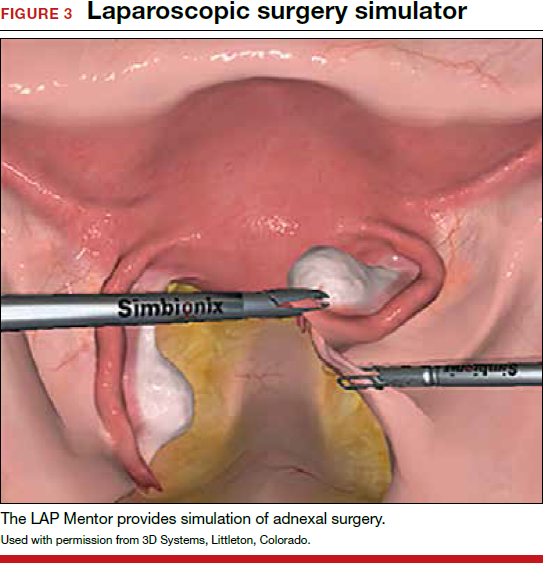
Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.