User login
HCV Screening Decision Tool
HCV SCREENING DECISION TOOL
The initial screening tool for HCV infection is an HCV antibody test. A positive anti-HCV antibody result can signify either current or resolved infection, followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present. Screening should be offered to all individuals falling within one or more of the following at-risk categories or behaviors.1-4
HIGH-RISK INDIVIDUALS HAVE/ARE…
• Been born between 1945 and 1965, regardless of other risk factors; should be screened one time
• Currently or formerly used injection drugs, including injecting only once many years ago; current injection drug users should be screened annually
• Received clotting factor concentrates made before 1987 (before more advanced methods for manufacturing those products were developed)
• Received blood transfusions or solid organ transplants before July 1992 (before better testing of blood donors became available)
• Born to HCV-positive mothers (If diagnosis is required for children younger than 18 months, use the HCV ribonucleic acid (RNA) test at 1 to 2 months.)
• Ever received long-term hemodialysis
• Human immunodeficiency virus (HIV) infection
• Known exposures to HCV
• Health care workers after needle stick injuries involving HCV-positive blood
• Recipients of blood or organs from donors who tested positive for HCV
• HIV-positive men who have sex with men; should be screened annually
• Signs and symptoms of liver disease (elevated transaminase levels)
LOWER-RISK INDIVIDUALS HAVE...
• Heterosexual intercourse with an HCV-infected person or multiple sexual partners
• Shared personal items that may contain blood, such as razors and toothbrushes
• Other invasive health care procedures, such as injections
• Cosmetic procedures, such as tattoos and piercings, where infection control is substandard
• Used intranasal drugs, cocaine, or marijuana
The following HCV screening algorithm, which includes the points at which referrals to specialists are indicated, is useful for determining the right test to use for a specific risk group.
Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease.
Read Sturm D, Gurevitz SL, Davidson D, Fritchley A, Wagaman A. Chronic hepatitis C infection: Bane of baby boomers. 2014;24(11):24-32 to earn 1 hour AAPA Category 1 CME credit, expires November 31, 2015.
URL: http://www.clinicianreviews.com/articles/cecme-activities/article/chronic-hepatitis-c-infection-bane-of-baby-boomers/73b211cce27d01b93463584dd2c44083.html
REFERENCES
4. [1.] The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed January 7, 2015.
8. [2.] CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. [3.] Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. [4.] American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed January 7, 2015.
HCV SCREENING DECISION TOOL
The initial screening tool for HCV infection is an HCV antibody test. A positive anti-HCV antibody result can signify either current or resolved infection, followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present. Screening should be offered to all individuals falling within one or more of the following at-risk categories or behaviors.1-4
HIGH-RISK INDIVIDUALS HAVE/ARE…
• Been born between 1945 and 1965, regardless of other risk factors; should be screened one time
• Currently or formerly used injection drugs, including injecting only once many years ago; current injection drug users should be screened annually
• Received clotting factor concentrates made before 1987 (before more advanced methods for manufacturing those products were developed)
• Received blood transfusions or solid organ transplants before July 1992 (before better testing of blood donors became available)
• Born to HCV-positive mothers (If diagnosis is required for children younger than 18 months, use the HCV ribonucleic acid (RNA) test at 1 to 2 months.)
• Ever received long-term hemodialysis
• Human immunodeficiency virus (HIV) infection
• Known exposures to HCV
• Health care workers after needle stick injuries involving HCV-positive blood
• Recipients of blood or organs from donors who tested positive for HCV
• HIV-positive men who have sex with men; should be screened annually
• Signs and symptoms of liver disease (elevated transaminase levels)
LOWER-RISK INDIVIDUALS HAVE...
• Heterosexual intercourse with an HCV-infected person or multiple sexual partners
• Shared personal items that may contain blood, such as razors and toothbrushes
• Other invasive health care procedures, such as injections
• Cosmetic procedures, such as tattoos and piercings, where infection control is substandard
• Used intranasal drugs, cocaine, or marijuana
The following HCV screening algorithm, which includes the points at which referrals to specialists are indicated, is useful for determining the right test to use for a specific risk group.
Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease.
Read Sturm D, Gurevitz SL, Davidson D, Fritchley A, Wagaman A. Chronic hepatitis C infection: Bane of baby boomers. 2014;24(11):24-32 to earn 1 hour AAPA Category 1 CME credit, expires November 31, 2015.
URL: http://www.clinicianreviews.com/articles/cecme-activities/article/chronic-hepatitis-c-infection-bane-of-baby-boomers/73b211cce27d01b93463584dd2c44083.html
REFERENCES
4. [1.] The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed January 7, 2015.
8. [2.] CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. [3.] Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. [4.] American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed January 7, 2015.
HCV SCREENING DECISION TOOL
The initial screening tool for HCV infection is an HCV antibody test. A positive anti-HCV antibody result can signify either current or resolved infection, followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present. Screening should be offered to all individuals falling within one or more of the following at-risk categories or behaviors.1-4
HIGH-RISK INDIVIDUALS HAVE/ARE…
• Been born between 1945 and 1965, regardless of other risk factors; should be screened one time
• Currently or formerly used injection drugs, including injecting only once many years ago; current injection drug users should be screened annually
• Received clotting factor concentrates made before 1987 (before more advanced methods for manufacturing those products were developed)
• Received blood transfusions or solid organ transplants before July 1992 (before better testing of blood donors became available)
• Born to HCV-positive mothers (If diagnosis is required for children younger than 18 months, use the HCV ribonucleic acid (RNA) test at 1 to 2 months.)
• Ever received long-term hemodialysis
• Human immunodeficiency virus (HIV) infection
• Known exposures to HCV
• Health care workers after needle stick injuries involving HCV-positive blood
• Recipients of blood or organs from donors who tested positive for HCV
• HIV-positive men who have sex with men; should be screened annually
• Signs and symptoms of liver disease (elevated transaminase levels)
LOWER-RISK INDIVIDUALS HAVE...
• Heterosexual intercourse with an HCV-infected person or multiple sexual partners
• Shared personal items that may contain blood, such as razors and toothbrushes
• Other invasive health care procedures, such as injections
• Cosmetic procedures, such as tattoos and piercings, where infection control is substandard
• Used intranasal drugs, cocaine, or marijuana
The following HCV screening algorithm, which includes the points at which referrals to specialists are indicated, is useful for determining the right test to use for a specific risk group.
Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease.
Read Sturm D, Gurevitz SL, Davidson D, Fritchley A, Wagaman A. Chronic hepatitis C infection: Bane of baby boomers. 2014;24(11):24-32 to earn 1 hour AAPA Category 1 CME credit, expires November 31, 2015.
URL: http://www.clinicianreviews.com/articles/cecme-activities/article/chronic-hepatitis-c-infection-bane-of-baby-boomers/73b211cce27d01b93463584dd2c44083.html
REFERENCES
4. [1.] The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed January 7, 2015.
8. [2.] CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. [3.] Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. [4.] American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed January 7, 2015.
Chronic Hepatitis C Infection: Bane of Baby Boomers
CE/CME No: CR-1411
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the risk factors for HCV infection.
• Identify who should be screened for HCV infection.
• Discuss the symptoms, clinical course, diagnosis, and complications of chronic HCV infection.
• Differentiate between the treatment of acute and chronic HCV infection.
• Describe the challenges of treating HCV infection in patients who are coinfected with HIV.
FACULTY
Daniel Sturm and Samuel L. Gurevitz are Assistant Professors in the Physician Assistant Program, College of Pharmacy and Health Sciences, at Butler University in Indianapolis. Cassidy Davidson, Abigail Fritchley, and Audrey Wagaman are students in the PA Program at Butler University. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Several million Americans, primarily those in their fifties and sixties, contracted hepatitis C virus (HCV) many years ago and are unaware of their infection and their risk for HCV-related liver disease. Screening at-risk patients is important because newer treatment regimens are curative and can reduce associated morbidity and mortality.
Chronic infection with hepatitis C virus (HCV) is a major cause of liver disease in the United States. National Health and Nutrition Examination Survey data indicates that 2.7 million people have chronic HCV infection; the CDC estimates 3.2 million.1-3 Yet these individuals may be asymptomatic for years, despite slow progression of sequelae (eg, chronic liver disease, cirrhosis, hepatocellular carcinoma [HCC]) that may silently unfold. Left untreated, chronic HCV infection is associated with a 15% to 30% risk for cirrhosis within 20 years, which subsequently confers an annual risk for HCC of 2% to 4%.4 Additionally, chronic HCV infection is now the leading indication for liver transplantation.3
Of note, 70% of those with chronic HCV infection were born between 1945 and 1965.2 This is believed to be attributable to viral transmission via contaminated blood, blood products, and organ transplants prior to the implementation of universal precautions for blood supply screening in 1992; and to past injection drug use, even if it occurred only once.3
In a recent analysis, it was determined that the clinical and economic burdens of chronic HCV infection increased in the past decade, and this trend is likely to continue during the next decade.5 For example, HCV was responsible for 15,106 US deaths in 2007, surpassing deaths caused by HIV for the first time.6 Since then, the number of HCV-related deaths has continued to increase, to 17,721 in 2011.7 Economic costs solely attributable to HCV infection are difficult to calculate, but estimates range from several hundred million dollars to $30 billion annually.5 The World Health Organization (WHO) estimates that more than 185 million people worldwide are infected with HCV and that HCV is responsible for 350,000 deaths annually.4
The main goal of treatment is to achieve a sustained virologic response (SVR), defined as undetectable HCV RNA in serum 12 to 24 weeks after completion of treatment and thereby prevent or reduce the complications of HCV infection.3 Proactive screening, diagnosis, monitoring, and treatment of HCV infection can significantly reduce long-term morbidity and mortality.
INDICATIONS FOR HCV SCREENING
Recommendations for HCV screening have been developed by numerous organizations, including the WHO, CDC, US Preventive Services Task Force (USPSTF), American Association for the Study of Liver Diseases (AASLD), and the Infectious Diseases Society of America (IDSA) (see Table 1).3,4,8,9-11 Screening should be offered to all individuals meeting one or more of the criteria. A 2012 CDC update calls for one-time testing for all persons born between 1945 and 1965 because of the disproportionately high prevalence of HCV infection in this cohort, which is five times greater than in the general population.11
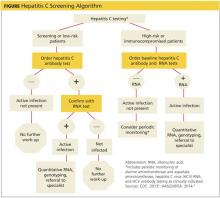
The initial screening tool for HCV infection is an HCV antibody test. A positive or reactive anti-HCV antibody result can signify either current or resolved infection, so a positive result should be followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present.4,8-10 See Figure on previous page for an HCV screening algorithm, which includes the points at which referrals to specialists are indicated.
A rapid HCV antibody test was approved by the FDA in 2011 and is favored because of its widespread availability, ease of use, rapid results, and low cost. Point-of-care testing is comparable in sensitivity and specificity to laboratory-based HCV assays and can utilize blood obtained via fingerstick or venipuncture.8 This form of testing facilitates HCV screening in a variety of settings, such as health fairs and emergency departments, as well as in high-risk settings such as methadone clinics.
Populations at increased risk for HCV infection are also at increased risk for hepatitis B virus (HBV), HIV, and tuberculosis infections; therefore, screening for these may also be warranted.4
PROGRESSION OF UNTREATED INFECTION
Just as with chronic HCV infection, patients newly infected with HCV are typically asymptomatic; the illness manifests with clinical symptoms in only 20% to 30% of cases.3 If symptoms do appear, they include fever, fatigue, dark urine, clay-colored stool, abdominal pain, loss of appetite, nausea, vomiting, joint pain, and jaundice. These acute phase symptoms may last two to 12 weeks.12
Spontaneous clearance of the virus occurs in only 15% to 25% of cases (see further discussion under “Acute HCV.”).2,3 Even if a patient clears an HCV infection, if he or she falls within one of the at-risk categories (see Table 1), then periodic screening should continue because prior HCV infection does not protect against future infection.3
The progression of chronic HCV is indolent and often subclinical, with fatigue being the most common complaint. Other nonspecific symptoms may include nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. One study noted that symptoms do not correlate with disease severity.13 In a 10-year prospective study, chronic HCV infection increased mortality when the infection was acquired at an early age (younger than 50) and/or when cirrhosis developed.14 Patients with cirrhosis progress to liver decompensation at a rate of 3% to 6% annually.15
In addition to liver disease, HCV infection is associated with an increased risk for non-Hodgkin lymphoma.3 HCV may also induce insulin resistance, which increases the risk for hepatic fibrosis. Other studies document HCV-induced cognitive impairment, but little scientific data is yet available as to its pathogenesis.16
DIAGNOSIS
Diagnostic testing for suspected HCV infection begins with an antibody test.8 A positive antibody test indicates one of three possibilities: active infection, resolved infection, or a false-positive result. Two drawbacks of HCV antibody testing are that immunocompromised patients may falsely test negative and that antibodies cannot be detected until eight to 12 weeks after the infection is acquired.11 A positive antibody test should be confirmed with an HCV polymerase chain reaction (PCR) RNA test.8,10
HCV RNA testing can detect the virus earlier than antibody testing—as early as two weeks after infection. Although a positive HCV RNA test confirms current HCV infection, its higher cost precludes its use as an initial diagnostic test for lower-risk patients.
In patients who test negative but for whom there is a high index of suspicion for HCV infection (eg, jaundiced patient with an elevated alanine aminotransferase level [ALT]), or for a health care worker with a recent bloodborne HCV exposure, testing for HCV antibodies, HCV RNA, and ALT levels should be ordered at regular intervals for a period of six months.10
Acute versus chronic infection
Distinguishing acute and chronic HCV is difficult. A determination that HCV is newly acquired requires a documented negative baseline antibody test, followed by laboratory evidence of seroconversion. This is typically only seen in cases where there has been a recent, known exposure to the virus.
Both HCV antibody and RNA testing are recommended when screening high-risk patients, including those who are immunocompromised, on hemodialysis, or have had a recent exposure to HCV-positive blood.10 Rapid PCR HCV RNA tests can assess both viral load and genotype (see discussion of HCV genotypes under “Chronic HCV”).17 This information helps guide and measure patient response to treatment.
Liver disease severity
Liver fibrosis and cirrhosis are serious complications of HCV; hepatomegaly or splenomegaly may or may not be present on physical examination and patients may require liver biopsies to evaluate disease stage.18 An assessment of the severity of liver damage can determine the urgency of treatment and predict treatment efficacy.
Biopsy results permit the grading of inflammation and nodularity and the staging of septal fibrosis, which can reliably predict future progression of the patient’s disease.19 However, liver biopsy is invasive, painful, and may contain sampling errors; complications may include bleeding, infection, and occasionally accidental injury to a nearby organ. An initial noninvasive assessment may be performed using vibration-controlled transient liver elastography, an ultrasound-based technology that measures liver stiffness, which correlates well with the degree of fibrosis or cirrhosis. Elastography, along with measurement of direct serum biomarkers that are produced by activated hepatic stellate cells involved in fibrosis, affords an accurate, noninvasive means of assessing liver damage.10
Continue for treatment options >>
TREATMENT
Acute HCV
To prevent progression of disease from acute to chronic infection, patients diagnosed with acute disease should be treated if
• They are likely to adhere to the treatment plan
• They have no contraindications to pegylated interferon a (PEG-IFN a) treatment.
Contraindications to PEG-IFN a treatment include uncontrolled depression, psychosis, or epilepsy; pregnancy; couples' unwillingness to use effective contraception during treatment; severe concurrent medical disease; and decompensated liver disease.20
For acute HCV infection, treatment with PEG-IFN a-2a 180 µg/wk or PEG-IFN a-2b 1.5 µg/kg/wk for 24 weeks is recommended.20 Treatment results in an SVR greater than 80%.21 Combination therapy with ribavirin (RBV) does not increase SVR in the treatment of acute HCV infection.22
The appropriate time to begin treatment has not been firmly established because, as previously noted, 15% to 25% of those infected will spontaneously clear the virus. The European Association for the Study of the Liver (EASL) suggests that patients who remain HCV positive at 12 weeks from the time of suspected infection should be treated.23
Chronic HCV
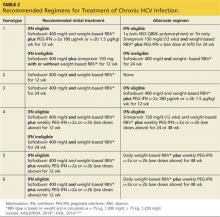
Treatment of chronic HCV is based on multiple considerations, many of them patient-specific (see Table 2). One key element in treatment choice is the HCV genotype with which the patient is infected.
The most prevalent in the US are genotypes 1 through 6; further, genotype 1 has two subtypes: 1a and 1b. In the US, genotype 1 is most common, infecting about 70% of patients; genotype 2, 16%; genotype 3, 12%; genotype 4, 1%; genotype 5, < 1%; and genotype 6, 1%.24 Infection with one genotype does not protect an individual from future infection with the same or a different HCV genotype.3
Other factors influencing treatment include viral load, ALT levels, coinfection (eg, HIV, HBV), comorbidities, and treatment contraindications.21 Treatment is recommended for those who have detectable HCV RNA levels, elevated ALT levels, progressive liver disease on biopsy, and the absence of any serious comorbid conditions or contraindications. ALT levels, however, can fluctuate and do not always correlate with disease severity.
For years, the standard treatment for chronic HCV infection has been PEG-IFN a/RBV for 48 weeks. This combination produced an SVR of 50% to 80%, depending on genotype.3,25 Recently, however, treatment protocols have changed considerably with the introduction of two new and very effective direct-acting antiviral agents (DAAs): simeprevir, an HCV NS3/4A protease inhibitor, and sofosbuvir, an HCV NS5B polymerase inhibitor.
Simeprevir was approved by the FDA in October 2013 for use in combination with PEG-IFN/RBV for treatment of chronic HCV genotype 1 infection in adults with compensated liver disease.27 Because the efficacy of simeprevir is reduced in patients with HCV genotype 1a with an NS3 Q80K polymorphism, screening for NS3 Q80K is recommended; alternative therapy should be considered when this mutation is present.27
Sofosbuvir received FDA approval in December 2013 for use in combination with other antiviral drugs for treatment of chronic HCV infection, with established efficacy for treatment of genotypes 1, 2, 3, and 4 and for HCV/HIV coinfection.28 For genotype 1, simeprevir and sofosbuvir achieve SVRs of 80% and 90%, respectively.27,28
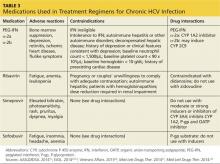
Table 3 lists the current medications for treatment of chronic HCV, including their adverse effects, contraindications, and drug interactions.
Updated treatment guidelines
AASLD, IDSA, EASL, WHO, and the Department of Veterans Affairs National Hepatitis C Resource Center Program recently issued updated evidence-based recommendations for the treatment of chronic HCV.4,10,23,26 Highlights of the changes to the guidelines include
• With the introduction of simeprevir and sofosbuvir, the guidelines no longer recommend combination PEG-IFN a/RBV for 48 weeks as the standard treatment for chronic HCV infection11,23,26
• Treatment regimens utilizing the protease inhibitors telaprevir and boceprevir are no longer recommended (with the exception of the WHO guidelines, which include them in a “conditional” recommendation).4
Regimens that include telaprevir and boceprevir are associated with higher rates of serious adverse effects, such as skin reactions and anemia, and involve longer treatment duration, higher pill burden, several drug interactions, frequent dosing, intensive monitoring, and the need to be taken with food (for telaprevir, high-fat food).10,23,26
Finally, the EASL recommends an additional agent, daclatasvir, an NS5A replication complex inhibitor, as an option for treating HCV genotype 1, 3, and 4.23 At this time, it is approved for use in Europe but has not been approved by the FDA.
Regardless of the treatment regimen, all patients receiving HCV antiviral therapy should be tested regularly to assess effectiveness of treatment and to monitor for the occurrence of adverse effects. Recommended periodic laboratory testing should include HCV RNA, complete blood count with differential, liver function, TSH level, renal function, comprehensive metabolic panel, bilirubin level, and pregnancy.29
SPECIAL POPULATIONS
Patients with HIV/HCV coinfection, a history of injection drug use, and renal impairment require management tailored to their individual circumstances.4,23,29
HIV/HCV-coinfected patients
Approximately 25% to 33% of patients infected with HIV are coinfected with HCV. HCV infection progresses more rapidly in HIV-infected patients, and coinfected patients are at greater risk for cirrhosis, liver cancer, and liver failure.30
For patients with HIV/HCV coinfection, the decision to start treatment is more complex because of the high pill burden, overlapping toxicities, and interactions among the drugs used to treat HIV and HCV infections.4 HIV antiviral therapy should be started before HCV treatment to improve immune function, thereby decreasing the risks for both further infections and HIV transmission. This also allows the patient to adjust gradually to each regimen.
One exception to this is when an HIV treatment–naïve patient has a CD4 count > 500 cells/mL.30 In this situation, HCV treatment is sometimes completed prior to the start of HIV treatment.
Recommended treatment for HIV/HCV coinfection, by HCV genotype, is outlined below. For all regimens, the weight-based RBV dosage is calculated as follows:
• Weight < 75 kg, 1,000 mg/d
• Weight ≥ 75 kg, 1,200 mg/d10,30
• Genotypes 1 and 4 (IFN eligible): Sofosbuvir 400 mg/d and weight-based RBV plus weekly PEG-IFN a for 12 weeks
• Genotypes 1 and 4 (IFN ineligible): Sofosbuvir 400 mg/d and weight-based RBV for 24 weeks
• Genotypes 2 and 3 (regardless of IFN eligibility): Sofosbuvir 400 mg/d and weight-based RBV for 12 weeks for genotype 2 and 24 weeks for genotype 3
• Genotypes 5 and 6 (IFN eligible): Sofosbuvir 400 mg/d plus weight-based RBV plus weekly PEG-IFN a for 12 weeks.
Injection drug users
HCV infection among young injection drug users (IDUs) is an emerging epidemic that must be addressed by recognizing at-risk populations, screening for early disease, and providing treatment and education. Globally, it is estimated that approximately 67% of IDUs—approximately 10 million people—are infected with HCV.31 Treatment of HCV in IDUs requires integration of many services and health care professionals, including addiction specialists. Dependence on opiates, alcohol, or other substances is common in this patient population. Patients should be counseled on the importance of abstaining from alcohol. IDUs are at risk for hepatitis A (HAV) and HBV infections and should be vaccinated against these diseases.4
Treatment decisions should be based on an individualized evaluation of the patient’s social, lifestyle, and clinical factors.4,23,29 Consideration must also be given to potential drug interactions.4,23,29 In IDUs, treatment with PEG-IFN a/RBV should be considered because DAA studies have excluded active users.20 Evaluation of the safety and efficacy of new regimens containing PEG-IFN a, as well as PEG-IFN-a–free regimens, in IDUs is needed.20
Renal impairment
Both PEG-IFN a and RBV require dose adjustments in patients with a creatinine clearance less than 30 mL/min.4,10,23,26 Further, simeprevir and sofosbuvir have not been studied in HCV patients with creatinine clearance less than 30 mL/min.10,26
On the next page: Barriers to therapy >>
BARRIERS TO THERAPY
Patient-related
Barriers to treatment include lack of acceptance of treatment due to the absence of symptoms; lengthy duration of treatment; adverse effects of HCV drugs; and treatment costs.10 Potential strategies to overcome such obstacles include patient education; simplified dosing; better-tolerated treatments; and collaboration with pharmaceutical companies that offer patient assistance programs.
Drugs for HCV treatment can cause unpleasant adverse effects. Clinicians should encourage adherence for the entire duration of treatment and provide practical advice for coping with adverse effects such as fatigue, headache and other flulike symptoms, injection site reactions, cough, bad taste in mouth, oral ulcers, dry mouth, anorexia, nausea or vomiting, skin reactions, hair thinning or hair loss, and insomnia.10,32
Strategies that may help alleviate these undesirable effects include regular low-impact exercise; drinking plenty of fluids; eating a well-balanced diet; maintaining good sleep hygiene; taking acetaminophen or ibuprofen for myalgias or headaches; and rotating PEG-IFN a injection sites.
Substance abuse and psychiatric disorders are common in patients with HCV infection. These patients should be referred to mental health or substance abuse services.10
Clinician-related
Obstacles to successfully treating patients with chronic HCV infection include patient-related barriers; lack of expertise in HCV management; practitioner bias against or resistance to treating patients who use illicit drugs or abuse alcohol; and concerns about the costs of treatment.
Potential strategies to overcome clinician barriers include collaboration with specialists (eg, hepatologists), utilizing telemedicine if necessary; availability of accessible, clear HCV treatment guidelines; and use of computer-based clinical decision support tools (eg, pop-up reminders and standing orders).10
PATIENT COUNSELING
Patients undergoing treatment for chronic HCV infection should be counseled on the following topics33
• Risk for transmission to sex partners
• Not sharing personal items that might have blood on them, such as toothbrushes or razors, and covering any bleeding wounds to keep from spreading infectious blood or secretions
• Need for vaccinations against HAV and HBV if not immune
• Not donating blood, organs, tissue, or semen
• Stop using illicit drugs
• If continuing to inject drugs, avoid reusing or sharing syringes, needles, water, or drug preparation equipment
• Clean the injection site with a new swab prior to injection
• Safely dispose of syringes after one use
• Consider the benefits of joining a support group
• Avoid alcohol because it can accelerate cirrhosis and end-stage liver disease
• Not to start any new medicines, including OTC and herbal medicines, without checking with their health care professional.
FUTURE TREATMENTS
Treatments for HCV infection are evolving rapidly, and IFN-free options with excellent SVRs are emerging. Below are brief summaries of some of the current research that is focused on the study of IFN-free options. Other new regimens are awaiting approval by the FDA. These include
• The combination of sofosbuvir plus ledipasvir (an NS5A inhibitor) with and without RBV, for HCV genotype 1 infection for 8 or 12 weeks. The SVR in both groups was 93% to 95%. RBV had no effect on SVR.33
• An all-oral combination therapy of daclatasvir (an HCV NS5A replication complex inhibitor) plus sofosbuvir, with or without RBV, for HCV genotypes 1, 2, and 3 for 24 weeks. The SVR varied from 98% with genotype 1, 92% with genotype 2, and 89% for genotype 3. Patients who received RBV had an SVR of 94%; those who did not achieved an SVR of 98%.34
• The combination of ABT-450 (a protease inhibitor boosted with ritonavir), ombitasvir (NS5A inhibitor), and dasabuvir (a nonnucleoside inhibitor) with RBV in patients with HCV genotype 1 and no cirrhosis. At 12 weeks, an SVR of 96% was achieved.35
Despite years of research, a vaccine to prevent HCV infection has not yet been developed, although research continues. The major challenge is the number of genotypes and subtypes of HCV. A vaccine to prevent HCV infection will need to induce immunity to all genotypes and subtypes.36
CONCLUSION
Patients with chronic HCV infection are frequently unaware of this fact, even though the majority of them acquired the liver disease decades ago. Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease. Identification of infected patients enables treatment initiation and, in most cases, cure of the infection. All patients at risk for infection should be counseled about risk reduction and screened periodically.
Thanks to newer, more effective treatment options, patients with HCV have an excellent chance today of clearing the virus and ultimately being cured. This could lead to a dramatic reduction in future HCV-associated morbidity and mortality. Since most of those infected today have never been treated, screening of at-risk patients is essential.
* Editor's note: At press time, the FDA had announced approval of a combination pill (ledipasvir/sofosbuvir) for the treatment of patients with chronic HCV.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. CDC. Hepatitis C FAQs for Health Professionals. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed October 19, 2014.
4. The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed October 19, 2014.
5. Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39(5):518-531.
6. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
7. CDC. Surveillance for Viral Hepatitis—United States, 2012. www.cdc.gov/
hepatitis/Statistics/2012Surveillance/index.htm. Accessed October 19, 2014.
8. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. Moyer VA, on behalf of the US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed October 19, 2014.
11. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR. 2012;61(RR-04):1-18.
12. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006;174(5):649-659.
13. Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med. 1993;86(2):119-125.
14. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687-1695.
15. Marinho RT, Vitor S, Velosa J. Benefits of curing hepatitis C infection.
J Gastrointestin Liver Dis. 2014;23(1):85-90.
16. Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369-3374.
17. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int. 2011;31(suppl 1): 3-12.
18. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
19. Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23(6):1334-1340.
20. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed October 19, 2014.
21. Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207.
22. Wiegand J, Buggisch P, Boecher W, et al; German HEP-NET Acute HCV Study Group. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-256.
23. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392-420.
24. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. September 2014. Published online ahead of print.
25. Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
26. Department of Veterans Affairs. National Hepatitis C Resource Center Program and the Office of Public Health. Chronic hepatitis C virus infection: treatment considerations. www.hepatitis.va.gov/pdf/2014hcv.pdf. Accessed October 19, 2014.
27. Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1-3.
28. Sofosbuvir (Sovaldi) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1434):5-6.
29. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
30. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aids info.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed October 19, 2014.
31. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systemic reviews. Lancet. 2011;378(9791):571-583.
32. US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration. A treatment improvement protocol: addressing viral hepatitis in people with substance abuse disorders. Tip 53. 2011.
33. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8-12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879-1888.
34. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211-221.
35. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):
1594-1603.
36. Drummer HE. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol. 2014;5:329.
CE/CME No: CR-1411
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the risk factors for HCV infection.
• Identify who should be screened for HCV infection.
• Discuss the symptoms, clinical course, diagnosis, and complications of chronic HCV infection.
• Differentiate between the treatment of acute and chronic HCV infection.
• Describe the challenges of treating HCV infection in patients who are coinfected with HIV.
FACULTY
Daniel Sturm and Samuel L. Gurevitz are Assistant Professors in the Physician Assistant Program, College of Pharmacy and Health Sciences, at Butler University in Indianapolis. Cassidy Davidson, Abigail Fritchley, and Audrey Wagaman are students in the PA Program at Butler University. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Several million Americans, primarily those in their fifties and sixties, contracted hepatitis C virus (HCV) many years ago and are unaware of their infection and their risk for HCV-related liver disease. Screening at-risk patients is important because newer treatment regimens are curative and can reduce associated morbidity and mortality.
Chronic infection with hepatitis C virus (HCV) is a major cause of liver disease in the United States. National Health and Nutrition Examination Survey data indicates that 2.7 million people have chronic HCV infection; the CDC estimates 3.2 million.1-3 Yet these individuals may be asymptomatic for years, despite slow progression of sequelae (eg, chronic liver disease, cirrhosis, hepatocellular carcinoma [HCC]) that may silently unfold. Left untreated, chronic HCV infection is associated with a 15% to 30% risk for cirrhosis within 20 years, which subsequently confers an annual risk for HCC of 2% to 4%.4 Additionally, chronic HCV infection is now the leading indication for liver transplantation.3
Of note, 70% of those with chronic HCV infection were born between 1945 and 1965.2 This is believed to be attributable to viral transmission via contaminated blood, blood products, and organ transplants prior to the implementation of universal precautions for blood supply screening in 1992; and to past injection drug use, even if it occurred only once.3
In a recent analysis, it was determined that the clinical and economic burdens of chronic HCV infection increased in the past decade, and this trend is likely to continue during the next decade.5 For example, HCV was responsible for 15,106 US deaths in 2007, surpassing deaths caused by HIV for the first time.6 Since then, the number of HCV-related deaths has continued to increase, to 17,721 in 2011.7 Economic costs solely attributable to HCV infection are difficult to calculate, but estimates range from several hundred million dollars to $30 billion annually.5 The World Health Organization (WHO) estimates that more than 185 million people worldwide are infected with HCV and that HCV is responsible for 350,000 deaths annually.4
The main goal of treatment is to achieve a sustained virologic response (SVR), defined as undetectable HCV RNA in serum 12 to 24 weeks after completion of treatment and thereby prevent or reduce the complications of HCV infection.3 Proactive screening, diagnosis, monitoring, and treatment of HCV infection can significantly reduce long-term morbidity and mortality.
INDICATIONS FOR HCV SCREENING
Recommendations for HCV screening have been developed by numerous organizations, including the WHO, CDC, US Preventive Services Task Force (USPSTF), American Association for the Study of Liver Diseases (AASLD), and the Infectious Diseases Society of America (IDSA) (see Table 1).3,4,8,9-11 Screening should be offered to all individuals meeting one or more of the criteria. A 2012 CDC update calls for one-time testing for all persons born between 1945 and 1965 because of the disproportionately high prevalence of HCV infection in this cohort, which is five times greater than in the general population.11
The initial screening tool for HCV infection is an HCV antibody test. A positive or reactive anti-HCV antibody result can signify either current or resolved infection, so a positive result should be followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present.4,8-10 See Figure on previous page for an HCV screening algorithm, which includes the points at which referrals to specialists are indicated.
A rapid HCV antibody test was approved by the FDA in 2011 and is favored because of its widespread availability, ease of use, rapid results, and low cost. Point-of-care testing is comparable in sensitivity and specificity to laboratory-based HCV assays and can utilize blood obtained via fingerstick or venipuncture.8 This form of testing facilitates HCV screening in a variety of settings, such as health fairs and emergency departments, as well as in high-risk settings such as methadone clinics.
Populations at increased risk for HCV infection are also at increased risk for hepatitis B virus (HBV), HIV, and tuberculosis infections; therefore, screening for these may also be warranted.4
PROGRESSION OF UNTREATED INFECTION
Just as with chronic HCV infection, patients newly infected with HCV are typically asymptomatic; the illness manifests with clinical symptoms in only 20% to 30% of cases.3 If symptoms do appear, they include fever, fatigue, dark urine, clay-colored stool, abdominal pain, loss of appetite, nausea, vomiting, joint pain, and jaundice. These acute phase symptoms may last two to 12 weeks.12
Spontaneous clearance of the virus occurs in only 15% to 25% of cases (see further discussion under “Acute HCV.”).2,3 Even if a patient clears an HCV infection, if he or she falls within one of the at-risk categories (see Table 1), then periodic screening should continue because prior HCV infection does not protect against future infection.3
The progression of chronic HCV is indolent and often subclinical, with fatigue being the most common complaint. Other nonspecific symptoms may include nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. One study noted that symptoms do not correlate with disease severity.13 In a 10-year prospective study, chronic HCV infection increased mortality when the infection was acquired at an early age (younger than 50) and/or when cirrhosis developed.14 Patients with cirrhosis progress to liver decompensation at a rate of 3% to 6% annually.15
In addition to liver disease, HCV infection is associated with an increased risk for non-Hodgkin lymphoma.3 HCV may also induce insulin resistance, which increases the risk for hepatic fibrosis. Other studies document HCV-induced cognitive impairment, but little scientific data is yet available as to its pathogenesis.16
DIAGNOSIS
Diagnostic testing for suspected HCV infection begins with an antibody test.8 A positive antibody test indicates one of three possibilities: active infection, resolved infection, or a false-positive result. Two drawbacks of HCV antibody testing are that immunocompromised patients may falsely test negative and that antibodies cannot be detected until eight to 12 weeks after the infection is acquired.11 A positive antibody test should be confirmed with an HCV polymerase chain reaction (PCR) RNA test.8,10
HCV RNA testing can detect the virus earlier than antibody testing—as early as two weeks after infection. Although a positive HCV RNA test confirms current HCV infection, its higher cost precludes its use as an initial diagnostic test for lower-risk patients.
In patients who test negative but for whom there is a high index of suspicion for HCV infection (eg, jaundiced patient with an elevated alanine aminotransferase level [ALT]), or for a health care worker with a recent bloodborne HCV exposure, testing for HCV antibodies, HCV RNA, and ALT levels should be ordered at regular intervals for a period of six months.10
Acute versus chronic infection
Distinguishing acute and chronic HCV is difficult. A determination that HCV is newly acquired requires a documented negative baseline antibody test, followed by laboratory evidence of seroconversion. This is typically only seen in cases where there has been a recent, known exposure to the virus.
Both HCV antibody and RNA testing are recommended when screening high-risk patients, including those who are immunocompromised, on hemodialysis, or have had a recent exposure to HCV-positive blood.10 Rapid PCR HCV RNA tests can assess both viral load and genotype (see discussion of HCV genotypes under “Chronic HCV”).17 This information helps guide and measure patient response to treatment.
Liver disease severity
Liver fibrosis and cirrhosis are serious complications of HCV; hepatomegaly or splenomegaly may or may not be present on physical examination and patients may require liver biopsies to evaluate disease stage.18 An assessment of the severity of liver damage can determine the urgency of treatment and predict treatment efficacy.
Biopsy results permit the grading of inflammation and nodularity and the staging of septal fibrosis, which can reliably predict future progression of the patient’s disease.19 However, liver biopsy is invasive, painful, and may contain sampling errors; complications may include bleeding, infection, and occasionally accidental injury to a nearby organ. An initial noninvasive assessment may be performed using vibration-controlled transient liver elastography, an ultrasound-based technology that measures liver stiffness, which correlates well with the degree of fibrosis or cirrhosis. Elastography, along with measurement of direct serum biomarkers that are produced by activated hepatic stellate cells involved in fibrosis, affords an accurate, noninvasive means of assessing liver damage.10
Continue for treatment options >>
TREATMENT
Acute HCV
To prevent progression of disease from acute to chronic infection, patients diagnosed with acute disease should be treated if
• They are likely to adhere to the treatment plan
• They have no contraindications to pegylated interferon a (PEG-IFN a) treatment.
Contraindications to PEG-IFN a treatment include uncontrolled depression, psychosis, or epilepsy; pregnancy; couples' unwillingness to use effective contraception during treatment; severe concurrent medical disease; and decompensated liver disease.20
For acute HCV infection, treatment with PEG-IFN a-2a 180 µg/wk or PEG-IFN a-2b 1.5 µg/kg/wk for 24 weeks is recommended.20 Treatment results in an SVR greater than 80%.21 Combination therapy with ribavirin (RBV) does not increase SVR in the treatment of acute HCV infection.22
The appropriate time to begin treatment has not been firmly established because, as previously noted, 15% to 25% of those infected will spontaneously clear the virus. The European Association for the Study of the Liver (EASL) suggests that patients who remain HCV positive at 12 weeks from the time of suspected infection should be treated.23
Chronic HCV
Treatment of chronic HCV is based on multiple considerations, many of them patient-specific (see Table 2). One key element in treatment choice is the HCV genotype with which the patient is infected.
The most prevalent in the US are genotypes 1 through 6; further, genotype 1 has two subtypes: 1a and 1b. In the US, genotype 1 is most common, infecting about 70% of patients; genotype 2, 16%; genotype 3, 12%; genotype 4, 1%; genotype 5, < 1%; and genotype 6, 1%.24 Infection with one genotype does not protect an individual from future infection with the same or a different HCV genotype.3
Other factors influencing treatment include viral load, ALT levels, coinfection (eg, HIV, HBV), comorbidities, and treatment contraindications.21 Treatment is recommended for those who have detectable HCV RNA levels, elevated ALT levels, progressive liver disease on biopsy, and the absence of any serious comorbid conditions or contraindications. ALT levels, however, can fluctuate and do not always correlate with disease severity.
For years, the standard treatment for chronic HCV infection has been PEG-IFN a/RBV for 48 weeks. This combination produced an SVR of 50% to 80%, depending on genotype.3,25 Recently, however, treatment protocols have changed considerably with the introduction of two new and very effective direct-acting antiviral agents (DAAs): simeprevir, an HCV NS3/4A protease inhibitor, and sofosbuvir, an HCV NS5B polymerase inhibitor.
Simeprevir was approved by the FDA in October 2013 for use in combination with PEG-IFN/RBV for treatment of chronic HCV genotype 1 infection in adults with compensated liver disease.27 Because the efficacy of simeprevir is reduced in patients with HCV genotype 1a with an NS3 Q80K polymorphism, screening for NS3 Q80K is recommended; alternative therapy should be considered when this mutation is present.27
Sofosbuvir received FDA approval in December 2013 for use in combination with other antiviral drugs for treatment of chronic HCV infection, with established efficacy for treatment of genotypes 1, 2, 3, and 4 and for HCV/HIV coinfection.28 For genotype 1, simeprevir and sofosbuvir achieve SVRs of 80% and 90%, respectively.27,28
Table 3 lists the current medications for treatment of chronic HCV, including their adverse effects, contraindications, and drug interactions.
Updated treatment guidelines
AASLD, IDSA, EASL, WHO, and the Department of Veterans Affairs National Hepatitis C Resource Center Program recently issued updated evidence-based recommendations for the treatment of chronic HCV.4,10,23,26 Highlights of the changes to the guidelines include
• With the introduction of simeprevir and sofosbuvir, the guidelines no longer recommend combination PEG-IFN a/RBV for 48 weeks as the standard treatment for chronic HCV infection11,23,26
• Treatment regimens utilizing the protease inhibitors telaprevir and boceprevir are no longer recommended (with the exception of the WHO guidelines, which include them in a “conditional” recommendation).4
Regimens that include telaprevir and boceprevir are associated with higher rates of serious adverse effects, such as skin reactions and anemia, and involve longer treatment duration, higher pill burden, several drug interactions, frequent dosing, intensive monitoring, and the need to be taken with food (for telaprevir, high-fat food).10,23,26
Finally, the EASL recommends an additional agent, daclatasvir, an NS5A replication complex inhibitor, as an option for treating HCV genotype 1, 3, and 4.23 At this time, it is approved for use in Europe but has not been approved by the FDA.
Regardless of the treatment regimen, all patients receiving HCV antiviral therapy should be tested regularly to assess effectiveness of treatment and to monitor for the occurrence of adverse effects. Recommended periodic laboratory testing should include HCV RNA, complete blood count with differential, liver function, TSH level, renal function, comprehensive metabolic panel, bilirubin level, and pregnancy.29
SPECIAL POPULATIONS
Patients with HIV/HCV coinfection, a history of injection drug use, and renal impairment require management tailored to their individual circumstances.4,23,29
HIV/HCV-coinfected patients
Approximately 25% to 33% of patients infected with HIV are coinfected with HCV. HCV infection progresses more rapidly in HIV-infected patients, and coinfected patients are at greater risk for cirrhosis, liver cancer, and liver failure.30
For patients with HIV/HCV coinfection, the decision to start treatment is more complex because of the high pill burden, overlapping toxicities, and interactions among the drugs used to treat HIV and HCV infections.4 HIV antiviral therapy should be started before HCV treatment to improve immune function, thereby decreasing the risks for both further infections and HIV transmission. This also allows the patient to adjust gradually to each regimen.
One exception to this is when an HIV treatment–naïve patient has a CD4 count > 500 cells/mL.30 In this situation, HCV treatment is sometimes completed prior to the start of HIV treatment.
Recommended treatment for HIV/HCV coinfection, by HCV genotype, is outlined below. For all regimens, the weight-based RBV dosage is calculated as follows:
• Weight < 75 kg, 1,000 mg/d
• Weight ≥ 75 kg, 1,200 mg/d10,30
• Genotypes 1 and 4 (IFN eligible): Sofosbuvir 400 mg/d and weight-based RBV plus weekly PEG-IFN a for 12 weeks
• Genotypes 1 and 4 (IFN ineligible): Sofosbuvir 400 mg/d and weight-based RBV for 24 weeks
• Genotypes 2 and 3 (regardless of IFN eligibility): Sofosbuvir 400 mg/d and weight-based RBV for 12 weeks for genotype 2 and 24 weeks for genotype 3
• Genotypes 5 and 6 (IFN eligible): Sofosbuvir 400 mg/d plus weight-based RBV plus weekly PEG-IFN a for 12 weeks.
Injection drug users
HCV infection among young injection drug users (IDUs) is an emerging epidemic that must be addressed by recognizing at-risk populations, screening for early disease, and providing treatment and education. Globally, it is estimated that approximately 67% of IDUs—approximately 10 million people—are infected with HCV.31 Treatment of HCV in IDUs requires integration of many services and health care professionals, including addiction specialists. Dependence on opiates, alcohol, or other substances is common in this patient population. Patients should be counseled on the importance of abstaining from alcohol. IDUs are at risk for hepatitis A (HAV) and HBV infections and should be vaccinated against these diseases.4
Treatment decisions should be based on an individualized evaluation of the patient’s social, lifestyle, and clinical factors.4,23,29 Consideration must also be given to potential drug interactions.4,23,29 In IDUs, treatment with PEG-IFN a/RBV should be considered because DAA studies have excluded active users.20 Evaluation of the safety and efficacy of new regimens containing PEG-IFN a, as well as PEG-IFN-a–free regimens, in IDUs is needed.20
Renal impairment
Both PEG-IFN a and RBV require dose adjustments in patients with a creatinine clearance less than 30 mL/min.4,10,23,26 Further, simeprevir and sofosbuvir have not been studied in HCV patients with creatinine clearance less than 30 mL/min.10,26
On the next page: Barriers to therapy >>
BARRIERS TO THERAPY
Patient-related
Barriers to treatment include lack of acceptance of treatment due to the absence of symptoms; lengthy duration of treatment; adverse effects of HCV drugs; and treatment costs.10 Potential strategies to overcome such obstacles include patient education; simplified dosing; better-tolerated treatments; and collaboration with pharmaceutical companies that offer patient assistance programs.
Drugs for HCV treatment can cause unpleasant adverse effects. Clinicians should encourage adherence for the entire duration of treatment and provide practical advice for coping with adverse effects such as fatigue, headache and other flulike symptoms, injection site reactions, cough, bad taste in mouth, oral ulcers, dry mouth, anorexia, nausea or vomiting, skin reactions, hair thinning or hair loss, and insomnia.10,32
Strategies that may help alleviate these undesirable effects include regular low-impact exercise; drinking plenty of fluids; eating a well-balanced diet; maintaining good sleep hygiene; taking acetaminophen or ibuprofen for myalgias or headaches; and rotating PEG-IFN a injection sites.
Substance abuse and psychiatric disorders are common in patients with HCV infection. These patients should be referred to mental health or substance abuse services.10
Clinician-related
Obstacles to successfully treating patients with chronic HCV infection include patient-related barriers; lack of expertise in HCV management; practitioner bias against or resistance to treating patients who use illicit drugs or abuse alcohol; and concerns about the costs of treatment.
Potential strategies to overcome clinician barriers include collaboration with specialists (eg, hepatologists), utilizing telemedicine if necessary; availability of accessible, clear HCV treatment guidelines; and use of computer-based clinical decision support tools (eg, pop-up reminders and standing orders).10
PATIENT COUNSELING
Patients undergoing treatment for chronic HCV infection should be counseled on the following topics33
• Risk for transmission to sex partners
• Not sharing personal items that might have blood on them, such as toothbrushes or razors, and covering any bleeding wounds to keep from spreading infectious blood or secretions
• Need for vaccinations against HAV and HBV if not immune
• Not donating blood, organs, tissue, or semen
• Stop using illicit drugs
• If continuing to inject drugs, avoid reusing or sharing syringes, needles, water, or drug preparation equipment
• Clean the injection site with a new swab prior to injection
• Safely dispose of syringes after one use
• Consider the benefits of joining a support group
• Avoid alcohol because it can accelerate cirrhosis and end-stage liver disease
• Not to start any new medicines, including OTC and herbal medicines, without checking with their health care professional.
FUTURE TREATMENTS
Treatments for HCV infection are evolving rapidly, and IFN-free options with excellent SVRs are emerging. Below are brief summaries of some of the current research that is focused on the study of IFN-free options. Other new regimens are awaiting approval by the FDA. These include
• The combination of sofosbuvir plus ledipasvir (an NS5A inhibitor) with and without RBV, for HCV genotype 1 infection for 8 or 12 weeks. The SVR in both groups was 93% to 95%. RBV had no effect on SVR.33
• An all-oral combination therapy of daclatasvir (an HCV NS5A replication complex inhibitor) plus sofosbuvir, with or without RBV, for HCV genotypes 1, 2, and 3 for 24 weeks. The SVR varied from 98% with genotype 1, 92% with genotype 2, and 89% for genotype 3. Patients who received RBV had an SVR of 94%; those who did not achieved an SVR of 98%.34
• The combination of ABT-450 (a protease inhibitor boosted with ritonavir), ombitasvir (NS5A inhibitor), and dasabuvir (a nonnucleoside inhibitor) with RBV in patients with HCV genotype 1 and no cirrhosis. At 12 weeks, an SVR of 96% was achieved.35
Despite years of research, a vaccine to prevent HCV infection has not yet been developed, although research continues. The major challenge is the number of genotypes and subtypes of HCV. A vaccine to prevent HCV infection will need to induce immunity to all genotypes and subtypes.36
CONCLUSION
Patients with chronic HCV infection are frequently unaware of this fact, even though the majority of them acquired the liver disease decades ago. Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease. Identification of infected patients enables treatment initiation and, in most cases, cure of the infection. All patients at risk for infection should be counseled about risk reduction and screened periodically.
Thanks to newer, more effective treatment options, patients with HCV have an excellent chance today of clearing the virus and ultimately being cured. This could lead to a dramatic reduction in future HCV-associated morbidity and mortality. Since most of those infected today have never been treated, screening of at-risk patients is essential.
* Editor's note: At press time, the FDA had announced approval of a combination pill (ledipasvir/sofosbuvir) for the treatment of patients with chronic HCV.
CE/CME No: CR-1411
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the risk factors for HCV infection.
• Identify who should be screened for HCV infection.
• Discuss the symptoms, clinical course, diagnosis, and complications of chronic HCV infection.
• Differentiate between the treatment of acute and chronic HCV infection.
• Describe the challenges of treating HCV infection in patients who are coinfected with HIV.
FACULTY
Daniel Sturm and Samuel L. Gurevitz are Assistant Professors in the Physician Assistant Program, College of Pharmacy and Health Sciences, at Butler University in Indianapolis. Cassidy Davidson, Abigail Fritchley, and Audrey Wagaman are students in the PA Program at Butler University. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Several million Americans, primarily those in their fifties and sixties, contracted hepatitis C virus (HCV) many years ago and are unaware of their infection and their risk for HCV-related liver disease. Screening at-risk patients is important because newer treatment regimens are curative and can reduce associated morbidity and mortality.
Chronic infection with hepatitis C virus (HCV) is a major cause of liver disease in the United States. National Health and Nutrition Examination Survey data indicates that 2.7 million people have chronic HCV infection; the CDC estimates 3.2 million.1-3 Yet these individuals may be asymptomatic for years, despite slow progression of sequelae (eg, chronic liver disease, cirrhosis, hepatocellular carcinoma [HCC]) that may silently unfold. Left untreated, chronic HCV infection is associated with a 15% to 30% risk for cirrhosis within 20 years, which subsequently confers an annual risk for HCC of 2% to 4%.4 Additionally, chronic HCV infection is now the leading indication for liver transplantation.3
Of note, 70% of those with chronic HCV infection were born between 1945 and 1965.2 This is believed to be attributable to viral transmission via contaminated blood, blood products, and organ transplants prior to the implementation of universal precautions for blood supply screening in 1992; and to past injection drug use, even if it occurred only once.3
In a recent analysis, it was determined that the clinical and economic burdens of chronic HCV infection increased in the past decade, and this trend is likely to continue during the next decade.5 For example, HCV was responsible for 15,106 US deaths in 2007, surpassing deaths caused by HIV for the first time.6 Since then, the number of HCV-related deaths has continued to increase, to 17,721 in 2011.7 Economic costs solely attributable to HCV infection are difficult to calculate, but estimates range from several hundred million dollars to $30 billion annually.5 The World Health Organization (WHO) estimates that more than 185 million people worldwide are infected with HCV and that HCV is responsible for 350,000 deaths annually.4
The main goal of treatment is to achieve a sustained virologic response (SVR), defined as undetectable HCV RNA in serum 12 to 24 weeks after completion of treatment and thereby prevent or reduce the complications of HCV infection.3 Proactive screening, diagnosis, monitoring, and treatment of HCV infection can significantly reduce long-term morbidity and mortality.
INDICATIONS FOR HCV SCREENING
Recommendations for HCV screening have been developed by numerous organizations, including the WHO, CDC, US Preventive Services Task Force (USPSTF), American Association for the Study of Liver Diseases (AASLD), and the Infectious Diseases Society of America (IDSA) (see Table 1).3,4,8,9-11 Screening should be offered to all individuals meeting one or more of the criteria. A 2012 CDC update calls for one-time testing for all persons born between 1945 and 1965 because of the disproportionately high prevalence of HCV infection in this cohort, which is five times greater than in the general population.11
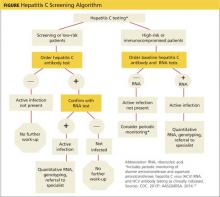
The initial screening tool for HCV infection is an HCV antibody test. A positive or reactive anti-HCV antibody result can signify either current or resolved infection, so a positive result should be followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present.4,8-10 See Figure on previous page for an HCV screening algorithm, which includes the points at which referrals to specialists are indicated.
A rapid HCV antibody test was approved by the FDA in 2011 and is favored because of its widespread availability, ease of use, rapid results, and low cost. Point-of-care testing is comparable in sensitivity and specificity to laboratory-based HCV assays and can utilize blood obtained via fingerstick or venipuncture.8 This form of testing facilitates HCV screening in a variety of settings, such as health fairs and emergency departments, as well as in high-risk settings such as methadone clinics.
Populations at increased risk for HCV infection are also at increased risk for hepatitis B virus (HBV), HIV, and tuberculosis infections; therefore, screening for these may also be warranted.4
PROGRESSION OF UNTREATED INFECTION
Just as with chronic HCV infection, patients newly infected with HCV are typically asymptomatic; the illness manifests with clinical symptoms in only 20% to 30% of cases.3 If symptoms do appear, they include fever, fatigue, dark urine, clay-colored stool, abdominal pain, loss of appetite, nausea, vomiting, joint pain, and jaundice. These acute phase symptoms may last two to 12 weeks.12
Spontaneous clearance of the virus occurs in only 15% to 25% of cases (see further discussion under “Acute HCV.”).2,3 Even if a patient clears an HCV infection, if he or she falls within one of the at-risk categories (see Table 1), then periodic screening should continue because prior HCV infection does not protect against future infection.3
The progression of chronic HCV is indolent and often subclinical, with fatigue being the most common complaint. Other nonspecific symptoms may include nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. One study noted that symptoms do not correlate with disease severity.13 In a 10-year prospective study, chronic HCV infection increased mortality when the infection was acquired at an early age (younger than 50) and/or when cirrhosis developed.14 Patients with cirrhosis progress to liver decompensation at a rate of 3% to 6% annually.15
In addition to liver disease, HCV infection is associated with an increased risk for non-Hodgkin lymphoma.3 HCV may also induce insulin resistance, which increases the risk for hepatic fibrosis. Other studies document HCV-induced cognitive impairment, but little scientific data is yet available as to its pathogenesis.16
DIAGNOSIS
Diagnostic testing for suspected HCV infection begins with an antibody test.8 A positive antibody test indicates one of three possibilities: active infection, resolved infection, or a false-positive result. Two drawbacks of HCV antibody testing are that immunocompromised patients may falsely test negative and that antibodies cannot be detected until eight to 12 weeks after the infection is acquired.11 A positive antibody test should be confirmed with an HCV polymerase chain reaction (PCR) RNA test.8,10
HCV RNA testing can detect the virus earlier than antibody testing—as early as two weeks after infection. Although a positive HCV RNA test confirms current HCV infection, its higher cost precludes its use as an initial diagnostic test for lower-risk patients.
In patients who test negative but for whom there is a high index of suspicion for HCV infection (eg, jaundiced patient with an elevated alanine aminotransferase level [ALT]), or for a health care worker with a recent bloodborne HCV exposure, testing for HCV antibodies, HCV RNA, and ALT levels should be ordered at regular intervals for a period of six months.10
Acute versus chronic infection
Distinguishing acute and chronic HCV is difficult. A determination that HCV is newly acquired requires a documented negative baseline antibody test, followed by laboratory evidence of seroconversion. This is typically only seen in cases where there has been a recent, known exposure to the virus.
Both HCV antibody and RNA testing are recommended when screening high-risk patients, including those who are immunocompromised, on hemodialysis, or have had a recent exposure to HCV-positive blood.10 Rapid PCR HCV RNA tests can assess both viral load and genotype (see discussion of HCV genotypes under “Chronic HCV”).17 This information helps guide and measure patient response to treatment.
Liver disease severity
Liver fibrosis and cirrhosis are serious complications of HCV; hepatomegaly or splenomegaly may or may not be present on physical examination and patients may require liver biopsies to evaluate disease stage.18 An assessment of the severity of liver damage can determine the urgency of treatment and predict treatment efficacy.
Biopsy results permit the grading of inflammation and nodularity and the staging of septal fibrosis, which can reliably predict future progression of the patient’s disease.19 However, liver biopsy is invasive, painful, and may contain sampling errors; complications may include bleeding, infection, and occasionally accidental injury to a nearby organ. An initial noninvasive assessment may be performed using vibration-controlled transient liver elastography, an ultrasound-based technology that measures liver stiffness, which correlates well with the degree of fibrosis or cirrhosis. Elastography, along with measurement of direct serum biomarkers that are produced by activated hepatic stellate cells involved in fibrosis, affords an accurate, noninvasive means of assessing liver damage.10
Continue for treatment options >>
TREATMENT
Acute HCV
To prevent progression of disease from acute to chronic infection, patients diagnosed with acute disease should be treated if
• They are likely to adhere to the treatment plan
• They have no contraindications to pegylated interferon a (PEG-IFN a) treatment.
Contraindications to PEG-IFN a treatment include uncontrolled depression, psychosis, or epilepsy; pregnancy; couples' unwillingness to use effective contraception during treatment; severe concurrent medical disease; and decompensated liver disease.20
For acute HCV infection, treatment with PEG-IFN a-2a 180 µg/wk or PEG-IFN a-2b 1.5 µg/kg/wk for 24 weeks is recommended.20 Treatment results in an SVR greater than 80%.21 Combination therapy with ribavirin (RBV) does not increase SVR in the treatment of acute HCV infection.22
The appropriate time to begin treatment has not been firmly established because, as previously noted, 15% to 25% of those infected will spontaneously clear the virus. The European Association for the Study of the Liver (EASL) suggests that patients who remain HCV positive at 12 weeks from the time of suspected infection should be treated.23
Chronic HCV
Treatment of chronic HCV is based on multiple considerations, many of them patient-specific (see Table 2). One key element in treatment choice is the HCV genotype with which the patient is infected.
The most prevalent in the US are genotypes 1 through 6; further, genotype 1 has two subtypes: 1a and 1b. In the US, genotype 1 is most common, infecting about 70% of patients; genotype 2, 16%; genotype 3, 12%; genotype 4, 1%; genotype 5, < 1%; and genotype 6, 1%.24 Infection with one genotype does not protect an individual from future infection with the same or a different HCV genotype.3
Other factors influencing treatment include viral load, ALT levels, coinfection (eg, HIV, HBV), comorbidities, and treatment contraindications.21 Treatment is recommended for those who have detectable HCV RNA levels, elevated ALT levels, progressive liver disease on biopsy, and the absence of any serious comorbid conditions or contraindications. ALT levels, however, can fluctuate and do not always correlate with disease severity.
For years, the standard treatment for chronic HCV infection has been PEG-IFN a/RBV for 48 weeks. This combination produced an SVR of 50% to 80%, depending on genotype.3,25 Recently, however, treatment protocols have changed considerably with the introduction of two new and very effective direct-acting antiviral agents (DAAs): simeprevir, an HCV NS3/4A protease inhibitor, and sofosbuvir, an HCV NS5B polymerase inhibitor.
Simeprevir was approved by the FDA in October 2013 for use in combination with PEG-IFN/RBV for treatment of chronic HCV genotype 1 infection in adults with compensated liver disease.27 Because the efficacy of simeprevir is reduced in patients with HCV genotype 1a with an NS3 Q80K polymorphism, screening for NS3 Q80K is recommended; alternative therapy should be considered when this mutation is present.27
Sofosbuvir received FDA approval in December 2013 for use in combination with other antiviral drugs for treatment of chronic HCV infection, with established efficacy for treatment of genotypes 1, 2, 3, and 4 and for HCV/HIV coinfection.28 For genotype 1, simeprevir and sofosbuvir achieve SVRs of 80% and 90%, respectively.27,28
Table 3 lists the current medications for treatment of chronic HCV, including their adverse effects, contraindications, and drug interactions.
Updated treatment guidelines
AASLD, IDSA, EASL, WHO, and the Department of Veterans Affairs National Hepatitis C Resource Center Program recently issued updated evidence-based recommendations for the treatment of chronic HCV.4,10,23,26 Highlights of the changes to the guidelines include
• With the introduction of simeprevir and sofosbuvir, the guidelines no longer recommend combination PEG-IFN a/RBV for 48 weeks as the standard treatment for chronic HCV infection11,23,26
• Treatment regimens utilizing the protease inhibitors telaprevir and boceprevir are no longer recommended (with the exception of the WHO guidelines, which include them in a “conditional” recommendation).4
Regimens that include telaprevir and boceprevir are associated with higher rates of serious adverse effects, such as skin reactions and anemia, and involve longer treatment duration, higher pill burden, several drug interactions, frequent dosing, intensive monitoring, and the need to be taken with food (for telaprevir, high-fat food).10,23,26
Finally, the EASL recommends an additional agent, daclatasvir, an NS5A replication complex inhibitor, as an option for treating HCV genotype 1, 3, and 4.23 At this time, it is approved for use in Europe but has not been approved by the FDA.
Regardless of the treatment regimen, all patients receiving HCV antiviral therapy should be tested regularly to assess effectiveness of treatment and to monitor for the occurrence of adverse effects. Recommended periodic laboratory testing should include HCV RNA, complete blood count with differential, liver function, TSH level, renal function, comprehensive metabolic panel, bilirubin level, and pregnancy.29
SPECIAL POPULATIONS
Patients with HIV/HCV coinfection, a history of injection drug use, and renal impairment require management tailored to their individual circumstances.4,23,29
HIV/HCV-coinfected patients
Approximately 25% to 33% of patients infected with HIV are coinfected with HCV. HCV infection progresses more rapidly in HIV-infected patients, and coinfected patients are at greater risk for cirrhosis, liver cancer, and liver failure.30
For patients with HIV/HCV coinfection, the decision to start treatment is more complex because of the high pill burden, overlapping toxicities, and interactions among the drugs used to treat HIV and HCV infections.4 HIV antiviral therapy should be started before HCV treatment to improve immune function, thereby decreasing the risks for both further infections and HIV transmission. This also allows the patient to adjust gradually to each regimen.
One exception to this is when an HIV treatment–naïve patient has a CD4 count > 500 cells/mL.30 In this situation, HCV treatment is sometimes completed prior to the start of HIV treatment.
Recommended treatment for HIV/HCV coinfection, by HCV genotype, is outlined below. For all regimens, the weight-based RBV dosage is calculated as follows:
• Weight < 75 kg, 1,000 mg/d
• Weight ≥ 75 kg, 1,200 mg/d10,30
• Genotypes 1 and 4 (IFN eligible): Sofosbuvir 400 mg/d and weight-based RBV plus weekly PEG-IFN a for 12 weeks
• Genotypes 1 and 4 (IFN ineligible): Sofosbuvir 400 mg/d and weight-based RBV for 24 weeks
• Genotypes 2 and 3 (regardless of IFN eligibility): Sofosbuvir 400 mg/d and weight-based RBV for 12 weeks for genotype 2 and 24 weeks for genotype 3
• Genotypes 5 and 6 (IFN eligible): Sofosbuvir 400 mg/d plus weight-based RBV plus weekly PEG-IFN a for 12 weeks.
Injection drug users
HCV infection among young injection drug users (IDUs) is an emerging epidemic that must be addressed by recognizing at-risk populations, screening for early disease, and providing treatment and education. Globally, it is estimated that approximately 67% of IDUs—approximately 10 million people—are infected with HCV.31 Treatment of HCV in IDUs requires integration of many services and health care professionals, including addiction specialists. Dependence on opiates, alcohol, or other substances is common in this patient population. Patients should be counseled on the importance of abstaining from alcohol. IDUs are at risk for hepatitis A (HAV) and HBV infections and should be vaccinated against these diseases.4
Treatment decisions should be based on an individualized evaluation of the patient’s social, lifestyle, and clinical factors.4,23,29 Consideration must also be given to potential drug interactions.4,23,29 In IDUs, treatment with PEG-IFN a/RBV should be considered because DAA studies have excluded active users.20 Evaluation of the safety and efficacy of new regimens containing PEG-IFN a, as well as PEG-IFN-a–free regimens, in IDUs is needed.20
Renal impairment
Both PEG-IFN a and RBV require dose adjustments in patients with a creatinine clearance less than 30 mL/min.4,10,23,26 Further, simeprevir and sofosbuvir have not been studied in HCV patients with creatinine clearance less than 30 mL/min.10,26
On the next page: Barriers to therapy >>
BARRIERS TO THERAPY
Patient-related
Barriers to treatment include lack of acceptance of treatment due to the absence of symptoms; lengthy duration of treatment; adverse effects of HCV drugs; and treatment costs.10 Potential strategies to overcome such obstacles include patient education; simplified dosing; better-tolerated treatments; and collaboration with pharmaceutical companies that offer patient assistance programs.
Drugs for HCV treatment can cause unpleasant adverse effects. Clinicians should encourage adherence for the entire duration of treatment and provide practical advice for coping with adverse effects such as fatigue, headache and other flulike symptoms, injection site reactions, cough, bad taste in mouth, oral ulcers, dry mouth, anorexia, nausea or vomiting, skin reactions, hair thinning or hair loss, and insomnia.10,32
Strategies that may help alleviate these undesirable effects include regular low-impact exercise; drinking plenty of fluids; eating a well-balanced diet; maintaining good sleep hygiene; taking acetaminophen or ibuprofen for myalgias or headaches; and rotating PEG-IFN a injection sites.
Substance abuse and psychiatric disorders are common in patients with HCV infection. These patients should be referred to mental health or substance abuse services.10
Clinician-related
Obstacles to successfully treating patients with chronic HCV infection include patient-related barriers; lack of expertise in HCV management; practitioner bias against or resistance to treating patients who use illicit drugs or abuse alcohol; and concerns about the costs of treatment.
Potential strategies to overcome clinician barriers include collaboration with specialists (eg, hepatologists), utilizing telemedicine if necessary; availability of accessible, clear HCV treatment guidelines; and use of computer-based clinical decision support tools (eg, pop-up reminders and standing orders).10
PATIENT COUNSELING
Patients undergoing treatment for chronic HCV infection should be counseled on the following topics33
• Risk for transmission to sex partners
• Not sharing personal items that might have blood on them, such as toothbrushes or razors, and covering any bleeding wounds to keep from spreading infectious blood or secretions
• Need for vaccinations against HAV and HBV if not immune
• Not donating blood, organs, tissue, or semen
• Stop using illicit drugs
• If continuing to inject drugs, avoid reusing or sharing syringes, needles, water, or drug preparation equipment
• Clean the injection site with a new swab prior to injection
• Safely dispose of syringes after one use
• Consider the benefits of joining a support group
• Avoid alcohol because it can accelerate cirrhosis and end-stage liver disease
• Not to start any new medicines, including OTC and herbal medicines, without checking with their health care professional.
FUTURE TREATMENTS
Treatments for HCV infection are evolving rapidly, and IFN-free options with excellent SVRs are emerging. Below are brief summaries of some of the current research that is focused on the study of IFN-free options. Other new regimens are awaiting approval by the FDA. These include
• The combination of sofosbuvir plus ledipasvir (an NS5A inhibitor) with and without RBV, for HCV genotype 1 infection for 8 or 12 weeks. The SVR in both groups was 93% to 95%. RBV had no effect on SVR.33
• An all-oral combination therapy of daclatasvir (an HCV NS5A replication complex inhibitor) plus sofosbuvir, with or without RBV, for HCV genotypes 1, 2, and 3 for 24 weeks. The SVR varied from 98% with genotype 1, 92% with genotype 2, and 89% for genotype 3. Patients who received RBV had an SVR of 94%; those who did not achieved an SVR of 98%.34
• The combination of ABT-450 (a protease inhibitor boosted with ritonavir), ombitasvir (NS5A inhibitor), and dasabuvir (a nonnucleoside inhibitor) with RBV in patients with HCV genotype 1 and no cirrhosis. At 12 weeks, an SVR of 96% was achieved.35
Despite years of research, a vaccine to prevent HCV infection has not yet been developed, although research continues. The major challenge is the number of genotypes and subtypes of HCV. A vaccine to prevent HCV infection will need to induce immunity to all genotypes and subtypes.36
CONCLUSION
Patients with chronic HCV infection are frequently unaware of this fact, even though the majority of them acquired the liver disease decades ago. Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease. Identification of infected patients enables treatment initiation and, in most cases, cure of the infection. All patients at risk for infection should be counseled about risk reduction and screened periodically.
Thanks to newer, more effective treatment options, patients with HCV have an excellent chance today of clearing the virus and ultimately being cured. This could lead to a dramatic reduction in future HCV-associated morbidity and mortality. Since most of those infected today have never been treated, screening of at-risk patients is essential.
* Editor's note: At press time, the FDA had announced approval of a combination pill (ledipasvir/sofosbuvir) for the treatment of patients with chronic HCV.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. CDC. Hepatitis C FAQs for Health Professionals. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed October 19, 2014.
4. The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed October 19, 2014.
5. Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39(5):518-531.
6. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
7. CDC. Surveillance for Viral Hepatitis—United States, 2012. www.cdc.gov/
hepatitis/Statistics/2012Surveillance/index.htm. Accessed October 19, 2014.
8. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. Moyer VA, on behalf of the US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed October 19, 2014.
11. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR. 2012;61(RR-04):1-18.
12. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006;174(5):649-659.
13. Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med. 1993;86(2):119-125.
14. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687-1695.
15. Marinho RT, Vitor S, Velosa J. Benefits of curing hepatitis C infection.
J Gastrointestin Liver Dis. 2014;23(1):85-90.
16. Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369-3374.
17. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int. 2011;31(suppl 1): 3-12.
18. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
19. Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23(6):1334-1340.
20. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed October 19, 2014.
21. Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207.
22. Wiegand J, Buggisch P, Boecher W, et al; German HEP-NET Acute HCV Study Group. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-256.
23. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392-420.
24. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. September 2014. Published online ahead of print.
25. Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
26. Department of Veterans Affairs. National Hepatitis C Resource Center Program and the Office of Public Health. Chronic hepatitis C virus infection: treatment considerations. www.hepatitis.va.gov/pdf/2014hcv.pdf. Accessed October 19, 2014.
27. Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1-3.
28. Sofosbuvir (Sovaldi) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1434):5-6.
29. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
30. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aids info.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed October 19, 2014.
31. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systemic reviews. Lancet. 2011;378(9791):571-583.
32. US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration. A treatment improvement protocol: addressing viral hepatitis in people with substance abuse disorders. Tip 53. 2011.
33. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8-12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879-1888.
34. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211-221.
35. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):
1594-1603.
36. Drummer HE. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol. 2014;5:329.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. CDC. Hepatitis C FAQs for Health Professionals. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed October 19, 2014.
4. The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed October 19, 2014.
5. Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39(5):518-531.
6. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
7. CDC. Surveillance for Viral Hepatitis—United States, 2012. www.cdc.gov/
hepatitis/Statistics/2012Surveillance/index.htm. Accessed October 19, 2014.
8. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. Moyer VA, on behalf of the US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed October 19, 2014.
11. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR. 2012;61(RR-04):1-18.
12. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006;174(5):649-659.
13. Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med. 1993;86(2):119-125.
14. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687-1695.
15. Marinho RT, Vitor S, Velosa J. Benefits of curing hepatitis C infection.
J Gastrointestin Liver Dis. 2014;23(1):85-90.
16. Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369-3374.
17. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int. 2011;31(suppl 1): 3-12.
18. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
19. Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23(6):1334-1340.
20. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed October 19, 2014.
21. Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207.
22. Wiegand J, Buggisch P, Boecher W, et al; German HEP-NET Acute HCV Study Group. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-256.
23. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392-420.
24. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. September 2014. Published online ahead of print.
25. Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
26. Department of Veterans Affairs. National Hepatitis C Resource Center Program and the Office of Public Health. Chronic hepatitis C virus infection: treatment considerations. www.hepatitis.va.gov/pdf/2014hcv.pdf. Accessed October 19, 2014.
27. Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1-3.
28. Sofosbuvir (Sovaldi) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1434):5-6.
29. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
30. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aids info.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed October 19, 2014.
31. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systemic reviews. Lancet. 2011;378(9791):571-583.
32. US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration. A treatment improvement protocol: addressing viral hepatitis in people with substance abuse disorders. Tip 53. 2011.
33. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8-12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879-1888.
34. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211-221.
35. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):
1594-1603.
36. Drummer HE. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol. 2014;5:329.