User login
Clinical Progress Note: Direct Oral Anticoagulants for Treatment of Venous Thromboembolism in Children
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
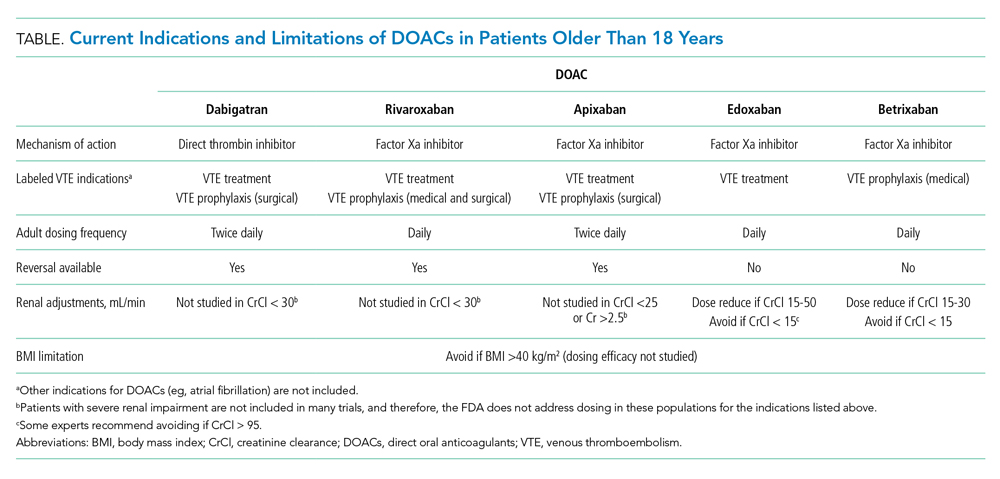
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.
The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.

The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.

The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
© 2021 Society of Hospital Medicine