User login
What Is Your Diagnosis? Spitzoid Melanoma
Crohn's Disease of the Penis Masquerading as Pyoderma Gangrenosum: A Case Report and Review of the Literature
Pyoderma gangrenosum (PG) is an idiopathic, progressive, ulcerative skin disease. Half of all cases are associated with identifiable systemic diseases including arthritis,1 hepatitis,2 blood dyscrasia,3 and immunosuppression.4-6 PG is most frequently associated with inflammatory bowel disease (IBD).4,7
Crohn’s disease (regional enteritis) is a chronic, granulomatous, inflammatory bowel disease that may affect the entire gastrointestinal (GI) tract, as well as the skin. Draining sinuses or fistulas may form between the GI tract and contiguous skin. Cutaneous manifestations associated with regional enteritis include PG, erythema nodosum, and metastatic Crohn’s disease in noncontiguous skin.
There are several differential diagnoses that should be considered: (1) infections, such as Fournier’s gangrene, bacterial pyoderma, deep fungal infection, chronic herpetic ulcer, anaerobic erosive balanitis in uncircumcised men, mycobacterial infection, gummatous syphilis, and amebiasis cutis; (2) neoplastic conditions, such as squamous cell carcinoma, metastatic gastrointestinal or genitourinary carcinoma, extramammary Paget’s disease; and (3) miscellaneous conditions, such as metastatic Crohn’s disease, PG, erosive lichen planus, halogenodermas, necrotizing vasculitis, antiphospholipid syndrome, brown recluse spider bite, and factitial dermatitis. Differentiating between PG and cutaneous Crohn’s disease (CCD) may be difficult, as the clinical and pathologic features often are similar.
CASE REPORT
A 52-year-old circumcised white man was diagnosed with Crohn’s disease in 1974. The condition periodically was responsive to sulfasalazine, metronidazole, and prednisone. A small bowel resection was performed 1 and 10 years after the diagnosis; pathology showed mucosal thickening and cobblestoning confined to the small bowel and proximal colon. Histologic examination revealed inflammation of all layers of the intestinal wall and evidence of granulomas.
In January 1998, the patient developed a small nodule on the distal lateral shaft of the penis. The lesion ulcerated, and biopsies were performed. Histologically, the tissues showed epidermal spongiosis and ulceration with a mixed dermal inflammatory infiltrate containing numerous neutrophils, compatible with PG. Results of cultures and special stains (Gomori methenamine-silver, Brown-Brenn, Fite) were negative. The Crohn’s disease was inactive, evinced by a normal upper GI study in March 1998. However, the penile nodule ulcerated further and then remained under control on a therapeutic regimen of prednisone, amoxicillin, isoniazid, and sulfamethoxasole/trimethoprim.
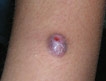
In March 1998, a papule arose on the patient’s right thigh that soon became ulcerated. A biopsy revealed an inflammatory cell infiltrate with giant cells and palisading granulomas consistent with metastatic Crohn’s disease (Figure 1).
In January 1999, the penile lesion became increasingly erythematous, edematous, ulcerative, and painful. Burning developed with urination. The ulcer measured 3x2.5 cm with a depth of 1 cm and involved the distal lateral neck of the glans, corona, and glans penis (Figure 2). A violaceous rolled border was present inferiorly. The ulcer produced a sparse purulent exudate. No inguinal lymphadenopathy was discerned. A urethral fistula was discovered in March 1999.
Due to the refractory nature of the condition, immunosuppressive medications were considered. The patient refused cyclosporine, and mycophenolate mofetil 500 mg twice a day was initiated. The purulent drainage, urine leakage, pain, and induration experienced by the patient decreased in 3 weeks. Complete blood count, differential blood count, and electrolyte count test results were unremarkable. A second lesion arose on the left corona, and the mycophenolate mofetil was increased to 1 gm twice a day. Inflammation and pain again subsided.
With progression of the disease process and development of a fistula, characteristic of Crohn’s disease, a partial penectomy was subsequently performed, leading to resolution of the disease process.
Comment
Pyoderma Gangrenosum—PG presents as tender erythematous vesicles, pustules, or nodules that may ulcerate. The 4 types of PG recognized are vegetative, ulcerative, pustular, and bullous.8 Ulcerative PG begins as tender pustules that rapidly enlarge. Mature lesions have characteristic blue, rolled, undermined borders and are frequently surrounded by erythema. Classic ulcers are aseptic, though superinfection may occur. Healing results in an atrophic cribriform scar. The trunk and lower extremities are most frequently affected,4 but lesions also may occur on the upper extremities, head, and neck. Trauma and cutaneous compromise, including surgery, may precipitate or exacerbate lesions (pathergy phenomenon).
IBD is present in up to one third of patients with ulcerative PG.4,9 Conversely, PG occurs in 1.5% to 5.0% of patients with IBD.4 Ulcerative PG may occur before, after, or concurrently with IBD, and the condition typically demonstrates an independent course.8
A diagnosis of PG is made with clinical and pathologic correlation because the histologic features are not specific. In advanced lesions, histology shows epidermal necrosis and a mixed dermal infiltrate with prominent neutrophils. Late-stage PG can have a mononuclear cell infiltrate and scarring but little or no evidence of granulomas.10 Excluding other conditions is important (see differential diagnosis). Early recognition may avert surgical debridement, which may lead to increased tissue loss and disease progression (pathergy).11-14
Eight cases of penile PG have been reported (Table).11-18 The 2 associated diseases, chronic lymphocytic leukemia12 and ulcerative colitis,13 were present in 2 cases. The scrotum was affected in 4 cases (50%).12-15 One case had perianal involvement,13 and one case had a urethral fistula.16 It is likely the latter case was incorrectly diagnosed because fistula formation is a feature of Crohn’s disease, not PG.
Crohn’s Disease—Crohn’s disease infrequently involves sites outside the GI tract.19 Although oral,31 perineal, and perianal involvement in Crohn’s disease has been described, involvement of sites noncontiguous with the GI tract is rare.
Fourteen patients (including the present case) of CCD with penile involvement have been reported (Table).19-30 Eleven patients (79%) had concomitant perineal or perianal involvement; 8 patients (57%) noted fistula or sinus formation; 5 patients (36%) experienced cutaneous abscesses; and 2 patients (14%) had scrotal lesions.
Fistulas and perineal ulcerations are frequent complications (7/11 or 64%).32 CCD most commonly affects the lower extremities; however, genital, abdominal, and facial involvement also have been reported. Although Crohn’s disease frequently involves the small bowel exclusively (40%), all documented cases of CCD have arisen in patients with disease of the colon or rectum.32 A temporal correlation between the severity of bowel involvement and the presence of cutaneous lesions has not been observed.32
Histopathology results reveal a dermal infiltrate with noncaseating granulomas formed by aggregating epithelioid histiocytes and multinucleated giant cells. Lymphocytes and plasma cells often are present. Involvement of the fat produces a granulomatous panniculitis.33
The cause of CCD is unknown. Two theories have been proposed: CCD is a form of granulomatous vasculitis precipitated by sensitized T-lymphocytes reacting to a circulating antigen,34,35 or CCD may represent a granulomatous perivasculitis with inflammatory cells responding to cutaneous antigens.36 Immunofluorescence studies have suggested a T-lymphocyte–mediated type IV hypersensitivity reaction.20
Conclusion
Differentiating Crohn’s disease from PG may be difficult in sites that are potentially contiguous with the GI tract. Histologically, fibrinoid necrosis of dermal vessels may be present in both PG and CCD. However, CCD is more likely to demonstrate a granulomatous reaction as opposed to the sterile pyodermatous reaction seen in PG. CCD characteristically demonstrates persistent cutaneous fistulas and sinuses despite successful therapy to halt the inflammatory reaction in the bowel. Patients with CCD tend to be younger than patients with PG and have anal, perineal, or perianal involvement. Recurrent or persistent penile ulcers should be cultured for opportunistic pathogens. A biopsy should be performed to rule out other causes and to help differentiate these entities.
- Stolman LP, Rosenthal D, Yaworsky R, et al. Pyoderma gangrenosum and rheumatoid arthritis. Arch Dermatol. 1975;111:1020-1023.
- Byrne JPH, Hewitt M, Summerly R. Pyoderma gangrenosum associated with active chronic hepatitis: report of two cases. Arch Dermatol. 1976;112:1297-1301.
- Tay CH. Pyoderma gangrenosum and leukemia. Arch Dermatol. 1973;108:580-581.
- Powell FC, Schroeter AL, Su WPD, et al. Pyoderma gangrenosum: a review of 86 patients. Q J Med. 1985;55:173-186.
- Ko CB, Walton S, Wyatt EH. Pyoderma gangrenosum: associations revisited. Int J Dermatol. 1992;131:574-577.
- Hickman JG, Lazarus GS. Pyoderma gangrenosum: a reappraisal of associated systemic diseases. Br J Dermatol. 1980;102:235-237.
- Johnson RB, Lazarus GS. Pulse therapy: therapeutic efficacy in the treatment of pyoderma gangrenosum. Arch Dermatol. 1982;118:76-84.
- Powell FC, Su WPD, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Von Den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
- Sanders S, Tahan SR, Kwan T, et al. Giant cells in pyoderma gangrenosum. J Cutan Pathol. 2001;28:97-100.
- Sanusi ID, Gonzalez E, Venable DD. Pyoderma gangrenosum of penile and scrotal skin. J Urol. 1982;127:547-549.
- Harto A, Sanz-Gadea G, Vives R, et al. Pioderma gangrenoso en pene. Actas Urol Esp. 1985;9:263-266.
- Baskin LS, Dixon C, Stoller ML, et al. Pyoderma gangrenosum presenting as Fournier’s gangrene. J Urol. 1990;144:984-986.
- Chow RKP, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol. 1996;34:1046-1060.
- Wahba A, Cohen HA. Herpes simplex virus isolation from pyoderma gangrenosum lesions in a patient with chronic lymphatic leukaemia. Dermatologica. 1979;158:373-378.
- Farrell AM, Black MM, Bracka A, et al. Pyoderma gangrenosum of the penis. Br J Dermatol. 1998;138:337-340.
- Sanchez MH, Sanchez SR, del Cerro Heredero M, et al. Pyoderma gangrenosum of penile skin. Int J Dermatol. 1997;36:638-639.
- Soto LD. Diaminodiphenylsulfone and steroids in the treatment of pyoderma gangrenosum. Int J Derm. 1970;9:293-300.
- Mountain JC. Cutaneous ulceration in Crohn’s disease. Gut. 1970;11:18-26.
- Dantzig PI. Pyoderma gangrenosum. New Engl J Med. 1975;292:47-48.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Atherton DJ, Massam M, Wells RS, et al. Genital Crohn’s disease in a 6-year old boy. Br Med J. 1978;1:552.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Levine N, Bangert J. Cutaneous granulomatosis in Crohn’s disease. Arch Dermatol. 1982;118:1006-1009.
- Sumathipala AHT. Penile ulcer in Crohn’s disease. J R Soc Med. 1984;77:966-967.
Pyoderma gangrenosum (PG) is an idiopathic, progressive, ulcerative skin disease. Half of all cases are associated with identifiable systemic diseases including arthritis,1 hepatitis,2 blood dyscrasia,3 and immunosuppression.4-6 PG is most frequently associated with inflammatory bowel disease (IBD).4,7
Crohn’s disease (regional enteritis) is a chronic, granulomatous, inflammatory bowel disease that may affect the entire gastrointestinal (GI) tract, as well as the skin. Draining sinuses or fistulas may form between the GI tract and contiguous skin. Cutaneous manifestations associated with regional enteritis include PG, erythema nodosum, and metastatic Crohn’s disease in noncontiguous skin.
There are several differential diagnoses that should be considered: (1) infections, such as Fournier’s gangrene, bacterial pyoderma, deep fungal infection, chronic herpetic ulcer, anaerobic erosive balanitis in uncircumcised men, mycobacterial infection, gummatous syphilis, and amebiasis cutis; (2) neoplastic conditions, such as squamous cell carcinoma, metastatic gastrointestinal or genitourinary carcinoma, extramammary Paget’s disease; and (3) miscellaneous conditions, such as metastatic Crohn’s disease, PG, erosive lichen planus, halogenodermas, necrotizing vasculitis, antiphospholipid syndrome, brown recluse spider bite, and factitial dermatitis. Differentiating between PG and cutaneous Crohn’s disease (CCD) may be difficult, as the clinical and pathologic features often are similar.
CASE REPORT
A 52-year-old circumcised white man was diagnosed with Crohn’s disease in 1974. The condition periodically was responsive to sulfasalazine, metronidazole, and prednisone. A small bowel resection was performed 1 and 10 years after the diagnosis; pathology showed mucosal thickening and cobblestoning confined to the small bowel and proximal colon. Histologic examination revealed inflammation of all layers of the intestinal wall and evidence of granulomas.
In January 1998, the patient developed a small nodule on the distal lateral shaft of the penis. The lesion ulcerated, and biopsies were performed. Histologically, the tissues showed epidermal spongiosis and ulceration with a mixed dermal inflammatory infiltrate containing numerous neutrophils, compatible with PG. Results of cultures and special stains (Gomori methenamine-silver, Brown-Brenn, Fite) were negative. The Crohn’s disease was inactive, evinced by a normal upper GI study in March 1998. However, the penile nodule ulcerated further and then remained under control on a therapeutic regimen of prednisone, amoxicillin, isoniazid, and sulfamethoxasole/trimethoprim.
In March 1998, a papule arose on the patient’s right thigh that soon became ulcerated. A biopsy revealed an inflammatory cell infiltrate with giant cells and palisading granulomas consistent with metastatic Crohn’s disease (Figure 1).
In January 1999, the penile lesion became increasingly erythematous, edematous, ulcerative, and painful. Burning developed with urination. The ulcer measured 3x2.5 cm with a depth of 1 cm and involved the distal lateral neck of the glans, corona, and glans penis (Figure 2). A violaceous rolled border was present inferiorly. The ulcer produced a sparse purulent exudate. No inguinal lymphadenopathy was discerned. A urethral fistula was discovered in March 1999.
Due to the refractory nature of the condition, immunosuppressive medications were considered. The patient refused cyclosporine, and mycophenolate mofetil 500 mg twice a day was initiated. The purulent drainage, urine leakage, pain, and induration experienced by the patient decreased in 3 weeks. Complete blood count, differential blood count, and electrolyte count test results were unremarkable. A second lesion arose on the left corona, and the mycophenolate mofetil was increased to 1 gm twice a day. Inflammation and pain again subsided.
With progression of the disease process and development of a fistula, characteristic of Crohn’s disease, a partial penectomy was subsequently performed, leading to resolution of the disease process.
Comment
Pyoderma Gangrenosum—PG presents as tender erythematous vesicles, pustules, or nodules that may ulcerate. The 4 types of PG recognized are vegetative, ulcerative, pustular, and bullous.8 Ulcerative PG begins as tender pustules that rapidly enlarge. Mature lesions have characteristic blue, rolled, undermined borders and are frequently surrounded by erythema. Classic ulcers are aseptic, though superinfection may occur. Healing results in an atrophic cribriform scar. The trunk and lower extremities are most frequently affected,4 but lesions also may occur on the upper extremities, head, and neck. Trauma and cutaneous compromise, including surgery, may precipitate or exacerbate lesions (pathergy phenomenon).
IBD is present in up to one third of patients with ulcerative PG.4,9 Conversely, PG occurs in 1.5% to 5.0% of patients with IBD.4 Ulcerative PG may occur before, after, or concurrently with IBD, and the condition typically demonstrates an independent course.8
A diagnosis of PG is made with clinical and pathologic correlation because the histologic features are not specific. In advanced lesions, histology shows epidermal necrosis and a mixed dermal infiltrate with prominent neutrophils. Late-stage PG can have a mononuclear cell infiltrate and scarring but little or no evidence of granulomas.10 Excluding other conditions is important (see differential diagnosis). Early recognition may avert surgical debridement, which may lead to increased tissue loss and disease progression (pathergy).11-14
Eight cases of penile PG have been reported (Table).11-18 The 2 associated diseases, chronic lymphocytic leukemia12 and ulcerative colitis,13 were present in 2 cases. The scrotum was affected in 4 cases (50%).12-15 One case had perianal involvement,13 and one case had a urethral fistula.16 It is likely the latter case was incorrectly diagnosed because fistula formation is a feature of Crohn’s disease, not PG.
Crohn’s Disease—Crohn’s disease infrequently involves sites outside the GI tract.19 Although oral,31 perineal, and perianal involvement in Crohn’s disease has been described, involvement of sites noncontiguous with the GI tract is rare.
Fourteen patients (including the present case) of CCD with penile involvement have been reported (Table).19-30 Eleven patients (79%) had concomitant perineal or perianal involvement; 8 patients (57%) noted fistula or sinus formation; 5 patients (36%) experienced cutaneous abscesses; and 2 patients (14%) had scrotal lesions.
Fistulas and perineal ulcerations are frequent complications (7/11 or 64%).32 CCD most commonly affects the lower extremities; however, genital, abdominal, and facial involvement also have been reported. Although Crohn’s disease frequently involves the small bowel exclusively (40%), all documented cases of CCD have arisen in patients with disease of the colon or rectum.32 A temporal correlation between the severity of bowel involvement and the presence of cutaneous lesions has not been observed.32
Histopathology results reveal a dermal infiltrate with noncaseating granulomas formed by aggregating epithelioid histiocytes and multinucleated giant cells. Lymphocytes and plasma cells often are present. Involvement of the fat produces a granulomatous panniculitis.33
The cause of CCD is unknown. Two theories have been proposed: CCD is a form of granulomatous vasculitis precipitated by sensitized T-lymphocytes reacting to a circulating antigen,34,35 or CCD may represent a granulomatous perivasculitis with inflammatory cells responding to cutaneous antigens.36 Immunofluorescence studies have suggested a T-lymphocyte–mediated type IV hypersensitivity reaction.20
Conclusion
Differentiating Crohn’s disease from PG may be difficult in sites that are potentially contiguous with the GI tract. Histologically, fibrinoid necrosis of dermal vessels may be present in both PG and CCD. However, CCD is more likely to demonstrate a granulomatous reaction as opposed to the sterile pyodermatous reaction seen in PG. CCD characteristically demonstrates persistent cutaneous fistulas and sinuses despite successful therapy to halt the inflammatory reaction in the bowel. Patients with CCD tend to be younger than patients with PG and have anal, perineal, or perianal involvement. Recurrent or persistent penile ulcers should be cultured for opportunistic pathogens. A biopsy should be performed to rule out other causes and to help differentiate these entities.
Pyoderma gangrenosum (PG) is an idiopathic, progressive, ulcerative skin disease. Half of all cases are associated with identifiable systemic diseases including arthritis,1 hepatitis,2 blood dyscrasia,3 and immunosuppression.4-6 PG is most frequently associated with inflammatory bowel disease (IBD).4,7
Crohn’s disease (regional enteritis) is a chronic, granulomatous, inflammatory bowel disease that may affect the entire gastrointestinal (GI) tract, as well as the skin. Draining sinuses or fistulas may form between the GI tract and contiguous skin. Cutaneous manifestations associated with regional enteritis include PG, erythema nodosum, and metastatic Crohn’s disease in noncontiguous skin.
There are several differential diagnoses that should be considered: (1) infections, such as Fournier’s gangrene, bacterial pyoderma, deep fungal infection, chronic herpetic ulcer, anaerobic erosive balanitis in uncircumcised men, mycobacterial infection, gummatous syphilis, and amebiasis cutis; (2) neoplastic conditions, such as squamous cell carcinoma, metastatic gastrointestinal or genitourinary carcinoma, extramammary Paget’s disease; and (3) miscellaneous conditions, such as metastatic Crohn’s disease, PG, erosive lichen planus, halogenodermas, necrotizing vasculitis, antiphospholipid syndrome, brown recluse spider bite, and factitial dermatitis. Differentiating between PG and cutaneous Crohn’s disease (CCD) may be difficult, as the clinical and pathologic features often are similar.
CASE REPORT
A 52-year-old circumcised white man was diagnosed with Crohn’s disease in 1974. The condition periodically was responsive to sulfasalazine, metronidazole, and prednisone. A small bowel resection was performed 1 and 10 years after the diagnosis; pathology showed mucosal thickening and cobblestoning confined to the small bowel and proximal colon. Histologic examination revealed inflammation of all layers of the intestinal wall and evidence of granulomas.
In January 1998, the patient developed a small nodule on the distal lateral shaft of the penis. The lesion ulcerated, and biopsies were performed. Histologically, the tissues showed epidermal spongiosis and ulceration with a mixed dermal inflammatory infiltrate containing numerous neutrophils, compatible with PG. Results of cultures and special stains (Gomori methenamine-silver, Brown-Brenn, Fite) were negative. The Crohn’s disease was inactive, evinced by a normal upper GI study in March 1998. However, the penile nodule ulcerated further and then remained under control on a therapeutic regimen of prednisone, amoxicillin, isoniazid, and sulfamethoxasole/trimethoprim.
In March 1998, a papule arose on the patient’s right thigh that soon became ulcerated. A biopsy revealed an inflammatory cell infiltrate with giant cells and palisading granulomas consistent with metastatic Crohn’s disease (Figure 1).
In January 1999, the penile lesion became increasingly erythematous, edematous, ulcerative, and painful. Burning developed with urination. The ulcer measured 3x2.5 cm with a depth of 1 cm and involved the distal lateral neck of the glans, corona, and glans penis (Figure 2). A violaceous rolled border was present inferiorly. The ulcer produced a sparse purulent exudate. No inguinal lymphadenopathy was discerned. A urethral fistula was discovered in March 1999.
Due to the refractory nature of the condition, immunosuppressive medications were considered. The patient refused cyclosporine, and mycophenolate mofetil 500 mg twice a day was initiated. The purulent drainage, urine leakage, pain, and induration experienced by the patient decreased in 3 weeks. Complete blood count, differential blood count, and electrolyte count test results were unremarkable. A second lesion arose on the left corona, and the mycophenolate mofetil was increased to 1 gm twice a day. Inflammation and pain again subsided.
With progression of the disease process and development of a fistula, characteristic of Crohn’s disease, a partial penectomy was subsequently performed, leading to resolution of the disease process.
Comment
Pyoderma Gangrenosum—PG presents as tender erythematous vesicles, pustules, or nodules that may ulcerate. The 4 types of PG recognized are vegetative, ulcerative, pustular, and bullous.8 Ulcerative PG begins as tender pustules that rapidly enlarge. Mature lesions have characteristic blue, rolled, undermined borders and are frequently surrounded by erythema. Classic ulcers are aseptic, though superinfection may occur. Healing results in an atrophic cribriform scar. The trunk and lower extremities are most frequently affected,4 but lesions also may occur on the upper extremities, head, and neck. Trauma and cutaneous compromise, including surgery, may precipitate or exacerbate lesions (pathergy phenomenon).
IBD is present in up to one third of patients with ulcerative PG.4,9 Conversely, PG occurs in 1.5% to 5.0% of patients with IBD.4 Ulcerative PG may occur before, after, or concurrently with IBD, and the condition typically demonstrates an independent course.8
A diagnosis of PG is made with clinical and pathologic correlation because the histologic features are not specific. In advanced lesions, histology shows epidermal necrosis and a mixed dermal infiltrate with prominent neutrophils. Late-stage PG can have a mononuclear cell infiltrate and scarring but little or no evidence of granulomas.10 Excluding other conditions is important (see differential diagnosis). Early recognition may avert surgical debridement, which may lead to increased tissue loss and disease progression (pathergy).11-14
Eight cases of penile PG have been reported (Table).11-18 The 2 associated diseases, chronic lymphocytic leukemia12 and ulcerative colitis,13 were present in 2 cases. The scrotum was affected in 4 cases (50%).12-15 One case had perianal involvement,13 and one case had a urethral fistula.16 It is likely the latter case was incorrectly diagnosed because fistula formation is a feature of Crohn’s disease, not PG.
Crohn’s Disease—Crohn’s disease infrequently involves sites outside the GI tract.19 Although oral,31 perineal, and perianal involvement in Crohn’s disease has been described, involvement of sites noncontiguous with the GI tract is rare.
Fourteen patients (including the present case) of CCD with penile involvement have been reported (Table).19-30 Eleven patients (79%) had concomitant perineal or perianal involvement; 8 patients (57%) noted fistula or sinus formation; 5 patients (36%) experienced cutaneous abscesses; and 2 patients (14%) had scrotal lesions.
Fistulas and perineal ulcerations are frequent complications (7/11 or 64%).32 CCD most commonly affects the lower extremities; however, genital, abdominal, and facial involvement also have been reported. Although Crohn’s disease frequently involves the small bowel exclusively (40%), all documented cases of CCD have arisen in patients with disease of the colon or rectum.32 A temporal correlation between the severity of bowel involvement and the presence of cutaneous lesions has not been observed.32
Histopathology results reveal a dermal infiltrate with noncaseating granulomas formed by aggregating epithelioid histiocytes and multinucleated giant cells. Lymphocytes and plasma cells often are present. Involvement of the fat produces a granulomatous panniculitis.33
The cause of CCD is unknown. Two theories have been proposed: CCD is a form of granulomatous vasculitis precipitated by sensitized T-lymphocytes reacting to a circulating antigen,34,35 or CCD may represent a granulomatous perivasculitis with inflammatory cells responding to cutaneous antigens.36 Immunofluorescence studies have suggested a T-lymphocyte–mediated type IV hypersensitivity reaction.20
Conclusion
Differentiating Crohn’s disease from PG may be difficult in sites that are potentially contiguous with the GI tract. Histologically, fibrinoid necrosis of dermal vessels may be present in both PG and CCD. However, CCD is more likely to demonstrate a granulomatous reaction as opposed to the sterile pyodermatous reaction seen in PG. CCD characteristically demonstrates persistent cutaneous fistulas and sinuses despite successful therapy to halt the inflammatory reaction in the bowel. Patients with CCD tend to be younger than patients with PG and have anal, perineal, or perianal involvement. Recurrent or persistent penile ulcers should be cultured for opportunistic pathogens. A biopsy should be performed to rule out other causes and to help differentiate these entities.
- Stolman LP, Rosenthal D, Yaworsky R, et al. Pyoderma gangrenosum and rheumatoid arthritis. Arch Dermatol. 1975;111:1020-1023.
- Byrne JPH, Hewitt M, Summerly R. Pyoderma gangrenosum associated with active chronic hepatitis: report of two cases. Arch Dermatol. 1976;112:1297-1301.
- Tay CH. Pyoderma gangrenosum and leukemia. Arch Dermatol. 1973;108:580-581.
- Powell FC, Schroeter AL, Su WPD, et al. Pyoderma gangrenosum: a review of 86 patients. Q J Med. 1985;55:173-186.
- Ko CB, Walton S, Wyatt EH. Pyoderma gangrenosum: associations revisited. Int J Dermatol. 1992;131:574-577.
- Hickman JG, Lazarus GS. Pyoderma gangrenosum: a reappraisal of associated systemic diseases. Br J Dermatol. 1980;102:235-237.
- Johnson RB, Lazarus GS. Pulse therapy: therapeutic efficacy in the treatment of pyoderma gangrenosum. Arch Dermatol. 1982;118:76-84.
- Powell FC, Su WPD, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Von Den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
- Sanders S, Tahan SR, Kwan T, et al. Giant cells in pyoderma gangrenosum. J Cutan Pathol. 2001;28:97-100.
- Sanusi ID, Gonzalez E, Venable DD. Pyoderma gangrenosum of penile and scrotal skin. J Urol. 1982;127:547-549.
- Harto A, Sanz-Gadea G, Vives R, et al. Pioderma gangrenoso en pene. Actas Urol Esp. 1985;9:263-266.
- Baskin LS, Dixon C, Stoller ML, et al. Pyoderma gangrenosum presenting as Fournier’s gangrene. J Urol. 1990;144:984-986.
- Chow RKP, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol. 1996;34:1046-1060.
- Wahba A, Cohen HA. Herpes simplex virus isolation from pyoderma gangrenosum lesions in a patient with chronic lymphatic leukaemia. Dermatologica. 1979;158:373-378.
- Farrell AM, Black MM, Bracka A, et al. Pyoderma gangrenosum of the penis. Br J Dermatol. 1998;138:337-340.
- Sanchez MH, Sanchez SR, del Cerro Heredero M, et al. Pyoderma gangrenosum of penile skin. Int J Dermatol. 1997;36:638-639.
- Soto LD. Diaminodiphenylsulfone and steroids in the treatment of pyoderma gangrenosum. Int J Derm. 1970;9:293-300.
- Mountain JC. Cutaneous ulceration in Crohn’s disease. Gut. 1970;11:18-26.
- Dantzig PI. Pyoderma gangrenosum. New Engl J Med. 1975;292:47-48.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Atherton DJ, Massam M, Wells RS, et al. Genital Crohn’s disease in a 6-year old boy. Br Med J. 1978;1:552.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Levine N, Bangert J. Cutaneous granulomatosis in Crohn’s disease. Arch Dermatol. 1982;118:1006-1009.
- Sumathipala AHT. Penile ulcer in Crohn’s disease. J R Soc Med. 1984;77:966-967.
- Stolman LP, Rosenthal D, Yaworsky R, et al. Pyoderma gangrenosum and rheumatoid arthritis. Arch Dermatol. 1975;111:1020-1023.
- Byrne JPH, Hewitt M, Summerly R. Pyoderma gangrenosum associated with active chronic hepatitis: report of two cases. Arch Dermatol. 1976;112:1297-1301.
- Tay CH. Pyoderma gangrenosum and leukemia. Arch Dermatol. 1973;108:580-581.
- Powell FC, Schroeter AL, Su WPD, et al. Pyoderma gangrenosum: a review of 86 patients. Q J Med. 1985;55:173-186.
- Ko CB, Walton S, Wyatt EH. Pyoderma gangrenosum: associations revisited. Int J Dermatol. 1992;131:574-577.
- Hickman JG, Lazarus GS. Pyoderma gangrenosum: a reappraisal of associated systemic diseases. Br J Dermatol. 1980;102:235-237.
- Johnson RB, Lazarus GS. Pulse therapy: therapeutic efficacy in the treatment of pyoderma gangrenosum. Arch Dermatol. 1982;118:76-84.
- Powell FC, Su WPD, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Von Den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
- Sanders S, Tahan SR, Kwan T, et al. Giant cells in pyoderma gangrenosum. J Cutan Pathol. 2001;28:97-100.
- Sanusi ID, Gonzalez E, Venable DD. Pyoderma gangrenosum of penile and scrotal skin. J Urol. 1982;127:547-549.
- Harto A, Sanz-Gadea G, Vives R, et al. Pioderma gangrenoso en pene. Actas Urol Esp. 1985;9:263-266.
- Baskin LS, Dixon C, Stoller ML, et al. Pyoderma gangrenosum presenting as Fournier’s gangrene. J Urol. 1990;144:984-986.
- Chow RKP, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol. 1996;34:1046-1060.
- Wahba A, Cohen HA. Herpes simplex virus isolation from pyoderma gangrenosum lesions in a patient with chronic lymphatic leukaemia. Dermatologica. 1979;158:373-378.
- Farrell AM, Black MM, Bracka A, et al. Pyoderma gangrenosum of the penis. Br J Dermatol. 1998;138:337-340.
- Sanchez MH, Sanchez SR, del Cerro Heredero M, et al. Pyoderma gangrenosum of penile skin. Int J Dermatol. 1997;36:638-639.
- Soto LD. Diaminodiphenylsulfone and steroids in the treatment of pyoderma gangrenosum. Int J Derm. 1970;9:293-300.
- Mountain JC. Cutaneous ulceration in Crohn’s disease. Gut. 1970;11:18-26.
- Dantzig PI. Pyoderma gangrenosum. New Engl J Med. 1975;292:47-48.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Atherton DJ, Massam M, Wells RS, et al. Genital Crohn’s disease in a 6-year old boy. Br Med J. 1978;1:552.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Levine N, Bangert J. Cutaneous granulomatosis in Crohn’s disease. Arch Dermatol. 1982;118:1006-1009.
- Sumathipala AHT. Penile ulcer in Crohn’s disease. J R Soc Med. 1984;77:966-967.