User login
Buried Deep
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
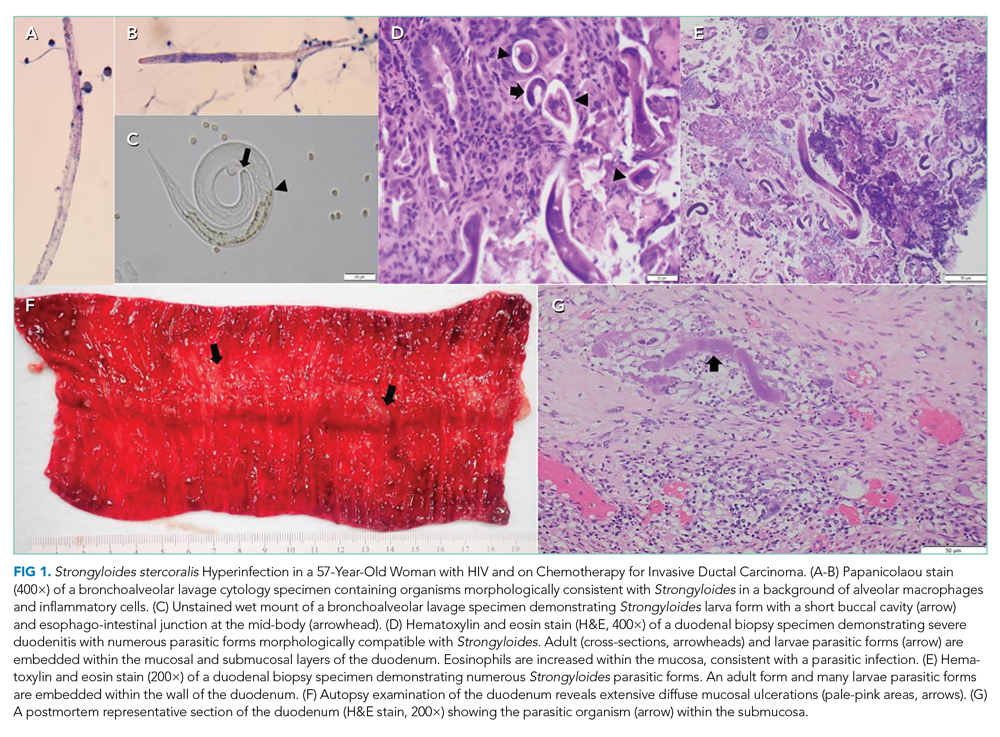
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
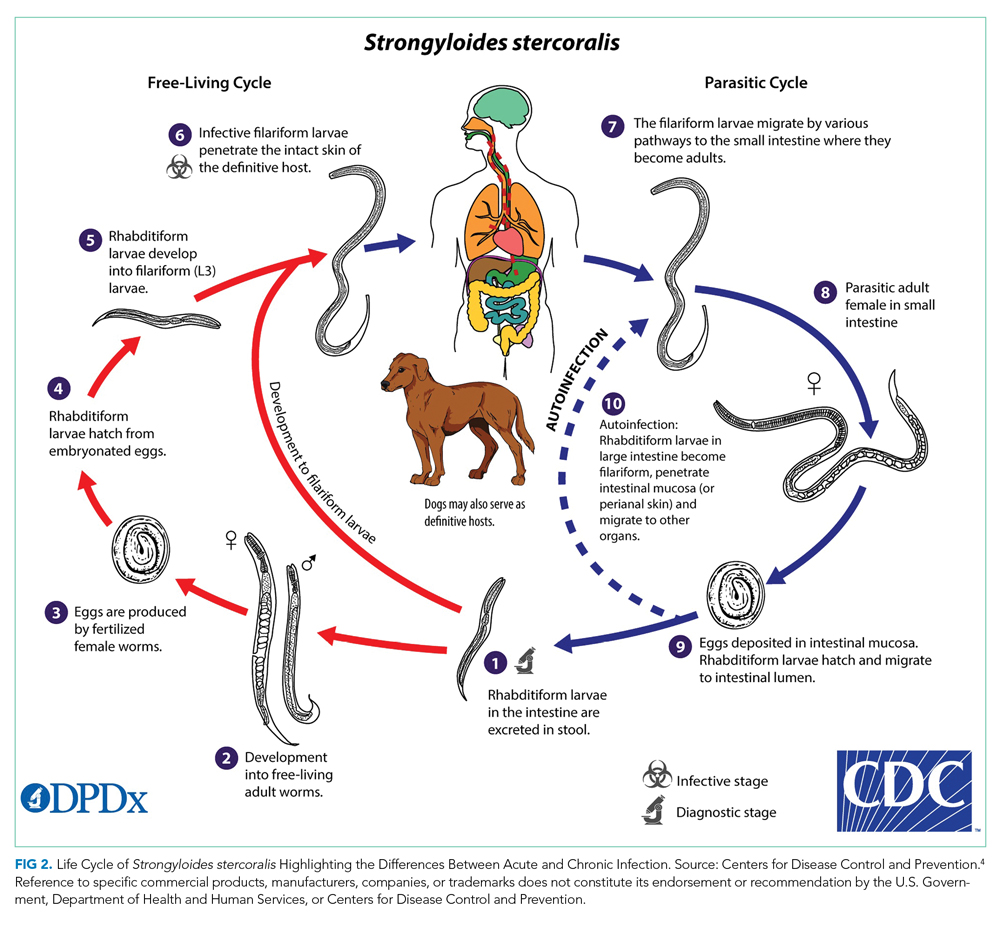
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
© 2021 Society of Hospital Medicine
All in the Stream
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
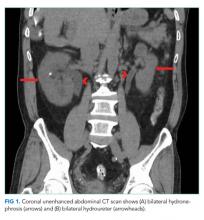
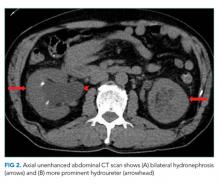
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
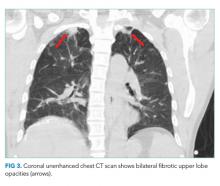
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
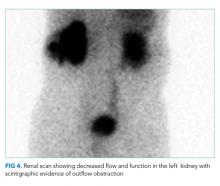
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
© 2019 Society of Hospital Medicine
Betting the Farm
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.